Abstract
Marichromatium gracile: YL28 (M. gracile YL28) is an anoxygenic phototrophic bacterial strain that utilizes ammonia, nitrate, or nitrite as its sole nitrogen source during growth. In this study, we investigated the removal and transformation of ammonium, nitrate, and nitrite by M. gracile YL28 grown in a combinatorial culture system of sodium acetate-ammonium, sodium acetate-nitrate and sodium acetate-nitrite in response to different initial dissolved oxygen (DO) levels. In the sodium acetate-ammonium system under aerobic conditions (initial DO = 7.20–7.25 mg/L), we detected a continuous accumulation of nitrate and nitrite. However, under semi-anaerobic conditions (initial DO = 4.08–4.26 mg/L), we observed a temporary accumulation of nitrate and nitrite. Interestingly, under anaerobic conditions (initial DO = 0.36–0.67 mg/L), there was little accumulation of nitrate and nitrite, but an increase in nitrous oxide production. In the sodium acetate-nitrite system, nitrite levels declined slightly under aerobic conditions, and nitrite was completely removed under semi-anaerobic and anaerobic conditions. In addition, M. gracile YL28 was able to grow using nitrite as the sole nitrogen source in situations when nitrogen gas produced by denitrification was eliminated. Taken together, the data indicate that M. gracile YL28 performs simultaneous heterotrophic nitrification and denitrification at low-DO levels and uses nitrite as the sole nitrogen source for growth. Our study is the first to demonstrate that anoxygenic phototrophic bacteria perform heterotrophic ammonia-oxidization and denitrification under anaerobic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In past decades, an increased interest in studying microorganisms that metabolize nitrogen has increased our understanding of nitrogen cycling in terrestrial and marine ecosystems and has led to improved technology for the biological removal of nitrogen in these ecosystems (Fowler et al. 2013). The most common mechanisms for biological removal of nitrogen are nitrification by autotrophs (ammonium-oxidizing bacteria and nitrite-oxidizing bacteria) under aerobic conditions and denitrification by heterotrophs (denitrifying bacteria) under anaerobic conditions.
Previous studies have shown that aerobic denitrification occurs at high-dissolved oxygen (DO) levels and nitrification occurs in response to low-DO levels (Huang et al. 2015; Ma et al. 2015; Zheng et al. 2014; Critchley et al. 2014). Certain groups of heterotrophic-nitrifying bacteria, such as Pseudomonas stutzeri (Zhang et al. 2011), Bacillus strains (Yang et al. 2011), Alcaligenes faecalis (Joo et al. 2005), Alcaligenes sp. S84S3, Proteus sp. S19 (Angar et al. 2016), Acinetobacter sp. (Yao et al. 2013), Paracoccus denitrificans (Stouthamer et al. 1997), Thiosphaera pantotropha (Arts et al. 1995), and Comamonas sp. (Patureau et al. 1997), perform heterotrophic nitrification and aerobic denitrification. Some nitrifying reactors operate stably at DO concentrations below 0.5 mg/L (Bellucci et al. 2011; Liu and Wang 2013; Park and Noguera 2004); however, the microorganisms that exist at low-DO nitrifying reactors are not well defined. Ammonia-oxidizing archaea and ammonia-oxidizing bacteria are present in low-DO nitrifying reactors (Park et al. 2006; Labrenz et al. 2010; Erguder et al. 2009), but their abundance in the reactors is not known. Anaerobic ammonia-oxidizing (anammox) bacteria (De Clippeleir et al. 2011) and heterotrophic nitrifiers (Zhang et al. 2011) have also been suggested to be important for nitrification in low-DO nitrifying reactors, but the role these bacteria play and their interrelationship in the microbial community of the reactors is unclear. Strains of Pseudomonas, Xanthomonadaceae, Rhodococcus, and Sphingomonas were shown to be involved in ammonia oxidation in a low-DO lab-scale reactor (Fitzgerald et al. 2015). These strains are the first microorganisms demonstrated to perform nitrification at low-DO levels; however, their underlying metabolic mechanisms remain unknown.
Anoxygenic phototrophic bacteria (APB) have gained widespread application in aquaculture due to their metabolic diversity, their roles in inorganic nitrogen removal and water purification, and their utility as fish feed (Qi et al. 2009). APB, which include purple sulfur bacteria, purple nonsulfur bacteria, green sulfur bacteria, and green nonsulfur bacteria, convert light energy into chemical energy by anoxygenic photosynthesis. Some APB species also grow in darkness as either chemoorganotrophs or chemolithotrophs (Pfennig and Trüper 1989). In addition, APB are widely involved in nitrogen fixation, ammonification, ammonia assimilation, nitrification, and denitrification (reported in purple nonsulfur bacteria but not yet in purple sulfur bacteria). Ammonium is the preferred nitrogen source for APB. In the presence of ammonium, some APB strains consume nitrate and nitrite through denitrification (Alef et al. 1985; Chen et al. 2011). Other APB strains use nitrate as the sole nitrogen source in the absence of ammonium (Shapleigh 2008). However, APB rarely use nitrite as the sole nitrogen source during growth (Madigan 1995).
In this study, we present the novel finding that an anoxygenic phototrophic bacterium, Marichromatium gracile YL28, (M. gracile YL28) can perform simultaneous heterotrophic nitrification and denitrification at low-DO as well as utilize nitrite as the sole nitrogen source during growth.
Materials and methods
Bacterial strains
Marichromatium gracile YL28 was isolated from the mangrove wetlands. The 16S rRNA gene was sequenced (GenBank Accession Number JF719917) as previously described (Zhao et al. 2011). M. gracile YL28 was identified as a purple sulfur bacterium, which converts light energy into chemical energy by anoxygenic photosynthesis.
Growth conditions
Modified Pfennig medium (Pfennig and Trüper 1992) was supplemented with 24.38 mmol/L sodium acetate as the carbon source and was used as the base growth medium for M. gracile YL28. Different concentrations of ammonium chloride, representing high, medium and low concentrations, sodium nitrate, or sodium nitrite were added to the base medium as the source of nitrogen, according to the experimental design. The medium pH was adjusted to 7.0. To ensure that each culture was inoculated with equal bacterial numbers, the cell densities of the inocula were adjusted to an OD600 = 0.15. The bacterial cultures were incubated at 28 °C in light with surface irradiance of 3000 lux and at one of three initial DO levels - aerobic, semi-anaerobic, or anaerobic - as described below:
Aerobic growth
500 mL flasks containing 100 mL of sterile medium were used and static incubated in an illuminated incubator with jiggling once a day for homogenization.
Semi-anaerobic growth
We used sterilized 250 mL screw-cap bottles containing 200 mL of sterile medium. After inoculation, additional sterile medium was added to the bottles for a total volume of 310 mL, eliminating any additional room for air in the bottles. The bottles were static incubated in an illuminated incubator with jiggling once a day for homogenization.
Anaerobic growth
We added resazurin sodium salt (0.2%) and l-cysteine hydrochloride (0.5 g/L) to the culture medium. Sterilized screw-cap bottles containing the medium were transferred to an anaerobic chamber (PLAS-LAB 855-AC, USA) immediately after sterilization, and the medium was aliquoted either to 50 in 100 mL serum bottles or to 5 in 20 mL headspace sampling bottles for gas kinetics analysis. Unless otherwise specified, the anaerobic chambers were filled with argon. After inoculation, the serum bottles or headspace sampling bottles were sealed with rubber septa and aluminum caps and transferred to an illuminated incubator with jiggling once a day for homogenization. When nitrogen gas was exchanged for argon we used jars that were covered with eight layers of gauze (instead of rubber stoppers). Since density of nitrogen gas is lower than that of argon, the nitrogen gas is then released from the jars. The jars were moved to the transfer chamber under vacuum every 8 h to deplete the jars of nitrogen gas.
Growth was determined via turbidity measurements at a wavelength of 600 nm using a spectrophotometer (UV-2100, UNICO, USA).
Chemical analyses
The nitrate concentration was determined using a previously described UV spectrophotometric method (Critchley et al. 2014) that calculates the difference between measurements taken at OD220 and at OD275 (OD220 − 2 × OD275). The nitrite concentration was measured by colorimetry at a wavelength of 540 nm as previously described (Critchley et al. 2014). The ammonium concentration was measured using Nessler’s reagent according to a previously described protocol (Gebre et al. 2016). Chemical analysis data were subjected to one-way analysis of variance (ANOVA) followed by a multiple comparison test using the LSD-t test to determine the difference in mean values. Data analysis was conducted with SPSS software. Significance was set at P < 0.05. The initial DO levels in the medium were measured using a handheld optical DO meter (ProODO, YSI, USA) that utilizes luminescent technology.
Kinetics of gas production
The headspace sampling bottles were moved at set intervals to a semiautomatic system for gas measurements (Molstad et al. 2007). The gas from the headspace was sampled by an autosampling system connected to a peristaltic pump, which pumped the sample into a PLOT gas chromatography column (Agilent GC - 7890A, USA) to separate CH4, CO2 and N2O. N2O was measured by a thermal conductivity detector (TCD) and an electron capture detector (ECD).
Results
Ammonium removal and heterotrophic nitrification
We incubated the bacteria with three concentrations of ammonium as the sole nitrogen source and create aerobic, semi-anaerobic and anaerobic conditions for incubating. The culture density, ammonium, nitrate, and nitrite concentrations were measured.
Under aerobic growth conditions, the initial DO range in the medium was 7.20–7.25 mg/L. In response to this DO range, NH4 +-N removal and culture density increased exponentially during the first few days of growth until maximum NH4 +-N removal was achieved (Fig. 1). When the ammonium concentration was 48.06 mmol/L, the removal of ammonium reached 7.16 mmol/L (14.90%). M. gracile YL28 grew to a maximum OD600 ranging from 2.13 to 2.42, with initial ammonium concentrations ranging from 4.23 to 48.06 mmol/L. Continuous accumulation of nitrate and nitrite was concomitantly observed with ammonium removal, and no apparent decrease in nitrite and nitrate concentrations was observed. The maximum concentration of nitrate and nitrite was 0.08–0.12 and 0.05–0.09 mmol/L, respectively.
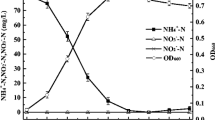
Under semi-anaerobic growth conditions, the initial DO range of the medium was 4.08–4.26 mg/L. Using these growth conditions, the maximum concentration of NH4 +-N removed was 8.80 mmol/L (18.24%) with an initial ammonium concentration of 48.23 mmol/L (Fig. 2). The amount of ammonium removed during semi-anaerobic growth was similar to that during aerobic growth; however, the culture density was greater under semi-anaerobic conditions compared to aerobic conditions (P < 0.05). Nitrate and nitrite temporarily accumulated in the first few days but declined thereafter. The maximum nitrate concentration was 0.58–0.63 mmol/L on day 2, and this level declined to the background level by day 7. When the initial ammonium concentration was 4.30 and 24.04 mmol/L, the nitrite concentration peaked on day 4, at 0.11 mmol/L (2.56%) and 0.14 mmol/L (0.58%), respectively, and was eventually depleted; when the initial ammonium concentration was 48.06 mmol/L, the nitrite concentration continued to accumulate for 5 days and peaked at 0.09 mmol/L (0.19%). Trace amounts of gas production were observed as bubbles during growth.
Heterotrophic nitrification in a sodium acetate-ammonium system with aerobic conditions; initial DO levels of 7.20–7.25 mg/L; surface irradiance of 3000 lux; 24.38 mmol/L of sodium acetate as the carbon source; and 4.23, 23.76, and 48.06 mmol/L NH4 +-N as the initial nitrogen sources. Error bars show the standard deviations of three replicate experiments. a Removal of NH4 +-N, b OD600, c accumulated concentration of nitrate, and d accumulated nitrite concentration
Heterotrophic nitrification in a sodium acetate-ammonium system with semi-anaerobic conditions; initial DO levels of 4.08–4.26 mg/L; surface irradiance of 3000 lux; 24.38 mmol/L sodium acetate as the carbon source; and 4.30, 24.04, and 48.23 mmol/L NH4 +-N as the initial nitrogen sources. Error bars show the standard deviations of three replicate experiments. a Removal of NH4 +-N, b OD600, c accumulated concentration of nitrate, and d accumulated concentration of nitrite
Under anaerobic growth conditions, the initial DO range of the medium was reduced to 0.36–0.67 mg/L. The maximum amount of NH4 +-N removed during anaerobic growth increased as the initial ammonium concentrations increased (P < 0.05). When the initial ammonium concentration was 48.18 mmol/L (Fig. 3), a maximum of 22.15 mmol/L (45.97%) of ammonium was removed. This ammonium concentration was much higher than the concentrations removed under aerobic and semi-anaerobic conditions. The culture density reached a maximum OD600 of 3.79–3.98 on day 9, the last day of the experiment, and growth still had not plateaued. Neither nitrate nor nitrite had accumulated during growth, but gas production was observed as bubbles in the medium.
Heterotrophic nitrification in sodium acetate-ammonia system with anaerobic conditions; initial DO levels of 0.36–0.67 mg/L; surface irradiance of 3000 lux; 24.38 mmol/L sodium acetate as the carbon source; and 4.25, 23.89, and 48.18 mmol/L NH4 +-N as the initial nitrogen sources. Error bars show the standard deviations of three replicate experiments. a Removal of NH4 +-N, b OD600, c accumulated concentration of nitrate, and d accumulated concentration of nitrite
These results suggest that in anaerobic conditions M. gracile YL28 growth increases and its ability to remove high ammonium concentrations is enhanced. M. gracile YL28 transformed ammonium to nitrate and nitrite by heterotrophic nitrification under aerobic and semi-anaerobic conditions. We previous published that M. gracile YL28 can remove nitrite at a high rate (Jiang et al. 2014). Thus, the absence of nitrate and nitrite under anaerobic conditions can be explained by denitrification. Based on these results we proposed the following mechanisms. First, the sustained accumulation of nitrate and nitrite under aerobic conditions might be attributed to the inhibition of denitrification by high DO levels. Second, the temporary accumulation of nitrate and nitrite under semi-anaerobic conditions may be the result of a dynamic balance of nitrification and denitrification, as the inhibition by oxygen was alleviated to a certain extent. Third, under anaerobic conditions, nitrate and nitrite might be immediately transformed by denitrification, which would result in the absence of nitrate and nitrite.
Denitrification
To verify if denitrification occurs during semi-anaerobic and anaerobic growth, we replaced ammonium with 2.00 mmol/L of nitrite as the sole nitrogen source in batch cultures grown under aerobic, semi-anaerobic, and anaerobic conditions. As shown in Fig. 4, nitrite removal was minimal after 6 days of aerobic growth. However, nitrite was almost completely removed by day 4 under the semi-anaerobic growth conditions and day 2 under the anaerobic conditions, with gas production observed as bubbles in both conditions. These results indicate that denitrification occurs under both semi-anaerobic and anaerobic growth conditions and that oxygen might inhibit the rate of denitrification.
Denitrification in a sodium acetate-nitrite system with either aerobic, semi-anaerobic, or anaerobic conditions; an initial nitrite concentration of 2.00, 1.97 and 1.96 mmol/L, respectively; surface irradiance of 3000 lux and 24.38 mmol/L sodium acetate as the carbon source. Error bars show the standard deviations of three replicate experiments
Gas kinetics
As described above, we observed increased gas production in M. gracile YL28 grown under semi-anaerobic and anaerobic conditions. To further verify this finding we determined the composition and volume of the gas produced. To elucidate the heterotrophic nitrification in response to low-DO levels, bacterial growth was measured in 5 mL of growth medium in sampling bottles with a 20 mL headspace instead of 100 mL of growth medium in serum bottles. All other growth conditions were similar to the experiments described above for anaerobic growth. Nitrate, nitrite and ammonium were added separately at initial concentrations of 5.25, 5.36 and 5.21 mmol/L, respectively, and 24.38 mmol/L sodium acetate was supplemented as the carbon source. The headspace sampling bottles were moved at set intervals to a semiautomatic system for gas measurement. The gas sample from the headspace was removed by an autosampler system and N2O was separated and measured by chromatography. As shown in Fig. 5, in the presence of nitrate and nitrite, the N2O concentration increased to 821.78 and 2622.32 ppm, respectively, by day 3 of culture, indicating that denitrification converts nitrate and nitrite to N2O. These levels declined to 87.56 and 242.12 ppm after 9 days of growth. However, when ammonium was used as the sole nitrogen source, the N2O concentration increased from 2.63 to 286.66 ppm during the first 3 days and gradually declined to 10.35 ppm after 9 days of growth. With the exception of ammonium consumption during the growth of the bacteria, some ammonium was converted to nitrate and nitrite through heterotrophic nitrification and consequently converted to N2O by denitrification. These data confirm that M. gracile YL28 converts ammonium to nitrate or nitrite by heterotrophic nitrification at low DO levels and converts nitrate or nitrite to N2O by denitrification. In summary, our findings explain the absence of nitrate and nitrite accumulation during growth under anaerobic condition (Fig. 3).
Gas kinetics during growth with nitrate, nitrite and ammonium at initial concentrations of 5.25, 5.36 and 5.21 mmol/L respectively, as sole nitrogen sources. Sodium acetate (24.38 mmol/L) was added as the carbon source and the initial DO levels was 0.37–0.66 mg/L. Sampling bottles with 20 mL headspace and 5 mL sterile medium were incubated with surface irradiance of 3000 lux; the headspace sampling bottles were moved at intervals to a semiautomatic system for gas measurement to detect N2O production. Error bars show the standard deviations of three replicate experiments
Growth with different nitrogen sources
In earlier experiments, a slow but steady increase in culture density was observed as nitrite was removed. To further investigate the growth requirements of M gracile YL28 when nitrite is the sole nitrogen source, batch cultures of M. gracile YL28 were grown with different nitrogen sources in the presence and absence of light.
When grown at an initial nitrite concentration of 4.85 mmol/L under anaerobic conditions, the M. gracile YL28 culture density increased from a starting OD600 of 0.15 to an OD600 of 0.425 when grown in light and an OD600 of 0.417 when grown in the dark (Fig. 6a). In contrast, when the nitrogen source was replaced with ammonium at an initial concentration of 5.12 mmol/L, the culture density reached an OD600 of 3.717 in the presence of light; we did not observe any change in growth when grown in the dark (Fig. 6b). These results demonstrate that M. gracile YL28 prefers ammonium over nitrite as the sole nitrogen source when grown in light. The finding that M. gracile YL28 did not grow in the dark with ammonium as the sole nitrogen source could likely be attributed to a lack of photosynthesis and a limited amount of energy produced via nitrification. In contrast, when nitrite was used as the sole nitrogen source, M. gracile YL28 acquired energy via denitrification and, therefore was capable of growing in the absence of light.
Growth of M. gracile YL28 under anaerobic conditions in the presence and absence of light with different nitrogen sources. Nitrogen sources are as follows: a 4.85 mmol/L nitrite, b 5.12 mmol/L ammonium, c nitrogen gas, and d 4.93 mmol/L nitrite while nitrogen gas produced by denitrification was eliminated during growth. Error bars show the standard deviations of three replicate experiments
Considering that many species of phototrophic bacteria can fix nitrogen, we investigated if M. gracile YL28 utilizes nitrite to produce nitrous oxide via denitrification and acquires nitrogen through nitrogen fixation. To test whether M. gracile YL28 can fix nitrogen, we exchanged the nitrogen gas with argon gas in the anaerobic chamber and no nitrogen source was added to the growth medium. As shown in Fig. 6c, the culture density increased to an OD600 of 2.709 in the presence of light, but no growth was observed when the strain was grown in the dark, indicating that M. gracile YL28 can fix nitrogen under light conditions, but not under dark conditions due to the lack of energy production.
Since our data indicated that M. gracile YL28 can fix nitrogen, we removed nitrogen gas produced by denitrification to test if the strain can assimilate nitrogen via nitrate reduction. To test this, argon gas was added in the anaerobic chamber and nitrite was added as the sole nitrogen source at an initial concentration of 4.93 mmol/L. As shown in Fig. 6d, when the nitrogen gas produced by denitrification, M. gracile YL28 still grew to a maximum OD600 of 0.435 and 0.422 in the presence and absence of light, respectively, indicating that M. gracile YL28 can utilize nitrite as the sole nitrogen source through assimilatory nitrate reduction and that it can acquire energy through denitrification both in light and dark conditions.
Discussion
In recent years, bacteria capable of heterotrophic nitrification and their mechanisms of nitrogen metabolism have been intensively studied (Su et al. 2015; Angar et al. 2016). The enzymatic pathways for heterotrophic nitrification are similar to those of autotrophic nitrification, in which ammonium is transformed to hydroxylamine by ammonia monooxygenase (AMO), and hydroxylamine is subsequently metabolized to nitrite and/or nitrate by hydroxylamine oxidoreductase (HAO) and nitrite oxidoreductase (NXR) (Tran et al. 2013). APB have the ability to perform nitrogen fixation, ammonification, and ammonia assimilation (Madigan 1995; Bast 1977; Kranz and Cullen 2004). Furthermore, heterotrophic nitrification has been detected in APB. Griffin et al. (Griffin et al. 2007) first reported that the purple sulfur bacterium Thiocapsa roseopersicina could oxidize nitrite to nitrate in the presence of light under anaerobic conditions, and the Thiocapsa sp. strain KS1 and Rhodopseudomonas sp. strain LQ17 were subsequently found to have common capabilities (Schott et al. 2010). Therefore, APB were thought to be nitrite-oxidizing bacteria (NOB) (Sorokin et al. 2012). However, evidence was lacking showing that APB could convert ammonium to nitrite through heterotrophic pathways. In the present study, we found that the APB M. gracile YL28 can convert ammonium to nitrite via heterotrophic pathways, providing evidence that APB are ammonia-oxidizing bacteria that utilize heterotrophic nitrification.
Denitrification by APB has been widely reported for purple non-sulfur bacteria but has not yet been reported in purple sulfur bacteria. Denitrification generally occurs under anaerobic conditions and is inhibited under aerobic conditions. In this study, we detected a sustained accumulation of nitrate and nitrite in M. gracile YL28 grown aerobically with ammonium as the sole nitrogen source, although we did not observe a significant change in bacterial growth. Under semi-anaerobic conditions, we detected temporary accumulation of nitrate and nitrite, which then gradually depleted. In this anaerobic condition we did not detect an accumulation of nitrate and nitrite, but we did observe an increase in N2O production, indicating that denitrification occurred. Nitrogen metabolism under the aerobic, semi-anaerobic, and anaerobic growth conditions might be explained by the occurrence of simultaneous nitrification and denitrification.
Heterotrophic nitrification of M. gracile YL28 at low-DO levels was consistent with a report by Griffin et al. (Griffin et al. 2007). However, in this study, not only was nitrite oxidized to nitrate, but ammonium was oxidized to nitrite. The heterotrophic nitrification at low-DO was detected only in the presence of light since ammonium was oxidized to nitrite and further to nitrate by anoxygenic photosynthesis. Microbial communities involved in ammonia oxidation under low-DO levels have generally been investigated using chemostat reactors, and the microorganisms involved in nitrogen metabolism were not known until recently. Pseudomonas, Xanthomonadaceae, Rhodococcus, and Sphingomonas were proposed to be involved in nitrification under low-DO levels (Fitzgerald et al. 2015). In the present study, M. gracile YL28 performed simultaneous phototrophic nitrification and denitrification at low-DO concentrations, furthering our understanding of nitrogen metabolism.
Many APB species use nitrate and nitrite in the environment; however, denitrification is a dissimilatory nitrate reduction and the energy produced cannot support bacterial growth. Nitrate and nitrite need be transformed to ammonia through assimilatory nitrate reduction to allow for bacterial growth (Madigan and Jung 2008). Ammonia is usually the preferred nitrogen source of APB, but when ammonia is absent, some APB strains can assimilate nitrate or nitrite. The genetics and biochemistry involved in assimilatory nitrate reduction have been intensively studied in Rhodopseudomonas capsulata AD2 (Alef and Klemme 1979) and Rhodobacter capsulatus E1F1 (Pino et al. 2006) during growth using nitrate as the sole nitrogen source. In some nitrite-oxidizing bacterium, such as Nitrobacter winogradskyi (Sayavedra-Soto et al. 2015) and Acinetobacter baumannii (Fan et al. 2015), nitrite serves as the sole nitrogen source through assimilatory nitrite reduction. However, in APB, only the Thiocapsa sp. strain KS1, Rhodopseudomonas sp. strain LQ17, and T. roseopersicina strains DSM 217 and DSM 221 can oxidize nitrite to nitrate through anoxygenic photosynthesis (Griffin et al. 2007; Schott et al. 2010). Fixation of molecular nitrogen is common among most APB, in which case nitrogen is assimilated from nitrate to nitrite through denitrification and then molecular nitrogen undergoes fixation. In this study, M. gracile YL28 assimilated nitrogen through nitrogen fixation and continued to grow with nitrite as the sole nitrogen source in the presence and absence of light using assimilatory nitrate reduction.
In conclusion, M. gracile YL28 simultaneously performs heterotrophic nitrification and denitrification at low-DO levels and uses nitrite as the sole nitrogen source. This is the first report of an APB that performs heterotrophic ammonia-oxidization and ammonia-oxidization at low-DO levels and the first report of denitrification in purple sulfur bacteria. These results further our understanding of microorganisms involved in the nitrogen cycle.
Abbreviations
- APB:
-
Anoxygenic phototrophic bacteria
- DO:
-
Dissolved oxygen
References
Alef K, Klemme JH (1979) Assimilatory nitrate reductase of Rhodopseudomonas capsulata AD2: a molybdo-hemeprotein. Z Naturforsch 34:33–37
Alef K, Jackson JB, McEwan AG, Ferguson SJ (1985) The activities of two pathways of nitrate reduction in Rhodopseudomonas capsulata. Arch Microbiol 142:403–408
Angar Y, Kebbouche-Gana S, Djelali NE, Khemili-Talbi S (2016) Novel approach for the ammonium removal by simultaneous heterotrophic nitrification and denitrification using a novel bacterial species co-culture. World J Microbiol Biotechnol 32:36
Arts PAM, Robertson LA, Kuenen JG (1995) Nitrification and denitrification by Thiosphaera pantotropha in aerobic chemostat cultures. FEMS Microbiol Ecol 18:305–316
Bast E (1977) Utilization of nitrogen compounds and ammonia assimilation by Chromatiaceae. Arch Microbiol 113:91–94
Bellucci M, Ofiteru ID, Graham DW, Head IM, Curtis TP (2011) Low-dissolved-oxygen nitrifying systems exploit ammonia-oxidizing bacteria with unusually high yields. Appl Environ Microbiol 77:7787–7796
Chen H, Zhang DM, Wang LG, Pan ZC (2011) Biological characteristics and phylogenetic analysis of a denitrifying photosynthetic bacterium. Acta Microbiol Sin 51:249–255
Critchley K, Rudolph DL, Devlin JF, Schillig PC (2014) Stimulating in situ denitrification in an aerobic, highly permeable municipal drinking water aquifer. J Contam Hydrol 171:66–80
De Clippeleir H, Yan XG, Verstraete W, Vlaeminck SE (2011) OLAND is feasible to treat sewage-like nitrogen concentrations at low hydraulic residence times. Appl Microbiol Biotechnol 90:1537–1545
Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W (2009) Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol Rev 33:855–869
Fan LM, Chen JZ, Liu Q, Wu W, Meng SL, Song C, Qu JH, Xu P (2015) Exploration of three heterotrophic nitrifying strains from a tilapia pond for their characteristics of inorganic nitrogen use and application in aquaculture water. J Biosci Bioeng 119:303–309
Fitzgerald CM, Camejo P, Oshlag JZ, Noguera DR (2015) Ammonia-oxidizing microbial communities in reactors with efficient nitrification at low-dissolved oxygen. Water Res 70:38–51
Fowler D, Coyle M, Skiba U, et al. (2013) The global nitrogen cycle in the twenty-first century. Phil Trans R Soc B 368:91–97
Gebre AE, Demissie HF, Mengesha ST, Segni MT (2016) The pollution profile of Modjo River due to industrial wastewater discharge, in Modjo Town, Oromia, Ethiopia. J Environ Anal Toxicol 6:363
Griffin BM, Schott J, Schink B (2007) Nitrite, an electron donor for anoxygenic photosynthesis. Science 326:1870
Huang TL, Zhou SL, Zhang HH, Zhou N, Guo L, Di SY, Zhou ZZ (2015) Nitrogen removal from micro-polluted reservoir water by indigenous aerobic denitrifiers. Int J Mol Sci 16:8008–8026
Jiang P, Zhao CG, Jia YQ, Yang SP (2014) Inorganic nitrogen removal by a marine purple sulfur bacterium capable of growth on nitrite as sole nitrogen source. Microbiol China 41:824–831
Joo HS, Hirai M, Shoda M (2005) Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis No. 4. J Biosci Bioeng 100:184–191
Kranz RG, Cullen PJ (2004) Regulation of nitrogen fixation genes. Anoxygenic photosynthetic bacteria. Springer, New York, pp 1191–1208
Labrenz M, Sintes E, Toetzke F, Zumsteg A, Herndl GJ, Seidler M, Jürgens K (2010) Relevance of a crenarchaeotal subcluster related to Candidatus Nitrosopumilus maritimus to ammonia oxidation in the suboxic zone of the central Baltic Sea. ISME J 4:1496–1508
Liu GQ, Wang JM (2013) Long-term low DO enriches and shifts nitrifier community in activated sludge. Environ Sci Technol 47:5109–5117
Ma F, Sun YL, Li A, Zhang XN, Yang JX (2015) Activation of accumulated nitrite reduction by immobilized Pseudomonas stutzeri T13 during aerobic denitrification. Bioresour Technol 187:30–36
Madigan MT (1995) Microbiology of nitrogen fixation by anoxygenic photosynthetic bacteria. Anoxygenic photosynthetic bacteria. Springer, New York, pp 915–928
Madigan MT, Jung DO (2008) An overview of purple bacteria: systematics, physiology, and habitats. In The purple phototrophic bacteria. Springer, New York, pp 1–15
Molstad L, Dörsch P, Bakken LR (2007) Robotized incubation system for monitoring gases (O2, NO, N2O, N2) in denitrifying cultures. J Microbiol Methods 71:202–211
Park HD, Noguera DR (2004) Evaluating the effect of dissolved oxygen on ammonia-oxidizing bacterial communities in activated sludge. Water Res 38:3275–3286
Park HD, Wells GF, Bae H, Criddle CS, Francis CA (2006) Occurrence of ammonia-oxidizing archaea in wastewater treatment plant bioreactors. Appl. Environ Microbiol 72:5643–5647
Patureau D, Bernet N, Moletta R (1997) Combined nitrification and denitrification in a single aerated reactor using the aerobic denitrifier Comamonas sp. strain SGLY2. Water Res 31:1363–1370
Pfennig N, Trüper H (1989) Anoxygenic phototrophic bacteria. Bergey’s Man Syst Bacteriol 3:1635–1709
Pfennig N, Trüper H (1992) The prokaryotes. Springer, New York, pp 3203–3205
Pino C, Olmo-Mira F, Cabello P, Martínez-Luque M, Castillo F, Roldán MD, Moreno-Vivián C (2006) The assimilatory nitrate reduction system of the phototrophic bacterium Rhodobacter capsulatus E1F1. Biochem Soc Trans 34:127–129
Qi Z, Zhang XH, Boon N, Bossier P (2009) Probiotics in aquaculture of China-Current state, problems and prospect. Aquaculture 290:15–21
Sayavedra-Soto L, Ferrell R, Dobie M, Mellbye B, Chaplen F, Buchanan A, Chang J, Bottomley P, Arp D (2015) Nitrobacter winogradskyi transcriptomic response to low and high ammonium concentrations. FEMS Microbiol Lett 362:1–7
Schott J, Griffin BM, Schink B (2010) Anaerobic phototrophic nitrite oxidation by Thiocapsa sp. strain KS1 and Rhodopseudomonas sp. strain LQ17. Microbiology 156:2428–2437
Shapleigh JP (2008) Dissimilatory and assimilatory nitrate reduction in the purple photosynthetic bacteria. In The purple phototrophic bacteria. Springer, New York, pp 623–642
Sorokin DY, Lücker S, Vejmelkova D, Kostrikina NA, Kleerebezem R, Rijpstra WI, Damsté JS, Le Paslier D, Muyzer G, Wagner M, van Loosdrecht MC, Daims H (2012) Nitrification expanded: discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME J 6:2245–2256
Stouthamer AH, de Boer APN., van der Oost J, van Spanning RJM (1997) Emerging principles of inorganic nitrogen metabolism in Paracoccus denitrificans and related bacteria. Antonie Van Leeuwenhoek 71:33–41
Su JF, Zhang K, Huang TL, Wen G, Guo L, Yang SF (2015) Heterotrophic nitrification and aerobic denitrification at low nutrient conditions by a newly isolated bacterium, Acinetobacter sp. SYF26. Microbiology 161:829–837
Tran NH, Urase T, Ngo HH, Hu JY, Ong SL (2013) Insight into metabolic and cometabolic activities of autotrophic and heterotrophic microorganisms in the biodegradation of emerging trace organic contaminants. Bioresour Technol 146:721–731
Yang XP, Wang SM, Zhang DW, Zhou LX (2011) Isolation and nitrogen removal characteristics of an aerobic heterotrophic nitrifying-denitrifying bacterium, Bacillus subtilis A1. Bioresour Technol 102:854–862
Yao S, Ni JR, Ma T, Li C (2013) Heterotrophic nitrification and aerobic denitrification at low temperature by a newly isolated bacterium, Acinetobacter sp. HA2. Bioresour Technol 139:80–86
Zhang JB, Wu PX, Hao B, Yu ZN (2011) Heterotrophic nitrification and aerobic denitrification by the bacterium Pseudomonas stutzeri YZN-001. Bioresour Technol 102:9866–9869
Zhao JY, Fu YN, Zhao CG, Yang SP, Qu Y, Jiao NZ (2011) Identification and characterization of a purple sulfur bacterium from mangrove with rhodopin as predominant carotenoid. Acta Microbiol Sin 51:1318–1325
Zheng MS, He D, Ma T, Chen Q, Liu S, Ahmad M, Gui M, Ni J (2014) Reducing NO and N2O emission during aerobic denitrification by newly isolated Pseudomonas stutzeri PCN-1. Bioresour Technol 162:80–88
Acknowledgements
This study was supported by National Marine Public Industry Research, China (No. 201505026), Natural Science Foundation of Fujian Province, China (No. 2015J01137), Scientific Research Project of Xiamen Southern Ocean Center, China (14GYY74NF38) and Science and Technology Project of Xiamen, China (No. 3502Z20163020). We thank Lars Molstad from the Norwegian UMB-Nitrogen-group (http://www.umb.no/nitrogengroup/) for designing and programming our robotized system for analyzing gas kinetics, and Dr. Xiaoru Yang from the Institute of Urban Environment, Chinese Academy of Science for the gas kinetics analysis.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hong, X., Chen, Z., Zhao, C. et al. Nitrogen transformation under different dissolved oxygen levels by the anoxygenic phototrophic bacterium Marichromatium gracile . World J Microbiol Biotechnol 33, 113 (2017). https://doi.org/10.1007/s11274-017-2280-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-017-2280-z