Abstract
The serodiagnosis of canine visceral leishmaniasis (CVL) presents problems related to its sensitivity and/or specificity. In the present study, a new Leishmania-specific hypothetical protein, LiHyD, was produced as a recombinant protein (rLiHyD) and evaluated in ELISA experiments for the CVL serodiagnosis. LiHyD was characterized as antigenic in a recent immunoproteomic search performed with Leishmania infantum proteins and the sera of dogs developing visceral leishmaniasis (VL). Aiming to compare the efficacy between whole proteins and synthetic peptides, two linear and one conformational B cell epitopes of LiHyD were synthesized and also evaluated as diagnostic markers. The four antigens were recognized by the sera of dogs suffering VL. On the contrary, low reactivity was observed when they were assayed with sera from non-infected healthy dogs living in endemic or non-endemic areas of leishmaniasis. In addition, no reactivity was found against them using sera from dogs experimentally infected by Trypanosoma cruzi, Babesia canis, or Ehrlichia canis, or sera from animals vaccinated with the Leish-Tec® vaccine, a prophylactic preparation commercially available for CVL prevention in Brazil. As comparative diagnostic tools, a recombinant version of the amastigote-specific A2 protein and a soluble crude Leishmania extract were studied. Both antigens presented lower sensitivity and/or specificity values than the LiHyD-based products. The rLiHyD presented better results for the CVL serodiagnosis than its linear epitopes, although the peptide recreating the conformational epitope resulted also appropriate as a diagnostic marker of CVL. To the best of our knowledge, this is the first study showing the use of a conformational epitope derived from a Leishmania protein for serodiagnosis of CVL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is a disease complex that presents a high morbidity and mortality in the world, where about 380 million people are at risk in 98 countries, with approximately 1.5 to 2.0 million new cases being registered annually (Alvar et al. 2012). Zoonotic visceral leishmaniasis (VL) is a disease caused by Leishmania infantum in the Mediterranean area, Middle East, Africa, Asian countries, and Latin America (WHO 2010), and dogs are considered important domestic reservoirs of parasites (Petersen 2009). The disease is also emerging in the USA, Canada, Northern Italy, and Germany and in the Americas, with about 95 % of the cases registered in Brazil (Ready 2010; Alvar et al. 2012).
Upon infection, dogs can develop asymptomatic or symptomatic forms of disease (Solano-Gallego et al. 2011). In symptomatic canine VL (CVL), cutaneous and organic alterations are observed correlating with the presence of high parasite burdens, and the disease usually results in the death of the infected animals (Ciaramella et al. 1997). For diagnosis, parasitological methods based on direct demonstration of amastigote forms by direct staining or amplification of the parasite DNA by the polymerase chain reaction (PCR) in collected samples from infected organs or tissues can be employed. PCR-based tests applied for Leishmania detection are more reliable than direct observation of parasites (Antinori et al. 2007). However, these methods require invasive procedures for sample collection and false negative results can be obtained when diagnosing individuals with low parasite burdens, like in asymptomatic patients (Coura-Vital et al. 2011). In addition, for PCR-based diagnosis, a careful standardization of protocols is needed in terms of design of primers and DNA extraction procedures (Alvar et al. 2004; Baneth & Aroch 2008; Deborggraeve et al. 2008; Maia & Campino 2008).
Serological tests have been recommended for the CVL diagnosis due to the fact that they use less invasive methods of sample collection. Infected dogs can present a moderate to strong humoral response, which generally accompanies the development of disease (Porrozzi et al. 2007; Maia & Campino 2008). However, antigens used present cross-reactivity with antibodies generated against proteins of other pathogens, leading to the occurrence of false positive results (Coura-Vital et al. 2011; Almeida-Leal et al. 2014). Moreover, in areas in which CVL is endemic, non-infected animals can also develop an antileishmanial serology, and they can be confused with infected dogs (Courtenay et al. 2002; Moshfe et al. 2009).
The Brazilian Ministry of Health has recommended that, for a CVL serological diagnosis, the “Dual Path Platform” (DPP®; Bio-Manguinhos, Fiocruz, Rio de Janeiro, Brazil) combined with the “Canine Leishmaniasis ELISA Kit” (EIE-LVC kit; BioManguinhos, Fiocruz, Rio de Janeiro, Brazil) should be employed (Coura-Vital et al. 2014; Laurenti et al. 2014). However, their efficacy has been hampered by factors affecting their sensitivity and/or specificity, mainly related to the antigens employed. Thus, it is necessary to find new antigenic proteins that serve to design serodiagnostic systems with higher degree of sensitivity and specificity than current kits. Different recombinant proteins have been evaluated as diagnostic markers of disease (Soto et al. 1998; Candido et al. 2008; Martins et al. 2013), although a precise antigen does not exist. In parallel to the use of recombinant proteins, synthetic peptides could be also considered, since these antigens are simpler, stable, and cheaper to produce (Noya et al. 2003; Chávez-Fumagalli et al. 2013).
In a recent immunoproteomic search performed with L. infantum proteins, a Leishmania-specific hypothetical protein was recognized by CVL sera (Coelho et al. 2012). This protein, namely, LiHyD (LinJ.33.3150), was obtained as a recombinant molecule in the present study (rLiHyD). It was chosen because it is only present in the Leishmania genus, it is highly conserved among Leishmania species, and it is predicted to have B cell epitopes. Aiming to evaluate new candidates for the CVL serodiagnosis, this study employed the rLiHyD protein and three of its specific B cell epitopes (two linear and one conformational) contained in three different synthetic peptides. Also, to the best of our knowledge, this study evaluates for the first time the diagnostic properties of a conformational epitope derived from a Leishmania hypothetical protein.
Materials and methods
Ethics statement
This study was approved by Committee on the Ethical Handling of Research Animals from Federal University of Minas Gerais (UFMG), Belo Horizonte, Brazil, under the protocol number 043/2011.
Canine sera
The sample size used was composed of 177 domestic animals (Canis familiaris) and consisted of males (n = 100) and females (n = 77), of different breeds and ages. CVL-positive animals presented positive parasitological results for L. infantum DNA assayed by a PCR technique (Reis et al. 2013). All of them presented positive serological results in two commercial tests (IFAT-LVC Bio-Manguinhos kit and EIE-LVC Bio-Manguinhos kit). Symptomatic VL dogs (CVLS, n = 44) were those with positive parasitological and serological results, as well as showing three or more clinical signals and/or symptoms. Asymptomatic VL dogs (CVLA, n = 9) were those presenting positive parasitological and serological results, but without any clinical signals of leishmaniasis. Non-infected dogs were selected from endemic (HEA, n = 44; Belo Horizonte, Minas Gerais, Brazil) or non-endemic (HNEA, n = 20; Poços de Caldas, Minas Gerais, Brazil) areas of leishmaniasis. All of them presented negative serological results, as well as were free of any signal of disease. In this study were also included sera samples of healthy animals vaccinated with Leish-Tec® (HV, n = 30), and sera from dogs infected with Ehrlichia canis (EC, n = 10), Babesia canis (BC, n = 10), or Trypanosoma cruzi (TC, n = 10). These last animals were maintained in kennels to prevent their contact with transmitting vectors of leishmaniasis.
Parasite
The L. infantum (MHOM/BR/1970/BH46) strain was used. Parasites were grown at 24 °C in Schneider’s medium (Sigma, St. Louis, MO, USA) supplemented with 10 % inactivated fetal bovine serum (FBS, Sigma), 20 mM L-glutamine, 200 U/mL penicillin, and 100 μg/mL streptomycin, at pH 7.4. The soluble Leishmania antigenic extract (SLA) was prepared from 1 × 109 stationary-phase promastigote cultures (5–7 days old), as described (Coelho et al. 2003).
Sequence analysis of the LiHyD protein
The process of in silico analysis of the L. infantum LiHyD sequence consisted of (i) the search for similarity among sequences deposited in non-redundant protein databases, (ii) comparison with the databases of other trypanosomatids whose genomes have been sequenced completely or are in the phase of annotation, i.e., Leishmaniamajor, Leishmania mexicana, Leishmania braziliensis, T. cruzi, Trypanosoma brucei, and Trypanosoma congolenses (all available at www.genedb.org), and (iii) evaluation of the sequence for analysis of its physicochemical properties using the ProtParam tool in the ExPASy server (Gasteiger et al. 2005). The parameters computed by the program and reported here include the molecular weight, theoretical isoelectric point, amino acid composition, total number of positive and negative residues, extinction coefficient, instability index, aliphatic index, and grand average of hydropathicity (GRAVY).
Cloning, expression, and purification of recombinant LiHyD protein
The cloning, expression, and purification of the LiHyD protein were performed as described by Lage et al. (2015). The L. donovani A2 recombinant protein used as an antigen control was produced as described by Zhang et al. (1996). After purification, the recombinant proteins were passed through a polymyxin-agarose column (Sigma), in order to remove residual endotoxin content (<10 ng of LPS per 1 mg of recombinant protein, measured by the Quantitative Chromogenic Limulus Amebocyte Assay QCL-1000, BioWhittaker, MD, USA).
Mapping of specific B cell epitopes of the LiHyD protein
Two linear and one conformational B cell epitope of LiHyD were synthesized. Two peptides containing the linear sequences (Peptide-1, PQPGYQPPPPMEHALP, 262-277 positions; and Peptide-2, SSLRRQNSMRRNE, 296-307 positions) were predicted using the ABCpred Prediction Server software (www.imtech.res.in/raghava/abcpred/), as described by Saha and Raghava (2006). The second epitope was also predicted using the Emini Surface Accessibility Scale algorithm, based on the program IEDB (Immune Epitope DataBase and Analysis Resource; available at www.iedb.org), as described by Emini et al. (1985). The conformational epitope (Peptide-3) was predicted using a combination of three algorithms, ABCpred Prediction Server, Bepipred Linear Epitope Prediction (www.tools.immuneepitope.org/bcell), and Kolaskar and Tongaonkar antigenicity scale (www.tools.immuneepitope.org/bcell/), following technical protocols described by Kolaskar and Tongaonkar (1990), Larsen et al. (2006), and Saha and Raghava (2006). This epitope contains a combination of amino acids from two different protein regions of LiHyD: LYHPAPSSL (221-229 positions) and PQPGYQPP (262-269 positions). All peptides were synthesized by the F-moc technique of Merrifield (1963), with modifications following Machado-de-Ávila et al. (2011). Briefly, peptides were released from the amine resin by trifluoracetic acid treatment in the presence of the appropriate scavengers. Then, they were diluted in Milli-Q water and purified by high-performance liquid chromatography (HPLC) on a C18 reverse phase column (flow rate 1.0 mL/min; Vydac). Finally, they were submitted to a MALDI-TOF-TOF analysis.
ELISA for CVL serodiagnosis
Previous titration curves were performed to determine the most appropriate concentration of antigens and sera sample dilutions to be used in the ELISA experiments. Microtiter immunoassay plates (Falcon) were coated with rLiHyD, rA2, Peptide-1, Peptide-2, Peptide-3, or L. infantum SLA (1.0, 1.0, 20.0, 20.0, 20.0, and 2.0 μg per well, respectively), dissolved in 100 μL coating buffer (50 mM carbonate buffer, pH 9.6), for 18 h at 4 °C. Next, free binding sites were blocked using 200 μL of PBS-T (phosphate-buffered saline plus Tween 20 0.05 %), containing 5 % albumin, for 1 h at 37 °C. After washing the plates three times with PBS-T, they were incubated with 100 μL of canine sera (1:100, diluted in PBS-T), for 1 h at 37 °C. Plates were subsequently washed four times in PBS-T and incubated with anti-dog IgG horseradish-peroxidase-conjugated antibody (1:5000, diluted in PBS-T; catalog A6792, Sigma Aldrich, USA), for 1 h at 37 °C. After washing the plates five times with PBS-T, the reactions were developed by incubation with 100 μL per well of a solution consisting of 2 μL H2O2, 2 mg orto-phenylenediamine, and 10 mL citrate-phosphate buffer at pH 5.0, for 30 min and in the dark. Reaction was stopped by adding 25 μL 2 N H2SO4. The optical density was read in an ELISA microplate spectrophotometer (Molecular Devices, Spectra Max Plus, Canada) at 492 nm.
Statistical analysis
The results were entered into Microsoft Excel (version 10.0) spreadsheets and analyzed using GraphPad PrismTM (version 6.0 for Windows). The mean optical density (OD) value was calculated by subtracting the mean blank OD from the mean OD for each individual sample. The lower limits of positivity (cutoff) for the diagnostic antigens were established for optimal sensitivity and specificity using the receiver operating characteristic (ROC) analysis. The curves were plotted with the values from symptomatic and asymptomatic CVL groups versus the control groups, following a sick/non-sick rating method. The result of the division between the mean OD obtained for the sample and its respective cutoff was called “optical density index” (ODI). The D’Agostino & Pearson normality test was used to determine whether a variable was normally distributed. An unpaired Students t test was also used, and significant differences were considered with P < 0.05. The diagnostic capacity of each antigen was measured by assessing its sensitivity (95 % confidence interval, CI 95%), specificity (CI 95%), area under the curve (AUC), and accuracy (AC). The degree of agreement between the assays was determined by kappa (κ) index (with CI 95%) and classified according to the Fleiss scale: 0.00–0.20 (poor), 0.21–0.40 (fair), 0.41–0.60 (moderate), 0.61–0.80 (good), 0.81–0.99 (very good), and 1.00 (perfect).
Results
Sequence database and physicochemical evaluation of LiHyD protein
In the present study, the hypothetical LiHyD protein was defined as a Leishmania spp. specific protein, and its identity degree found was 60, 79, and 80 % for the L. braziliensis, L. major, and L. mexicana species, respectively. No orthologue sequence was found in the T. cruzi, T. brucei, Trypanosoma vivax, and T. congolenses species. Based on the fact that LiHyD is a hypothetical protein, a physicochemical evaluation was in silico performed (Table 1). The results showed that it presents 327 amino acids in its primary sequence, having a molecular weight of 36 kDa, and an isoelectric point of 9.49.
Evaluation of the rLiHyD protein for CVL serodiagnosis
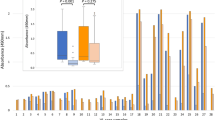
The rLiHyD protein was evaluated for CVL serodiagnosis (Fig. 1). For comparison, a recombinant version of the A2 protein and the L. infantum SLA were also employed in the ELISA assays. The results showed that 100 % of the CVL sera presented OD values over the cutoff, when rLiHyD was employed as an antigenic source (Fig. 1a). The ODI value of reactivity of rLiHyD was calculated and is also shown (Fig. 1b). The cutoff values for accessing the sensitivity and specificity of antigens were determined using receiver operating characteristic (ROC) analysis, and the area under the curve (AUC) was calculated to assess the accuracy of the tests (Fig. 1c). When the rA2 protein was used as a comparative diagnostic marker, its sensitivity and specificity values were 100 and 72.6 %, respectively (Fig. 2). The L. infantum SLA was not able to identify the asymptomatic animals. In addition, a poor specificity was observed, since it was recognized by 30 % of the sera from T. cruzi-infected dogs (Fig. 2a). The ODI values (Fig. 2b) and the result of the ROC analysis for rA2 and SLA (Fig. 2c) are also shown. The individual evaluation of each antigen for the CVL serodiagnosis was performed (Table 2). The AUC was used to compare the efficacy between the different evaluated diagnostic antigens. The rLiHyD protein presented the highest AUC value (1.000), followed by rA2 (0.992) and L. infantum SLA (0.964). The maximum sensitivity and specificity values (100.0 % in both cases), the maximum accuracy value (100.0%), and a total agreement were obtained using the rLiHyD protein. On the other hand, when the rA2 and L. infantum SLA were used, they presented sensitivity values of 98.1 and 83.0 %, respectively, and accuracy values of 96.0 and 93.0 %, respectively.
Evaluation of ELISA reactivity with the rLiHyD protein using a canine serological panel. The recombinant LiHyD protein was employed as an antigen for ELISA assays performed with canine sera samples obtained by the next animal groups: symptomatic (CVLS, n = 44) or asymptomatic (CVLA, n = 9) visceral leishmaniasis (VL) dogs, healthy dogs living in endemic (HEA, n = 44) or non-endemic (HNEA, n = 20) areas of leishmaniasis, dogs immunized with Leish-Tec® vaccine (HV, n = 30), and animals infected with Trypanosoma cruzi (TC, n = 10), Ehrlichia canis (EC, n = 10), or Babesia canis (BC, n = 10). The individual OD values are shown (a). The dotted line represents the cutoff value calculated by a ROC analysis. The box and whisker plots of the ODI values from sera grouped in non-infected (control) or CVL (Leishmania-infected) groups are also shown (b), as well as the ROC curves obtained for LiHyD protein (x). Statistically significant differences (***P < 0.001) were observed between the CVL group and the control groups
Evaluation of ELISA reactivity using the rA2 and L. infantum SLA against a canine serological panel. Two antigenic preparations were studied: the recombinant A2 protein (upper panels) and a soluble L. infantum antigenic (SLA) preparation (bottom panels). ELISA assays were performed using sera samples obtained by the next animal groups: symptomatic (CVLS, n = 44) or asymptomatic (CVLA, n = 9) visceral leishmaniasis (VL) dogs, healthy dogs living in endemic (HEA, n = 44) or non-endemic (HNEA, n = 20) areas of leishmaniasis, dogs immunized with Leish-Tec® vaccine (HV, n = 30), and animals infected with Trypanosoma cruzi (TC, n = 10), Ehrlichia canis (EC, n = 10), or Babesia canis (BC, n = 10). The individual OD values are shown (a). The dotted line represents the cutoff value calculated by a ROC analysis. The box and whisker plots of the ODI values from sera grouped in non-infected (control) or CVL (Leishmania-infected) groups are also shown (b), as well as the ROC curves obtained for LiHyD protein (c). Statistically significant differences (***P < 0.001) were observed between the CVL group and the control groups
Comparative efficacy between the rLiHyD protein and its linear and conformational B cell epitopes for CVL serodiagnosis
Next, the antigenicity of the linear and conformational epitopes was investigated by an ELISA assay. Results are shown in Fig. 3. The three peptides were recognized by CVL sera. However, an unexpected reactivity against Peptide-1 and Peptide-2 was observed when sera from non-infected dogs living in endemic area of leishmaniasis were employed. Of note, Peptide-3 was able to react specifically with CVL samples, irrespective of the presence of clinical signs (Fig. 3a). In fact, this antigen presented the best results to distinguish the CVL sera from the other samples, since all sera reactivities were higher than the cutoff value calculated by ROC analysis. The ODI values from L. infantum-infected dogs and those from non-infected animals were also determined (Fig. 3b). The AUC was used to compare the efficacy between the different peptides (Fig. 3c). Peptide-3 presented the highest AUC value (1.000), followed by Peptide-2 (0.885) and Peptide-1 (0.844). Parameters related to the sensitivity and specificity of these antigens applied in the CVL serodiagnosis were determined and are also shown (Table 3). Peptide-3 presented the maximum sensitivity and accuracy values (100.0 % in both cases), similar to the values obtained using the rLiHyD protein. Peptide-1 and Peptide-2 showed sensitivity values of 92.5 and 81.1 %, respectively, and an accuracy of 72.8 and 80.8 %, respectively (Table 3). Evaluating the specificity of the synthetic epitopes, Peptide-3 showed the highest value (100.0 %), followed by Peptide-2 (80.7 %) and Peptide-1 (64.5 %).
Analysis of the specific B cell epitopes derived from LiHyD protein for CVL serodiagnosis. ELISA assays were performed using three synthetic epitopes designed on the basis of a computation analysis looking for linear (Peptide-1 [upper panels] and Peptide-2 [middle panels]) and the conformational epitopes (Peptide-3). Sera samples were obtained from symptomatic (CVLS; n = 44) or asymptomatic (CVLA; n = 9) visceral leishmaniasis (VL) dogs, from healthy dogs living in endemic (HEA; n = 44) or non-endemic (HNEA; n = 20) areas of leishmaniasis, and from dogs immunized with Leish-Tec® vaccine (HV; n = 30) or from animals infected with Trypanosoma cruzi (TC; n = 10), Ehrlichia canis (EC; n = 10), or Babesia canis (BC; n = 10). The individual OD values are shown (a). The dotted line represents the cut-off value calculated by a ROC analysis. The box and whisker plots of the ODI values from sera grouped in non-infected (control) or CVL (Leishmania-infected) groups are also shown (b), as well as the ROC curves obtained from the same groups (c). Statistically significant differences (***P < 0.001) were observed between the CVL group and the control groups
Discussion
The development of a cost-effective and affordable diagnostic tool for CVL is still needed. It will allow the establishment of field assays within the national control strategic programs of the endemic countries for detecting canine infections (Desjeux 2004). Similarly, some problems have been reported for an accurate CVL serodiagnosis. The first one is the variable sensitivity of the tests, especially in determining asymptomatic but infected animals that can present low titers of antileishmanial antibodies. An additional difficulty is the low specificity of the tests when they are employed in areas endemic for other pathogens related or not to Leishmania spp. This lack of specificity usually produces false positive results (Coelho et al. 2009; Laurenti et al. 2014; Wolf et al. 2014; Peixoto et al. 2015).
In recent years, studies have been developed aiming to improve the quality of the CVL serodiagnosis. Some of these were directed to produce and characterize recombinant versions of parasite individual antigens to be employed for CVL diagnosis (Celeste et al. 2004; Fonseca et al. 2014; Menezes-Souza et al. 2015). Derived from this line of investigation, detection of the major antigenic determinants within these proteins and construction of synthetic peptides able to diagnose the disease are also the focus of current research (Costa et al. 2011; Martins et al. 2015). It should be noted that peptides are usually more stable, easier to produce, and cheaper than recombinant proteins (Chávez-Fumagalli et al. 2013). In this light, the present study evaluated the antigenic properties of a Leishmania hypothetical protein that was recently identified by an immunoproteomic study performed with L. infantum total extracts (Coelho et al. 2012). Aiming to compare the efficacy between different antigenic compositions based on the same protein, studies were completed using three putative B cell epitopes derived from LiHyD, two being linear and the other a conformational epitope.
SLA-based ELISA has been evaluated in the CVL serodiagnosis (Coelho et al. 2009; Chávez-Fumagalli et al. 2013). The main inconvenience is that total Leishmania preparations share common epitopes with other microbial antigens, resulting in the cross-reaction with serum samples from dogs infected with related diseases (De Arruda et al. 2013; Kubar & Fragaki 2005). Another limitation has been related to the standardization of the production of these extracts, affecting the reproducibility of the tests. In fact, the commercial EIE-LVC® kit can present false positive results. Marcondes et al. (2011) reported a high degree of cross-reactivity between Leishmania spp. and T. cruzi (57%) species, as well as between Leishmania spp. and E. canis (57%) species. Zanette et al. (2014), using three serological methods for the CVL serodiagnosis, showed cross-reactivity among the sera from dogs infected with E. canis, B. canis, Toxoplasma gondii, Neospora caninum, and T. cruzi.
In the present study, it was observed that all CVL sera recognized the rLiHyD protein. In addition, a null cross-reactivity was observed when sera of T. cruzi-, B. canis-, or E. canis-infected dogs were evaluated. When the putative B cell epitopes were studied, different results were obtained. Although the three peptides were clearly antigenic, only Peptide-3, designed to contain a conformational epitope, presented the same sensitivity and specificity values as the recombinant protein. The development and use of a new generation of biotechnological products has been based on identification of linear or conformational epitopes. Peptides recognized by the antibodies present in sera of patients developing different diseases can be employed for their diagnosis (Chávez-Fumagalli et al. 2013; Menezes-Souza et al. 2014). Also, peptides have emerged as vaccine candidates against rotavirus infection (Jafarpour et al. 2015) or dengue (Amat-ur-Rasool et al. 2015). However, to the best of our knowledge, the present study is the first to employ a peptide containing a conformational Leishmania epitope for CVL serodiagnosis. The better results observed for the conformational peptide relative to the linear ones offers an alternative approach to find new antigenic molecules that can be easily constructed and reproduced for diagnostic purposes.
Aiming to compare the diagnostic efficacy of the rLiHyD with other known diagnostic antigenic markers, the rA2 protein (Porrozzi et al. 2007; Akhoundi et al. 2013) was included in the analysis. This antigen is expressed in the amastigote stage of some Leishmania species and belongs to a protein family that displays a variable number of repeated sequences of 10 amino acid residues (Zhang et al. 1996). In 2007, the Brazilian Ministry of Agriculture licensed the use of Leish-Tec® vaccine (based the recombinant A2 protein) to prevent CVL. One problem associated with this vaccination is that about 30.9 % of the vaccinated dogs tested seropositive when an SLA-based ELISA is employed (Fernandes et al. 2014). Data obtained here showed that the rA2-based ELISA failed not only to distinguish between Leishmania-infected dogs from those vaccinated with Leish-Tec® but also between Leishmania and E. canis-infected dogs. The possibility to distinguish Leish-Tec® vaccinated dogs from the Leishmania-infected ones is another advantage of using the rLiHyD protein or Peptide-3. Both molecules have improved the diagnostic values found with other antigens, such as other recombinant single proteins (Fonseca et al. 2014; Rodríguez-Cortés et al. 2013) or chimeric proteins (Boarino et al. 2005; Faria et al. 2015), synthetic linear peptides (Chávez-Fumagalli et al. 2013), and phage-derived mimotopes (Costa et al. 2013).
One drawback of this work is that we have not demonstrated the presence of the LiHyD protein in Leishmania. However, the presence of antibodies recognizing this protein in the sera of infected dogs may be taken as an indication that it is expressed by the parasites during the active disease. Database searches performed in this study demonstrated the presence of LiHyD encoding genes in different Leishmania species. The protein is highly conserved in Leishmania spp. and no orthologue form was found in other Trypanosomatidae. This specificity together with its high antigenicity allows its use as a diagnostic tool for VL. However, further studies should be performed to understand its expression pattern, as well as the biological function that the protein plays in the parasite.
Although nearly 200 serum samples had been used in the present work, other studies are also necessary to evaluate a larger canine serological panel, in order to further corroborate the efficacy of these diagnostic markers for the CVL serodiagnosis. For instance, our panel did not contain samples from L. braziliensis-infected dogs, although in Brazil there are endemic areas for both tegumentary and visceral leishmaniasis (Courtenay et al. 2002; Coura-Vital et al. 2011). In this context, the present study should be taken as a proof-of-concept of the capacity of the proposed antigens for the CVL serodiagnosis and may well serve as a reference for further assays. However, due to scarcity of antigens to diagnose this important neglected disease, this study irradiates new possibilities to use both the rLiHyD protein and its conformational epitope as possible diagnostic markers for CVL.
References
Akhoundi B, Mohebali M, Shojaee S, Jalali M, Kazemi B, Bandehpour M, Keshavarz H, Edrissian GH, Eslami MB, Malekafzali H, Kouchaki A (2013) Rapid detection of human and canine visceral leishmaniasis: assessment of a latex agglutination test based on the A2 antigen from amastigote forms of Leishmania infantum. Exp Parasitol 133:307–313
Almeida-Leal GG, Roatt BM, Aguiar-Soares RDO, Carneiro CM, Giunchetti RC, Teixeira-Carvalho A, Martins-Filho OA, Francisco AF, Cardoso JM, Mathias FA, Correa-Oliveira R, Carneiro M, Coura-Vital W, Reis AB (2014) Immunological profile of resistance and susceptibility in naturally infected dogs by Leishmania infantum. Vet Parasitol 205:472–482
Alvar J, Canavate C, Molina R, Moreno J, Nieto J (2004) Canine leishmaniasis. Adv Parasitol 57:1–88
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J (2012) WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671
Amat-ur-Rasool H, Saghir A, Idrees M (2015) Computational prediction and analysis of envelop glycoprotein epitopes of DENV-2 and DENV-3 Pakistani isolates: a first step towards Dengue vaccine development. PLoS One 10:e0119854
Antinori S, Calattini S, Longhi E, Bestetti G, Piolini R, Magni C, Orlando G, Gramiccia M, Acquaviva V, Foschi A, Corvasce S, Colomba C, Titone L, Parravicini C, Cascio A, Corbellino M (2007) Clinical use of polymerase chain reaction performed on peripheral blood and bone marrow samples for the diagnosis and monitoring of visceral leishmaniasis in HIV-infected and HIV-uninfected patients: a single-center, 8-year experience in Italy and review of the literature. Clin Infect Dis 44:1602–1610
Baneth G, Aroch I (2008) Canine leishmaniasis: a diagnostic and clinical challenge. Vet J 175:14–15
Boarino A, Scalone A, Gradoni L, Ferroglio E, Vitale F, Zanatta R, Giuffrida MG, Rosati S (2005) Development of recombinant chimeric antigen expressing immunodominant B epitopes of Leishmania infantum for serodiagnosis of visceral leishmaniasis. Clin Diagn Lab Inmunol 12:647–653
Candido TC, Perri SHV, Gerzoschkwitz TDO, Luvizotto MCR, Lima VMF (2008) Comparative evaluation of enzyme-linked immunosorbent assay based on crude and purified antigen in the diagnosis of canine visceral leishmaniasis in symptomatic and oligosymptomatic dogs. Vet Parasitol 157:175–181
Celeste BJ, Angel SO, Castro LGM, Gidlund M, Goto H (2004) Leishmania infantum heat shock protein 83 for the serodiagnosis of tegumentary leishmaniasis. Braz J Med Biol Res 37:1591–1593
Chávez-Fumagalli MA, Martins VT, Testasicca MCS, Lage DP, Costa LE, Lage PS, Duarte MC, Ker HG, Ribeiro TG, Carvalho FA, Régis WC, Reis AB, Tavares CA, Soto M, Fernandes AP, Coelho EA (2013) Sensitive and specific serodiagnosis of Leishmania infantum infection in dogs by using peptides selected from hypothetical proteins identified by an immunoproteomic approach. Clin Vaccine Immunol 20:835–841
Ciaramella P, Oliva G, Luna RD, Gradoni L, Ambrosio R, Cortese L, Scalone A, Persechino A (1997) A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet Rec 141:539–543
Coelho EAF, Tavares CAP, Carvalho FAA, Chaves KF, Teixeira KN, Rodrigues RC, Charest H, Matlashewski G, Gazzinelli RT, Fernandes AP (2003) Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect Immun 71:3988–3994
Coelho EAF, Ramírez L, Costa MAF, Coelho VTS, Martins VT, Chávez-Fumagalli MA, Oliveira DM, Tavares CA, Bonay P, Nieto CG, Abánades DR, Alonso C, Soto M (2009) Specific serodiagnosis of canine visceral leishmaniasis using Leishmania species ribosomal protein extracts. Clin Vaccine Immunol 16:1774–1780
Coelho VTS, Oliveira JS, Valadares DG, Chávez-Fumagalli MA, Duarte MC, Lage PS, Soto M, Santoro MM, Tavares CA, Fernandes AP, Coelho EA (2012) Identification of proteins in promastigote and amastigote-like Leishmania using an immunoproteomic approach. PLoS Negl Trop Dis 6:e1430
Costa MM, Andrade HM, Bartholomeu DC, Freitas LM, Pires SF, Chapeaurouge AD, Perales J, Ferreira AT, Giusta MS, Melo MN, Gazzinelli RT (2011) Analysis of Leishmania chagasi by 2-D difference gel electrophoresis (2-D DIGE) and immunoproteomic: identification of novel candidate antigens for diagnostic tests and vaccine. J Proteome Res 10:2172–2184
Costa LE, Lima MIS, Chávez-Fumagalli MA, Menezes-Souza D, Martins VT, Duarte MC, Lage PS, Lopes EG, Lage DP, Ribeiro TG, Andrade PH, Magalhães-Soares DF, Soto M, Tavares CA, Goulart LR, Coelho EA (2013) Subtractive phage display selection from canine visceral leishmaniasis identifies novel epitopes that mimic Leishmania infantum antigens with potential serodiagnosis applications. Clin Vaccine Immunol 21:96–106
Coura-Vital W, Marques MJ, Veloso VM, Roatt BM, Aguiar-Soares RDDO, Reis LES, Braga SL, Morais MH, Reis AB, Carneiro M (2011) Prevalence and factors associated with Leishmania infantum infection of dogs from an urban area of Brazil as identified by molecular methods. PLoS Negl Trop Dis 5:1–10
Coura-Vital W, Ker HG, Roatt BM, Aguiar-Soares RDO, Leal GGDA, Moreira NDD, Oliveira LA, Menezes-Machado EM, Morais MH, Corrêa-Oliveira R, Carneiro M, Reis AB (2014) Evaluation of change in canine diagnosis protocol adopted by the visceral leishmaniasis control program in Brazil and a new proposal for diagnosis. PLoS One 9:1–6
Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C (2002) Infectiousness in a cohort of Brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J Infect Dis 186:1314–1320
De Arruda MM, Figueiredo FB, Cardoso FA, Hiamamoto RM, Brazuna JCM, Oliveira MR, Noronha EF, Romero GA (2013) Validity and reliability of enzyme immunoassays using Leishmania major or L. infantum antigens for the diagnosis of canine visceral leishmaniasis in Brazil. PLoS One 8:8–13
Deborggraeve S, Laurent T, Espinosa D, Van der Auwera G, Mbuchi M, Wasunna M, El-Safi S, Al-Basheer AA, Arévalo J, Miranda-Verástegui C, Leclipteux T, Mertens P, Dujardin JC, Herdewijn P, Büscher P (2008) A simplified and standardized polymerase chain reaction format for the diagnosis of leishmaniasis. J Infect Dis 198:1565–1572
Desjeux P (2004) Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27:305–318
Emini EA, Hughes JV, Perlow DS, Boger J (1985) Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol 55:836–839
Faria R, Veloso LDC, Coura-Vital W, Reis AB (2015) Novel recombinant multiepitope proteins for the diagnosis of asymptomatic Leishmania infantum-infected dogs. PLoS Negl Trop Dis 9:e3429
Fernandes AP, Canavaci AM, McCall LI, Matlashewski G (2014) A2 and other Visceralizing proteins of Leishmania: role in pathogenesis and application for vaccine development. Subcell Biochem 74:77–101
Fonseca AM, Faria AR, Rodrigues FTG, Nagem RAP, Magalhães RDM, Cunha JL, Bartholomeu DC, Andrade HM (2014) Evaluation of three recombinant Leishmania infantum antigens in human and canine visceral leishmaniasis diagnosis. Acta Tropica 137:25–30
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A (2005) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788
Jafarpour S, Ayat H, Ahadi AM (2015) Design and antigenic epitopes prediction of a new trial recombinant multiepitopic rotaviral vaccine: in silico analyses. Viral Immunol 28:325–330
Kolaskar AS, Tongaonkar PC (1990) A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett 276:172–174
Kubar J, Fragaki K (2005) Recombinant DNA-derived Leishmania proteins: from the laboratory to the field. Lancet Infect Dis 5:107–114
Lage DP, Martins VT, Duarte MC, Garde E, Chávez-Fumagalli MA, Menezes-Souza D, Roatt BM, Tavares CA, Soto M, Coelho EA (2015) Prophylactic properties of a Leishmania-specific hypothetical protein in a murine model of visceral leishmaniasis. Parasite Immunol 37:646–656
Larsen JE, Lund O, Nielsen M (2006) Improved method for predicting linear B-cell epitopes. Immunome Res 2:2
Laurenti MD, Leandro MVDS, Tomokane TY, Lucca HRL, Aschar M, Souza CSF, Silva RM, Marcondes M, Matta VLR (2014) Veterinary parasitology comparative evaluation of the DPP® CVL rapid test for canine serodiagnosis in area of visceral leishmaniasis. Vet Parasitol 205:444–450
Machado-de-Ávila RA, Stransky S, Velloso M, Castanheira P, Schneider FS, Kalapothakis E, Sanchez EF, Nguyen C, Molina F, Granier C, Chávez-Olórtegui C (2011) Mimotopes of mutalysin-II from Lachesis muta snake venom induce hemorrhage inhibitory antibodies upon vaccination of rabbits. Peptides 32:1640–1646
Maia C, Campino L (2008) Methods for diagnosis of canine leishmaniasis and immune response to infection. Vet Parasitol 158:274–287
Marcondes M, Biondo AW, Gomes AAD, Silva ARS, Vieira RFC, Camacho AA, Quinn J, Chandrashekar R (2011) Validation of a Leishmania infantum ELISA rapid test for serological diagnosis of Leishmania chagasi in dogs. Vet Parasitol 175:15–19
Martins VT, Chávez-Fumagalli MA, Costa LE, Martins AMCC, Lage PS, Duarte MC, Valadares DG, Magalhães RD, Ribeiro TG, Nagem RA, Da-Rocha WD, Régis WC, Soto M, Coelho EA, Fernandes AP, Tavares CA (2013) Antigenicity and protective efficacy of a Leishmania amastigote-specific protein, member of the super-oxygenase family, against visceral leishmaniasis. PLoS Negl Trop Dis 7, e2148
Martins VT, Duarte MC, Chávez-Fumagalli MA, Menezes-Souza D, Coelho CS, Magalhães-Soares DF, Fernandes AP, Soto M, Tavares CA, Coelho EA (2015) A Leishmania-specific hypothetical protein expressed in both promastigote and amastigote stages of Leishmania infantum employed for the serodiagnosis of, and as a vaccine candidate against, visceral leishmaniasis. Parasite Vectors 8:363
Menezes-Souza D, Mendes TA, Gomes MS, Reis-Cunha JL, Nagem RA, Carneiro CM, Coelho EA, Galvão LM, Fujiwara RT, Bartholomeu DC (2014) Epitope Mapping of the HSP83.1 Protein of Leishmania braziliensis Discloses novel targets for immunodiagnosis of tegumentary and visceral clinical forms of leishmaniasis. Clin Vaccine Immunol 21:949–959
Menezes-Souza D, Oliveira-Mendes TA, Araújo-Leão AC, Souza-Gomes M, Fujiwara RT, Bartholomeu DC (2015) Linear B-cell epitope mapping of MAPK3 and MAPK4 from Leishmania braziliensis: implications for the serodiagnosis of human and canine leishmaniasis. Appl Microbiol Biotechnol 99:1323–1336
Merrifield RB (1963) Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol 32:221–296
Moshfe A, Mohebali M, Edrissian G, Zarei Z, Akhoundi B, Kazemi B, Jamshidi S, Mahmoodi M (2009) Canine visceral leishmaniasis: asymptomatic infected dogs as a source of L. infantum infection. Acta Tropica 112:101–105
Noya O, Patarroyo ME, Guzmán F, Alarcón-de-Noya B (2003) Immunodiagnosis of parasitic diseases with synthetic peptides. Curr Protein Pept Sci 4:299–308
Peixoto HM, Oliveira MR, Romero GA (2015) Serological diagnosis of canine visceral leishmaniasis in Brazil: systematic review and meta-analysis. Trop Med Int Health 20:334–352
Petersen CA (2009) Leishmaniasis, an emerging disease found in companion animals in the United States. Top Companion Anim Med 24:182–188
Porrozzi R, Costa MVS, Teva A, Falqueto A, Ferreira AL, Santos CD, Fernandes AP, Gazzinelli RT, Campos-Neto A, Grimaldi G Jr (2007) Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic Leishmania infantum visceral infections in dogs. Clin Vaccine Immunol 14:544–548
Ready PD (2010) Leishmaniasis emergence in Europe. Euro Surveill 15:19505
Reis LE, Coura-Vital W, Roatt BM, Bouillet LE, Ker HG, Fortes-de-Brito RC, Resende DM, Carneiro M, Giunchetti RC, Marques MJ, Carneiro CM, Reis AB (2013) Molecular diagnosis of canine visceral leishmaniasis: a comparative study of three methods using skin and spleen from dogs with natural Leishmania infantum infection. Vet Parasitol 197:498–503
Rodríguez-Cortés A, Ojeda A, Todolí F, Alberola J (2013) Performance of commercially available serological diagnostic tests to detect Leishmania infantum infection on experimentally infected dogs. Vet Parasitol 191:363–366
Saha S, Raghava GPS (2006) Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 65:40–48
Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, Bourdeau P, Oliva G, Baneth G (2011) LeishVet guidelines for the practical management of canine leishmaniosis. Parasite Vectors 4:86
Soto M, Requena JM, Quijada L, Alonso C (1998) Multicomponent chimeric antigen for serodiagnosis of canine visceral leishmaniasis. J Clin Microbiol 36:58–63
Zanette MF, Lima VM, Laurenti MD, Rossi CN, Vides JP, Vieira RF, Biondo AW, Marcondes M (2014) Serological cross-reactivity of Trypanosoma cruzi, Ehrlichia canis, Toxoplasma gondii, Neospora caninum and Babesia canis to Leishmania infantum chagasi tests in dogs. Rev Soc Bras Med Trop 47:105–107
Zhang WW, Charest H, Ghedin E, Matlashewski G (1996) Identification and overexpression of the A2 amastigote-specific protein in Leishmania donovani. Mol Bioch Parasitol 78:79–90
Wolf D, Failing K, Taubert A, Pantchev N (2014) Serological diagnosis of canine leishmaniosis: comparison of three commercially available tests. Parasitol Res 113:1997–2002
World Health Organization (2010) Control of the leishmaniases: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22-26 March 2010. WHO Technical Report Series, no. 949.WHO, Geneva, Switzerland
Acknowledgments
This work was supported by grants from Instituto Nacional de Ciência e Tecnologia em Nano-biofarmacêutica (INCT-NanoBiofar), FAPEMIG (CBB-APQ-00496-11, CBB-APQ-00819-12, and CBB-APQ-01778-2014), and CNPq (APQ-472090/2011-9 and APQ-488237/2013-0). In addition, this study was partially funded in Madrid by the Spanish grant from Ministerio de Economía y Competitividad-FEDER (FISPI14/00366 from the Instituto de Salud Carlos III), and from Universidade do Extremo Sul Catarinense (UNESC). MACF is a grant recipient of FAPEMIG/CAPES. EAFC is a grant recipient of CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that they have no conflicts of interest in relation to this work.
Rights and permissions
About this article
Cite this article
Lage, D.P., Martins, V.T., Duarte, M.C. et al. A new Leishmania-specific hypothetical protein and its non-described specific B cell conformational epitope applied in the serodiagnosis of canine visceral leishmaniasis. Parasitol Res 115, 1649–1658 (2016). https://doi.org/10.1007/s00436-016-4904-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-4904-x