Abstract
(S)-4-chloro-3-hydroxybutanoate ((S)-CHBE) is an important chiral intermediate to synthesize the side chain of cholesterol-lowering drug atorvastatin. To biosynthesize the (S)-CHBE, a recombinant Escherichia coli harboring the carbonyl reductase and glucose dehydrogenase was successfully constructed. The recombinant E. coli was cultured in a 500-L fermentor; after induction and expression, the enzyme activity and cell biomass were increased to 23,661.65 U/L and 13.90 g DCW/L which was 3.24 and 2.60-folds compared with those in the 50 L fermentor. The biocatalytic process for the synthesis of (S)-CHBE in an aqueous-organic solvent system was constructed and optimized with a substrate fed-batch strategy. The ethyl 4-chloro-3-oxobutanoate concentration reached to 1.7 M, and the (S)-CHBE with yield of 97.2 % and enantiomeric excess (e.e.) of 99 % was obtained after 4-h reaction in a 50-L reactor. In this study, the space-time yield and space-time yield per gram of biomass (dry cell weight, DCW) were 413.17 mM/h and 27.55 mM/h/g DCW for (S)-CHBE production, respectively, which were the highest values as compared to previous reports. Finally, (S)-CHBE was extracted from the reaction mixture with 82 % of yield and 95 % of purity. This study paved the foundation for the upscale production of (S)-CHBE by biocatalysis method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ethyl (S)-4-chloro-3-hydroxybutanoate ((S)-CHBE), one of the optically active alcohols, is a key chiral intermediate to synthesize the side chain of cholesterol-lowering drug atorvastatin (Lipitor), which is the hydroxymethylglutaryl CoA reductase inhibitor (Roth 2002; Ye et al. 2011). (S)-CHBE also can be used in the synthesis of Slagenins B and C and 1,4-dihydropyridine type β-blockers (Kaliaperumal et al. 2010). The chemically asymmetric synthesis of (S)-CHBE by Ruthenium as catalyst was well studied. Qiu et al. (2002) synthesized (S)-CHBE with enantiomeric excess (e.e.) of 96.7 % using ethyl 4-chloro-3-oxobutanoate (COBE) and RuLCl2(DMF)n as substrate and catalyst with ratio of 667:1 at high pressure condition for 24 h. Madec et al. (2001) screened a novel Ruthenium catalyst named RuCl3/((R)-Meo-BIPHEP) and applied it into the synthesis of (S)-CHBE with e.e. of 92 % using COBE as substrate. Though the chemically asymmetric synthesis method has advantages in productivity and downstream processing, the requirement of high pressure and expensive metal catalyst limits its industrial application (Hoff and Anthonsen 1999).
The enzymatically asymmetric reduction of COBE, which can be easily synthesized from diketene (Hoff and Anthonsen 1999; Sundby et al. 2003), has drawn more attentions and become a promising way to produce (S)-CHBE (Matsuda et al. 2009; Ye et al. 2011). It was reported that there are two main routes to synthesize (S)-CHBE by lipase and carbonyl reductases (Ye et al. 2011). The lipase catalyzed synthesis reactions including kinetic resolution and esterification resulted in a low productivity and the e.e. value of product cannot meet the requirements from industry (Matsuda et al. 2009). Carbonyl reductases (CBRs, EC 1.1.1.148) are the alternatives and the best suitable catalysts for biosynthesis of (S)-CHBE (Ye et al. 2011). CBRs as the powerful biocatalytic tools for preparation of chiral pharmaceutical intermediates, including antidepression drugs (Soni and Banerjee 2005), anticholesterol drugs (You et al. 2014), β-lactams antibiotics (Shimoda et al. 2009), and anticancer drugs (Applegate et al. 2011), have become valuable alternatives to traditional chemical catalysts. Until now, several CBRs have been found exhibiting high catalytic activity and exquisite selectivity to COBE (Kaliaperumal et al. 2010; Wang et al. 2011; Yasohara et al. 2001). Some of CBRs have been cloned and overexpressed in Escherichia coli or Pichia pastoris to prepare the recombinant enzymes for asymmetric synthesis (S)-CHBE (An et al. 2012; Engelking et al. 2004; Ye et al. 2009). However, in most reported studies, the synthesis of (S)-CHBE using CBRs needs to add extra NAD+ or NADP+, which limits its application in industry because of the high level of cost (Ye et al. 2011).
Kizaki et al. (2001) cloned a CBR from Candida magnolia and applied it into preparation of (S)-CHBE using glucose dehydrogenase from Bacillus megaterium to regenerate NADPH. The concentration of (S)-CHBE could reach to 208 g/L in aqueous system and the concentration of (S)-CHBE was improved to 430 g/L after 34-h reaction, when aqueous and organic solvent was used as reaction system. Since the price of NAD+ is much lower than that of NADP+, to reduce the cost of production of (S)-CHBE, Morikawa et al. (2005) engineered B. megaterium CBR by site directed mutagenesis method to exchange the NADP+ cofactor dependence to NAD+, the final concentration of (S)-CHBE was achieved at 163 g/L when using NAD+ as cofactor. An et al. (2012) obtained a novel CBR from Candida albicans by genome mining method. COBE (4,800 mM) was completely biotransformed to (S)-CHBE with molar productivity of 93.8 % and e.e. >99 % by whole cells harboring this newly obtained CBR combined with GDH for 25 h, without addition of NAD+ or NADP+, which is the highest productivity with industrial potentials that have been ever reported. This approach is the most attractive deracemization method allowing complete transformation of a racemate into a single stereoisomeric product. However, there are no any reports on the upsclae production of (S)-CHBE by recombinnat CBR combined with GDH.
In this study, to establish a feasible system for the practical application in the production of (S)-CHBE, the genes encoding CBR and GDH were cloned and simultaneously expressed in E. coli. The fermentation in 500 L fermentor was carried out to simultaneously produce CBR and GDH. The biosynthesis process of COBE to produce (S)-CHBE were investigated and optimized. Relatively high concentration and enantioselectivity of (S)-CHBE was achieved in the aqueous-organic solvent system. After biotransformation, the (S)-CHBE was isolated and purified, high purity of (S)-CHBE with yield of 82 % was obtained. This study provided a new route and paved a foundation for upscale production of (S)-CHBE by CBR coupled with GDH.
Materials and methods
Microorganisms, mediums, and chemicals
E. coli BL21(DE3) (Invitrogen, Carlsbad, CA, USA) and pCDFDuet-1 (Novagen, Darmstadt, Germany) were used for co-expression of the recombinant CBR and glucose dehydrogenase (GDH). Luria–Bertani (LB) medium containing (per liter) 5 g of yeast extract, 10 g of tryptone, and 10 g of NaCl (Sambrook and Russell 2001) was used for E. coli cultivation. 2× YT medium composed of 10 g/L yeast extract, 16 g/L tryptone, and 5 g/L NaCl was used for seeds cultivation in the fermentation process. Fermentation medium consisted of peptone 15.0 g/L, yeast extract 12.0 g/L, NaCl 10.0 g/L, (NH4)2SO4 5 g/L, glycerol 15 g/L, KH2PO4 1.36 g/L, K2HPO4·3H2O 2.28 g/L, MgSO4·7H2O 0.375 g/L, and defoamer 0.1 % (v/v). Streptomycin sulfate (SM, 50 μg/mL) was added to provide selective pressure during cultivation. COBE and (S)-CHBE were purchased from J&K Scientific Ltd. (Shanghai, China). All other chemicals were of analytical grade purity and commercially available.
Construction of co-expression plasmid
The CBR gene (GenBank accession no. ACQ99345) from Candida parapsilosis (Nie et al. 2011) and GDH gene (GenBank accession no. ACB59697.1) from Exiguobacterium sibiricum were artificially synthesized after optimization of the codons by PCR assembly method (You et al. 2014). The codon-optimized CBR and GDH genes were, respectively, deposited in the GenBank database under the accession numbers KC91439 and KM817194. After synthesized, both genes were subcloned into the vector pGEM-T, respectively, to construct the recombinant plasmids, named pGEM-T-CBR and pGEM-T-GDH. The GDH gene obtained from pGEM-T-GDH digested with Nde I and Xho I restriction enzymes was cloned into Nde I/Xho I site of pCDFDuet-1 vector (Novagen) MCSII (multiple cloning sites-2) to construct the recombinant plasmid, pCDF-GDH. The CBR gene obtained from pGEM-T-CBR digested with Nco I and Hind III restriction enzymes was subsequently cloned into Nco I/Hind III site of recombinant pCDF-GDH vector MCSI (multiple cloning sites-1 of pCDFDuet-1 vector). The resulting recombinant plasmid (pCDF-GDH-SCR) carrying the GDH and CBR genes was transformed into E. coli BL21(DE3) competent cells by the heat-shock method (Chung et al. 1989). Details on the construction of co-expression plasmid were shown in Fig. 1.
Expression and SDS-PAGE analysis of recombinant co-expressing E. coli
Recombinant E. coli cells harboring the CBR and GDH genes were pre-cultured in LB medium. Incubations were carried out at 37 °C on a rotary shaker at 150 rpm and protein expression was induced at 28 °C by the addition of 1.5 % (w/v) lactose when the OD600 reached to 1.2. After induction for 12 h, cells were harvested by centrifugation at 4 °C, 8,000×g for 10 min, and then stored at −20 °C for further use.
The cells were suspended in physiological saline solution, and disrupted by sonication for a period of 30 min on ice. After centrifuging 10 min at 15,000 rpm, the supernatant was used as crude enzyme. The coexpression of the recombinant CBR and GDH were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis, which was carried out with a 5 % acrylamide stacking gel (pH 6.8) and 12 % separating gel (pH 8.8) as described by Laemmli (1970).
Enzyme assay
Activity of the CBR was determined with COBE as substrate in a reaction mixture containing 100 mM K2HPO4-KH2PO4 buffer (pH 7.0), 100 mM COBE, 145 mM glucose, and 0.05 mM NADP+. The reduction of COBE was followed by measuring the decrease in the absorption of NADPH using a universal microplate spectrophotometer (Spectramax plus, Molecular Devices, Sunnyvale, CA, USA) at 340 nm and 45 °C. One unit (U) of CBR activity is defined as the amount of enzyme that consumes 1 μmol of NADPH per minute under the above assay conditions.
Fermentation of the recombinant E. coli co-expressing CBR and GDH genes
Recombinant E. coli BL21(DE3) harboring the CBR and GDH genes was maintained on LB agar slants and inoculated into 500 mL flasks containing 2 × YT medium and SM 50 μg/mL. After 4 h cultivation, the cultures were then inoculated into 50 L fermentor (Shanghai BaoXing Bio-engineering Equipment Co., LTD., Shanghai, China) containing 30 L 2 × YT medium and cultured at 37 °C, 500 rpm for 5 h. The airflow was 2 m3 and pH was adjusted to 7.0. Then the cultures were inoculated into 500 L fermentor (Shanghai BaoXing Bio-engineering Equipment Co., LTD., Shanghai, China) at 37 °C, 240 rpm. The airflow was 25 m3 and pH was adjusted to 7.0. When the value of OD600 reached about 10, 10 g/L lactose was added to induce the enzymes expressed at 28 °C. To keep the highest biomass and enzyme activity, the end point of fermentation was set at the time when the biomass and enzyme activity reached maxima.
Biosynthesis of (S)-CHBE in aqueous-organic solvent system
30 L of the reaction mixture containing 600 mM of COBE and 870 mM of glucose in 15 L of K2HPO4-KH2PO4 buffer (pH 7.0, 100 mM) was mixed with 15 L of butyl acetate in a 50-L bioreactor (Shanghai BaoXing Bio-engineering Equipment Co., LTD., Shanghai, China) with two six-bladed disc turbine impellers and electrodes for measuring temperature and pH. The reaction was carried out at 35 °C with agitation speed of 350 rpm. The pH was adjusted to 7.0 with 2 M NaOH during reaction. The crude enzymes (equivalent to 15 g DCW/L) were then added into the reactor and the incubation continued until the end of the reaction. Six hundred millimeter COBE and 870 mM glucose were added to the mixture at 30 min while 500 mM COBE and 725 mM glucose were added at 90 min.
Analytical methods
The amount of COBE and (S)-CHBE was measured by gas chromatography (GC; Agilent 7890A system, Santa Clara, CA, USA) equipped with a flame ionization detector and an HP-5 capillary column (30 m × 0.32 mm, 0.25 μm film thickness). Nitrogen was used as carrier gas at a flow rate of 1.5 mL/min. The inlet and detector temperatures were set at 230 °C and 250 °C, respectively. The temperature of column was held for 2.5 min at 120 °C, elevated at 30 °C/min to 165 °C, and held for 1.2 min at 165 °C. The retention times of COBE and (S)-CHBE were 3.99 and 4.21 min, respectively. Optical purity was determined by GC-14C gas chromatography (Shimadzu, Nakagyo-ku, Kyoto, Japan) equipped with FID detector and a BGB-174 chiral capillary column (30 m × 0.25 mm, 0.25 μm film thickness) using helium as carrier gas. The inlet and detector temperatures were all set at 220 °C. The temperature of column was programmed from 110 °C and raised to 125 °C at a rate of 0.5 °C/min. The retention times of (R)-CHBE and (S)-CHBE were 23.7 and 24.2 min, respectively.
Isolation and identification of (S)-CHBE
Two grams per liter activated carbon was added to the reaction mixture and filtrated after stirring 30 min at 100 rpm under the ambient temperature. The (S)-CHBE in aqueous phase of filtrate was extracted by ethyl acetate for five times, the volume ratio of ethyl acetate to the aqueous phase was 0.2 in every extraction. After extraction, the extracted phases containing (S)-CHBE were combined and used to extract residue (S)-CHBE from the activated carbon. Amber product was obtained by vacuum distillation at 50–60 °C from the organic phase after washed by NaHCO3 or NaCl saturated solution, and subsequently dried by anhydrous Na2SO4. The product was analyzed by gas chromatography-mass spectrum (GC-MS), Fourier transform infrared spectrum (FTIR), nuclear magnetic resonance (NMR), and chiral gas chromatography (GC).
The FTIR spectrum for (S)-CHBE was recorded on a Fourier transform infrared spectrometer (Nicolet 6700; Thermo, Waltham, MA, USA) with Contimuμm FTIR Micro system in the region of 4,000–400 cm−1 using KBr pellets. The GC-MS spectrum for (S)-CHBE was recorded on a GC-MS spectrometer (Agilent Technologies 1200 Series, 6210 Time-of-Flight MS spectrometer; Agilent Technologies, Waldbronn, Germany), and the experimental parameters were used as follows: ionization mode, −ESI scan; gas temp, 300 °C; fragmentor, 80 V; drying gas, 3.5 L/min; skimmer, 65 V; nebulizer, 20 psig; OCTIRFV pp, 250 V; and Vcap, 3,000 V. The 13C and 1H NMR spectra of (S)-CHBE were obtained on a NMR spectrometer (AVANCE 500 MHz, Bruker, Fallanden, Switzerland) with DMSO as the solvent using 500 and 125 MHz for carbon and proton determinations, respectively.
Results
Co-expressing recombinant CBR and GDH in E. coli
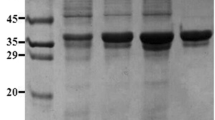
The CBR requires a coenzyme to produce (S)-CHBE with the stoichiometric reagents, which makes the process too expensive and impossible for the upscale production. To lower the cost, CBR and GDH were co-expressed to create a coenzyme regeneration system. In this system, not only the GDH can regenerate NADP+, but also the GDH substrate, glucose, is inexpensive. After codons optimization, the synthesized CBR gene with the length of 837 bp encoding a 279-amino acid protein and GDH gene with 786 bp encoding a 262-amino acid protein were, respectively, cloned into the MCSI and MCSII sites of pCDFDuet-1 vector. After induced by lactose, the recombinant CBR and GDH were successfully expressed in the same E. coli host. The bands with molecular weights of 30 and 28 kDa, respectively, for CBR and GDH appeared in the SDS-PAGE (Fig. 2) showed that soluble proteins were obtained, which indicated that the expressed products are consistent with the predicted proteins.
SDS-PAGE analysis of recombinant proteins. M protein molecular weight marker, 1 E. coli BL21(DE3), 2 E. coli BL21(DE3)/pET28b-CBR without induction, 3 E. coli BL21(DE3)/pET28b-CBR with 0.1 mM IPTG, 4 E. coli BL21(DE3)/pET28b-GDH without induction, 5 E. coli BL21(DE3)/pET28b-GDH with 0.1 mM IPTG, 6 E. coli BL21(DE3)/pCDFDuet-1-GDH-CBR without induction, 7 E. coli BL21(DE3)/pCDFDuet-1-GDH- CBR with 0.1 mM IPTG
Fermentation of the recombinant co-expressing E. coli
After cultured for 5 h, 30 L culture as seeds was inoculated into 500 L fermentor. The pH was adjusted to 7.0 by ammonium hydroxide or 50 % (v/v) phosphoric acid solution. The dissolved oxygen was decreased sharply after 6 h which means the strain growth was into logarithmic phase (Fig. 3). At this phase, the recombinant E. coli grow quickly and much of the nutrition was consumed. When cultured at 37 °C for 8.5 h, the OD600 reached around 10; after which, 10 g/L lactose was added to induce enzymes expressed at 28 °C. After 3 h of induction, the catalytic activity was tested, and the results showed that the CBR and GDH were successfully expressed in E. coli (Fig. 4). In the 19.5th hour (11th hour of induction), the cultivation process was in the stationary phase, and the biomass of cells and CBR activity reached their maxima with values of 13.90 g DCW/L and 23,661.65 U/L, respectively. The biomass and enzyme activity respectively increased to 3.24 and 2.60-folds compared with those in the 50 L fermentor because of the higher dissolved oxygen and the faster mass transfer in the 500-L fermentor which were superior to the 50 L (data not shown). After 21 h of cultivation, the biomass, enzyme activity, and specific activity were all descended simultaneously, which indicated that equilibration period of cells was arrival, resulting in the cells autolyzed.
Biosynthesis of (S)-CHBE in aqueous-organic solvent system
In this study, to increase the solubility of COBE and (S)-CHBE and to improve the stability of COBE, the aqueous-organic solvent system was constructed and used for biotransformation to produce the (S)-CHBE. In this system, it is ideal that the COBE and (S)-CHBE with high concentrations were dissolved in organic solvent phase, and the cells were in aqueous phase avoiding the toxic effects generated from the high concentration of COBE and (S)-CHBE. Therefore, the selected organic solvent used in this study must have excellent distribution coefficient to make more COBE and (S)-CHBE dissolved in the organic phase. To obtain the appropriate organic solvent, 25 kinds of organic solvents were tested to construct the aqueous-organic solvent system (Table 1). Among these organic solvents, butyl acetate was finally selected for its excellent distribution coefficient for COBE and (S)-CHBE, as well as its lower boiling point and toxicity to the cells.
The extra addition of NADP to the reaction system is the main bottleneck for biosynthesis of (S)-CHBE to be applied in industrial scale, because the NADP+ are too expensive to upscale application (An et al. 2012). In this study, the biosynthesis of (S)-CHBE by whole cells containing recombinant CBR and GDH without adding NADP+, which eliminated the barrier of mass transfer between COBE and glucose. The fed-batch strategy for COBE addition was used to reduce the substrate inhibition and increase the substrate concentration for industrial application. The results shown in Fig. 5 indicated that (S)-CHBE with a yield of 95.6 % and 99 % of e.e. was obtained after a 180-min reaction, and the amount of (S)-CHBE was 1,147.48 mM, which indicated that (S)-CHBE can be produced efficiently without adding extra NADP+. The concentration of COBE could reach 1.7 M by 2 times addition and the yield of (S)-CHBE was 97.2 % in 4 h (Fig. 6) under the optimized reaction conditions. The space-time yield and the space-time yield per gram of biomass of this asymmetric reduction were 413.2 mM/h and 27.6 Mm/h/g DCW, respectively, which is by far the highest space-time yield ever reported.
Extraction and identification of (S)-CHBE
After the reaction, activated carbon was added to the reaction mixture to absorb the cellular debris and proteins. The added amount of activated carbon was optimized (Fig. S1 in the Supporting Information). When the added amount of activated carbon exceeded 2 g/L, the clarity of solution after filtration process can reach 99 %, and the mixture was separated into two phases (Fig. S2 in the Supporting Information). Then the organic phase was used to extract (S)-CHBE. Amber product was obtained by vacuum distillation from the organic phase after washed by NaHCO3 or NaCl saturated solution, and subsequently dried by anhydrous Na2SO4. The whole yield of (S)-CHBE was 82 %, and the product purity was >95 %.
The purified (S)-CHBE was characterized by GC-MS, FTIR and NMR spectra. The determined result of the purified (S)-CHBE by chiral GC showed that there is only one peak at 24.2 min appeared (Fig. 7), which further confirmed that the purified product was (S)-CHBE with high optical purity and its e.e. was above 99 %。The GC-MS spectrum of the purified (S)-CHBE (Fig. 8) showed that the molecular ion peak of (S)-CHBE was found at m/z 167.0, which was in accordance with the calculated mass of (S)-CHBE with m/z 166.6. The isotopic peak of the corresponding Cl37 was shown at m/z 169.0. The (S)-CHBE showed fragment ions obtained by loss of a -OH group and a -OC2H5 group, which were observed at m/z 149.0 and m/z 121.0, respectively. The peak on the GC-MS spectrum at m/z 103.0 could be assigned to fragment ion after (S)-CHBE losing both -OH and -OC2H5. The loss of both -OC2H5 and -CH2Cl yielded a fragment ion at m/z 71.0. The FTIR spectrum of the separated (S)-CHBE was presented in Fig. 9a, b. The results showed that the two strong bands presented at 3,446.9 and 1,731.3 cm−1 could be assigned to O-H and C=O stretching vibrations, respectively. The bands arising from C-H and C-O-C stretching vibrations were observed at 2,983.8 and 1,375.7 cm−1, respectively. All the absorption bands of purified (S)-CHBE are in accordance with those of the standard (S)-CHBE. The results of 1H NMR spectra (Fig. S3a in the Supporting Information) showed that five different kinds of H atoms corresponded to the structure of (S)-CHBE. Resonances of H atoms belonging to -CH3 group were found at 1.17–1.19 ppm. The split peaks at chemical shifts between δ = 5.00 ppm and δ = 4.95 ppm represented the -OH group proton and the peak at chemical shift between δ = 4.08 ppm and δ = 4.04 ppm referred to the protons of -CH- and -OCH2- groups. Moreover, the split peaks at chemical shifts between δ = 3.61 ppm and δ = 3.54 ppm represented the H positions on the -CH2Cl group and the peak at chemical shift δ = 2.59 ppm and δ = 2.35 referred to the H position of -CH2CO- group. The data (Fig. S3b in the Supporting Information) of 13C NMR indicated that the purified (S)-CHBE gave signals between 170.46 and 169.19 ppm, which referred to the C positions of carboxyl group. The absorption bands between 68.98 and 67.00 ppm were due to the C positions on the -O-CH2- group. The signals between 60.24 and 59.76 ppm could be assigned to the C positions of -CH(−OH)- group. The signals between 48.84 and 48.70 ppm were due to the C positions on the -CH2Cl group. The signal at 18.37 ppm referred to the C positions of -CH2- group, and signals between 13.89 and 13.79 ppm could be assigned to the C positions of -CH3. Accordingly, it was concluded that the isolated product was (S)-CHBE.
Discussions
(S)-CHBE is the key intermediate to synthesize the side chain of atorvastatin (Roth 2002; Ye et al. 2011). The production of (S)-CHBE with high enantiopurity has been a hot issue in preparation of side chains of atorvastatin (You et al. 2014). Though the process for the chemically asymmetric synthesis of (S)-CHBE with high yield by hydrogenation is simple, it needs high pressure for the hydrogenation, which has special requirements for the equipments and reactors, besides using the expensive metal as catalyst (Madec et al. 2001; Qiu et al. 2002). To avoid using the expensive metal catalyst, another chemical method using (S)-epichlorohydrin as a substrate was developed, in which (S)-4-chloro-3-hydroxy-butyronitrile was obtained by NaCN through the ring opening, and then (S)-CHBE was synthesized after ethanol solution was added into the reaction mixture containing (S)-4-chloro-3-hydroxy-butyronitrile (Cho et al. 2002). In this method, the overall yield was 83.7 % after two step reactions. However, besides the chemical processes need to be at low temperatures, the use of toxic and hazardous reagent and high energy consumption made the processes unfriendly to the environment, which does not comply with the concept of green chemistry (Clark 1999; Anastas and Eghbali 2010). Compared with conventional chemical synthesis, the asymmetric reduction of COBE to (S)-CHBE using biocatalysis method has several advantages, including low cost, mild reaction conditions, high yield, and excellent enantioselectivity (Nakamura et al. 2003).
Though the bioproduction of (S)-CHBE was well studied, the problems including the low solubility and stability of substrate and the toxic influence of substrate or product to the biocatalyst in aqueous phase limited the upscale application to produce the (S)-CHBE (You et al. 2014). The application of the aqueous-organic solvent phase system in the production of (S)-CHBE made it possible for biocatalyst in aqueous phase and substrate and product in organic solvent phase which could avoid the toxic influences of substrate and product on the biocatalyst, meanwhile the concentration and stability of substrate and product were improved. In addition, the reaction happened in the interface between aqueous and organic solvent phases, because most of the substrate and product was in organic solvent phase which greatly induce the inhibition on biocatalyst from substrate and product with high concentration. Besides the advantages mentioned above, two-phase system used in this study has merits as follows: (1) The stability and concentration of substrate and product were improved in the organic solvent phase. (2) The product was extracted into organic solvent phase in situ, and the thermodynamic equilibrium of the reaction shifts towards product formation. (3) The biocatalysis in the two-phase system can be combined with isolation of the (S)-CHBE, which simplified the downstream processes, e.g., separation and purification. To lower the toxic influence of organic solvent on the biocatalyst, butyl acetate was used as the targeted organic solvent to construct the two-phase system to produce the (S)-CHBE in this study. The concentration of substrate can be improved from 100 mM in aqueous phase to 1.7 M in butyl acetate without affecting the conversion and e.e. Compared to previous reports, the highest the time-space yield (413.17 mM/L/h) of (S)-CHBE was achieved in this study.
The system using two recombinant cells receptively harboring CBR and GDH was seriously emulsified caused by the cellular debris and proteins which provides the problems in downstream process, especially in (S)-CHBE extraction, isolation, and purification processes. Though the emulsion effect can be solved by centrifugation or filtration, it is very hard to be applied in the industry. Especially, using separately cloned CBR and GDH in E. coli as catalysts to produce the (S)-CHBE needs extra addition of NAD+ or NADP+, which increased the cost of the production (Yamamoto et al. 2004; Ye et al. 2010; An et al. 2012). In this study, to break the barrier of H+ cycle between two cells by self-regeneration of NADP+ in E. coli cell, the CBR and GDH were coexpressed in one E. coli cell. Without addition of the expensive NAD+ or NADP+, the (S)-CHBE with high yield was obtained which greatly decreased the cost and simplified the downstream processing. This coexpression system with CBR and GDH in one E. coli cell resolved the problem of cost and regeneration of NAD+ or NADP+ to prepare the (S)-CHBE, which is promising for the upscale production of (S)-CHBE (Yamamoto et al. 2004).
In summary, a recombinant E. coli harboring the CBR and GDH was successfully constructed in this study. The recombinant E. coli was cultured in a 500-L fermentor, and the enzyme activity and cell biomass were obtained at 23,661.65 U/L and 13.90 g DCW/L. The biocatalytic process for the synthesis of (S)-CHBE in an aqueous-organic solvent system was constructed and optimized with a substrate fed-batch strategy. The COBE concentration reached to 1.7 M, and the (S)-CHBE with yield of 97.2 % and e.e. of 99 % was obtained in a 50-L bioreactor. In this study, the highest space-time yield and space-time yield per gram of biomass were 413.17 mM/h and 27.55 mM/h/g DCW. This study paved the foundation for the upscale production of (S)-CHBE by biocatalysis method.
References
An M, Cai P, Yan M, Hao N, Wang S, Liu H, Li Y, Xu L (2012) A novel reductase from Candida albicans for the production of ethyl (S)-4-chloro-3-hydroxybutanoate. Biosci Biotechnol Biochem 76:1210–1212
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301–312
Applegate GA, Cheloha RW, Nelson DL, Berkowitz DB (2011) A new dehydrogenase from Clostridium acetobutylicum for asymmetric synthesis: dynamic reductive kinetic resolution entry into the Taxotere side chain. Chem Commun 47:2420–2422
Cho CW, Cho YHG, Chun JP, Roh KR, Shin JH, Yu HS (2002) Process for producing optically pure δ-hydroxy-β-ketoester derivatives. WO 2002096915 A1
Chung CT, Niemela SL, Miller RH (1989) One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci 86:2172–2175
Clark JH (1999) Green chemistry: challenges and opportunities. Green Chem 1:1–8
Engelking H, Pfaller R, Wich G, Weuster-Botz D (2004) Stereoselective reduction of ethyl 4-chloro acetoacetate with recombinant Pichia pastoris. Tetrahedron Asymmetry 15:3591–3593
Hoff BH, Anthonsen T (1999) Lipase-catalyzed resolution of esters of 4-chloro-3-hydroxybutanoic acid: effects of the alkoxy group and solvent on the enantiomeric ratio. Tetrahedron Asymmetry 10:1401–1412
Kaliaperumal T, Kumar S, Gummadi SN, Chadha A (2010) Asymmetric synthesis of (S)-ethyl-4-chloro-3-hydroxybutanoate using Candida parapsilosis ATCC 7330. J Ind Microbiol Biotechnol 37:159–165
Kizaki N, Yasohara Y, Hasegawa J, Wada M, Kataoka M, Shimizu S (2001) Synthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 55:590–595
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Madec J, Pfister X, Phansavath P, Ratovelomanana-Vidal V, Genet JP (2001) Asymmetric hydrogenation reactions using a practical in situ generation of chiral ruthenium-diphosphine catalysts from anhydrous RuCl3. Tetrahedron 57:2563–2568
Matsuda T, Yamanaka R, Nakamura K (2009) Recent progress in biocatalysis for asymmetric oxidation and reduction. Tetrahedron Asymmetry 20:513–557
Morikawa S, Nakai T, Yasohara Y, Nanba H, Kizaki N, Hasegawa J (2005) Highly active mutants of carbonyl reductase S1 with inverted coenzyme specificity and production of optically active alcohols. Biosci Biotechnol Biochem 69:544–552
Nakamura K, Yamanaka R, Matsuda T, Harada T (2003) Recent developments in asymmetric reduction of ketones with biocatalysts. Tetrahedron Asymmetry 14(18):2659–2681
Nie Y, Xiao R, Xu Y, Montelione GT (2011) Novel anti-Prelog stereospecific carbonyl reductases from Candida parapsilosis for asymmetric reduction of prochiral ketones. Org Biomol Chem 9:4070–4078
Qiu L, Qi J, Pai CC, Chan S, Zhou Z, Choi MC, Chan AS (2002) Synthesis of novel diastereomeric diphosphine ligands and their applications in asymmetric hydrogenation reactions. Org Lett 4:4599–4602
Roth BD (2002) The discovery and development of atorvastatin, a potent novel hypolipidemic agent. Prog Med Chem 40:1–22
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press
Shimoda K, Kubota N, Hamada H, Kobayashi T, Hamada H, Shafi SM, Nakajima N (2009) Production of (2R, 3S)-2-benzamidomethyl-3-hydroxybutanoates by immobilized plant cells of parthenocissus tricuspidata. Biochem Insights 2:1–3
Soni P, Banerjee UC (2005) Biotransformations for the production of the chiral drug (S)-duloxetine catalyzed by a novel isolate of Candida tropicalis. Appl Microbiol Biotechnol 67:771–777
Sundby E, De Zotti M, Anthonsen T (2003) The enantioselectivity of reduction of ethyl 4-halo-3-oxobutanoate catalyzed by Geotrichum candidum depends on the cofactor. J Mol Catal B Enzym 21:63–66
Wang LJ, Li CX, Ni Y, Zhang J, Liu X, Xu JH (2011) Highly efficient synthesis of chiral alcohols with a novel NADH-dependent reductase from Streptomyces coelicolor. Bioresour Technol 102:7023–7028
Yamamoto H, Mitsuhashi K, Kimoto N, Matsuyama A, Esaki N, Kobayashi Y (2004) A novel NADH-dependent carbonyl reductase from Kluyveromyces aestuarii and comparison of NADH-regeneration system for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate. Biosci Biotechnol Biochem 68:638–649
Yasohara Y, Kizaki N, Hasegawa J, Wada M, Kataoka M, Shimizu S (2001) Stereoselective reduction of alkyl 3-oxobutanoate by carbonyl reductase from Candida magnoliae. Tetrahedron Asymmetry 12:1713–1718
Ye Q, Yan M, Yao Z, Xu L, Cao H, Li ZJ, Chen Y, Li SY, Bai JX, Xiong J, Ying HJ, Ouyang PK (2009) A new member of the short-chain dehydrogenases/reductases superfamily: Purification, characterization and substrate specificity of a recombinant carbonyl reductase from Pichia stipitis. Bioresour Technol 100:6022–6027
Ye Q, Cao H, Zang GL, Mi L, Yan M, Wang Y, Zhang YY, Li XM, Li JA, Xu L, Xiong JA, Ouyang PK, Ying HJ (2010) Biocatalytic synthesis of (S)-4-chloro-3-hydroxybutanoate ethyl ester using a recombinant whole-cell catalyst. Appl Microbiol Biotechnol 88:1277–1285
Ye Q, Ouyang PK, Ying H (2011) A review-biosynthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate ester: recent advances and future perspectives. Appl Microbiol Biotechnol 89:513–522
You ZY, Liu ZQ, Zheng YG (2014) Characterization of a newly synthesized carbonyl reductase and construction of a biocatalytic process for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate with high space-time yield. Appl Microbiol Biotechnol 98:1671–1680
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program) (No. 2011CB710800), Natural Science Foundation of Zhejiang Province (No. R3110155) and Qianjiang Talent Project of Zhejiang Province.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 131 kb)
Rights and permissions
About this article
Cite this article
Liu, ZQ., Ye, JJ., Shen, ZY. et al. Upscale production of ethyl (S)-4-chloro-3-hydroxybutanoate by using carbonyl reductase coupled with glucose dehydrogenase in aqueous-organic solvent system. Appl Microbiol Biotechnol 99, 2119–2129 (2015). https://doi.org/10.1007/s00253-014-6245-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6245-y