Abstract
During our search for novel prenyltransferases, a putative gene ATEG_04218 from Aspergillus terreus raised our attention and was therefore amplified from strain DSM 1958 and expressed in Escherichia coli. Biochemical investigations with the purified recombinant protein and different aromatic substrates in the presence of dimethylallyl diphosphate revealed the acceptance of all the tested tryptophan-containing cyclic dipeptides. Structure elucidation of the main enzyme products by NMR and MS analyses confirmed the attachment of the prenyl moiety to C-7 of the indole ring, proving the identification of a cyclic dipeptide C7-prenyltransferase (CdpC7PT). For some substrates, reversely C3- or N1-prenylated derivatives were identified as minor products. In comparison to the known tryptophan-containing cyclic dipeptide C7-prenyltransferase CTrpPT from Aspergillus oryzae, CdpC7PT showed a much higher substrate flexibility. It also accepted cyclo-l-Tyr-l-Tyr as substrate and catalyzed an O-prenylation at the tyrosyl residue, providing the first example from the dimethylallyltryptophan synthase (DMATS) superfamily with an O-prenyltransferase activity towards dipeptides. Furthermore, products with both C7-prenyl at tryptophanyl and O-prenyl at tyrosyl residue were detected in the reaction mixture of cyclo-l-Trp-l-Tyr. Determination of the kinetic parameters proved that (S)-benzodiazepinedione consisting of a tryptophanyl and an anthranilyl moiety was accepted as the best substrate with a K M value of 204.1 μM and a turnover number of 0.125 s−1. Cyclo-l-Tyr-l-Tyr was accepted with a K M value of 1,411.3 μM and a turnover number of 0.012 s−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
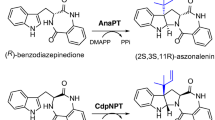
Prenyltransferases are an ubiquitous and intriguing class of enzymes involved in the biosynthesis of both primary and secondary metabolites (Heide 2009; Li 2010; Lu et al. 2009; Yazaki et al. 2009; Yu and Li 2012). One of the most investigated subgroup is the DMATS superfamily, which shares more or less sequence similarity with dimethylallyltryptophan synthase in the biosynthesis of ergot alkaloids (Tsai et al. 1995; Yu and Li 2012). Bioinformatic analysis in 2011 revealed that about 200 putative genes, mainly from genome sequencing projects, belong to this superfamily (Bonitz et al. 2011). The number of these genes has increased in the last years with the releasing of new genome sequences. So far, more than 30 such putative genes have been experimentally proven to be prenyltransferases and characterized biochemically (Pockrandt et al. 2014; Tarcz et al. 2014; Winkelblech and Li 2014; Yu and Li 2012). The majority of the DMATS superfamily uses indole derivatives including tryptophan and tryptophan-containing cyclic dipeptides as substrates and is involved in the biosynthesis of prenylated indole alkaloids. They catalyze transfer reactions of a prenyl moiety from prenyl diphosphate, usually dimethylallyl diphosphate (DMAPP) to different positions of the indole ring (Yu and Li 2012). In comparison to their nonprenylated precursors, prenylated indole alkaloids show distinct and in general more potent biological and pharmacological activities (Jain et al. 2008; Li 2010; Williams et al. 2000; Wollinsky et al. 2012a). The resulting products are therefore important sources of drugs or drug candidates (Li 2010; Williams et al. 2000). These characteristics open numerous multidisciplinary research opportunities for scientists from different disciplines. As a consequence, enzymes that catalyze transfer reactions of a prenyl moiety onto each position of the indole ring have been identified in the last decade and intensively studied on the molecular biological, biochemical and structural biological level. Tryptophan prenyltransferases such as FgaPT2, 5-DMATS, 6-DMATS, and 7-DMATS use logically l-tryptophan as natural substrate and catalyze prenylation reactions at C-4, C-5, C-6, and C-7 of the indole ring (Kremer et al. 2007; Unsöld and Li 2005; Winkelblech and Li 2014; Yu et al. 2012), respectively (Fig. 1). Cyclic dipeptide prenyltransferases accept in turn tryptophan-containing cyclic dipeptides as natural (BrePT, FtmPT1, and AnaPT) (Grundmann and Li 2005; Yin et al. 2009, 2013a) or best substrates (CdpNPT, CdpC2PT, and CdpC3PT) (Mundt and Li 2013; Schuller et al. 2012; Yin et al. 2010b). These enzymes mainly catalyze prenylations at C-2 or C-3 of the indole nucleus (Yu and Li 2012). The cyclic dipeptide prenyltransferase CTrpPT with its preferable substrate cyclo-l-Trp-l-Trp catalyzed simultaneously both N1- and C7-prenylation (Fig. 1) (Zou et al. 2010). CTrpPT is the sole cyclic dipeptide prenyltransferase, which catalyzes a prenylation at the benzene ring in the presence of its natural prenyl donor DMAPP.
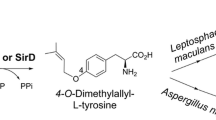
In addition to indole prenyltransferases, some members of the DMATS superfamily use nonindole compounds as substrates. For example, SirD from Leptosphaeria maculans catalyzes an O-prenylation of l-tyrosine (Kremer and Li 2010; Rudolf and Poulter 2013; Zou et al. 2011). However, an enzymatic O-prenylation of tyrosyl residue in a dipeptide has not been reported prior to this study.
Analysis of the genome sequence of Aspergillus terreus NIH2624 revealed the presence of ten putative prenyltransferase genes from the DMATS superfamily. Functions of five such genes have already been characterized molecular biologically or biochemically. We report here the cloning and expression of a putative prenyltransferase gene ATEG_04218 as well as the identification of the overproduced enzyme as a tryptophan-containing cyclic dipeptide C7-prenyltransferase (CdpC7PT) with an O-prenyltransferase activity for tyrosine-containing cyclic dipeptides.
Materials and methods
Chemicals
DMAPP was prepared according to the method described for geranyl diphosphate (Woodside et al. 1988). Cyclo-l-Tyr-l-Tyr was synthesized according to a protocol described previously (Jeedigunta et al. 2000). Other cyclic dipeptides were prepared as described elsewhere (Wollinsky et al. 2012a; Yu et al. 2013) or purchased from Bachem (Bubendorf, Switzerland).
Computer-assisted sequence analysis
Sequence analysis and intron prediction were performed with FGENESH-M (Softberry, Mount Kisco, NY; www.softberry.com) and geneid (http://genome.crg.es/geneid.html). Alignments of amino acid sequences were carried out by using the programs “BLAST 2 SEQUENCES” (http://blast.ncbi.nlm.nih.gov) and ClustalX2 (http://www.clustal.org/clustal2/).
Bacterial strains, plasmids, and culture conditions
pGEM-T Easy and pQE-60 were purchased from Promega (Mannheim, Germany) and QIAGEN (Hilden, Germany) and used for cloning and expression experiments, respectively. Escherichia coli XL1 Blue MRF’ (Agilent Technologies, Amsterdam, the Netherlands) and M15 (pREP4) cells (QIAGEN, Hilden, Germany) were used as cloning and expression hosts. Cultivation of E. coli was carried out at 30 or 37 °C in liquid or on solid LB medium with 1.5 % (w/v) agar (Sambrook and Russell 2001). Kanamycin (25 μg ml−1) and carbenicillin (50 μg ml−1) were used for selection of recombinant strains.
Cultivation of A. terreus for DNA isolation
A. terreus DSM 1958 was obtained from German Collection of Microorganisms and Cell Cultures (DSMZ) and cultivated in 300-ml cylindrical flasks containing 100 ml YME medium (Yeast extract [4.0 g l−1], glucose monohydrate [4.0 g l−1], and Malt Extract [10.0 g l−1] at 30 °C for 5 days in darkness for DNA isolation.
DNA propagation in E. coli and DNA isolation from fungi
Standard procedures for DNA isolation and manipulation in E. coli were performed as described elsewhere (Sambrook and Russell 2001).
To isolate genomic DNA from A. terreus, the mycelia of a 5-day-old culture were collected and washed with phosphate-buffered saline consisting of 137 mM NaCl, 2.7 mM KCl, 1 mM Na2HPO4, and 0.18 mM KH2PO4 (pH 7.3). Genomic DNA was isolated according to a method described previously (Yu and Li 2012).
PCR amplification and gene cloning
For PCR amplification, an iCycler from BioRad was used. Two primer pairs ATEG_04218_1fw (5′-AACC ATGGCCAACCGCGTGTCTAACG-3′) and ATEG_04218_3rv (5′-CGACTACTCTGAGACAAATCCACATCGGGAAATATATCTTTCAGCATCTG-3′) were used for amplification of the first exon and ATEG_04218_2fw (5′-CAGATGCTGAAAGATATATTTCCCGATGTGGATTTGTCTCAGAGTAGTCG-3′) and ATEG_04218_4rv (5′-GCAGATCTCTCTGCTAAGAATTCAGTAGAGCGCG-3′) for the second exon. Bold letters in ATEG_04218_1fw and ATEG_04218_4rv represent mutations inserted in comparison to the original genome sequence in order to get the underlined restriction sites NcoI and BglII for cloning in pQE-60, respectively. The underlined letters in ATEG_04218_2fw and ATEG_04218_3rv indicate sequences at 3′-end of exon 1 and 5′-end of exon 2 for fusion of the two exons. A PCR program of 35 cycles with an annealing temperature at 59 °C for 60 s and elongation at 72 °C for 120 s was used for PCR amplification. After amplification of both exons separately, the PCR products were mixed in a molar ratio of 1:1 and used for the second round PCR to get the fusion product with the expected size of 1,350 bp. The obtained PCR fragment was cloned into pGEM-T Easy vector resulting in plasmid pCaW16, which was sequenced by Eurofins Genomics GmbH (Ebersberg, Germany) to confirm the sequence. After digestion of pCaW16 with the restriction enzymes NcoI, BglII, and DraI, the resulting NcoI-BglII fragment of 1,344 bp was directly ligated into pQE-60, which had been digested with NcoI and BglII, previously. The resulting plasmid pCaW18 was used for protein overproduction.
Overproduction and purification of CdpC7PT-His6
For cdpC7PT expression with pCaW18, E. coli M15 [pREP] was cultivated in a 2,000-ml cylindrical flask containing 1,000 ml liquid LB medium supplemented with carbenicillin (50 μg ml−1) and kanamycin (25 μg ml−1). After growing at 37 °C to an optical density at 600 nm of 0.6, gene expression was induced with isopropyl thiogalactoside at a final concentration of 0.3 mM at 37 °C for 16 h. The bacterial cultures were harvested by centrifugation and resuspended in lysis buffer (10 mM imidazole, 50 mM NaH2PO4, 300 mM NaCl, pH 8.0) at 2–5 ml g−1 wet weight. After incubation for 30 min on ice with 1 mg ml−1 lysozyme, the cells were sonicated six times for 10 s each at 200 W. To separate the cellular debris from the soluble proteins, the lysate was centrifuged at 14,000×g for 30 min at 4 °C. One-step purification of the recombinant His6-tagged fusion protein by affinity chromatography with Ni-NTA agarose resin (Macherey-Nagel, Düren, Germany) was carried out according to the manufacturer’s instructions. The protein was eluted with 250 mM imidazole in 50 mM NaH2PO4 and 300 mM NaCl, pH 8.0. In order to change the buffer, the protein fraction was passed through a NAP-5 column (GE Healthcare, Freiburg, Germany), which had been equilibrated with 50 mM Tris/HCl and 15 % (v/v) glycerol, pH 7.5. CdpC7PT-His6 was eluted with the same buffer as for column equilibration and stored at −80 °C for enzyme assays.
Protein analysis and determination of molecular mass of active CdpC7PT-His6
CdpC7PT was analyzed on SDS-PAGE according to the method described previously (Laemmli 1970) and stained with Coomassie Brilliant Blue G-250. The molecular mass of the recombinant CdpC7PT-His6 was determined on a HiLoad 16/60 Superdex 200 column (GE Healthcare, Freiburg, Germany) by using 50 mM Tris–HCl buffer (pH 7.5) containing 150 mM NaCl as elution buffer. The column was calibrated with dextran blue 2000 (2,000 kDa), ferritin (440 kDa), aldolase (158 kDa), conalbumin (75 kDa), carbonic anhydrase (29 kDa), and ribonuclease A (13.7 kDa) (GE Healthcare, Freiburg, Germany). The molecular mass of the recombinant active CdpC7PT-His6 was determined as 62 kDa, proving that CdpC7PT acts as a monomer.
Assays for CdpC7PT activity
For determination of CdpC7PT activity, the reaction mixtures (100 μl) contained 50 mM Tris–HCl (pH 7.5), 5 mM CaCl2, 1 mM cyclic dipeptide (Table 1), 2 mM DMAPP, and 50 μg (9.0 μM) of purified recombinant CdpC7PT and were incubated at 37 °C for 16 h. The reactions were terminated by addition of 60 μl methanol. After removal of proteins by centrifugation at 13,000×g for 20 min, the enzyme products were analyzed on HPLC with the method described below. Duplicate values were determined routinely for quantitative measurement of the enzyme activity. The assays for determination of the kinetic parameters of (S)-benzodiazepinedione (1a), cyclo-l-Trp-l-Trp (3a), cyclo-l-Trp-l-Tyr (4a), cyclo-l-Trp-l-Phe (5a), cyclo-l-Trp-l-Leu (6a), cyclo-l-Trp-l-Ala (7a), and of cyclo-l-Tyr-l-Tyr (18a) contained 5 mM CaCl2, 2 mM DMAPP, 2.0 μM (1a, 3a), or 4.0 μM (4a–7a, 18a) CdpC7PT and aromatic substrates at final concentrations of up to 5.0 mM. For determination of the kinetic parameters of DMAPP, 2.0 μM CdpC7PT, 1 mM 1a, 5 mM CaCl2, and DMAPP at final concentrations of 0.01, 0.025, 0.05, 0.1, 0.25, 0.5, 1.0, 2.0, and 5.0 mM were used. Incubations were carried out at 37 °C for 60 min (DMAPP, 1a, 3a) or 120 min (other substrates).
For structural elucidation of the enzyme products, the isolation assays (20 ml) contained 50 mM Tris–HCl (pH 7.5), 1 mM DMAPP, 1 mM aromatic substrate, 4.0–6.0 μM of CdpC7PT, 5 mM CaCl2 and were incubated at 37 °C for 16 h.
HPLC analysis and product isolation
Enzyme assays of CdpC7PT were analyzed on HPLC with an Agilent series 1200 (Agilent, Foster City, CA) by using a Multospher 120 RP-18 column (250 × 4 mm, 5 μm, CS-Chromatographie Service, Langerwehe, Germany) and flow rate of 1 ml min−1. Water (solvent A) and methanol (solvent B) were used as solvents. For analysis of enzyme products, a linear gradient of 30–100 % (v/v) solvent B in A in 25 min was used. The column was then washed with 100 % solvent B for 5 min and equilibrated with 30 % solvent A for 5 min.
The assays for isolation of the enzyme products were extracted with ethyl acetate and concentrated on a rotation evaporator at 35 °C to dryness. The residues were dissolved in 800 μl methanol and purified on an HPLC with a Multospher 120 RP-18 column (250 × 10 mm, 5 μm) at a flow rate of 2.5 ml min−1. A linear gradient of 60–100 % of solvent B in A in 20 min was used for separation. The collected fractions were evaporated to dryness and subjected to high-resolution mass spectrometry (HR-MS) and NMR analyses.
Spectroscopic data
1H NMR spectra were recorded on a JEOL ECA-500 spectrometer (JEOL, Akishima, Tokyo, Japan). All Spectra were processed with MestReNova.6.0.2 (Metrelab Research, Santiago de Compostella, Spain) and chemical shifts were referenced to those of the solvent signals. The NMR data are given in Table 2 and spectra as Supplementary Material Information. The isolated products were also analyzed by mass spectrometry on a Q-Trap Quantum (Applied Biosystems, Darmstadt, Germany) by using a high-resolution electron impact (HR-EI) ionization mode. HR-EI-MS data are given in Table S2 in the Supplementary Material.
Cultivation of A. terreus and analysis of secondary metabolites by LC/MS analysis
A. terreus DSM 1958 were cultivated in 300-ml cylindrical flasks containing 100 ml AMM medium (6 g/l NaNO3; 0.52 g/l KCl, 0.52 g/l MgSO4x7H2O) at 30 °C and 120 rpm in darkness for 7 and 14 days. The culture filtrates were extracted twice with 100 ml ethyl acetate. The combined organic layers were dried over Na2SO4 and subsequently evaporated in vacuo to dryness. For LC/MS analysis, the extracts were dissolved in 1 ml of methanol. LC/MS analysis was carried out on an Agilent 1100 HPLC system (Agilent Technologies) coupled to a LTQ-FT Ultra ESI-MS instrument (Thermo Fischer Scientific) using a linear ion trap (MS) and an ICR cell (HR-MS), operated in positive ionization mode. Water (solvent A) and acetonitrile (solvent B) were used at a flow rate of 0.2 ml min−1. A linear gradient of 10–100 % (v/v) solvent B in 45 min was used. The column was then washed with 100 % solvent B for 5 min and equilibrated with 10 % (v/v) solvent B for 5 min.
Accession numbers
The nucleotide sequence of the genomic DNA from A. terreus reported in this paper has been deposited in the GenBank database under accession number CH476598.1. The coding region of cdpC7PT is available in GenBank under the accession number XM_0013396.1.
Results
Prenyltransferase genes in the genome of A. terreus
As aforementioned, five of the ten putative prenyltransferase genes of the DMATS superfamily from A. terreus NIH2624 had been characterized molecular biologically by gene deletion experiments or biochemically with recombinant proteins from A. terreus or with their orthologues from other fungi. They include one C3-prenyltransferase gene ATEG_10306 belonging to the acetylaszonalenin gene cluster and its orthologue AnaPT from Neosartorya fischeri NRRL 181 was proven to be responsible for the C3α-prenylation of (R)-benzodiazepinedione (Yin et al. 2009). 7-DMATS encoded by ATEG_08428 catalyzes the C7-prenylation of l-tryptophan and is involved in the biosynthesis of astechrome (Kremer et al. 2007; Yin et al. 2013b). AstPT encoded by ATEG_09980 functions as a prenyltransferase of methylated asterriquinone and catalyzes both reverse N1- and regular C2-prenylation (Tarcz et al. 2014). Gene deletion experiments suggested the involvement of ATEG_02823 and ATEG_00702 in the biosynthesis of butyrolactones and asterriquinone CT5, respectively (Guo et al. 2013). However, no biochemical data are available for the encoded enzymes. Other five putative genes ATEG_04999, ATEG_06111, ATEG_03092, ATEG_01730 and ATEG_04218 remained with unknown function prior to this work.
ATEG_04218 is located on the genomic sequence of A. terreus NIH2624 between bp 1888259 and 1889646 under the accession number CH476598.1. According to the prediction given in GenBank (http://ncbi.nlm.nih.gov), ATEG_04218 consists of two exons of 1,208 and 133 bp, respectively, interrupted by one intron of 47 bp. The deduced polypeptide EAU36020 (=CdpC7PT) has a length of 446 amino acids with a calculated molecular mass of 51.0 kDa. Blast search revealed clear sequence similarities between EAU36020 and several known cyclic dipeptide prenyltransferases of the DMATS superfamily from various fungi. For example, CdpC7PT shares a sequence identity of 34 % on the amino acid level with the reverse prenyltransferase CdpC2PT from N. fischeri (Mundt and Li 2013) and 32 % with the cyclo-l-Trp-l-Pro reverse C2-prenyltransferase BrePT from Aspergillus versicolor (Yin et al. 2013a). The sequence identity of CdpC7PT and the cyclo-l-Trp-l-Trp C7-prenyltransferase CTrpPT from Aspergillus oryzae (Zou et al. 2010) was found to be 29 %.
Analysis of the neighboring genes of ATEG_04218 on CH476598.1 did not indicate the presence of an entire putative gene cluster for secondary metabolites (Table S1 in the Supplementary Material). No genes encoding for nonribosomal peptide synthetases (NRPS), polyketide synthases (PKS), or other known secondary metabolite backbone genes have been identified in the vicinity of the prenyltransferase gene ATEG_04218. The natural substrate of ATEG_04218 can therefore not be predicted by sequence analysis. Based on its sequence similarities with cyclic dipeptide prenyltransferases, it might be speculated that these substances could be accepted by the enzyme as prenylation substrates.
Cloning of cdpC7PT, protein overproduction, and purification
Initial attempt to clone the coding sequence of ATEG_04218 from mRNA of A. terreus DSM 1958 was unsuccessful (data not shown). Its coding region consisting of two exons and was therefore amplified from genomic DNA by using two round fusion PCR and cloned into the expression vector pQE-60. Gene expression in E. coli and purification of the soluble protein resulted in a predominant band on SDS-PAGE with migration above the 45 kDa size marker (Fig. S1 in the Supplementary Material), corresponding well to the calculated mass of 51.8 kDa for CdpC7PT-His6. Protein yield was calculated to be 2.4 mg of purified protein per liter of culture. The molecular mass of the native recombinant CdpC7PT-His6 was determined by size exclusion chromatography as 62 kDa, indicating that CdpC7PT presumably acts as a monomer.
Acceptance of tryptophan-containing cyclic dipeptides by CdpC7PT as substrates
As mentioned above, CdpC7PT shares comparatively higher sequence similarities with cyclic dipeptide prenyltransferases, especially with CdpC2PT and BrePT, than with other prenyltransferases (Mundt and Li 2013; Yin et al. 2013a). Therefore, CdpC7PT was firstly incubated with 16 tryptophan-containing cyclic dipeptides (1a–16a, Table 1 and Fig. S2 in the Supplementary Material) and l-tryptophan (17a) in the presence of DMAPP as prenyl donor at 37 °C for 16 h. Enzyme assays with inactivated CdpC7PT by boiling the enzyme for 30 min were used as negative controls. HPLC analysis revealed product formation in all of the reaction mixtures with active CdpC7PT. (S)-benzodiazepinedione (1a) was found to be the best accepted substrate with a product yield of 46 %. Its enantiomer (2a), the natural substrate of AnaPT (Yin et al. 2009), was poorly accepted by CdpC7PT. Product yields of more than 20 % were detected for cyclo-l-Trp-l-Trp (3a), cyclo-l-Trp-l-Tyr (4a), cyclo-l-Trp-l-Phe (5a), cyclo-l-Trp-l-Leu (6a), cyclo-l-Trp-l-Pro (11a) and cyclo-l-Trp-Gly (15a) under the same conditions. As given in Table 1, cyclic dipeptides with d-configured amino acid moieties were poor substrates for CdpC7PT. Inspection of the HPLC chromatograms revealed the presence of one to three product peaks. l-Tryptophan was accepted only with a very low product yield of 3 %, excluding its role as natural substrate. Product formation was detected in the enzyme assays neither with geranyl nor farnesyl diphosphate, demonstrating its high specificity towards its prenyl donor DMAPP (Fig. S3 in the Supplementary Material).
C7-prenylation on the indole ring as the main reaction
To prove their structures, ten enzyme products (1b, 3b–7b, 3c, 4c, 7c, and 4d) were isolated on HPLC from incubation mixtures of six selected substrates (1a, 3a–7a; Fig. 2) in preparative scales and subjected to MS and NMR analyses. NMR data are given in Table 2 and spectra in Figs. S4–S25 in the Supplementary Material. MS data are given in Table S2 in the Supplementary Material. HR-EI-MS data proved that the molecular masses of all the isolated products are 68 Daltons larger than those of the respective substrates, indicating a monoprenylation in their structures. The b-series products (1b, 3b–7b), i.e., those with a smaller retention time on the RP-column than other products of a given substrate, were isolated from all the enzyme assays (1a, 3a–7a). Inspection of their 1H NMR spectra revealed clearly the presence of signals for a regular prenyl moiety each at δ H 3.41–3.54 (d, H-1′), 5.24–5.43 (t, H-2′), 1.58–1.79 (s, 3H-4′), and 1.49–1.75 ppm (s, 3H-5′). The chemical shifts of H-1′ proved its attachment to an aromatic C-atom (Winkelblech and Li 2014; Wollinsky et al. 2012b; Yu et al. 2012; Zou et al. 2010). This conclusion was also supported by the disappearance of signals for one aromatic proton at H-4 or H-7, which was deduced from the number and coupling pattern of the aromatic protons. Detailed comparison of the NMR spectra of 1b and 3b–7b with those of their substrates indicated that the doublet for H-7 has disappeared. The chemical shifts of the three remained coupling protons on the prenylated ring clearly differed from those of C4-prenylated and corresponded well to those of C7-prenylated derivatives (Steffan and Li 2009; Zou et al. 2010). H-H COSY, HSQC and HMBC (Fig. 3 and Figs. S5–S12 in the Supplementary Material) of 1b proved unequivocally the attachment of the prenyl moiety to C-7 of the indole ring. The NMR data of 3b and 5b in CDCl3 corresponded perfectly to those of the enzyme products of CTrpPT with 3a and 5a, respectively, whose structures have been elucidated by NMR analysis including HSQC and HMBC in that study (Zou et al. 2010). The NMR data of 7b are consistent well with those of terezine D (Wang et al. 1995). These compounds are therewith identified as C7-prenylated derivatives and therefore CdpC7PT functions as a cyclic dipeptide C7-prenyltransferase (Fig. 2).
Prenylations at N1 and C3 of the indole ring as well as O-prenylation on the tyrosyl residue as minor products
3c from the incubation mixture of cyclo-l-Trp-l-Trp (3a) was identified as reversely N1-prenylated derivative by comparison of its NMR data with those of an enzyme product of 3a with CTrpPT published previously (Zou et al. 2010). Signals of a reverse prenyl moiety attached to C-3 of an indoline ring were detected in the spectra of 4c and 7c, which is characterized by the signals of the two methyl groups at high magnetic field (approx. 1 ppm) (Yin et al. 2010a, b; Yu et al. 2013). Furthermore, 4c and 7c were easily identified as reversely C3ß-prenylated cyclo-l-Trp-l-Tyr and cyclo-l-Trp-l-Ala with a syn-cis configuration by comparison of their NMR data with those published previously (Yin et al. 2010b; Yu et al. 2013). Using NMR analysis including HSQC, HMBC and NOESY, C3ß-prenylated derivatives were identified as main products in the reaction mixtures of CdpNPT and CdpC3PT with tryptophan-containing cyclic dipeptides (Yin et al. 2010b; Yu et al. 2013). Identification of these compounds as enzyme products in the incubation mixtures of CdpC7PT demonstrated the decrease of the regioselectivity due to a putative unfavorable orientation of such substrates in the active site of the enzyme, as observed previously for other enzymes (Wollinsky et al. 2012b). In comparison, only one product 1b bearing a C7-prenyl moiety was detected with the best accepted substrate 1a (Fig. 2).
In the spectrum of the third product 4d from the incubation mixture of cyclo-l-Trp-l-Tyr (4a), signals for a regular prenyl moiety were observed at δ H 4.45 (d, H-1′), 5.37 (td, H-2′), 1.69 (s, 3H-4′), and 1.67 ppm (s, 3H-5′). The low chemical shift of H-1′ indicates a prenylation at a hetero atom, i.e., an oxygen or nitrogen atom. This is also confirmed by the same number and coupling pattern of the aromatic protons in the spectra of 4d and 4a. The chemical shift of H-1′ of 4d in CD3OD at δ H 4.45 ppm differed clearly from that of cyclo-N1-dimethylallyl-l-Trp-l-Tyr at δ H 4.68 ppm in the same solvent (Yin et al. 2007). 4d was therefore identified as an O-prenylated cyclo-l-Trp-l-Tyr derivative. To exclude the possibility of conversions between 4b, 4c, and 4d during the incubation, 4a was incubated with CdpC7PT for different times. As shown in Fig. 4, the three products are formed independently from each other during the incubation.
Acceptance and O-prenylation of cyclo-l-Tyr-l-Tyr by CdpC7PT
Identification of 4d with an O-prenylated tyrosyl residue as an enzyme product of cyclo-l-Trp-l-Tyr (4a) prompted us to test the acceptance of tyrosine-containing cyclic dipeptides lacking a tryptophanyl moiety such as cyclo-l-Tyr-l-Tyr (18a), cyclo-l-Tyr-l-Ser (19a), cyclo-l-Tyr-l-Pro (20a) and cyclo-l-Tyr-Gly (21a) as well as l-tyrosine (22a). Under the same conditions used for tryptophan-containing cyclic dipeptides, 18a was accepted by CdpC7PT with a product yield of 14.5 %. That is approximately 31.8 % of that of the best accepted 1a and 51.2 % of that of 4a. An acceptance of cyclo-l-Tyr-l-Tyr by a prenyltransferase has not been reported previously. No product formation was observed in the incubation mixtures of 19a–22a (Table 1). The sole product 18b of the reaction mixture with 18a was isolated on HPLC and its structure was elucidated by NMR and MS analyses (Fig. S25 and Table S2 in the Supplementary Material). As in the case of 4d, no significant changes were observed for the number and coupling pattern of the aromatic protons in 18b, in comparison to those of 18a. The chemical shift of H-1′ also proved the regular O-prenylation in 18b (Fig. 2).
Comparison of the substrate specificity of CdpC7PT with that of CTrpPT
As aforementioned, CTrpPT from A. oryzae catalyzes simultaneous C7- and N1-prenylation of cyclic dipeptides with cyclo-l-Trp-l-Trp as the preference substrate (Zou et al. 2010). To compare their behavior towards cyclic dipeptides as well as l-tryptophan and l-tyrosine, the same amount of CTrpPT (9.0 μM) was incubated with the substrates tested for CdpC7PT (Table 1) and reaction mixtures were analyzed under the same HPLC conditions. As shown in Fig. S2 in the Supplementary Material, cyclo-l-Trp-l-Trp was confirmed to be the best substrate for CTrpPT with a product yield of about 89 %, followed by cyclo-l-Trp-l-Tyr, cyclo-l-Trp-l-Leu and cyclo-l-Trp-l-Phe with 53, 34, and 11 %, respectively. These substrates were also well accepted by CdpC7PT. In comparison to the results obtained with CdpC7PT, CTrpPT had a clear preference for these substrates. A number of cyclic dipeptides, including cyclo-l-Tyr-l-Tyr and other tyrosine-containing cyclic dipeptides, were not converted by CTrpPT (Table 1). 1a, the best substrate of CdpC7PT, was only accepted by CTrpPT with a relative activity of 11 % of that of 3a. For a given substrate, the main product peak in both incubation mixtures with CdpC7PT and CTrpPT showed the same retention time, indicating the same main products of the two enzymes. In comparison to CTrpPT, CdpC7PT showed somewhat lower reaction velocity, but much higher flexibility towards tryptophan-containing cyclic dipeptides. For example, cyclo-l-Trp-l-Pro (11a) and cyclo-l-Trp-Gly (15a) were well accepted by CdpC7PT, but only poorly by CTrpPT.
Biochemical properties and kinetic parameters of CdpC7PT
Usually, the members of the DMATS superfamily are independent of the presence of metal ions in their reactions, although Ca2+ or Mg2+ enhances the reaction velocities (Steffan et al. 2009). For determination of the ion dependency of CdpC7PT, incubation mixtures with different divalent metal ions such as Ca2+, Mg2+, Mn2+, Cu2+, Zn2+, Fe2+, and also monovalent ions such as K+ and Na+ at final concentrations of 5 mM were carried out in the presence of 1a and DMAPP (Fig. S26 in the Supplementary Material). Incubations with the chelating agent EDTA or without additives were used as controls. Our results showed that addition of Ca2+, Mn2+, and Mg2+ resulted in a slight increase of the enzyme activity to 158, 131, and 120 % of that of the incubation mixture without additives. Addition of EDTA to the incubation mixture did not change the enzyme activity significantly. These results indicated that, similar to those of most the DMATS enzymes, the enzyme reaction of CdpC7PT was independent of metal ions, but can be enhanced by Ca2+, Mn2+, and Mg2+.
To find out suitable conditions for determination of kinetic parameters, dependency of product formation on incubation time was proven with 4.0 μM CdpC7PT in the presence of 1 mM 1a and 2 mM DMAPP. Linear dependency of up to 120 min was observed in this experiment. For seven selected cyclic dipeptides (1a, 3a–7a, and 18a), kinetic parameters including Michaelis-Menten constants (K M) and turnover numbers (k cat) were determined and calculated from Eadie-Hofstee, Hanes-Woolf, and Lineweaver-Burk plots (Table 3; Figs. S27–S34 in the Supplementary Material). The reactions catalyzed by CdpC7PT apparently followed Michaelis-Menten kinetics. CdpC7PT showed a high affinity to its prenyl donor DMAPP with a K M value of 78.5 μM and a k cat of 0.067 s−1. In the cases of the tested aromatic substrates, CdpC7PT showed the highest affinity to 1a with a K M value of 204.1 μM and a turnover number k cat of 0.125 s−1. For the other tryptophan-containing cyclic dipeptides (3a–7a), K M values between 239.3 and 746.9 μM and turnover numbers in the range of 0.009 and 0.049 s−1 were determined (Table 3). A significantly higher K M value of 1,411.3 μM was calculated for cyclo-l-Tyr-l-Tyr with a relative catalytic efficiency of 1.3 % of that of 1a.
Cultivation of A. terreus and screening for C7-prenylated cyclic dipeptides
For analysis of secondary metabolite accumulation, A. terreus DSM 1958 were cultivated as described in “Materials and methods” section. Culture filtrate extracts were analyzed by LC/MS for the presence of prenylated dipeptides by using enzyme assays of (S)-benzodiazepinedione with CdpC7PT and AnaPT as positive controls (Fig. S35 in the Supplementary Material). Initial analysis with extract from 14-day-old AMM culture revealed clearly the presence of [M+H]+ ion at m/z = 374.1863 (C23H24N3O2) for aszonalenin, the AnaPT product with (R)-benzodiazepinedione, and the absence of the product of CdpC7PT with (S)-benzodiazepinedione (Fig. S36 in the Supplementary Material). Detailed analysis of extracts from 7- to 14-day-old cultures confirmed the presence of aszonalenin and prenylated cyclo-l-Trp-l-Ala. Ions from prenylated cyclo-l-Trp-l-Trp, cyclo-l-Trp-l-Phe, cyclo-l-Trp-l-Leu and cyclo-l-Trp-l-Tyr were not detected (Figs. S37 and S38 in the Supplementary Material).
Discussions
In this study, we focused on a putative prenyltransferase from the ascomycete A. terreus. The coding sequence of the putative prenyltransferase gene ATEG_04218 was cloned into the expression vector pQE-60 and the soluble recombinant protein CdpC7PT was purified to near homogeneity after overproduction in E. coli. Biochemical investigations revealed the acceptance of all the 16 tested tryptophan-containing cyclic dipeptides. Structure elucidation of the isolated enzyme products from six selected substrates indicated regularly C7-prenylated derivatives as main products and proved that CdpC7PT functions as a cyclic dipeptide C7-prenyltransferase. In addition, N1- and C3-prenylated derivatives were also identified as minor enzyme products.
(S)-benzodiazepinedione (1a) was accepted by CdpC7PT as the best substrate. Interestingly, this compound was also found to be the best substrate of the reverse C2-prenyltransferase CdpC2PT from N. fischeri (Mundt and Li 2013). However, neither a C7- nor a C2-prenylated (S)-benzodiazepinedione or a derivative thereof was isolated from nature. Its enantiomer (R)-benzodiazepinedione (2a) is involved in the biosynthesis of acetylaszonalenin, which has been intensively studied in the last years (Ames and Walsh 2010; Yin et al. 2009). AnaPT encoded by ATEG_10306 is responsible for 2a prenylation (Yin et al. 2009).
As aforementioned, the natural substrate of CdpC7PT cannot be predicted by sequence analysis of the genetic context in the genome because this gene is not clustered with necessary backbone genes of secondary metabolism (Table S1 in the Supplementary Material). HPLC-MS analysis of the fungal cultures reveal the presence of aszonalenin, the product of AnaPT, but not C7-prenylated benzodiazepinedione, cyclo-l-Trp-l-Trp, cyclo-l-Trp-l-Tyr, cyclo-l-Trp-l-Phe or cyclo-l-Trp-l-Leu (Figs. S35–S38 in the Supplementary Material). This demonstrated that cdpC7PT was not expressed under our tested conditions or the expression level was very low, so that the accumulated product could not be detected by LC-MS analysis. This might be also the reason for unsuccessful PCR amplification of this gene from mRNA mentioned above.
A few fungal natural products including astechrome, terezine D, and echinulins (Arai et al. 1981; Birch et al. 1961; Wang et al. 1995, 2007) are derived from C7-prenylated cyclo-l-Trp-l-Ala. The biosynthetic gene cluster of astechrome containing a NRPS gene has been identified in Aspergillus fumigatus, N. fischeri, and A. terreus (Kremer et al. 2007; Yin et al. 2013b). Astechrome biosynthesis in A. fumigatus begins with the C7-prenylation of l-tryptophan catalyzed by 7-DMATS and terezine D was detected as a shunt product of the astechrome pathway (Yin et al. 2013b). Similar reaction steps are also expected for the astechrome biosynthesis in A. terreus and the product of ATEG_08428 would catalyze the prenylation of tryptophan. The detected prenylated cyclo-l-Trp-l-Ala by LC-MS could be terezine D. For the biosynthesis of echinulins, a NRPS would be necessary for the formation of the cyclic dipeptide cyclo-l-Trp-l-Ala. As shown in Table S1 in the Supplementary Material, no such gene is located in the vicinity of cdpC7PT in the genome of A. terreus. Furthermore, cyclo-l-Trp-l-Ala was poorly accepted by CdpC7PT with a K M value of 744.7 μM and a turnover number of 0.009 s−1 (Fig. 2). Therefore, cyclo-l-Trp-l-Ala seems not to be the natural substrate for CdpC7PT and consequently, CdpC7PT is not involved in the biosynthesis of echinulins. Other C7-prenylated cyclic dipeptides or derivatives thereof are not isolated from nature. Therefore, the natural substrate of CdpC7PT and its role in the fungal strain remained unknown.
In comparison to the known C7- and N1-prenyltransferase CTrpPT from A. oryzae (Zou et al. 2010), CdpC7PT showed somewhat lower reaction velocity, but much higher flexibility towards tryptophan-containing cyclic dipeptides. Furthermore, we demonstrated in this study that CdpC7PT also accepted cyclo-l-Tyr-l-Tyr as substrate and catalyzes an O-prenylation on the phenolic hydroxyl group (Fig. 2). An O-prenylated derivative was also isolated as minor product from reaction mixture of CdpC7PT with cyclo-l-Trp-l-Tyr. To the best of our knowledge, this is the first report on an enzymatic O-prenylation of tyrosine-containing cyclic dipeptides. The known O-prenyltransferase SirD catalyzes O-prenylation of l-tyrosine, but not cyclic dipeptides (Kremer and Li 2010). SirD also prenylates l-tryptophan and several derivatives at C-7 of the indole ring, assuming the relationship of C7- and O-prenyltransferases in the evolution (Kremer and Li 2010; Rudolf and Poulter 2013; Zou et al. 2011). O-prenylation of tyrosyl moiety in cyclo-l-Trp-l-Tyr and cyclo-l-Tyr-l-Tyr by a tryptophan-containing cyclic dipeptide C7-prenyltransferase demonstrated similar behavior of cyclic dipeptide prenyltransferases and supported this hypothesis.
References
Ames BD, Walsh CT (2010) Anthranilate-activating modules from fungal nonribosomal peptide assembly lines. Biochemistry 49:3351–3365
Arai K, Sato S, Shimizu S, Nitta K, Yamamoto Y (1981) Metabolic products of Aspergillus terreus. VII. Astechrome: an iron-containing metabolite of the strain IFO 6123. Chem Pharm Bull 29:1510–1517
Birch AJ, Blance GE, David S, Smith H (1961) Studies in relation to biosynthesis. XXIV. Some remarks on the structure of echinuline. J Chem Soc 3128–3131
Bonitz T, Alva V, Saleh O, Lupas AN, Heide L (2011) Evolutionary relationships of microbial aromatic prenyltransferases. PLoS One 6:e27336
Grundmann A, Li S-M (2005) Overproduction, purification and characterization of FtmPT1, a brevianamide F prenyltransferase from Aspergillus fumigatus. Microbiology 151:2199–2207
Guo CJ, Knox BP, Sanchez JF, Chiang YM, Bruno KS, Wang CCC (2013) Application of an efficient gene targeting system linking secondary metabolites to their biosynthetic genes in Aspergillus terreus. Org Lett 3562–3565
Heide L (2009) Prenyl transfer to aromatic substrates: genetics and enzymology. Curr Opin Chem Biol 13:171–179
Jain HD, Zhang C, Zhou S, Zhou H, Ma J, Liu X, Liao X, Deveau AM, Dieckhaus CM, Johnson MA, Smith KS, Macdonald TL, Kakeya H, Osada H, Cook JM (2008) Synthesis and structure-activity relationship studies on tryprostatin A, a potent inhibitor of breast cancer resistance protein. Bioorg Med Chem 16:4626–4651
Jeedigunta S, Krenisky JM, Kerr RG (2000) Diketopiperazines as advanced intermediates in the biosynthesis of ecteinascidins. Tetrahedron 56:3303–3307
Kremer A, Li S-M (2010) A tyrosine O-prenyltransferase catalyses the first pathway-specific step in the biosynthesis of sirodesmin PL. Microbiology 156:278–286
Kremer A, Westrich L, Li S-M (2007) A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: overproduction, purification and biochemical characterization. Microbiology 153:3409–3416
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li S-M (2010) Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat Prod Rep 27:57–78
Lu YP, Liu HG, Liang PH (2009) Different reaction mechanisms for cis- and trans-prenyltransferases. Biochem Biophys Res Commun 379:351–355
Mundt K, Li S-M (2013) CdpC2PT, a reverse prenyltransferase from Neosartorya fischeri with distinct substrate preference from known C2-prenyltransferases. Microbiology 159:2169–2179
Pockrandt D, Sack C, Kosiol T, Li S-M (2014) A promiscuous prenyltransferase from Aspergillus oryzae catalyses C-prenylations of hydroxynaphthalenes in the presence of different prenyl donors. Appl Microbiol Biotechnol 98:4987–4994
Rudolf JD, Poulter CD (2013) Tyrosine O-prenyltransferase SirD catalyzes S-, C-, and N-prenylations on tyrosine and tryptophan derivatives. ACS Chem Biol 8:2707–2714
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Schuller JM, Zocher G, Liebhold M, Xie X, Stahl M, Li S-M, Stehle T (2012) Structure and catalytic mechanism of a cyclic dipeptide prenyltransferase with broad substrate promiscuity. J Mol Biol 422:87–99
Steffan N, Li S-M (2009) Increasing structure diversity of prenylated diketopiperazine derivatives by using a 4-dimethylallyltryptophan synthase. Arch Microbiol 191:461–466
Steffan N, Grundmann A, Yin W-B, Kremer A, Li S-M (2009) Indole prenyltransferases from fungi: a new enzyme group with high potential for the production of prenylated indole derivatives. Curr Med Chem 16:218–231
Tarcz S, Ludwig L, Li S-M (2014) AstPT catalyses both reverse N1- and regular C2-prenylation of a methylated bisindolyl benzoquinone. Chembiochem 15:108–116
Tsai HF, Wang H, Gebler JC, Poulter CD, Schardl CL (1995) The Claviceps purpurea gene encoding dimethylallyltryptophan synthase, the committed step for ergot alkaloid biosynthesis. Biochem Biophys Res Commun 216:119–125
Unsöld IA, Li S-M (2005) Overproduction, purification and characterization of FgaPT2, a dimethylallyltryptophan synthase from Aspergillus fumigatus. Microbiology 151:1499–1505
Wang Y, Gloer JB, Scott JA, Malloch D (1995) Terezines A-D: new amino acid-derived bioactive metabolites from the coprophilous fungus Sporormiella teretispora. J Nat Prod 58:93–99
Wang WL, Lu ZY, Tao HW, Zhu TJ, Fang YC, Gu QQ, Zhu WM (2007) Isoechinulin-type alkaloids, variecolorins A-L, from halotolerant Aspergillus variecolor. J Nat Prod 70:1558–1564
Williams RM, Stocking EM, Sanz-Cervera JF (2000) Biosynthesis of prenylated alkaloids derived from tryptophan. Top Curr Chem 209:97–173
Winkelblech J, Li S-M (2014) Biochemical investigations of two 6-DMATS enzymes from Streptomyces revealing novel features of L-tryptophan prenyltransferases. Chembiochem 15:1030–1039
Wollinsky B, Ludwig L, Hamacher A, Yu X, Kassack MU, Li S-M (2012a) Prenylation at the indole ring leads to a significant increase of cytotoxicity of tryptophan-containing cyclic dipeptides. Bioorg Med Chem Lett 22:3866–3869
Wollinsky B, Ludwig L, Xie X, Li S-M (2012b) Breaking the regioselectivity of indole prenyltransferases: identification of regular C3-prenylated hexahydropyrrolo[2,3-b]indoles as side products of the regular C2-prenyltransferase FtmPT1. Org Biomol Chem 10:9262–9270
Woodside AB, Huang Z, Poulter CD (1988) Trisammonium geranyl diphosphate. Org Synth 66:211–215
Yazaki K, Sasaki K, Tsurumaru Y (2009) Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 70:1739–1745
Yin W-B, Ruan H-L, Westrich L, Grundmann A, Li S-M (2007) CdpNPT, an N-prenyltransferase from Aspergillus fumigatus: overproduction, purification and biochemical characterisation. Chembiochem 8:1154–1161
Yin W-B, Grundmann A, Cheng J, Li S-M (2009) Acetylaszonalenin biosynthesis in Neosartorya fischeri: identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation. J Biol Chem 284:100–109
Yin W-B, Xie X-L, Matuschek M, Li S-M (2010a) Reconstruction of pyrrolo[2,3-b]indoles carrying an α-configured reverse C3-dimethylallyl moiety by using recombinant enzymes. Org Biomol Chem 8:1133–1141
Yin W-B, Yu X, Xie X-L, Li S-M (2010b) Preparation of pyrrolo[2,3-b]indoles carrying a ß-configured reverse C3-dimethylallyl moiety by using a recombinant prenyltransferase CdpC3PT. Org Biomol Chem 8:2430–2438
Yin S, Yu X, Wang Q, Liu XQ, Li S-M (2013a) Identification of a brevianamide F reverse prenyltransferase BrePT from Aspergillus versicolor with a broad substrate specificity towards tryptophan-containing cyclic dipeptides. Appl Microbiol Biotechnol 97:1649–1660
Yin W-B, Baccile JA, Bok JW, Chen Y, Keller NP, Schroeder FC (2013b) A nonribosomal peptide synthetase-derived iron(III) complex from the pathogenic fungus Aspergillus fumigatus. J Am Chem Soc 135:2064–2067
Yu X, Li S-M (2012) Prenyltransferases of the dimethylallyltryptophan synthase superfamily. Methods Enzymol 516:259–278
Yu X, Liu Y, Xie X, Zheng X-D, Li S-M (2012) Biochemical characterization of indole prenyltransferases: filling the last gap of prenylation positions by a 5-dimethylallyltryptophan synthase from Aspergillus clavatus. J Biol Chem 287:1371–1380
Yu X, Zocher G, Xie X, Liebhold M, Schütz S, Stehle T, Li S-M (2013) Catalytic mechanism of stereospecific formation of cis-configured prenylated pyrroloindoline diketopiperazines by indole prenyltransferases. Chem Biol 20:1492–1501
Zou H-X, Xie X-L, Linne U, Zheng X-D, Li S-M (2010) Simultaneous C7- and N1-prenylation of cyclo-L-Trp-L-Trp catalyzed by a prenyltransferase from Aspergillus oryzae. Org Biomol Chem 8:3037–3044
Zou H-X, Xie X, Zheng X-D, Li S-M (2011) The tyrosine O-prenyltransferase SirD catalyzes O-, N-, and C-prenylations. Appl Microbiol Biotechnol 89:1443–1451
Acknowledgments
This project was financially supported by a grant from the Deutsche Forschungsgemeinschaft (Li844/4-1 to S.-M. Li). We thank L. Ludwig for synthesis of DMAPP. We also thank analytic department of the faculty of Pharmacy for taking mass and NMR spectra, respectively.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 11725 kb)
Rights and permissions
About this article
Cite this article
Wunsch, C., Zou, HX., Linne, U. et al. C7-prenylation of tryptophanyl and O-prenylation of tyrosyl residues in dipeptides by an Aspergillus terreus prenyltransferase. Appl Microbiol Biotechnol 99, 1719–1730 (2015). https://doi.org/10.1007/s00253-014-5999-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5999-6