Abstract
The correct and early identification of humans and dogs infected with Leishmania are key steps in the control of leishmaniasis. Additionally, a method with high sensitivity and specificity at low cost that allows the screening of a large number of samples would be extremely valuable. In this study, we analyzed the potential of mitogen-activated protein kinase 3 (MAPK3) and mitogen-activated protein kinase 4 (MAPK4) proteins from Leishmania braziliensis to serve as antigen candidates for the serodiagnosis of human visceral and tegumentary leishmaniasis, as well as canine visceral disease. Moreover, we mapped linear B-cell epitopes in these proteins and selected those epitopes with sequences that were divergent in the corresponding orthologs in Homo sapiens, in Canis familiaris, and in Trypanosoma cruzi. We compared the performance of these peptides with the recombinant protein using ELISA. Both MAPK3 and MAPK4 recombinant proteins showed better specificity in the immunodiagnosis of human and canine leishmaniasis than soluble parasite antigens and the EIE-leishmaniose-visceral-canina-bio-manguinhos (EIE-LVC) kit. Furthermore, the performance of this serodiagnosis assay was improved using synthetic peptides corresponding to B-cell epitopes derived from both proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is a disease caused by vector-borne parasites of Leishmania genus that affects approximately 12 million people around the world causing approximately 50,000 deaths each year (Desjeux 2001). Although the most affected countries are in tropical or subtropical regions, the disease is expanding to non-endemic areas in North America and Europe (Gonzalez et al. 2010; Ready 2010; Shaw 2007). Leishmaniasis encompasses multiple clinical syndromes that are classified as cutaneous, mucosal, and visceral, and the development of each clinical form is associated with the genetic variability of both parasite and host. Cutaneous and mucosal leishmaniasis can cause substantial morbidity (Molina et al. 1994), whereas visceral leishmaniasis is frequently fatal if untreated (Wilson and Streit 1996); thus, the correct and early diagnosis of infection is a key step in the control of this disease. Moreover, visceral leishmaniasis surveillance programs depend on the identification of infected dogs that are important reservoirs that maintain the domestic cycle of the parasite (Molina et al. 1994).
As clinical manifestation lacks specificity, the diagnosis of visceral leishmaniasis should be performed by parasite detection in the infected tissue by microscopy, PCR, and/or serology (Chappuis et al. 2007; Sundar and Rai 2002). Most of the serological tests use crude Leishmania-antigen preparations that include soluble antigens. Those antigens show variable sensitivity due to antigenic differences among Leishmania isolates and lack specificity due to cross-reactivity with other diseases (Caballero et al. 2007; Zanette et al. 2014). The diagnosis of tegumentary leishmaniasis considers epidemiological data, clinical aspects, and laboratory tests (Goto and Lindoso 2010), which include the Montenegro skin test (MST) and the microscopic detection of the parasite in lesions (Alves et al. 2013). The MST identifies a delayed-type hypersensitivity response to parasite antigens in infected individuals, but does not discriminate between active and cured infection (Stockdale and Newton 2013). The correct and early identification of infected humans and dogs are key steps in disease control. Therefore, a low-cost method with high sensitivity and specificity that allows for the screening of a large number of samples would be extremely valuable.
Serological tests have significant advantages for the diagnosis of leishmaniasis, including their non-invasive nature, their capacity for the early detection of infection before the formation of lesions, and their capacity to be automated and quantitative. However, a small number of well-characterized antigens have been described in the literature, hindering the development of new serodiagnostic tests with improved performance.
Previous studies have demonstrated that conserved housekeeping genes and virulence factors have a high potential for the serodiagnosis of leishmaniasis (Lakhal-Naouar et al. 2009; Menezes-Souza et al. 2014a). Among these genes, mitogen-activated protein kinases (MAPKs) are proteins encoded in practically all eukaryote genomes and display signal transduction functions associated with cytoskeletal rearrangements, proliferation, differentiation, adaptation, and stress-response (Brumlik et al. 2011; Pearson et al. 2001). Genomic analyses revealed that there are 17 putative MAPKs in Leishmania spp., being one of the most expanded protein kinase families when compared to mammals (Brumlik et al. 2011; Wiese 2007). Several MAPKs, including mitogen-activated protein kinase 3 (MAPK3) and mitogen-activated protein kinase 4 (MAPK4), have been implicated in the differentiation process of promastigote into amastigote. Whereas MAPK3 displays control of flagellar length (Erdmann et al. 2006), the overexpression of MAPK4 increases stage-specific phosphorylase activity (Wang et al. 2005).
Due to the importance of MAPK3 and MAPK4 in host-parasite interaction, we analyzed the potential of both proteins from Leishmania braziliensis to be used as antigens for the serological diagnosis of visceral and tegumentary human leishmaniasis and visceral canine disease. We also evaluated different protein characteristics that could influence the serodiagnosis performance, such as expression levels in parasite stages present in mammalian hosts and sequence similarity between other phylogenetically related parasites and hosts. Additionally, we mapped linear B-cell epitopes in these proteins with sequences that were divergent from their orthologs in Homo sapiens, Canis familiaris, and Trypanosoma cruzi and compared their performance with recombinant protein using ELISA.
Materials and methods
Ethics statement and human and dog sera samples used
Experiments involving human samples were approved by the Ethics Committee of the Federal University of Minas Gerais (UFMG) under protocol CAAE: 00842112.2.0000.5149. Before blood collection, all subjects provided written informed consent. A total of 65 sera samples from tegumentary leishmaniasis patients with cutaneous (cutaneous leishmaniasis (CL), n = 45) or mucosal (mucosal leishmaniasis (ML), n = 20) clinical manifestations were obtained from the Centro de Referência em Leishmaniose (Januária, Minas Gerais state, Brazil), and 55 sera samples from visceral leishmaniasis (VL) patients were obtained from the University Hospital (Montes Claros, Minas Gerais state, Brazil). The parasitological infection was confirmed by the presence of amastigotes in microscopic analyses of cutaneous lesions or bone marrow aspirates and by PCR assays using specific primers for the Leishmania Kinetoplastid DNA (kDNA) sequence (de Bruijn and Barker 1992). All Leishmania-infected patients were found to be uninfected with T. cruzi. Sera from 20 patients with Chagas disease (CD) were collected, with T. cruzi infection being confirmed by hemoculture or the Chagatest® recombinant ELISA v.3.0 kit and the Chagatest® hemagglutination inhibition test; these samples were uninfected with Leishmania. Sera from 50 healthy humans from a non-endemic area to Leishmania or Trypanosoma were used as negative controls (CT).
The use of dog samples was carried out in agreement with the guidelines of the Brazilian College of Animal Experimentation, strictly following Brazilian law regarding “Procedures for the Scientific Use of Animals” (11.794/2008) and were approved by the Institutional Animal Care and Committee on Ethics of Animal Experimentation (CETEA) from the UFMG under protocol number 44/2012. Dog sera samples were obtained from the endemic area for canine visceral leishmaniasis (CVL) in Minas Gerais state, Brazil. Infection was confirmed by the presence of amastigotes in microscopic analyses of bone marrow aspirates from 30 animals. Samples from 15 dogs experimentally infected with T. cruzi (canine Chagas disease (CCD)) and negative for Leishmania were used to evaluate cross-reactivity. A total of 30 dogs from a non-endemic area for visceral leishmaniasis and negative to Leishmania and T. cruzi were included as a control group (canine control (CCT)).
Sequence analysis and linear B-cell epitope prediction
The sequences of L. braziliensis MAPK3 (LbMAPK3; ID: LbrM.10.0620) and L. braziliensis MAPK4 (LbMAPK4; ID: LbrM.19.1710) from L. braziliensis were retrieved from TriTrypDB (Aslett et al. 2010). Orthologous proteins in Leishmania infantum and T. cruzi were obtained from an orthologs list present in TriTrypDB and confirmed as the best hit using LbMAPK3 and LbMAPK4 as queries in a Protein Basic Local Alignment Search Tool (BLASTp) search (Altschul et al. 1990) against whole protein sequences of the two species. BLASTp was also used to identify the more similar human and dog protein sequences in the NCBI Reference Sequence (RefSeq) database, and global multiple sequence alignments were performed using ClustalX 2.0 (Thompson et al. 2002) with default parameters. Linear B-cell epitopes were identified by BepiPred program (Larsen et al. 2006) with a cutoff of 1.3, and epitopes were considered as those with at least nine continuous amino acids with individual prediction scores above the cutoff. Intrinsically unstructured/disordered regions (IURs) were considered as those with at least nine continuous amino acids with an individual score above 0.5 predicted by IUPred program (Dosztanyi et al. 2005).
Parasites
L. braziliensis (MHOM/BR/75/M2904) is a reference strain from the Evandro Chagas Institute, Belém, Brazil. This strain was isolated by direct culture from a lesion on the right side of the thorax of a man who had been performing survey work in Serra dos Carajás, Brazilian Amazonia. Parasites were gently provided by Prof. Maria Norma de Melo (UFMG). Promastigote stages were maintained in Schneider’s insect medium (Sigma-Aldrich) supplemented with 10 % inactivated fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin (Life Technologies) at 23 °C ± 1 °C. Metacyclic promastigote forms were obtained after the purification of stationary cultures of L. braziliensis with peanut lectin, as previously described (Alcolea et al. 2009). Intracellular amastigotes were obtained by infecting 1 × 105 macrophages with 1 × 106 stationary promastigotes for 24 to 60 h at 37 °C and 5 % CO2.
RNA extraction and quantitative real-time PCR (qRT-PCR) assays
Total RNA from the different developmental stages of L. braziliensis was extracted using the Total RNA isolation kit (Macherey-Nagel) according to the manufacturer’s recommendations. cDNA synthesis was carried out using the High Capacity cDNA Reverse Transcription kit (PE Applied Biosystems), and qRT-PCR was performed as previously described (Menezes-Souza et al. 2012). The primer sequences of the MAPK3 and MAPK4 genes as well as of five constitutive control genes (GAPDH, 18s, α-tubulin, β-tubulin, and Actin) are listed in Table S1. Logarithmic promastigotes were used as a calibrator group by the 2−∆∆Ct method (Bustin et al. 2009).
Soluble L. braziliensis antigen (SLbA)
A total of 1 × 1010 L. braziliensis promastigotes were washed three times with cold phosphate-buffered saline followed by three cycles of freezing in liquid nitrogen and thawing (42 °C). After ultrasonication with ten alternating cycles of 30 s at 35 MHz, the lysate was centrifuged at 6,000 × g at 4 °C for 15 min. The supernatant containing SLbA was collected, and the protein concentration was estimated using the Pierce™ BCA™ Protein Assay (Thermo Scientific).
Cloning, recombinant protein expression, and purification
LbMAPK3 and LbMAPK4 genes were amplified from the genomic DNA of L. braziliensis by PCR using the forward (GCTAGCATGCACAAGAGCAACCAGGA) and reverse (GGATCCTTAGTGATGGTGGGCGCTG) primers for MAPK3 and the forward (GCTAGCATGACTCAACTCGTCCCT) and reverse (CTCGAGCTATTCATTCGAATGTGAGTAAGC) primers for MAPK4. NheI and BamHI restriction sites were added to the forward and reverse primers for MAPK3 and NheI and XhoI for MAPK4. The 1,167-kb fragments of MAPK3 and 1,104 kb of MAPK4 were excised from the agarose gel, purified, digested with the restriction enzymes, and ligated to the pET28a-TEV vector previously digested with the same restriction enzymes (Coitinho et al. 2012). Electrocompetent Escherichia coli BL21 Arctic Express (DE3) (Agilent Technologies) cells were transfected with recombinant plasmids by electroporation using a MicroPulser Electroporation Apparatus (Bio-Rad Laboratories). Gene insertion was confirmed by colony PCR and sequencing using T7 primers (Macrogen). Recombinant LbMAPK3 and LbMAPK4 were expressed by adding 1.0 mM Isopropyl-β-D-thiogalactopyranoside (IPTG, Promega) for 24 h at 12 °C with shaking at 200 rpm. The cells were then lysed by sonication and centrifuged at 6,000 × g for 30 min at 4 °C. The recombinant proteins were purified using a HisTrap HP affinity column connected to the ÄKTAprime chromatography system (GE Healthcare). The eluted fractions containing recombinant LbMAPK3 (rLbMAPK3) and recombinant LbMAPK4 (rLbMAPK4) were concentrated using Amicon® Ultra 15 Centrifugal Filters 10,000 NMWL (Millipore) and further purified on a SuperdexTM 200 gel filtration column (GE Healthcare).
Peptide synthesis
Soluble peptides were manually synthesized in solid phase on a 30-μmol scale using 9-florenyl-methoxy-carbonyl (Fmoc) chemistry (Mendes et al. 2013b; Wellings and Atherton 1997). First, Fmoc-amino acids were activated with a 1:2 solution of Oxyme and DIC. The activated amino acids were incorporated into Rink amide resin with a substitution degree of 0.61. Fmoc deprotection was then performed using 25 % 4-methylpiperidine. These steps were repeated until the synthesis of the peptide was complete. The side chain was unprotected and released form the resin by treatment with a solution of 95.0 % trifluoroacetic acid, 2.5 % water, and 2.5 % triisopropylsilane. The peptide was precipitated with cold diisopropyl ether and purified by high-performance liquid chromatography on a C18 reverse-phase column using a gradient program of 0 to 25 % acetonitrile. The peptides were obtained with 90 % purity, as confirmed by mass spectrometry using Autoflex Speed MALDI/TOF equipment.
ELISA and depletion ELISA assay
Initially, recombinant proteins and SLbA were coated onto 96-well microplates (Nalge Nunc Intl.) overnight at 2–8 °C at a concentration of 250 ng/well for rLbMAPK3 and rLbMAPK4 and 50 ng/well for SLbA. For the peptides, flat-bottom plates (Costar®) were coated with 10 μg/well of soluble peptide overnight at 37 °C. After blocking with 5 % BSA in phosphate-buffered saline (PBS) for 1 h at 37 °C, followed by three washing steps with PBS containing 0.05% Tween 20 (PBS-T), the plates were incubated with human or dog serum (dilution 1:100). The plates were washed three times with PBS-T, and secondary HRP-conjugated antihuman or antidog IgG antibody 1:5,000 was added for 1 h at 37 °C, followed by four washes. TMB substrate (Sigma-Aldrich) in citrate buffer containing hydrogen peroxide was used for detection; the reaction was stopped after 30 min with 4 N H2SO4, and the absorbance was measured at 450 nm. For depletion ELISAs, the sera were previously incubated in peptide-coated and blocked plates at a 1:100 dilution and incubated overnight at 2–8 °C. Depleted and undepleted samples were transferred to plates coated overnight with recombinant proteins (50 ng/well) and blocked, and the ELISAs were performed as described above.
Statistical analysis
The cutoffs for rLbMAPK3, rLbMAPK4, SLbA, and peptide-1 and peptide-2 were established for optimal sensitivity and specificity using the receiver-operator curve (ROC) curve. The cutoff was chosen based on the point that provided the maximum of the sum of the sensitivity and specificity (Linnet et al. 2012). The EIE-leishmaniose-visceral-canina-bio-manguinhos (EIE-LVC) cutoff was obtained according to the manufacturer (twice the average of the negative control provided by the kit). The performance of each test was evaluated according to the sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV), area under the curve (AUC), and accuracy (AC). The degree of agreement between the ELISA assays using the recombinant proteins, SLbA, or the EIE-LVC kit with the parasitological test (biopsy, aspirate, or PCR) was determined by Kappa index (κ) values with 95% confidence intervals and interpreted according to the following Fleiss scale: 0.00–0.20, poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; 0.81–0.99, very good; and 1.00, perfect. The one-sample Kolmogorov-Smirnoff test was used to determine whether a variable was normally distributed. For depletion assays, significant differences were detected using a two-way ANOVA. The differences were considered statistically significant at p < 0.05. All of the statistical analyses were performed using GraphPad PrismTM (version 6.0).
Results
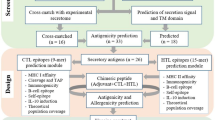
In the present work, we used a combination of in silico and experimental approaches to analyze the potential use of L. braziliensis MAPK3 and MAPK4 as antigens for serodiagnosis of visceral and tegumentary human leishmaniasis and visceral canine disease (Fig. 1). Initially, both proteins were submitted to linear B-cell epitope prediction, and Leishmania-specific epitopes were identified through sequence alignment of Leishmania, human, dog, and T. cruzi MAPK3 and MAPK4 orthologs. The expression profile of LbMAPK3 and LbMAPK4 transcripts in log-phase and metacyclic promastigotes and amastigotes were measured by real-time (RT)-PCR. The recombinant proteins produced by heterologous expression in E. coli were used in ELISA protocols for diagnosis of canine and human leishmaniasis. Synthetic peptides representing the linear B-cell epitopes were synthesized, and their contribution to the overall antigenicity of MAPK3 and MAPK4 was evaluated by depletion ELISAs. The performance of the synthetic peptides was also evaluated for the serodiagnosis of canine and human leishmaniasis.
Pipeline for the identification of new targets for the serodiagnosis of canine and human leishmaniasis The presence of linear B-cell epitopes in Leishmania protein targets (LbMAPK3 and LbMAPK4) were predicted by BepiPred program (a), and orthologous proteins of T. cruzi and mammalian hosts were retrieved from the TritrypDB and NCBI RefSeq using the BLASTp algorithm (b). Leishmania and other orthologous protein sequences were aligned using CLUSTALW (c), and linear B-cell epitopes specific for Leishmania parasites were identified (d and e). The RNA expression level of LbMAPK3 and LbMAPK4 in log-phase and metacyclic promastigotes and amastigotes were measured by real-time PCR (f) to identify proteins with increased expression in parasite forms present in mammalian host cells. Recombinant proteins produced by heterologous expression in E. coli (g) were used in ELISA protocols (h) to diagnose canine and human leishmaniasis. Synthetic peptides representing linear B-cell epitopes were synthesized by the Fmoc approach (i), and their contribution to the overall antigenic properties of the corresponding recombinant proteins were evaluated by depletion ELISA (j). The performance of specific peptides was also evaluated to serodiagnosis of canine and human leishmaniasis (k)
Analysis of orthologous sequences and identification of linear B-cell epitopes in LbMAPK3 and LbMAPK4
Although MAPK3 and MAPK4 are encoded in the genome of different parasites as well as in their hosts (Brumlik et al. 2011), differences among these protein sequences, in particular in the B-cell epitopes, may be sufficient to induce the production of Leishmania-specific antibodies (Mendes et al. 2013b; Menezes-Souza et al. 2014a). In this context, we compared the sequence of proteins and predicted B-cell epitopes of LbMAPK3 (Fig. 2a) and LbMAPK4 (Fig. 2b) with orthologous sequences from L. infantum, T. cruzi, H. sapiens, and C. familiaris. As expected, both LbMAPK3 and LbMAPK4 and their orthologs display high levels of identity (Table 1). However, both proteins are divergent at the C-terminal region compared to the T. cruzi, dog, and human orthologs (Fig. 2a, b). Moreover, the predicted B-cell epitope in LbMAPK3 co-occurs with an intrinsically unstructured region, which suggests that this protein region has an unfolded structure and is therefore potentially accessible for antibody binding.
Sequence divergence and prediction of B-cell linear epitopes and intrinsically unstructured/disordered regions in L. braziliensis MAPK3 and MAPK4 and its orthologs. a Alignment between L. braziliensis MAPK3 (TritrypDB ID: LbrM.10.0620) and orthologous proteins present in L. infantum (TritrypDB: LinJ.10.0540), T. cruzi (TritrypDB: LinJ.10.0540), H. sapiens (RefSeq ID: NP_620581.1), and C. familiaris (RefSeq ID: NP_001003206.1). b Alignment between L. braziliensis MAPK4 (TritrypDB ID: LbrM.19.1710) and orthologous proteins present in L. infantum (TritrypDB ID: LinJ.19.1480), T. cruzi (TritrypDB ID: TcCLB.511299.70), H. sapiens (RefSeq ID: NP_620581.1), and C. familiaris (RefSeq ID: NP_001003206.1). The yellow boxes mark predicted linear B-cell epitopes, and the gray boxes mark predicted disordered regions. The continuous black underlined amino acid sequences represent potential B-cell epitopes predicted by BepiPred in the MAPK3 and MAPK4 proteins, and green highlight amino acid conservations in the T. cruzi, C. familiaris, and H. sapiens sequences in relation to the L. braziliensis sequence
Expression of LbMAPK3 and LbMAPK4 in the different parasite stages
In addition to having B-cell epitopes, a good target for serodiagnosis should be expressed in the parasite stages present in the mammalian host. We evaluated the mRNA expression of LbMAPK3 and LbMAPK4 in different parasite stages using qRT-PCR. As is displayed in Fig. 3, both genes have increased expression during amastigote differentiation and proliferation.
Analyses of relative mRNA expression of MAPK3 and MAPK4 genes in the evolutive stages of the parasite. The assay was performed using specific MAPK3 and MAPK4 primers and promastigote in logarithmic growth phase as a calibrator group by the 2−∆∆Ct method. The experimental groups represented are metacyclic promastigote and intracellular amastigotes after 24 and 60 h of macrophage infection. The results are expressed as the mean of the relative copy number of mRNA (Log 2−ΔΔCt). Significant differences are indicated in the graph
Recombinant expression of LbMAPK3 and LbMAPK4
To assess the potential of MAPK3 and MAPK4 proteins from L. braziliensis to diagnose human and canine leishmaniasis, we expressed these proteins as His-tagged recombinant proteins (rLbMAPK3 and rLbMAPK4). First, the entire coding regions of the genes were amplified by PCR and cloned into the pET28a-TEV expression vector. The recombinant proteins with a predicted molecular weight of 43.7 (MAPK3) and 41.7 (MAPK4) kDa were successfully expressed in E. coli BL21 arctic express (DE3) cells as soluble proteins and obtained at a high level of purity (Supplementary Figure S1).
Performance of rLbMAPK3 and rLbMAPK4 for the serodiagnosis of human and canine leishmaniasis
ELISAs were performed to evaluate the reactivity of sera from patients with tegumentary (TL) and visceral leishmaniasis (VL) against rLbMAPK3, rLbMAPK4, and SLbA (Fig. 4). Samples from Chagasic patients and non-infected individuals were also used to measure the performance of each antigen. The results for each parameter (sensibility, specificity, positive and negative predictive values, and accuracy) are summarized in Table 2. For TL, the sensibility of recombinant protein rLbMAPK3 (83.08% for TL and 94.55% for VL) was higher than SLbA (70.77 % for TL and 52.73 % for VL), but the specificity was similar for TL (71.43 % for rLbMAPK3 and 68.57 % for SLbA) and worse (31.43 % for rLbMAPK3 and 50.00 % for SLbA) for VL resulting in little improvement in leishmaniasis diagnosis. The recombinant protein rLbMAPK4 showed increased accuracy for both TL and VL (96.67 % for TL and 81.60 % for VL) compared with SLbA (69.63 % for TL and 51.20 % for VL) due to discrete increases in sensitivity (72.73 %) and specificity (88.57 %) to the serodiagnosis of VL and the improvement of specificity (97.14 %) and a discrete increase in sensitivity (75.38 %) for the immunodiagnosis of TL (Fig. 4 and Table 2).
a Comparison of the reactivity of ELISAs against rLbMAPK3, rLbMAPK4, SLbA, and the EIE-LVC kit and b ROC curves obtained from TL and VL patients and from L. infantum-infected dogs (CVL). TL and VL: ELISAs were performed on samples from different groups of individuals (CT, control group, n = 50; CD, Chagas disease patients, n = 20; CL, cutaneous leishmaniasis, n = 45; ML, mucosal leishmaniasis, n = 20; VL, visceral leishmaniasis, n = 55). CVL: ELISAs were performed on samples from different groups of dogs (CCT, control group, n = 30; CCD, T. cruzi-infected dogs, n = 15; CVL, canine visceral leishmaniasis, n = 30). *Cutoff obtained by ROC curve. #Cutoff obtained according to the manufacturer
The recombinant proteins were also used to evaluate the potential to serodiagnosis canine visceral leishmaniasis (CVL). The reactivity of samples against these antigens was compared with the EIE-LVC kit (Fig. 4). The EIE-LVC kit showed high sensitivity (100.00 %) but low specificity (53.33 %). In contrast to the human samples, both rLbMAPK3 and rLbMAPK4 showed enormous improvement in specificity (95.56 % for both proteins), resulting in the improvement of accuracy from 72 % for the EIE-LVC kit to 94.67 % and 96.00 % for rLbMAPK3 and rLbMAPK4, respectively.
Contribution of the linear B-cell epitopes to the antigenicity of rLbMAPK3 and rLbMAPK4
The performance of recombinant proteins can be affected by the presence of a similar sequence in proteins of Leishmania species, T. cruzi, and the hosts. The use of specific epitopes derived from Leishmania sequences may potentially increase the performance of the serodiagnosis assay. To investigate this, peptide-1 (VGGGNSKNG) and peptide-2 (DPAEEADAP) were chemically synthesized. These peptides represent the predicted B-cell linear epitopes in LbMAPK3 and LbMAPK4 with sequences that are divergent in T. cruzi, human, and dog orthologs. Depletion ELISAs were performed to confirm that the synthetic peptides were recognized by Leishmania-specific antibodies present in the sera of infected human and dogs and to evaluate the contribution of these peptides to the overall antigenicity of both proteins. This technique is based on the reduction of serum reactivity due to depletion of peptide-specific antibodies by previously incubating the sample with the synthetic peptide coated on ELISA plates (Bueno et al. 2011; Santiago et al. 2011). The reactivity of the depleted sample against the recombinant protein was measured and the reduction was proportional to the titer of antibodies that bind to the peptide within the protein sequence.
The IgG reactivity against rLbMAPK3 and rLbMAPK4 after antibody depletion using synthetic peptide-1 and peptide-2, respectively, was reduced in both human and canine Leishmania-infected groups (Fig. 5). The reduction in the serum reactivity after depletion with peptide-2 derived from LbMAPK4 was similar in both human and canine leishmaniasis. Alternately, for peptide-1 derived from LbMAPK3, the decreases in reactivity in both human tegumentary and visceral leishmaniasis (13 %, p < 0.05 to cutaneous; 12 %, p < 0.05 to mucosal; and 15 %, p < 0.05 to visceral) were lower than in canine visceral disease (29%, p < 0.05). No difference between depleted and undepleted sera was observed in the control groups, confirming that these epitopes are specific to Leishmania parasites.
Immunodepletion assay showing specific IgG antibody recognition of the synthetic peptides with known reactivity to MAPK3 and MAPK4. Pools of sera (n = 10) from the different groups were depleted with peptide-1 (CT, control group; CD, Chagas disease; CL, cutaneous leishmaniasis; ML, mucosal leishmaniasis; VL, visceral leishmaniasis; CCT, control dogs; CCT, T. cruzi-infected dogs; CVL, canine visceral leishmaniasis). The mean antibody optical density (OD) values are shown on the y-axis, and the error bars indicate the standard deviation. Significant differences are indicated on the graphs with significant p values (*p < 0.05; **p < 0.01; ***p < 0.001)
Performance of synthetic peptide representing linear Leishmania-specific B-cell epitopes for serodiagnosis of human and canine leishmaniasis
After confirming that the synthetic peptides were partially responsible for the antigenicity of the recombinant proteins, we analyzed the performance of these Leishmania-specific B-cell epitopes in the serodiagnosis of human and canine leishmaniasis (Fig. 6). The use of peptide-1 derived from LbMAPK3 as an antigen resulted in increased accuracy for the serodiagnosis of TL and VL compared with rLbMAPK3. AUC analysis of the ROC curves (Table 3) confirmed the higher performance of synthetic peptide-1 (AUC for TL = 0.988 and for VL = 0.954) compared with rLbMAPK3 (AUC for TL = 0.823 and VL = 0.639) and SLbA (AUC for TL = 0.753 and VL = 0.510) (Table 2) for both clinical forms of human leishmaniasis. Furthermore, this epitope also showed very good agreement with the parasitological test (Table 3), the standard gold method for the diagnosis of TL. With respect to the serodiagnosis of canine visceral leishmaniasis, synthetic peptide-1 again displayed slightly higher accuracy than rLbMAPK3, a result supported by AUC analysis (Table 3).
a Comparison of the reactivity of ELISAs against peptide-1 and peptide-2 and b ROC curves obtained from TL and VL patients and from L. infantum-infected dogs (CVL). TL and VL: ELISAs were performed on samples from different groups of individuals (CT, control group, n = 50; CD, Chagas disease patients, n = 20; CL, cutaneous leishmaniasis, n = 45; ML, mucosal leishmaniasis, n = 20; VL, visceral leishmaniasis, n = 55). CVL: ELISAs were performed on samples from different groups of dogs (CCT, control group, n = 30; CCD, T. cruzi-infected dogs, n = 15; CVL, canine visceral leishmaniasis, n = 30). *Cutoff obtained by ROC curve. #Cutoff obtained according to the manufacturer
Synthetic peptide-2, which corresponds to a linear B-cell epitope of LbMAPK4, generally performed better than rLbMAPK4 in both TL and VL. This improvement was confirmed by an AUC analysis and a very good agreement with the parasitological test (Fig. 6, Table 3). In contrast, the recombinant protein rLbMAPK4 showed higher sensitivity, specificity, accuracy, and AUC than synthetic peptide-2 for canine visceral leishmaniasis. In agreement with these results, rLbMAPK4, but not synthetic peptide-2, presented very good concordance with the parasitological test of infected dogs.
Discussion
The use of serology for leishmaniasis diagnosis has significant advantages over parasitological methods as it is less invasive, allows the early detection of infection before the formation of lesions, and is a quantitative assay that is easily automated, allowing for the analysis of a large number of samples (Mabey et al. 2004; Mendes et al. 2013b; Souza et al. 2013). However, current methods have variable specificity and sensitivity due to antigen composition depending on the species and strain of the parasite, the antigen production protocol, and the experimental conditions used in serological tests (Sundar and Rai 2002; Zanette et al. 2014). Thus, antigen selection is a key step in improving the specificity and sensitivity of serodiagnostic techniques. The use of recombinant proteins allows higher standardization and technical safety than crude antigens, not requiring the maintenance and processing of live parasites. The availability of multiple genomes from Leishmania parasites and epitope prediction tools enable their protein sequences to be compared with the host and other pathogen proteomes to identify Leishmania-specific antigens (Mendes et al. 2013a). Proteins associated with the infection process and/or the intracellular survival of the parasite within the host may be attractive targets because they are expressed during parasite stages present in the mammalian host (Silverman et al. 2010). Among those proteins, MAPK3 and MAPK4 are potential diagnostic targets due to their involvement in the differentiation process of promastigote into amastigote (Brumlik et al. 2011; Erdmann et al. 2006) and their sequence divergence compared with host orthologs (Bahia et al. 2009; Parsons et al. 2005).
Although protein kinases are encoded by virtually all eukaryote genomes, there is a massive expansion of kinases in trypanosomatids (Naula et al. 2005). Protein kinases comprise approximately 2 % of trypanosomatid genomes, with the number of kinases approximately 33 % greater in these parasites than in Saccharomyces cerevisiae and at least twice that of malaria parasites (Parsons et al. 2005). This expansion is accompanied by the significant divergence in the protein sequences, resulting in many proteins with no strong similarity to any other recognized kinase family. These unique trypanosomatid kinases may differ in structure and function from the mammalian orthologs, including the presence of parasite-specific epitopes within their sequence.
The present study demonstrated that rLbMAPK3 and rLbMAPK4.1 could be among the target molecules for immunodiagnostics of human and canine leishmaniasis. Furthermore, two synthetic peptides corresponding to predicted B-cell epitopes derived from each one of these proteins presented high performance for the serodiagnosis of human and canine leishmaniasis. A better performance of synthetic peptides compared with the cognate recombinant protein has already been described in the literature (Chamekh et al. 1992; Menezes-Souza et al. 2014b; Oliveira et al. 2006). This difference is likely related to the fact that these epitopes may correspond to a highly immunogenic region of the pathogen protein that is absent in the host and in other organisms frequently associated with cross-reactivity in serodiagnosis. In fact, the depletion ELISA demonstrated that each one of these peptides contributes significantly to the overall antigenicity of the corresponding protein in all Leishmania-infected groups, but not in the control group. The reactivity, however, was not completely reduced, most likely because there are other unpredicted linear epitopes as well as conformational epitopes not accessed in our analysis.
Further studies involving samples from different geographic regions are necessary to better characterize the performance of the antigens identified in this study for the serodiagnosis of human and canine leishmaniasis as well as to evaluate their use in monitoring post-therapeutic TL and VL cures. Moreover, the antigen discovery approach present in this study can be applied to other infectious diseases.
References
Alcolea PJ, Alonso A, Sanchez-Gorostiaga A, Moreno-Paz M, Gomez MJ, Ramos I, Parro V, Larraga V (2009) Genome-wide analysis reveals increased levels of transcripts related with infectivity in peanut lectin non-agglutinated promastigotes of Leishmania infantum. Genomics 93(6):551–64
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–10
Alves CF, Figueiredo MM, Souza CC, Machado-Coelho GL, Melo MN, Tafuri WL, Raso P, Soares RP (2013) American tegumentary leishmaniasis: effectiveness of an immunohistochemical protocol for the detection of Leishmania in skin. PLoS One 8(5):e63343
Aslett M, Aurrecoechea C, Berriman M, Brestelli J, Brunk BP, Carrington M, Depledge DP, Fischer S, Gajria B, Gao X, Gardner MJ, Gingle A, Grant G, Harb OS, Heiges M, Hertz-Fowler C, Houston R, Innamorato F, Iodice J, Kissinger JC, Kraemer E, Li W, Logan FJ, Miller JA, Mitra S, Myler PJ, Nayak V, Pennington C, Phan I, Pinney DF, Ramasamy G, Rogers MB, Roos DS, Ross C, Sivam D, Smith DF, Srinivasamoorthy G, Stoeckert CJ Jr, Subramanian S, Thibodeau R, Tivey A, Treatman C, Velarde G, Wang H (2010) TriTrypDB: a functional genomic resource for the Trypanosomatidae. Nucleic Acids Res 38:457–62
Bahia D, Oliveira LM, Lima FM, Oliveira P, Silveira JF, Mortara RA, Ruiz JC (2009) The TryPIKinome of five human pathogenic trypanosomatids: Trypanosoma brucei, Trypanosoma cruzi, Leishmania major, Leishmania braziliensis and Leishmania infantum–new tools for designing specific inhibitors. Biochem Biophys Res Commun 390(3):963–70
Brumlik MJ, Pandeswara S, Ludwig SM, Murthy K, Curiel TJ (2011) Parasite mitogen-activated protein kinases as drug discovery targets to treat human protozoan pathogens. J Signal Transduct 2011:971968
Bueno LL, Lobo FP, Morais CG, Mourao LC, de Avila RA, Soares IS, Fontes CJ, Lacerda MV, Chavez Olortegui C, Bartholomeu DC, Fujiwara RT, Braga EM (2011) Identification of a highly antigenic linear B cell epitope within Plasmodium vivax apical membrane antigen 1 (AMA-1). PLoS One 6(6):e21289
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–22
Caballero ZC, Sousa OE, Marques WP, Saez-Alquezar A, Umezawa ES (2007) Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin Vaccine Immunol 14(8):1045–9
Chamekh M, Gras-Masse H, Bossus M, Facon B, Dissous C, Tartar A, Capron A (1992) Diagnostic value of a synthetic peptide derived from Echinococcus granulosus recombinant protein. J Clin Invest 89(2):458–64
Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M (2007) Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol 5(11):873–82
Coitinho JB, Costa DM, Guimaraes SL, de Goes AM, Nagem RA (2012) Expression, purification and preliminary crystallographic studies of NahF, a salicylaldehyde dehydrogenase from Pseudomonas putida G7 involved in naphthalene degradation. Acta Crystallogr Sect F: Struct Biol Cryst Commun 68(1):93–7
de Bruijn MH, Barker DC (1992) Diagnosis of New World leishmaniasis: specific detection of species of the Leishmania braziliensis complex by amplification of kinetoplast DNA. Acta Trop 52(1):45–58
Desjeux P (2001) Worldwide increasing risk factors for leishmaniasis. Med Microbiol Immunol 190(1–2):77–9
Dosztanyi Z, Csizmok V, Tompa P, Simon I (2005) IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 21(16):3433–4
Erdmann M, Scholz A, Melzer IM, Schmetz C, Wiese M (2006) Interacting protein kinases involved in the regulation of flagellar length. Mol Biol Cell 17(4):2035–45
Gonzalez C, Wang O, Strutz SE, Gonzalez-Salazar C, Sanchez-Cordero V, Sarkar S (2010) Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis 4(1):e585
Goto H, Lindoso JA (2010) Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther 8(4):419–33
Lakhal-Naouar I, Boussoffara T, Meddeb-Garnaoui A, Ben Achour-Chenik Y, Louzir H, Chenik M (2009) Cellular and humoral responses to Leishmania major virulence factors in healed cutaneous leishmaniasis and Mediterranean visceral leishmaniasis patients. Clin Vaccine Immunol 16(6):956–8
Larsen JE, Lund O, Nielsen M (2006) Improved method for predicting linear B-cell epitopes. Immunome Res 2:2
Linnet K, Bossuyt PM, Moons KG, Reitsma JB (2012) Quantifying the accuracy of a diagnostic test or marker. Clin Chem 58(9):1292–301
Mabey D, Peeling RW, Ustianowski A, Perkins MD (2004) Diagnostics for the developing world. Nat Rev Microbiol 2(3):231–40
Mendes TA, Lobo FP, Rodrigues TS, Rodrigues-Luiz GF, daRocha WD, Fujiwara RT, Teixeira SM, Bartholomeu DC (2013a) Repeat-enriched proteins are related to host cell invasion and immune evasion in parasitic protozoa. Mol Biol Evol 30(4):951–63
Mendes TA, Reis Cunha JL, de Almeida LR, Rodrigues Luiz GF, Lemos LD, dos Santos AR, da Camara AC, Galvao LM, Bern C, Gilman RH, Fujiwara RT, Gazzinelli RT, Bartholomeu DC (2013b) Identification of strain-specific B-cell epitopes in Trypanosoma cruzi using genome-scale epitope prediction and high-throughput immunoscreening with peptide arrays. PLoS Negl Trop Dis 7(10):e2524
Menezes-Souza D, Guerra-Sa R, Carneiro CM, Vitoriano-Souza J, Giunchetti RC, Teixeira-Carvalho A, Silveira-Lemos D, Oliveira GC, Correa-Oliveira R, Reis AB (2012) Higher expression of CCL2, CCL4, CCL5, CCL21, and CXCL8 chemokines in the skin associated with parasite density in canine visceral leishmaniasis. PLoS Negl Trop Dis 6(4):e1566
Menezes-Souza D, Mendes TA, Gomes MD, Reis-Cunha JL, Nagem RA, Carneiro CM, Coelho EA, Galvao LM, Fujiwara RT, Bartholomeu DC (2014a) Epitope mapping of the HSP83.1 protein of Leishmania braziliensis discloses novel targets for the immunodiagnosis of tegumentary and visceral clinical forms of leishmaniasis. Clin Vaccine Immunol 21(7):949–59
Menezes-Souza D, Mendes TA, Nagem RA, Santos TT, Silva AL, Santoro MM, de Carvalho SF, Coelho EA, Bartholomeu DC, Fujiwara RT (2014b) Mapping B-Cell Epitopes for the peroxidoxin of Leishmania (Viannia) braziliensis and its potential for the clinical diagnosis of tegumentary and visceral leishmaniasis. PLoS One 9(6):e99216
Molina R, Amela C, Nieto J, San-Andres M, Gonzalez F, Castillo JA, Lucientes J, Alvar J (1994) Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans R Soc Trop Med Hyg 88(4):491–3
Naula C, Parsons M, Mottram JC (2005) Protein kinases as drug targets in trypanosomes and Leishmania. Biochim Biophys Acta 1754(1–2):151–9
Oliveira EJ, Kanamura HY, Takei K, Hirata RD, Nguyen NY, Hirata MH (2006) Application of synthetic peptides in development of a serologic method for laboratory diagnosis of Schistosomiasis mansoni. Mem Inst Oswaldo Cruz 101(1):355–7
Parsons M, Worthey EA, Ward PN, Mottram JC (2005) Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics 6:127
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22(2):153–83
Ready PD (2010) Leishmaniasis emergence in Europe. Euro Surveill 15(10):19505
Santiago HC, Bennuru S, Boyd A, Eberhard M, Nutman TB (2011) Structural and immunologic cross-reactivity among filarial and mite tropomyosin: implications for the hygiene hypothesis. J Allergy Clin Immunol 127(2):479–86
Shaw J (2007) The leishmaniases—survival and expansion in a changing world. A mini-review. Mem Inst Oswaldo Cruz 102(5):541–7
Silverman JM, Clos J, De’Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE (2010) An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci 123(6):842–52
Souza AP, Soto M, Costa JM, Boaventura VS, de Oliveira CI, Cristal JR, Barral-Netto M, Barral A (2013) Towards a more precise serological diagnosis of human tegumentary leishmaniasis using Leishmania recombinant proteins. PLoS One 8(6):e66110
Stockdale L, Newton R (2013) A review of preventative methods against human leishmaniasis infection. PLoS Negl Trop Dis 7(6):e2278
Sundar S, Rai M (2002) Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol 9(5):951–8
Thompson JD, Gibson TJ, Higgins DG (2002) Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics Chapter 2:Unit 2 3
Wang Q, Melzer IM, Kruse M, Sander-Juelch C, Wiese M (2005) LmxMPK4, a mitogen-activated protein (MAP) kinase homologue essential for promastigotes and amastigotes of Leishmania mexicana. Kinetoplastid Biol Dis 4:6
Wellings DA, Atherton E (1997) Standard Fmoc protocols. Methods Enzymol 289:44–67
Wiese M (2007) Leishmania MAP kinases–familiar proteins in an unusual context. Int J Parasitol 37(10):1053–62
Wilson ME, Streit JA (1996) Visceral leishmaniasis. Gastroenterol Clin North Am 25(3):535–51
Zanette MF, Lima VM, Laurenti MD, Rossi CN, Vides JP, Vieira RF, Biondo AW, Marcondes M (2014) Serological cross-reactivity of Trypanosoma cruzi, Ehrlichia canis, Toxoplasma gondii, Neospora caninum and Babesia canis to Leishmania infantum chagasi tests in dogs. Rev Soc Bras Med Trop 47(1):105–7
Author information
Authors and Affiliations
Corresponding author
Additional information
Daniel Menezes-Souza and Tiago Antônio de Oliveira Mendes contributed equally to this work
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 296 kb)
Rights and permissions
About this article
Cite this article
Menezes-Souza, D., de Oliveira Mendes, T.A., de Araújo Leão, A.C. et al. Linear B-cell epitope mapping of MAPK3 and MAPK4 from Leishmania braziliensis: implications for the serodiagnosis of human and canine leishmaniasis. Appl Microbiol Biotechnol 99, 1323–1336 (2015). https://doi.org/10.1007/s00253-014-6168-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6168-7