Abstract
Phenazines represent a large group of nitrogen-containing heterocyclic compounds produced by the diverse group of bacteria including actinobacteria. In this study, a total of 197 actinobacterial strains were isolated from seven different marine sponge species in the South China Sea using five different culture media. Eighty-seven morphologically different actinobacterial strains were selected and grouped into 13 genera, including Actinoalloteichus, Kocuria, Micrococcus, Micromonospora, Mycobacterium, Nocardiopsis, Prauserella, Rhodococcus, Saccharopolyspora, Salinispora, Serinicoccus, and Streptomyces by the phylogenetic analysis of 16S rRNA gene. Based on the screening of phzE genes, ten strains, including five Streptomyces, two Nocardiopsis, one Salinispora, one Micrococcus, and one Serinicoccus were found to be potential for phenazine production. The level of phzE gene expression was highly expressed in Nocardiopsis sp. 13-33-15, 13-12-13, and Serinicoccus sp. 13-12-4 on the fifth day of fermentation. Finally, 1,6-dihydroxy phenazine (1) from Nocardiopsis sp. 13-33-15 and 13-12-13, and 1,6-dimethoxy phenazine (2) from Nocardiopsis sp. 13-33-15 were isolated and identified successfully based on ESI-MS and NMR analysis. The compounds 1 and 2 showed antibacterial activity against Bacillus mycoides SJ14, Staphylococcus aureus SJ51, Escherichia coli SJ42, and Micrococcus luteus SJ47. This study suggests that the integrated approach of gene screening and chemical analysis is an effective strategy to find the target compounds and lays the basis for the production of phenazine from the sponge-associated actinobacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine sponges (phylum Porifera), which are evolutionarily olden metazoans, multicellular invertebrate stalkless filter-feeders, represent a major component in benthic populations of the global oceans. Marine sponges are rich sources of novel compounds, which have multiple bioactivities with medicinal and pharmaceutical applications (Blunt et al. 2012). Sponges are an important resource for the marine drug development. Presently, five compounds or semisynthetic analogues produced from the sponges have been concluded as medicines, and 13 compounds are in clinical trials for several purposes, mostly as anticancer drugs, and 100 compounds are included in the assessment of preclinical trials (Mayer et al. 2010).
The interior of sponge species contains abundant microbial populations together with archaea, bacteria, fungi, and viruses (Fuerst 2014). Culture-dependent and culture-independent molecular approaches have demonstrated that at least 47 bacterial phyla and candidate phyla are associated with the marine sponges (Reveillaud et al. 2014). The majority of the phyla belong to Acidobacteria, Actinobacteria, Chloroflexi, Nitrospira, Cyanobacteria, Bacteriodetes, Gemmatimonadetes, Planctomycetes, Spirochaetes, and Proteobacteria (Webster and Taylor 2012). The microbes associated with marine sponges have the number of responsibilities such as nutrient acquirement, processing of metabolic waste, and the production of secondary metabolites. These microbes also mediate nutrient cycles such as carbon, nitrogen, and sulfur within the sponge tissues (Taylor et al. 2007). Additionally, the microbial mediated chemical defenses have also been detected within the sponges (Hochmuth et al. 2010). In a few instances, the chemicals produced by the sponge-associated microbes have been identified to be formerly produced by the host sponge (Konig et al. 2006).
Actinobacteria derived from the marine organisms are believed to be good sources for isolating new bioactive compounds (Zotchev 2012; Valliappan et al. 2014). The actinobacteria associated with marine sponges have attracted more attention due to their biotechnological potential (Montalvo et al. 2005; Hentschel et al. 2006; Piel 2006). The sponge-associated actinobacteria hold the pharmic activities such as antibacterial, antifungal, antiparasitic, antimalarial, immunomodulatory, anti-inflammatory, antioxidant, and anticancer, the active compounds are categorized into the chemical groups such as polyketides, peptides, alkaloids, isoprenoids, phenazines, indolocarbazoles, fatty acids, sterols, and terpenes (Abdelmohsen et al. 2014a; Valliappan et al. 2014).
Phenazines represent a large group of structurally diverse nitrogen-containing heterocyclic compounds with biological activities (Laursen and Nielsen 2004; Pierson and Pierson 2010; Gao et al. 2012; Mavrodi et al. 2013), such as antibiotic (Abken et al. 1998), antimalarial (Makgatho et al. 2000; de Andrade-Neto et al. 2004), antibacterial (Laursen and Nielsen 2004; Liu et al. 2007), and anticancer activities (Abdelfattah et al. 2011a, b; Gao et al. 2012). Additionally, phenazine is a small molecule, and it can invade easily into the tissues and organs and influence the multiple targets (Gao et al. 2012). Therefore, phenazine might be a potential compound for chemoprevention and has a possible role in both clinical and industrial processes.
Marine actinobacteria are known to produce phenazines with medicinal applications (Gao et al. 2012). The structurally different types of phenazines have been identified from the sponge-associated actinobacteria, including phenzine 1,6-dicarboxilic acid, streptophenazine A-H, and JBIR 46-48 (Khan et al. 2010; Izumikawa et al. 2010; Mitova et al. 2008; Schneemann et al. 2011). Therefore, it is the great value to isolate actinobacteria from the sponges to produce phenazines with biological activity. Since, only a few percentage of the actinobacteria are able to produce phenazine under the fermentation condition, the selection of positive phenazine producer based on the chemical screening is difficult and time consuming (Izumikawa et al. 2010). Thus, genetic screening of actinobacteria using a primer targeting the fragment of phzE gene (Schneemann et al. 2011) is a promising strategy to identify the phenazine-producing actinobacteria. Herein, we reported genetic approach at both DNA and RNA levels to detect the potential phenazine producers from the South China Sea sponge-associated actinobacteria, and finally test their ability for the production of phenazine by fermentation and chemical analysis under the guidance of phzE gene sequencing.
Materials and methods
Sample collection
Seven visually healthy sponges (Theonella swinhoei, Dysidea arenaria, Agelas sceptrum, Ircinia sp., Haliclona simulans, Smenospongia aurea, and Iotrochota sp.) were collected by SCUBA diving within a 15-m radius at ca. 10-m depths near Yongxing Island (112° 20′ E, 16° 50′ N) of the South China Sea (112° 20′ E, 16° 50′ N) in August 2013 and identified based on the 28S ribosomal ribonucleic acid sequencing (four species of sponges) and sponge morphology (three species of sponges) (Hooper and Van Soest 2002). Sponges were kept in plastic bags containing seawater, transported to the laboratory on ice, and immediately stored at −80 °C.
Isolation of actinobacteria
To remove the loosely attached bacteria from the surrounding seawater, sponge samples were thoroughly rinsed with sterile artificial seawater (ASW) (Sun et al. 2010) for at least three times, followed by 1 g of sponge material sliced into pieces about 1 cm3 and then homogenized in sterile mortars. Homogenates were pretreated by incubating in a water bath at 55 °C for 5 min, serially diluted and plated in triplicate on agar plates. Five different types of media including M1 (Mincer et al. 2002), M2 (Zhang et al. 2013), M5 (Zhang et al. 2013), Kusters (Poongodi et al. 2014), and arginine glycerin agar (Zhang et al. 2014) were used to isolate actinobacteria. All media were prepared in ASW and supplemented with nalidixic acid (25/mg) and nystatin (50/mg). The inoculated plates were incubated at 28 °C for 2–6 weeks. Colonies were counted and representative of distinct colonies were picked and restreaked on yeast extract-malt extract agar (ISP 2) (Shirling and Gottlieb 1966). Nocardiopsis sp. 13-33-15, Nocardiopsis sp. 13-12-13, and Serinicoccus sp. 13-12-4 were deposited in China Center for Type Culture Collection under the number CCTCC AA 2014034, CCTCC AA 2014033, and CCTCC AB 2014234, respectively.
Molecular identification and phylogenetic analysis
Eighty-seven actinobacterial strains were selected based on their source sponge, colony morphology, aerial and substrate mycelia for the molecular identification and phylogenetic analysis. Isolation of genomic DNA, PCR amplification, and sequencing of the 16S ribosomal RNA (rRNA) genes were performed according to Sun et al. (2010). The resulting 16S rRNA gene sequences were evaluated by comparing with those sequences previously submitted in the public databases using NCBI (http://www.ncbi.nlm.nih.gov/) Basic Local Alignment Search Tool (BLAST). The phylogenetic trees were constructed with related sequences retrieved from the public databases using the maximum likelihood algorithm with bootstrap values based on 1000 replications in MEGA version 5.1.
Amplification and identification of the phenazine gene fragments
The phzE gene fragment of phenazine pathway was PCR-amplified using the degenerated primers, phzEf (5′-GAAGGCGCCAACTTCGTYATCAA-3′) and phzEr (5′-GCCYTCGATGAAGTACTCGGTGTG-3′) (Schneemann et al. 2011). The PCR amplification was carried out in a 50-μl reactions containing 20 ng of DNA, 2× master mix, 5 % (v/v) dimethyl sulfoxide, and 0.2-μM concentrations of both primers. The PCR conditions were as follows: initial denaturation at 94 °C for 4 min, followed by 36 cycles of denaturation at 94 °C for 30 s, primer annealing at 54.7 °C for 60 s, and primer extensions at 72 °C for 120 s, followed by a final extension at 72 °C for 420 s was performed. The amplified products were recovered and purified using Agarose Gel DNA extraction kit (CWBio gel extraction kit) following the manufacturer’s instructions.
The PCR products were cloned using the TOPO TA cloning kit (TransGen) according to the manufacturer’s protocol. The positive clones were selected and sequenced using the M13F primer on ABI 3730xl capillary sequencers (Applied Biosystems). Sequences were analyzed using the NCBI BLAST.
Reverse transcription PCR
Total RNA was isolated from the culture of phzE-positive strains, incubated at different days in 28 °C using the CWBio RNA pure plant kit. The reverse transcriptase reactions were carried out at 50 °C for 30 min using 0.2 μg of the total RNA template using CWBio HiFi-Script cDNA kit. The phzE gene from cDNA was amplified according to the method given above.
Fermentation and chemical analysis
Actinobacteria with the expression of phzE genes were inoculated into 20 mL of ISP2 medium in 250-mL Erlenmeyer flasks and incubated at 28 °C on rotary shakers (280 rpm) for 36 h. Each seed culture was aseptically transferred to 1-L Erlenmeyer flasks containing 400 mL of the GYM medium and incubated at 28 °C on rotary shakers (280 rpm) for 7 days. After fermentation, pH of the medium (20 L) was reduced to pH 4 using concentrated HCl and extracted with equal volumes of ethyl acetate for three times. The organic phase was concentrated under reduced pressure to give a crude extract. The crude extract was subjected to semipreparative RP-C18 (X Aqua-C18 5 μm, 10 × 250 mm) HPLC (Agilent Technologies, USA) and eluted with 50 % methanol at a flow rate of 2 mL/min to afford phenazines from 13-12-13 (1) (tR = 30.7 min) and 13-33-15 (1, 2) (tR = 33.2 min; 34.7 min), respectively. Further, the fractions of F1 and F2 were repeatedly applied to semipreparative RP-C18 (Eclipse XDB-C18 5 μm, 4.6 × 150 mm) HPLC and eluted with a linear gradient from 10 to 100 % aqueous CH3CN over the course of 20 min and gave compounds 1 (tR 6.7 min) and 2 (tR 6.17 min).
For LC-QTOF-MS analysis, the methanol solution of compounds 1 and 2 was detected on an ultra-performance liquid and quadrupole time of flight mass spectroscopy (UPLC-QTOF-MS Premier, Waters Corporation, USA). The compounds 1 and 2 were separated on a C18 RP-column (ACQUITY BEH-C18 1.7 μm, 2.1 × 100 mm, Waters Co.), with the linear gradient elution from H2O to 100 % MeCN. Total ion chromatography (TIC) and mass spectrum of selected ion were acquired in positive electrospray ionization mass spectrum (ESI-MS) mode.
In the case of NMR analysis, the compounds 1 and 2 were dissolved in CD3OD and CDCl3, respectively. Proton and carbon nuclear magnetic resonance (1H and 13C NMR) spectrum was recorded on an ADVANCE III 400 spectrometer (400 MHz, Bruker, and 100 MHz, Bruker, respectively).
Antimicrobial tests of phenazines
To test antimicrobial activity, compounds 1 and 2 were suspended in DMSO (1 μg/μL) and 20 μL of the each sample was applied to a paper disk (d = 6 mm). The disks were then placed onto an agar plate inoculated with Bacillus mycoides SJ14, Staphylococcus aureus SJ51, Escherichia coli SJ42, Micrococcus luteus SJ47, Saccharomyces cerevisiae SJ32, and Rhodotorula sp. SJ24. Ampicillin (1 μg/μL) and kanamycin (1 μg/μL) were used as positive controls against the bacteria and yeast.
Nucleotide sequence accession numbers
The GenBank accession numbers obtained for 16S rRNA genes and phzE genes were KM886124 - KM886210 and KM923774 - KM923783, respectively. The GenBank accession numbers obtained for the 28S rRNA gene of sponges were as follows: Theonella swinhoei (13-2, JF506040), Dysidea arenaria (13-3, KJ675585), Ircinia sp. (13-17, KC774023), and Iotrochota sp. (13-36, KC762714).
Results
Diversity of culturable actinobacteria associated with sponges
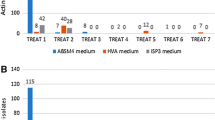
Totally, 197 actinobacterial strains were isolated on a range of selective media (M1, M2, M5, Kusters, and AGA) from the seven sponges. Highest numbers of actinobacterial isolates (16 isolates) were recovered from the M2 for Ircinia sp., whereas only one isolate was recovered from AGA for Haliclona simulans. The actinobacteria isolated from the seven sponge species were significantly different. Ircinia sp. yielded the highest number of isolates (61), followed by Theonella swinhoei (34), Dysidea arenaria (32), Smenospongia aurea (29), Agelas sceptrum (27), Haliclona simulans (15), and Iotrochota sp. (7) (Fig. 1).
According to the actinobacterial colony morphology and the source sponge, 87 strains were selected for 16S rRNA gene sequencing. Based on the BLAST analysis, 56 representative sequences were used to construct the phylogenetic trees. Sequence comparison with the previously submitted sequences in NCBI GenBank database revealed that these strains were affiliated with eight families and 13 genera. The highest numbers of isolates were found to be identified as genus Salinispora, which is a marine obligate actinobacterium (26), followed by Streptomyces (25), Nocardiopsis (7), Serinicoccus (6), Nocardia (5), Saccharopolyspora (4), Kocuria (4), Micrococcus (4), Rhodococcus (2), Prauserella (1), Micromonospora (1), Mycobacterium (1), and Actinoalloteichus (1) (Fig. 2). The sponge Dysidea arenaria provided the maximum of six genera, including Streptomyces, Salinispora, Saccharopolyspora, Kocuria, Prauserella, and Micromonospora in the medium M2.
Interestingly, 17 strains showed only 94–98 % sequence similarities with previously described species (Table S1), which was also supported by phylogenetic analysis (Figs. 3, 4, and 5). These strains were closely related to the genera Nocardia, Salinispora, Streptomyces, Micrococcus, and Saccharopolyspora. The phylogenetic relationships among the 56 actinobacterial strains are shown in Figs. 3, 4, and 5 along with their nearest NCBI (BLASTn) matches and type strains. The 19 Streptomyces strains were assembled into 16 clusters (Fig. 3). The isolate 13-18-23 showed 97 % similarity with Streptomyces sp. NCL 716 (FJ919811), which might be a new species within the genus Streptomyces. Similarly, Micrococcus sp.13-18-21 from Ircinia sp. also exhibited a distinct clade within Micrococcus (Fig. 4). Interestingly, phylogenetic analysis of the strains belonging to the family Micromonosporaceae, Pseudonocardiaceae, Mycobacteriaceae, and Nocardiaceae (Fig. 5) revealed that ten isolates, 13-18-9, 13-18-31, 13-18-20, 13-18-25, 13-18-24, 13-18-17, 13-18-8, 13-18-32, 13-18-33, and 13-3-44, belong to the genera Salinispora, which forms a different clade and the lowest sequence similarity, further proposing that these isolates might belong to a novel species within Salinispora. Similarly, the isolates Saccharopolyspora sp. 13-18-42 and Nocardia sp. 13-2-4 formed a separate clade indicating that it might be also a new species within the genera Saccharopolyspora and Nocardia, respectively.
Maximum likelihood phylogenetic tree of culturable Streptomycetaceae isolated from the South China Sea sponges and their NCBI (BLASTn) relatives based on the 16S rRNA gene sequences. Sequences obtained in this study are marked (●). Bootstrap values (1000 resamples) are given in percent at the nodes of the tree (>80). The outgroup is Escherichia coli ATCC 11775 T. The scale bar indicates 0.02 nucleotide substitution per nucleotide position
Maximum likelihood phylogenetic tree of culturable Nocardiopsaceae, Micrococcaceae, and Intrasporangiacea isolated from the South China Sea sponges and their NCBI (BLASTn) relatives based on the 16S rRNA gene sequences. Sequences obtained in this study are marked (●). Bootstrap values (1000 resamples) are given in percent at the nodes of the tree (>80). The outgroup is Escherichia coli ATCC 11775 T. The scale bar indicates 0.02 nucleotide substitution per nucleotide position
Maximum likelihood phylogenetic tree of culturable Micromonosporaceae, Pseudonocardiaceae, Mycobacteriaceae, and Nocardiaceae isolated from the South China Sea sponges and their NCBI (BLASTn) relatives based on the 16S rRNA gene sequences. Sequences obtained in this study are marked (●). Bootstrap values (1000 resamples) are given in percent at the nodes of the tree (>80). The outgroup is Escherichia coli ATCC 11775 T. The scale bar indicates 0.05 nucleotide substitution per nucleotide position
Screening of actinobacteria with phzE gene
The PCR amplification of phzE gene fragments showed that 10 out of 87 strains were positive for the presence of phenazines. phzE gene fragments were detected in the strains representing Streptomyces (5), Nocardiopsis (2), Micrococcus (1), Salinispora (1), and Serinicoccus (1). The sequence length of phzE gene fragments of these strains ranged from 423 to 444 bp. All these sequences showed the similarity in the range of 76 to 84 % with the phenazine gene cluster and phzE gene fragments related to Pseudomonas and Streptomyces. The summary of the BLAST searches of the sequence analysis is given in Table 1. Furthermore, we confirmed the expression of the phzE gene using reverse transcription PCR. As a result, the phzE gene was highly expressed in Serinicoccus sp. 13-12-4 (CCTCC AB 2014234), Nocardiopsis sp. 13-33-15 (CCTCC AA 2014034), and Nocardiopsis sp. 13-12-13 (CCTCC AA 2014033) on the fifth day of fermentation. Particularly, phzE gene was not expressed in strains Salinispora sp. 13-3-2 and Micrococcus sp. 13-18-21 during the incubation period, whereas the remaining five strains were poorly expressed on the fifth day of fermentation. Subsequently, their expression levels were gradiently decreased until the 12th day of fermentation (Fig. 6).
Expression of the phzE gene on different days of fermentation. (1) Streptomyces sp. 13-33-9, (2) Nocardiopsis sp. 13-33-15, (3) Streptomyces sp. 13-2-21, (4) Streptomyces sp. 13-2-25, (5) Streptomyces sp. 13-3-4,(6) Salinispora sp. 13-3-2, (7) Serinicoccus sp. 13-12-4, (8) Serinicoccus sp. 13-12-10, (9) Nocardiopsis sp. 13-12-13, (10) Micrococcus sp. 13-18-21, and (PC) DNA of Nocardiopsis sp. 13-12-13
Preparation of phenazine by actinobateria with phzE gene expression
Based on the phzE gene screening, reverse transcription PCR, UV absorption, and RP-HPLC separation, phenazines were isolated successfully from the Nocardiopsis sp. 13-33-15 and Nocardiopsis sp. 13-12-13. However, phenazine was not found in the fermentation broth of Serinicoccus sp. 13-12-4.
1,6-Dihydroxy phenazine (1) (Fig. 7) was isolated from Nocardiopsis sp. 13-33-15 and Nocardiopsis sp. 13-12-13 as yellow powder. ESI-MS showed the molecular ion clusters for [M + H] in positive mode with the molecular formula C12H9N2O2 and molecular ion peak at m/z 213.06 (Fig. S1). Consequently, the UV spectrum showed absorption at 270 nm. The 1H-NMR (400 MHz, Cd3Od) showed δ 7.20 (2H, m, 2, 7-H) and δ 7.71 ~ 7.78 (4H, m, 3,4,8,9 - H) (Fig. S2). 13C NMR (100 MHz, Cd3Od) showed δ 111.03 (C-2,7), 119.65 (C-4,9), 131.79 (C-3,8), 136.15 (C-5a, 10a), 142.55 (C-4a, 9a), 153.85 (C-1,6) (Fig. S3).
1,6-Dimethoxy phenazine (2) (Fig. 7) was isolated as yellow needles from Nocardiopsis sp. 13-33-15. The UV absorption spectrum was observed at 270 nm. The molecular formula was identified as C14H12O2N2 in ESI-MS, and molecular ion peak was observed at m/z 241.09 for [M + H] (Fig. S4). The 1H-NMR (400 MHz, CDCl3) displayed the methoxy protons (δ 4.21) and aromatic protons (δ 7.13–8.03) (Fig. S5). The 13C NMR (400 MHz, CDCl3) displayed seven spectrums among them six are aromatic carbons (δ 107.08 (C-2,7), 122.25 (C-4,9), 130.35 (C-3,8), 137.1 (C-5a, 10a), 143.23 (C-4a, 9a), 155.12 (C-1,6)), and one is methoxy carbon (56.7) (Fig. S6). The presence of phenazine and methoxy group was confirmed based on both molecular formula and NMR spectrum.
Antimicrobial activity of phenazines produced by actinobateria associated with sponges
The purified compounds 1and 2 (1 μg/μL) effectively inhibited the growth of Bacillus mycoides SJ14, Staphylococcus aureus SJ51, Escherichia coli SJ42, and Micrococcus luteus SJ47 with the inhibition zone ranging from 8 to 25 mm against 14 to 26 mm caused by ampicillin, and kanamycin in the above concentrations. The antibacterial activities of compounds 1 and 2 against Bacillus mycoides SJ14, Staphylococcus aureus SJ51, and Micrococcus luteus SJ47 were slightly higher than the kanamycin. No inhibition zone was observed against Saccharomyces cerevisiae SJ32 and Rhodotorula sp. SJ24 (Table 2).
Discussion
To date, more than 50 sponge species have been reported to be hosts for actinobacteria. One hundred twelve genera within 39 families of actinobacteria have been detected within different sponges based on culture-independent methods, and 44 genera of the culturable actinobacteria have been isolated within the marine sponges (Valliappan et al. 2014). Relatively, the actinobacteria showed to be randomly associated with sponges (Abdelmohsen et al. 2014a, b). However, it is obvious that actinobacteria are well adapted to survive within the marine sponges (Webster and Taylor 2012). Most of them are the major producers of bioactive compounds with medicinal application (Zotchev 2012; Valliappan et al. 2014).
In this study, for the first time, actinobacteria were isolated from the sponges Theonella swinhoei, Smenospongia aurea, and Ircinia sp. in the South China Sea. Totally, 13 genera, including Salinispora, Streptomyces, Nocardiopsis, Serinicoccus, Nocardia, Saccharopolyspora, Kocuria, Micrococcus, Rhodococcus, Prauserella, Micromonospora, Mycobacterium, and Actinoalloteichus were identified to be associated with these sponges. The total numbers of actinobacterial isolates varied between different sponges, e.g., Ircinia sp. harbored the highest number of actinobacterial isolates, while Dysidea arenaria had the highest number of actinobacterial genera. Among these sponges, Iotrochota sp. yielded only three representative isolates and each belongs to different genera, including Mycobacterium, Serinicoccus, and Micrococcus, which was not previously isolated from the Iotrochota sp. (Cellulosimicrobium, Nocardiopsis, and Streptomyces) (Jiang et al. 2008). This supported the fact that the number of actinobacteria and its genera varied among sponge species (Abdelmohsen et al. 2014a, b). Some studies also proved that the similar species of sponges from distinct geographic regions had different actinobacterial assemblages (Webster and Taylor 2012). Selvin et al. (2009) reported that Micromonospora, Saccharomonospora, and Streptomyces were the major groups in the marine sponge Dendrillanigra, whereas Xi et al. (2012) reported that Micromonospora and Streptomyces were the major groups of culturable actinobacteria associated with marine sponges. Similarly, abundant Streptomyces (Selvin et al. 2009; Xi et al. 2012; Valliappan et al. 2014) was isolated from the seven species of sponges tested in this study. Particularly, for the first time, genus Salinispora was found to be dominant in the sponge Ircinia sp. with lowest sequence similarity, which indicates that it might be a novel genus/species within the family Micromonosporaceae.
Based on the investigation of culture-independent strategy, 39 different genera have been detected from sponges (Khan et al. 2014; Han et al. 2012; Jackson et al. 2012; Simister et al. 2012; Li et al. 2011; Sun et al. 2010). However, 18 actinobacterial genera, i.e., Actinomyces, Aeromicrobium, Amycolatopsis, Dermacoccus, Dermatophilus, Gordonia, Iamia, Illumatobacter, Isoptericola, Leucobacter, Microlunatus, Millisia, Nitriliruptor, Nocardioides, Salinibacterium, Terrabacter, Tsukamurella, and Williamsia, detected by the culture-independent methods have not been isolated from the marine sponges. Therefore, it is essential to optimize the isolation strategies to improve the actinobacterial cultivation from the marine sponges.
In the present study, heat pretreatment and the serial dilution methods were applied successfully to isolate the actinobacteria from seven sponge samples using five different media. The intention of the heat treatment is to decrease the amount of bacteria and to enrich the actinobacteria (Jensen et al. 1991). This strategy has been used successfully to isolate rare actnobacteria (Mincer et al. 2002; Kim et al. 2005; Jensen et al. 2005; Öner et al. 2014). The results showed that media composed of rich nutrients, particularly the presence of yeast extract and peptone yield the highest number of culturable actinobacteria, which was consistent with previous results (Öner et al. 2014; Vicente et al. 2013; Abdelmohsen et al. 2014b). The isolation and consequent molecular identification of actinobacteria illustrated that the isolation rate and diversity of actinobacteria varied on different media. Therefore, the usages of different media are required to attain the maximum isolation of sponge-associated actinobacteria. Several rare and phylogenitically distinct actinobacteria were isolated in this study, e.g., Salinispora, Serinicoccus, Nocardia, Kocuria, Prauserella, and Actinoalloteichus. Meanwhile, strain 13-3-3 assigned to the genus Prauserella was isolated first time from the marine sponges, which was more closely related to the type strain of Prauserella aidingensis YIM 90636 isolated from the salt lake in Yunnan Province, China (Li et al. 2009). Even though, the new approaches of isolation such as encapsulation of cells in gel microdroplets (Zengler et al. 2002) or the employment of diffusion chambers (Lewis et al. 2010), microbial traps (Sizova et al. 2012), and isolation chips (Pahlow et al. 2013) can be used in the future to isolate the uncultivable actinobacteria from the marine sponges.
Mavrodi et al. (2010) designed four different pairs of primers targeting phzF gene to study the phenazine- producing bacteria from the plant and soil. Moreover, Schneemann et al. (2011) designed the universal primer targeting phzE gene to investigate the different groups of bacteria, including actinobacteria and Pseudomonas without any prior information on phylogenetic classification. Schneemann et al. (2011) identified 22 known phenazines from the actinobacteria based on the phzE gene detection. Hence, the results suggested that actinobacteria with phzE genes were possible to produce phenazines. In this study, the PCR screening of 87 marine sponge-associated actinobacteria revealed that the phzE genes were distributed unevenly among different taxa. The detection of phzE gene in the genera Micrococcus and Serinicoccus recommended that these less studied genera signify an unexplored source for natural products. Even though the actinobacteria contain phzE gene, it might not have the ability to produce phenazine in the laboratory conditions. Hence, the expressions of these genes using reverse transcription PCR are required to identify the highly active phenazine-producing strains.
The lowest sequence similarity with previously published phenazine biosynthetic genes revealed that the production of the compounds using this gene sequence might not be accurately predicted, and subsequently, it should be required to be confirmed by chemical analysis. Even though the genetic screening at both DNA and RNA levels of the phenazine is easy to detect the phenazine-producing actinobacteria, it is difficult to isolate phenazines from the fermentation broth e.g. Serinicoccus sp. 13-12-4 due to the difficulty in the detection of phenazine by UV detector and purifying the correct fractions using RP-HPLC. Further, the isolation and purification of phenazine in pure state is most important and time-consuming. Hence, the application of new discriminatory methods from extraction to purification is required to reduce the time and enhance the level of purity for identification.
Using the strategy above, three strains, i.e., Nocardiopsis sp. 13-33-15, Nocardiopsis sp. 13-12-13, and Serinicoccus sp. 13-12-4, were found to be having phzE gene activity. Consequently, two compounds 1,6-dihydroxy phenazine and 1, 6-dimethoxy phenazine were successfully obtained from Nocardiopsis sp. 13-33-15 and Nocardiopsis sp. 13-12-13. It might be considered that 1,6-dihydroxy phenazine was originated from two decarboxylative hydroxylations of phenazine-1,6-dicarboxylic catalyzed by Mpz9, which was indicated by the gene cluster of the strain Streptomyces sp. SpC080624SC-11 (Zeyhle et al. 2014) and the analogue to the PhzS of Pseudomonas aeruginosa (Greenhagen et al. 2008). Interestingly, 1,6-dihydroxy phenazine was identified in both Nocardiopsis sp. 13-33-15 and 13-12-13, indicating that 1,6-dihydroxy phenazine might act as the substrate for the synthesis of 1,6-dimethoxy phenazine in Nocardiopsis sp. 13-33-15 and also for other phenazines in Nocardiopsis sp. 13-12-13. In the future, genome-based approach will be helpful to identify the putative gene cluster and the biosynthetic pathway of these compounds (Heine et al. 2014; Zeyhle et al. 2014).
Streptomyces genome sequencing revealed that every strain has several genes to produce >20 possible secondary metabolites (Bentley et al. 2002; Ikeda et al. 2003; Ohnishi et al. 2008). However, only a part of them are expressed during fermentation. Likewise, phenazine biosynthetic gene cluster discovered from the Streptomyces tendae Tu1028 was inactive and it was activated by introducing a constitutive promoter in the upstream of the phenazine biosynthetic genes, which led to the synthesis of phenazine-1-carboxylic acid (PCA) and a new derivative of phenazine (Saleh et al. 2012). The results of phzE gene expression analysis revealed that the strains from Salinispora and Micrococcus were not active, while the strains Streptomyces sp. 13-3-4, Streptomyces sp. 13-12-10, Streptomyces sp. 13-2-25, Streptomyces sp. 13-2-21, and Streptomyces sp. 13-33-9 were weekly expressed in the fermentation medium. It indicated that these isolates were inactive or less active to produce phenazines in the fermentation medium. In theory, the related pathway could be activated by the suitable fermentation condition to produce phenazine. For example, the methods such as rifampcin resistance (rpoB) mutations, ribosome engineering, dasR–N-acetylglucosamine system, LAL regulatory system, metabolic engineering, and cell-to-cell interactions might be used to induce or enhance the expression of phenazine biosynthetic genes (Ochi and Hosaka 2013; Hosaka et al. 2009; van Wezel and McDowall 2011; Craney et al. 2012; Onaka et al. 2011).
In conclusion, seven species of South China Sea sponges were found to host abundant and diverse culturable actinobacteria. Comparatively, different sponges had considerably different actinobacterial species. Totally, 13 different genera and 17 putatively novel isolates were isolated from these sponges. To our knowledge, this study is the first report on the isolation of Prauserella from the marine sponges. The genetic screening of phzE gene revealed that ten actinobacterial strains have the potential to produce phenazines. Further, the gene expression at RNA level and chemical analysis showed that only two strains have the ability to produce phenazine in the fermentation medium. Finally, 1,6-dihydroxy phenazine and 1,6-dimethoxy phenazine were purified from Nocardiopsis sp. 13-33-15 and Nocardiopsis sp. 13-12-13. The antimicrobial test proved that these two phenazines were active against bacteria. These results suggested that the integrated approach of gene screening at both DNA and RNA levels, and chemical analysis is a valuable approach to guide the preparation of target compounds, and highlighted the potential of South China Sea sponges as a resource of novel actinobacteria for marine drugs development.
References
Abdelfattah MS, Kazufumi T, Ishibashi M (2011a) Isolation and structure elucidation of izuminosides A–C: a rare phenazine glycosides from Streptomyces sp. IFM 11260. J Antibiot 64:271–275
Abdelfattah MS, Kazufumi T, Ishibashi M (2011b) New pyranonaphthoquinones and a phenazine alkaloid isolated from Streptomyces sp., IFM 11307 with trail resistance-overcoming activity. J Antibiot 64:729–734
Abdelmohsen UR, Bayer K, Hentschel U (2014a) Diversity, abundance and natural products of marine sponge associated actinomycetes. Nat Prod Rep 31:381–399
Abdelmohsen UR, Yang C, Horn H, Hajjar D, Ravasi T, Hentschel U (2014b) Actinomycetes from Red Sea sponges: sources for chemical and phylogenetic diversity. Mar Drugs 12:2771–2789
Abken HJ, Tietze M, Brodersen J, Bäumer S, Beifuss U, Deppenmeier U (1998) Isolation and characterization of methanophenazine and function of phenazines in membrane-bound electron transport of Methanosarcina mazei Gö1. J Bacteriol 180:2027–2032
Bentley SD, Chater KF, Cerdeño-Tárraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3 (2). Nature 417:141–147
Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR (2012) Marine natural products. Nat Prod Rep 29:144–222
Craney A, Ozimok C, Pimentel-Elardo SM, Capretta A, Nodwell JR (2012) Chemical perturbation of secondary metabolism demonstrates important links to primary metabolism. Chem Biol 19:1020–1027
de Andrade-Neto V, Goulart MOF, da Silva Filho JF, da Silva MJ, Pinto MCFR, Pinto AV, Zalis MG, Carvalho LH, Krettli AU (2004) Antimalarial activity of phenazines from lapachol, β-lapachone and its derivatives against Plasmodium falciparum in vitro and Plasmodium berghei in vivo. Bioorg Med Chem Lett 14:1145–1149
Fuerst JA (2014) Diversity and biotechnological potential of microorganisms associated with marine sponges. Appl Microbiol Biotechnol 98:7331–7347
Gao X, Lu Y, Xing Y, Ma Y, Lu J, Bao W, Wang Y, Xi T (2012) A novel anticancer and antifungus phenazine derivative from a marine actinomycete BM-17. Microbiol Res 167:616–622
Greenhagen BT, Shi K, Robinson H, Gamage S, Bera AK, Ladner JE, Parsons JF (2008) Crystal structure of the pyocyanin biosynthetic protein PhzS. Biochemistry 47:5281–5289
Han M, Liu F, Zhang F, Li Z, Lin H (2012) Bacterial and archaeal symbionts in the South China Sea sponge Phakellia fusca: Community structure, relative abundance, and ammonia-oxidizing populations. Mar Biotechnol 14:701–713
Heine D, Martin K, Hertweck C (2014) Genomics-guided discovery of endophenazines from Kitasatospora sp. HKI 714. J Nat Prod 77:1083–1087
Hentschel U, Usher KM, Taylor MW (2006) Marine sponges as microbial fermenters. FEMS Microb Ecol 55:167–177
Hochmuth T, Niederkruger H, Gernert C, Siegl A, Taudien S, Platzer M, Crews P, Hentschel U, Piel J (2010) Linking chemical and microbial diversity in marine sponges: possible role for poribacteria as producers of methyl-branched fatty acids. Chembiochem 11:2572–2578
Hooper JNA, Van Soest RWM (2002) Systema Porifera: a guide to the classification of sponges. Kluwer Academic/ Plenum Publishers, Dordrecht, p 1756
Hosaka T, Ohnishi-Kameyama M, Muramatsu H, Murakami K, Tsurumi Y, Kodani S, Yoshida M, Fujie A, Ochi K (2009) Antibacterial discovery in actinomycetes strains with mutations in RNA polymerase or ribosomal protein S12. Nat Biotechnol 27:462–464
Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, Sakaki Y, Hattori M, Ōmura S (2003) Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat Biotechnol 21:526–531
Izumikawa M, Khan ST, Takagi M, Shin-ya K (2010) Sponge-derived Streptomyces producing isoprenoids via the mevalonate pathway. J Nat Prod 73:208–212
Jackson SA, Kennedy J, Morrissey JP, O’Gara F, Dobson ADW (2012) Pyrosequencing reveals diverse and distinct sponge-specific microbial communities in sponges from a single geographical location in Irish waters. Microb Ecol 64:105–116
Jensen PR, Dwight R, Fenical W (1991) Distribution of actinomycetes in near-shore tropical marine sediments. Appl Environ Microbiol 57:1102–1108
Jensen PR, Gontang E, Mafnas C, Mincer TJ, Fenical W (2005) Culturable marine actinomycete diversity from tropical Pacific ocean sediments. Environ Microbiol 7:1039–1048
Jiang S, Li X, Zhang L, Sun W, Dai S, Xie L, Liu Y, Lee KJ (2008) Culturable actinobacteria isolated from marine sponge Iotrochota sp. Mar Biol 153:945–952
Khan ST, Izumikawa M, Motohashi K, Mukai A, Takagi M, Shin-Ya K (2010) Distribution of the 3-hydroxyl-3-methylglutaryl coenzyme A reductase gene and isoprenoid production in marine-derived actinobacteria. FEMS Microbiol Lett 304:89–96
Khan ST, Musarrat J, Alkhedhairy AA, Kazuo S (2014) Diversity of bacteria and polyketide synthase associated with marine sponge Haliclona sp. Ann Microbiol 64:199–207
Kim TK, Garson MJ, Fuerst JA (2005) Marine actinomycetes related to the “Salinispora” group from the Great Barrier Reef sponge Pseudoceratina clavata. Environ Microbiol 7:509–518
Konig GM, Kehraus S, Seibert SF, Abdel-Lateff A, Muller D (2006) Natural products from marine organisms and their associated microbes. Chembiochem 7:229–238
Laursen JB, Nielsen J (2004) Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. Chem Rev 104:1663–1685
Lewis K, Epstein S, D'Onofrio A, Ling LL (2010) Uncultured microorganisms as a source of secondary metabolites. J Antibiot 63:468–476
Li C-Q, Liu W-C, Zhu P, Yang J-L, Cheng K-D (2011) Phylogenetic diversity of bacteria associated with the marine sponge Gelliodescarnosa collected from the Hainan island coastal waters of the South China Sea. Microb Ecol 62:800–812
Li Y, Tang S-K, Chen Y-G, Wu J-Y, Zhi X-Y, Zhang Y-Q, Li W-J (2009) Prauserella salsuginis sp. nov., Prauserella flava sp. nov., Prauserella aidingensis sp. nov. and Prauserella sediminis sp. nov., isolated from a salt lake. IJSEM 59:2923–2928
Liu H, He Y, Jiang H, Peng H, Huang X, Zhang X, Thomashow LS, Xu Y (2007) Characterization of a phenazine-producing strain Pseudomonas chlororaphis GP72 with broad- spectrum antifungal activity from green pepper rhizosphere. Curr Microbiol 54:302–306
Makgatho ME, Anderson R, O’Sullivan JF, Egan TJ, Freese JA, Cornelius N, van Rensburg CEJ (2000) Tetramethylpiperidine-substituted phenazines as novel anti-plasmodial agents. Drug Dev Res 50:195–202
Mavrodi DV, Parejko JA, Mavrodi OV, Kwak YS, Weller DM, Blankenfeldt W, Thomashow LS (2013) Recent insights into the diversity, frequency and ecological roles of phenazines in fluorescent Pseudomonas spp. Environ Microbiol 15:675–686
Mavrodi DV, Peever TL, Mavrodi OV, Parejko JA, Raaijmakers JM, Lemanceau P, Mazurier S, Heide L, Blankenfeldt W, Weller DM, Thomashow LS (2010) Diversity and evolution of the phenazine biosynthesis pathway. Appl Environ Microbiol 76:3866–3879
Mayer AM, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, McIntosh JM, Newman DJ, Potts BC, Shuster DE (2010) The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci 31:255–265
Mincer TJ, Jensen PR, Kauffman CA, Fenical W (2002) Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl Environ Microbiol 68:5005–5011
Mitova MI, Lang G, Wiese J, Imhoff JF (2008) Subinhibitory concentrations of antibiotics induce phenazine production in a marine Streptomyces sp. J Nat Prod 71:824–827
Montalvo NF, Mohamed NM, Enticknap JJ, Hill RT (2005) Novel actinobacteria from marine sponges. Antonie Van Leeuwenhoek 87:29–36
Ochi K, Hosaka T (2013) New strategies for drug discovery: activation of silent or weakly expressed microbial gene clusters. Appl Microbiol Biotechnol 97:87–98
Ohnishi Y, Ishikawa J, Hara H, Suzuki H, Ikenoya M, Ikeda H, Yamashita A, Hattori M, Horinouchi S (2008) Genome sequence of the streptomycin-producing microorganism Streptomyces griseus IFO 13350. J Bacteriol 190:4050–4060
Onaka H, Mori Y, Igarashi Y, Furumai T (2011) Mycolic acid-containing bacteria induce natural-product biosynthesis in Streptomyces species. Appl Environ Microbiol 77:400–406
Öner Ö, Ekiz G, Hameş EE, Demir V, Gübe Ö, Ozkaya FC, Yokeş MB, Uzel A, Bedir E (2014) Cultivable sponge-associated actinobacteria from coastal area of Eastern Mediterranean Sea. Adv Microbiol 4:306–316
Pahlow S, Kloss S, Blattel V, Kirsch K, Hubner U, Cialla D, Rosch P, Weber K, Popp J (2013) Isolation and enrichment of pathogens with a surface-modified aluminium chip for raman spectroscopic applications. Chem Phys Chem 14:3600–3605
Piel J (2006) Bacterial symbionts: prospects for the sustainable production of invertebrate-derived pharmaceuticals. Curr Med Chem 13:39–50
Pierson LS III, Pierson EA (2010) Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl Microbiol Biotech 86:1659–1670
Poongodi S, Karuppiah V, Sivakumar K, Kannan L (2014) Antioxidant activity of Nocardiopsis sp., a marine actinobacterium, isolated from the Gulf of Mannar Biosphere Reserve, India. Natl Acad Sci Lett 37:65–70
Reveillaud J, Maignien L, Eren AM, Huber JA, Apprill A, Sogin ML, Vanreusel A (2014) Host-specificity among abundant and rare taxa in the sponge microbiome. ISME J 8:1198–1209
Saleh O, Bonitz T, Flinspach K, Kulik A, Burkard N, Muhlenwe A, Vente A, Polnick S, Lammerhofer M, Gust B, Fiedler HP, Heide L (2012) Activation of a silent phenazine biosynthetic gene cluster reveals a novel natural product and a new resistance mechanism against phenazines. Med Chem Commun 3:1009–1019
Schneemann I, Wiese J, Kunz AL, Imhoff JF (2011) Genetic approach for the fast discovery of phenazine producing bacteria. Mar Drugs 9:772–789
Selvin J, Gandhimathi R, Kiran GS, Priya SS, Ravji TR, Hema TA (2009) Culturable heterotrophic bacteria from the marine sponge Dendrilla nigra: Isolation and phylogenetic diversity of actinobacteria. Helgol Mar Res 63:239–247
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340
Simister RL, Deines P, Botté ES, Webster NS, Taylor MW (2012) Sponge-specific clusters revisited: a comprehensive phylogeny of sponge-associated microorganisms. Environ Microbiol 14:517–524
Sizova MV, Hohmann T, Hazen A, Paster BJ, Halem SR, Murphy CM, Panikov NS, Epstein SS (2012) New approaches for isolation of previously uncultivated oral bacteria. Appl Environ Microbiol 78:194–203
Sun W, Dai SK, Jiang SM, Wang GH, Liu GH, Wu HB, Li X (2010) Culture-dependent and culture-independent diversity of Actinobacteria associated with the marine sponge Hymeniacidon perleve from the South China Sea. Antonie Van Leeuwenhoek 98:65–75
Taylor MW, Radax R, Steger D, Wagner M (2007) Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev 71:295–347
Valliappan K, Sun W, Li Z (2014) Marine actinobacteria associated with marine organisms and their potentials in producing pharmaceutical natural products. Appl Microbiol Biotechnol 98:7365–7377
van Wezel GP, McDowall KJ (2011) The regulation of the secondary metabolism of Streptomyces: new links and experimental advances. Nat Prod Rep 28:1311–1333
Vicente J, Stewart A, Song B, Hill R, Wright J (2013) Biodiversity of actinomycetes associated with Caribbean sponges and their potential for natural product discovery. Mar Biotechnol 15:413–424
Webster NS, Taylor MW (2012) Marine sponges and their microbial symbionts: Love and other relationships. Environ Microbiol 14:335–346
Xi L, Ruan J, Huang Y (2012) Diversity and biosynthetic potential of culturable actinomycetes associated with marine sponges in the China Seas. Int J Mol Sci 13:5917–5932
Zengler K, Toledo G, Rappe M, Elkins J, Mathur EJ, Short JM, Keller M (2002) Cultivating the uncultured. Proc Natl Acad Sci U S A 99:15681–15686
Zeyhle P, Bauer JS, Kalinowski J, Shin-ya K, Gross H, Heide L (2014) Genome-based discovery of a novel membrane-bound 1,6-dihydroxyphenazine prenyltransferase from a marine actinomycete. PLoS ONE 9:e99122
Zhang G, Yang Y, Cao T, Ma L, Ying J (2014) Diversity and novelty of actinobacteria in Arctic marine sediments. Antonie Van Leeuwenhoek 105:743–754
Zhang X-Y, Bao J, He F, Xu X-Y, Wang G-H, Qi S-H (2013) Diversity and antibacterial activity of culturable actinobacteria isolated from five species of the South China Sea gorgonian corals. World J Microbiol Biotechnol 29:1107–1116
Zotchev SB (2012) Marine actinomycetes as an emerging resource for the drug development pipelines. J Biotechnol 158:168–175
Acknowledgments
This study was financially supported by the High-Tech Research and Development Program of China (2013AA092901). Authors thank Dr. M. Gopi, Conservation of Coastal and Marine Resources Division, National Centre for Sustainable Coastal Management, Anna University Campus, Chennai, Tamil Nadu, India, for identifying three sponges based on sponge morphology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 414 kb)
Rights and permissions
About this article
Cite this article
Karuppiah, V., Li, Y., Sun, W. et al. Functional gene-based discovery of phenazines from the actinobacteria associated with marine sponges in the South China Sea. Appl Microbiol Biotechnol 99, 5939–5950 (2015). https://doi.org/10.1007/s00253-015-6547-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6547-8