Abstract
Economic and ecological reasons cause the industry to develop new innovative bio-based processes for the production of oil as renewable feedstock. Petroleum resources are expected to be depleted in the near future. Plant oils as sole substituent are highly criticized because of the competitive utilization of the agricultural area for food and energy feedstock production. Microbial lipids of oleaginous microorganisms are therefore a suitable alternative. To decrease production costs of microbial lipids and gain spatial independence from industrial sites of CO2 emission, a combination of heterotrophic and phototrophic cultivation with integrated CO2 recycling was investigated in this study. A feasibility study on a semi-pilot scale was conducted and showed that the cultivation of the oleaginous yeast Cryptococcus curvatus on a 1.2-L scale was sufficient to supply a culture of the oleaginous microalgae Phaeodactylum tricornutum in a 21-L bubble column reactor with CO2 while single cell oils were produced in both processes due to a nutrient limitation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concerns about the increasing emissions of greenhouse gases and the resulting global warming and the need to reduce our dependence from petroleum force the industry to develop innovative bio-based industrial processes with reduced CO2 emission (Lee et al. 2002). The substitution of petroleum is a long-term objective not only because of its importance as fuel for the transport sector, but it is also a main raw material for oleochemicals (Carlsson 2009). The introduction of plant-based first generation biofuels is increasingly criticized because of the rising competition between agricultural production for the food and for the energy sector. Against this backdrop, single cell oils from microorganisms seem promising substitutes. Heterotrophic oleaginous microorganisms are able to convert a carbon source, which is available in excess, into storage lipids inside the cells as soon as a concurrent nitrogen limitation stops cell growth. Advantages of such a microbial lipid production compared to petroleum production or agricultural production of plant oils are the independence from the season and from the location. However, oleaginous microorganisms require an organic carbon source. Microalgae fix CO2 and contribute to CO2 sequestration. Moreover, they can accumulate lipids to high specific contents up to more than 80 % of their dry weight (Spolaore et al. 2006; Tredici 2010). And most importantly, growth and lipid accumulation are more efficient than that of higher plants, which are cultivated as feedstock for biofuel production (Chisti 2007).
The establishment of economic processes for the production of microbial oil requires energy-efficient and cost-effective strategies. While algae harvesting and cell disruption of the resistant yeast cells are main cost drivers in downstream processing (Cerff et al. 2012; Chisti and Moo-Young 1986), the cultivation itself offers great potential for optimization. CO2 is generally assumed to be available for free in phototrophic processes as shown by studies assessing the economic viability (Singh et al. 2011). It can potentially be obtained from the flue gas of power plants (Brennan and Owende 2010), but its nitrous gases and sulphur oxides can entail negative effects on growth rates and volumetric productivity of algae (Lee et al. 2002). Moreover, optimal locations of plants for microalgae fuel production are often remote and distant from industrial sites of CO2 emission (Ratledge and Cohen 2008). In consequence, Feron and Hendriks (2005) suggest that a price in the range of 0.15 to 0.20 US$/kg should be attached to CO2 to account for the cost of its capture and transport. With a demand of approximately 1.8 kg CO2 for the production of 1 kg algae dry matter, this imposes cost on the process in a dimension that cannot be neglected.
In contrast to a complete Life Cycle Assessment (LCA), the study was conducted to investigate requirements and implications of the combination of a heterotrophic process and a phototrophic process with the purpose of producing single cell oils. Thereby, the heterotrophic process supplies the algae with CO2; thus, phototrophic conversion leads to a lower emission of CO2. On the other hand, the phototrophic lipid production benefits from the cost reduction in terms of the carbon source, but at the same time also gains independence from industrial plants in terms of location.
Cryptococcus curvatus is well-known oleaginous yeast, simple to cultivate in bioreactors with high growth rates, high lipid yields and cultivation duration of maximum 6 days. It was first isolated in 1978 by Moon and Hammond (1978) from cheese plant floors and floor drains during fermentation experiments on lactose. The lipid composition is similar to that of palm oil with high amount of oleic acid suitable for applications as biodiesel (Hassan et al. 1994). C. curvatus is able to convert several various carbon sources into fatty acids (Hassan et al. 1994), e.g. glucose, galactose, cellobiose, sucrose and lactose, and converts even waste substrates like whey as carbon and nitrogen sources (Ratledge and Cohen 2005). Phaeodactylum tricornutum, on the other hand, is suited as oleaginous microalgae since the organism accumulates lipids up to high specific contents. It grows with high growth rates at nutrient replete conditions and showed reasonable photosynthetic efficiency when nutrients were limiting (Dillschneider et al. 2013). Extensive research has been performed with P. tricornutum as a model organism, also because it is an interesting producer of polyunsaturated fatty acids, such as eicosapentaenoic acid (Yongmanitchai and Ward 1991).
Materials and methods
Microorganisms and culture medium
C. curvatus (ATCC 20509) was obtained from the American Type Culture Collection. P. tricornutum (SAG 1090-1a) was obtained from the Culture Collection of Algae (SAG), University of Göttingen, Germany.
The culture medium for the fermentation of the yeast was formulated on a phosphate buffer at pH 5 (8.99 g/L KH2PO4 and 0.12 g/L Na2HPO4·2H2O). The medium constituents were 0.1 g/L sodium citrate (C6H5O7Na3·2H2O), 0.1 g/L yeast extract, 0.2 g/L MgSO4·7H2O and 18.9 g/L (NH4)2SO4. The initial culture was supplemented with 2 mL trace elements solution (4 g/L CaCl2·2H2O, 0.55 g/L FeSO4·7H2O, 0.475 g/L citric acid, 0.1 g/L ZnSO4·7H2O, 0.076 g/L MnSO4·H2O, 100 μl 18 M H2SO4) and 1 mL salt solution (20 g/L MgSO4·7H2O, 10 g/L yeast extract) per 100 mL cultivation medium. The two former mentioned solutions were supplemented once a day to the culture broth during the whole cultivation time in the bioreactor. Glucose was fed via a stock solution with 500 g/L; initial glucose concentration was 50 g/L.
The first preculture was prepared in 20 mL medium in 100-mL conical shake flasks and was inoculated with 100 μL glycerol stock culture (15 % w/w, stored at −80 °C). The second preculture was prepared from the first preculture in 200 mL culture medium in 2-L shake flasks with an initial OD600 of 1. Both precultures were incubated at 28 °C with 120 rpm for approximately 24 h.
The culture medium for the phototrophic process was originally published by Mann and Myers (1968). The following modifications were made: NaCl concentration was 27.0 g/L, K2HPO4 concentration was 0.15 g/L and MgSO4·7H2O was reduced to a final concentration of 0.6 g/L. 30 mg/L Na2SiO3·5H2O were added. In order to attain a nitrogen limitation early in the process, the NaNO3 concentration was adjusted to 0.4 g/L. Tris buffer was present in the preculture medium but otherwise omitted in order to prevent growth of heterotrophic organisms (Fábregas et al. 1993). All chemicals, purchased from Carl Roth, were of analytical grade (p. a.).
The inoculum for the experiments was cultivated in shaking flask cultures incubated at 21 °C for 3 weeks with LED illumination adjusted to a PFD of 150 μmol/(m2·s). The pH of the preculture medium was adjusted to pH 7.5. During the process the pH was controlled at pH 7.7 as described below.
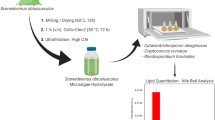
Set-up of the integrated bioprocess
The set-up of the integrated bioprocess is depicted in Fig. 1. The left side shows the 2.5-L stirred tank reactor (Infors Minifors) with 1.2 L liquid culture initial culture medium for the cultivation of the yeast C. curvatus. After inoculation, the initial OD600 was 1. Mixing was achieved by stirring with a rotation speed of 600 rpm. The cell suspension was aerated with compressed air at an aeration rate of 1 vvm and a slight overpressure of maximum 2 × 104 Pa. The pH was set to pH 5.0 by controlled addition of 4 M H3PO4 and 4 M NaOH in the bioreactor. Contraspum A 4050 HAC (Zschimmer und Schwarz) was applied as antifoam agent. Initial glucose concentration was 50 g/L. Each day, the glucose was measured and was replenished to a maximum concentration of 120 g/L if it was depleted in the culture.
The exhaust gas from the yeast bioreactor was filtered with a sterile filter (Millex-FG, 0.2 μm, Millipore) and directly fed into the photobioreactor. The latter was an annular bubble column reactor consisting of a 1.3-m long glass cylinder with 0.3 m diameter. The inner cylinder—made from stainless steel—contained the cooling water circulation system. The temperature was controlled at 21 °C. Before inoculation, the reactor was autoclaved with a SIP steam generator (DG 7/6, Zirbus) and filled under sterile conditions with the medium, which was separately autoclaved (Vakulab, MMM Group). The pH of the media was adjusted to pH 10.3 with NaNO3 prior to autoclaving but decreased in the course of the cultivation due to saturation with CO2. The pH was measured by an Easyferm Plus 425 Sensor (Hamilton) and controlled at pH 7.7. pH-control was implemented by addition of 4 M NaOH and CO2 by the additional mass flow controller (shown in Fig. 1) which was integrated to supply the algae culture with CO2 in case the volume fraction in the off-gas of the yeast culture was not sufficiently high. To account for fluctuations in the volume fraction of CO2, dead zones of the pH-controller were +0.5 and −0.3.
The volume fractions of O2 and CO2 in the off-gas were analyzed by an off-gas analyzer (Multor 610, Maihak). The annular column reactor was illuminated with 76 halogen spotlights (Decostar 20 W, Osram) which were adjusted circularly around the reactor so that the illumination was as homogenous as possible. The photon flux density amounted to 250 μE/(m2 · s).
Biomass concentration
Yeast dry biomass was analyzed gravimetrically. A 1 mL aliquot of the culture fluid was transferred into a predried and preweighed 1.5-mL reaction tube and centrifuged at 13,000 rpm for 5 min. The supernatant was collected and used for the determination of glucose and NH4 +. The cell pellet was washed with 800 μL saline (0.9 % NaCl), dried at 60 °C for 24 h and weighed.
Algae biomass concentration was determined by spectrophotometry. The absorbance was measured with a T60 U spectrophotometer (PG Instruments) at 750 nm, and the calculation of biomass concentration was based on a correlation factor of 0.376 g dry biomass/OD750 nm. The correlation factor was obtained from a calibration with six duplicate samples of 10 mL. The suspension was centrifuged, the pellet was washed, frozen and freeze-dried (ALPHA 1–2 LDplus, Christ). The weight of the dried biomass samples was determined gravimetrically.
Glucose concentration
Glucose was enzymatically measured in the supernatant of centrifuged samples for biomass concentration measurement with the D-Glucose kit (R-Biopharm). Glucose was measured in triplicate using microtiter plates.
Ammonium concentration
Ammonium concentration was measured photometrically with the Spectroquant kit (Merck) in the supernatant of samples for the measurement of biomass concentration. The assay was downsized to 300 μL per sample and measured in microtiter plates in triplicate.
Nitrate concentration
Nitrate concentration in the supernatant of samples were measured by ion chromatography (882 Compact IC plus, Metrohm) equipped with a conductivity detector (Metrohm). Samples were automatically diluted (1:10) and injected by an autosampler unit (Professional Sample Processor 858). The device was equipped with a Metrosep A Supp 5 column (Metrohm) consisting of polyvinyl alcohol with quaternary ammonium groups. The elution buffer consisted of 3.2 mM Na2CO3, 1.0 mM NaHCO3 and 12.5 % (v/v) acetonitrile in water.
Lipid quantification
Fatty acid concentrations and total lipid content were measured in a gas chromatograph (Agilent 6890N, Agilent Technologies) equipped with a 30-m DB-Wax column (l: 30 m d: 0.25 mm; Agilent Technologies) and FID detector. For sample preparation duplicates of 20 mg freeze-dried biomass samples were transesterified according to the method described by Meesters et al. (1996), but using hexane instead of chloroform. Methyl benzoate (FLUKA) was added as internal standard to a final concentration of 0.5 mg/mL in the lipophilic phase. The temperature program consisted of heating up at a rate of 8 °C/min starting from 40 °C. Final temperature was held at 250 °C to the end of the measurement. The analytical standards Marine Oil FAME Mix (Restek) and AOCS No. 3 (RM-3, Supelco) were used for calibration and fatty acid identification.
Due to the lower biomass concentration, algae lipid quantification was performed with a combination of nile red (9-diethylamino-5H-benzo[a]phenoxazine-5-one, Sigma-Aldrich) staining and fluorescence measurement and calibration with data gained from gas chromatography. The protocol was described in Dillschneider et al. (2013).
Exhaust gas composition
The volume fraction of O2 and CO2 in the exhaust gas was analyzed with a Multor 610 System (Maihak).
Results
Fatty acid profiles of the oleaginous microorganisms
The fatty acid profiles of both production strains were analyzed in order to state possible industrial applications of the microbial oil. The fatty acid profiles are presented in Fig. 2. The fatty acid profile of the microalgae P. tricornutum (Fig. 2b) contains 45 % palmitoleic acid (C16:1) as the main component followed by palmitic acid (C16:0) with 25 %. Apart from that, docosahexaenoic acid (C20:5) and oleic acid (C18:1) with 8.9 and 7.6 %, respectively, show interesting amounts. Myristic acid (C14:0), linoleic acid (C18:2) and arachidonic acid (C20:4) are rather negligible with less than 5 %.
Concerning the yeast C. curvatus (Fig. 2a), the main component of the stored lipid is oleic acid (C18:1) with 48.8 %. Palmitic acid (C16:0) and stearic acid (C18:0) reached values of 18.5 and 17.7 %, respectively. Linoleic acid (C18:2) with 8.6 % and lignoceric acid (C24:0) with 3 % were less represented. Myristic acid (C14:0), linolenic acid (C18:3), arachidic acid (C20:0) and behenic acid (C22:0) are negligible with less than 1 % of total fatty acids.
Preliminary study: characterization of the cultivation of C. curvatus
In order to plan a possible set-up for the combination of the yeast and algae cultivation considering the different cultivation durations, preliminary experiments with the oleaginous yeast C. curvatus were investigated to examine the course of the exhaust gas CO2 and its influence to carbon deprivation. The cultivation results of C. curvatus in a 2.5-L bioreactor are presented in Fig. 3. Figure 3a shows an overview of the lipid production process. The growth rate of biomass was maximal until the nitrogen source was totally consumed. The nitrogen limitation was reached after 35 h. After 40 h, the growth curve leveled off and the cells started to accumulate lipids. Figure 3b illustrates the course of the emitted gas CO2. Until the maximal growth rate, the CO2 increased to a maximum value of 1.49 % and leveled off continuously up to 1 % at the end of the cultivation. However, as soon as the glucose concentration reached a value of 0 g/L, the CO2 value dropped down immediately. As soon as new glucose was added, the CO2 increased again immediately up to the value which was measured before glucose deprivation. O2 and dissolved oxygen (pO2) (data not shown) ran counter-rotating to CO2. As soon as glucose was consumed, the values for O2 and pO2 increased immediately and decreased again as soon as new glucose was added. One further interesting aspect is the respiration coefficient during the process of the microbial lipid production, an online indicator for the metabolic state. The RQ value was calculated according to Eq. (1) and is shown in Fig. 3b.
Cultivation of the oleaginous yeast Cryptococcus curvatus in 2.5-L bioreactor. a Lipid percentage WL(%) (filled triangles), biomass concentration Cx (filled circles), glucose concentration CGLC (open diamonds)b Online data (solid line) : volume fraction of CO2 in the exhaust gas \( {\chi}_{{\mathrm{CO}}_2} \). Calculated data (dashed line): respiration coefficient RQ[−]; Arrows indicate begin of nitrogen limitation
As it was to be expected (Varma and Buscot 2005), the RQ value increased up to a maximum value of 1 at the time of the maximal growth rate during the growth phase on glucose. It is a parallel course compared to the production of CO2. After approximately 40 h, when lipid production started, the RQ value started to increase above 1 up to 1.3 and fluctuated between those two values until the total consumption of glucose was reached. At this time point, the RQ decreased immediately to values below 1. During the glucose deprivation phase up to the end of the process, the RQ value decreased to minimum values of 0.25. Summing up, the course of the RQ value could be used as an indication of the lipid production phase in oleaginous yeasts.
Process integration—combination of the heterotrophic and phototrophic processes
The heterotrophic and phototrophic processes were set up as described before. The yeast cultivation was conducted in a repeated fed-batch mode. C. curvatus was growing exponentially until day 1.5 where nitrogen limitation occurred for the first time and lipid accumulation started (Fig. 4). Afterwards, the culture was fed every day with a glucose solution to supply the culture with the carbon source to sustain lipid synthesis. At day 5, the culture reached a biomass concentration of 76.2 g/L and lipid concentration was 34.5 g/L when the first harvesting of biomass was conducted. One liter of the culture suspension was harvested and the same volume of fresh culture medium was supplied and glucose was added to a maximum value of 100 g/L. Therewith, a biomass concentration of 15.1 g/L was remaining in the culture suspension to continue metabolizing the carbon source and hence incessantly supply the algae culture with CO2. At this point, the yeast cells were also provided with ammonium. Renewed availability of the nitrogen source led to the cessation of lipid production, but adaption to a new growth phase of the yeast cells. The maximal volumetric productivity in this second cycle was slightly smaller than in the first cycle. During this exponential growth phase, the mass fractions of lipids in biomass decreased from initially 45.3 % to a minimum value of 18.2 %. When the culture entered again, the nitrogen limitation, a rapid switch to lipid production occurred. Lipids were then accumulated to a maximum value of 39.3 % before the second harvest was performed. Like in the first cycle, the intermittent glucose feeding was continued throughout the process until it was stopped at day 15. In the third cycle, the lipid content decreased first from 39.4 to 17.5 % during the growth phase and afterwards reached a maximum lipid content of 34.3 %.
Process integration by combination of the heterotrophic and phototrophic process. a Phototrophic process. b Heterotrophic process. Biomass concentration cX (filled circles). Lipid concentration cL (open triangles). Nitrate concentration \( {\mathrm{c}}_{{\mathrm{NO}}_3^{-}} \) in the photobioreactor (open squares). Ammonium concentration \( {\mathrm{c}}_{{\mathrm{NH}}_4^{+}} \) in the yeast bioreactor (open circles). Online data (solid line): volume fraction of CO2 in the exhaust gas \( {\chi}_{{\mathrm{CO}}_2} \) pH value in the photobioreactor (dashed line). Arrows indicate temporary supply with additional CO2 from an external source
From the comparison of the yield coefficients (lipid synthesis related to glucose consumption) as shown in Table 1, one can conclude that the lipid yields decreased slightly from 0.13 to 0.09 from the first to the third cycle. The values for the lipid production rate QL show a similar trend, because with an initial value 0.36 g/(L · h) in the first cycle they subsequently decrease to 0.21 g/(L · h) in the second and third cycle.
The phototrophic process was supplied with CO2 throughout the entire process by the exhaust gas from the yeast culture. The only exceptions were the time points of harvesting. At these time points, the pH value in the culture suspension of the phototrophic process reached the upper limits of the pH-controller which were defined as described in the section “Set-up of the integrated bioprocess”. The controller action then prevented the pH value from increasing more and supplied CO2 from a pressure cylinder through the action of a mass flow controller. At these harvesting points, CO2 had to be supplied from the external source for about 100 min with a flow rate of 2.8 mL/min (average value) during the start of the second cycle of yeast growth. Similarly, CO2 with an average flow rate of 4.1 mL/min had to be supplied for ca. 180 min at the start of the third cycle.
The phototrophic culture of P. tricornutum showed exponential growth during the first 7 days after a short initial lag phase. At day 7, the nitrate in the culture medium was completely consumed and lipid accumulation started. Biomass was ca. 1 g/L at the onset of the nitrogen limitation. An early limitation at low concentrations of biomass was aimed at during the planning stage of the experiment in order to shorten the process duration. In the following days, the biomass growth curve showed a linear evolution and storage lipids accumulated in this phase. Biomass increased to a final value of 2.3 g/L at day 15. The lipid concentration increased from initially 0.2 g/L at the onset of the nutrient limitation to ca. 1.0 g/L at the end of the process. Taking into account, the liquid volume of the reactor, which was 21 L, about 48 g biomass and ca. 21 g lipids were gained from the reactor at the relatively low light intensities which were impinging on the reactor surface.
The volume fraction of CO2 was measured in the exhaust gas of the photobioreactor. Therewith, the signal comprises the CO2 emission caused by the yeast respiration and also the effect of CO2 uptake of the algae. It was low in the beginning of the cultivation and increased subsequently with exponential growth of the yeast. A maximum value of ca. 2 % was reached at the onset of the first nitrogen depletion where metabolic rates slowed down compared to nutrient-replete growth. At the end of the first lipid accumulation phase, the volume fraction dropped below 1 %. In the subsequent two cycles, maximal values of 2.4 and 1.9 % were reached. Even though the variation of the volume fraction of CO2 in the gas stream was considerable, it never fell below a value of 1 % except for the time points of harvesting. Therefore, CO2 limitation was prevented throughout the entire process and the phototrophic process could be sufficiently supplied with CO2 by the heterotrophic yeast culture.
Due to the considerable changes in the volume fraction of CO2 in the gas stream entering the phototrophic reactor moderate changes in the pH value in the algae culture suspension occurred. The latter varied in a range between pH 7.5 and pH 8.2. Further variation of the pH was prevented due to the pH-controller settings. During the harvesting process of yeast and the subsequent adaption period, the CO2 flow temporarily decreased slightly so that the pH temporarily reached a value of pH 8.2, where additional CO2 was added for a short period as described above.
Discussion
Fatty acid composition and suitability for biodiesel production
In general, C16 and C18 fatty acids are well suited for the production of biodiesel. Those kinds of fatty acids are main component of plants used for biodiesel production (Ma and Hanna 1999) which fit well with the profiles of C. curvatus as well as that of P. tricornutum. Hence, microbial oil from those two strains can be used for the production of biodiesel. However, biodiesel from microbial oils cannot yet compete with those from plant oils. In addition to the use as biodiesel, the vast amounts of different fatty acids in the described process can find applications in other industrial sectors. The main fatty acid in the microalgae, palmitoleic acid, is known to prevent brain disease, cerebra- and cardiovascular diseases and enhances the function of vascular smooth muscle cells and can also be applied in the therapy against obesity. Therefore, this fatty acid can find novel and valuable uses in human nutrition and medicine (Yamori et al. 1986; Matsunaga et al. 1995; Yang et al. 2011). Oleic acid, the main fatty acid in the yeast lipid, finds application as lubricant, for cosmetics and cleaning agents, as food addition and for biodiesel production (Yin et al. 2009; Liebert 1987). Palmitic acid which is found in high concentrations in both cell types, is used for the production of biodiesel, soaps, cosmetics, lubricants, release agent and food additions (Carmo Jr et al. 2009; Liebert 1987).
Challenges of the process integration
The coupling of algae and yeast cultivation has been already described by Puanbut (Puanbut and Leesing 2012), but those results are based on shaking flask cultivations and the exhaust gas of the yeasts was supplemented by air flow. In contrast, this study focuses on the development of a scalable process strategy that is feasible to supply algae cultures with CO2. Furthermore, the study investigates on challenges and solutions with regard to an entirely automated process for biodiesel production with both organisms. The challenge of the process integration in order to achieve CO2 recycling was the adaptation of the diverging process duration of heterotrophic cultivations and phototrophic processes. Since yeast grows on average with a growth rate of 0.4 h−1 and therewith attains about 10 doublings per day algae usually attain significantly lower growth rates in the range of 1 day−1 for fast growing species. Another significant difference is that phototrophic processes are light dependent and the exponential growth is limited to low biomass concentrations due to shading of the cells. Once light is limiting the culture growth more or less linearly.
One possibility to match process durations to achieve constant supply of the algae cultures with CO2 would be the implementation of time delayed independent yeast bioprocesses. In such a set-up, several yeast bioreactors are started at different time points, and in phases of peak metabolic turnover, the exhaust gas could be connected with the photobioreactor. A switch would allow using the exhaust gas of the next bioreactor when a limitation occurs in one bioreactor. The obvious disadvantage of such a process would be the high investment required for the implementation.
The other option was a repeated fed-batch process which was conducted. The advantage of such a repeated fed-batch is the possibility to reduce the set-up and maintenance time because the bioreactor does not have to be cleaned and newly autoclaved after each batch process. Instead, just fresh medium needs to be added (Zhao et al. 2011). This strategy contributes to cost reduction in terms of cost of operation and besides reduces disbursement for the initial investment of the plant. Furthermore, studies have demonstrated that C. curvatus can attain higher lipid rates when cultured in a fed-batch process (Zhang et al. 2011).
Another challenge was the approach of changing volume fractions of CO2 in the gas stream. Through the chemical equilibrium of dissolved CO2, hydrogen-carbonate and carbonate changing partial pressures of CO2 affect the pH in solution (Camacho Rubio et al. 1999). This problem could be avoided in principle by the combination of a continuous yeast process and an autotrophic algae process. However, such a strategy was not feasible in the here presented study, since the objective was the production of lipids in both process stages. The approach to deal with the changing CO2 partial pressures was a pH-control in the photobioreactor that allows for minor variations of the pH due to a defined dead band of the controller.
Nevertheless, the mere adaptation of the pH-control in the photobioreactor is not sufficient to achieve constant supply of the algae cultures with their C-source. Therefore, the preliminary experiment was conducted in order to analyze the CO2 emission in a yeast bioprocess producing microbial lipids. The study was important to align the yeast process with the need of a relatively constant CO2 release in the exhaust gas. One basic necessity for continuous respiratory activity and additionally for the lipid production in yeast was that the carbon source has to be available in excess. This necessity entails that the carbon source is regularly added. On the other hand, a maximal concentration of 120 g/L should not be exceeded because of substrate inhibition (Zhang et al. 2011). An exhaustion of glucose leads to an immediate decrease of CO2 in the exhaust gas and needs to be avoided in the integrated process. Other than in the presented work, an automatic glucose feed could be installed to avoid such declines of CO2. Control of the glucose feed could be achieved by feeding of glucose as soon as the CO2 value decreases immediately. A rapid increase of the partial pressure of oxygen pO2 in the culture suspension could also be used as a controlled variable since it indicates declining respiratory activity. The latter would be advantageous because other than CO2 emission, which is measured in the exhaust gas of the phototrophic process, the pO2 can easily be measured independently of the second process stage. An automatic calculation of the RQ value with the online-measured values O2 and CO2 in the exhaust gas could be used as a direct indicator. Once an RQ value of 1 is reached, a decrease (RQ <1) could mean that the carbon source is exhausted, whereas an increase (RQ >1) depicts the lipid production phase.
Complementary to the here presented process strategy a semi-continuous fed-batch process with more frequent harvesting might be implemented for the heterotrophic process stage. It was shown that the two harvesting events had the strongest impact on the CO2 partial pressure. More frequent harvesting of smaller volumes would lead to a more constant CO2 volume fraction and peaks could be dampened.
Plant dimensioning
On an unclouded summer day, peak production rates of a photo-bioprocess are expected and should be in the range of 50 g/(m2 · d) according to Tredici (Tredici 2010). With a look at the carbon balance, a yield coefficient for biomass synthesis based on CO2 consumption of 0.55 g/g can be expected. For the underlying calculation, a carbon content of 50 % was assumed (Schlagermann et al. 2012). This would result in a CO2 requirement of 91.5 g/(m2 · d) for idealized conditions.
With the assumption of a yield factor of 0.5 g/g for aerobic growth of the yeast with glucose as a carbon and energy source (Babel et al. 1983) and a carbon content of roughly 50 % (Simmonds 1919), one can calculate that 37.5 % of the glucose is completely oxidized in the catabolism to generate ATP and reduction equivalents for growth. Therewith, 1.1 g CO2 are released per 1 g biomass synthesized in the heterotrophic process. This value depends on the carbon source, which is supplied in the culture medium and will be different if the yeast metabolizes another carbon source, such as e. g. the cheaper feedstock glycerol. Also, the accumulation of lipids affects the carbon content of biomass. However, the underlying assumptions give good approximations to estimate the minimum dimensioning of the yeast culture. Minimum volumetric productivities amounted to 21 g/(L · d) as depicted in Table 1. That corresponds to a CO2 release of 23.1 g/(L · d).
As shown above, heterotrophic fermentation and phototrophic production are coupled stoichiometrically with a postulated CO2 consumption of 91.5 g/(m2 · d). That requires for the presented cultivation conditions, a heterotrophic reactor volume of 40 m3 to supply CO2 for an optimized algae biomass production plant of 1 ha size. Beside the absolute amount, also, the concentrations are of importance. In the presented measurements, an off-gas concentration of about 3 % was obtained, while industrial production reactors reach about 10 % of CO2. That is the range for which photobioreactors are usually designed in terms of mass transfer. The off-gas contains about 1 % of CO2 according to the required partial pressure in the fluid phase, while the main part is converted to O2. While absolute amount and concentration of the gases are in the right range that consequently holds also for the absolute gas volume, so, a dilution, splitting or concentration of the gas stream is not necessary. A complete recycling of the gas phase between both reactors is possible assuming an adjustment of the mass transfer in the respective reactors will keep the system at the optimized working point during the hourly changing conditions. In future developments, also, an increase of CO2 and O2 on cost of N2 content in the respective parts of the gas phase could be envisaged leading to a further process intensification.
In the experimental set-up, the yeast reactor was smaller by a factor of ca. 3.3 compared to the dimensioning calculated from stoichiometry. The photobioreactor exhibited an illuminated surface area of ca. 1 m2 and the liquid volume of the heterotrophic process was 1.2 L. Nevertheless, the heterotrophic process was capable of supplying the algae with sufficient CO2 because the impinging photon flux density with 250 μE/(m2 · s) was low compared to peak intensities found under outdoor conditions.
In summary, it can be stated that dimensioning of the plants requires consideration of several factors. Most important points are the mass transfer coefficients in both reactors and the peak sunlight intensities which determine the CO2 requirement of the algae.
Potential benefits of the overall process
CO2 is often regarded as a free resource and this assumption often underlies assessments of the economic viability of biodiesel production with microalgae (Singh et al. 2011). It can potentially be obtained from the atmosphere, from the flue gas of power plants, from the by-product streams of other industrial plants or from soluble carbonates (Brennan and Owende 2010). Atmospheric partial pressures are usually too low and allow only for growth with reduced growth rates due to underlying kinetic coherences (Galloway et al. 1964; Doucha and Lívanský 2009). The suitability of flue gas is controversial (Clarens et al. 2010). Experiments were published that have shown a negative impact of nitric oxides and sulphur oxides on growth rates and the volumetric productivity of microalgae (Lee et al. 2002). In other experiments, the tolerance of limited contents of nitric oxides and sulphur oxides was demonstrated (Brown 1996). Even though the sequestration of CO2 emissions from power plants or other industrial plants is desirable, their location is often remote from potential locations of microalgae production facilities. Due to the specific requirements in terms of climate and price of land, optimal locations are often distant from urban areas or industrial bases (Ratledge and Cohen 2008). Consequently, the transport of CO2 to the site of its consumption would be necessary and increase the costs of operation of the biomass production (Feron and Hendriks 2005; García Sánchez et al. 2003). Beneman et al. (1987) estimate that—depending on the scale—the costs of CO2 could amount to 7 to 16 % of the cost of operation of a plant. Richardson et al. (2010) estimate with costs of CO2 at 0.15 to 0.20 US$/kg. With an annual production of 80 t/(ha · a), which is aimed at Wijffels and Barbosa (2010), potential savings of up to 30,000 US$/(ha · a) can be achieved only for the sequestered CO2. This calculation does not even comprise the losses of unexploited CO2 in the off-gas because a concentration difference needs to be sustained to obtain sufficient mass transfer from the gaseous phase into the liquid phase. The exact calculation of the cost saving potential would require exact knowledge of the mass transfer coefficient, which can substantially vary between different reactor designs and set-ups.
In summary, it can be stated that independence from sites of CO2 emission can be achieved by the scale-up of the integrated bioprocess with the combination of heterotrophic cultivation of yeast and CO2 recycling in a phototrophic process. Moreover, a significant cost saving potential can be accessed so that process integration can significantly improve the economic viability of biodiesel production with oleaginous microorganisms. However, further process scale-up experiments are required to demonstrate a viable process strategy on ecological scale.
References
Babel W, Muller RH, Markuske KD (1983) Improvement of growth-yield of yeast on glucose to the maximum by using an additional energy source. Arch Microbiol 136(3):203–208. doi:10.1007/bf00409845
Benemann JR, Tillett DM, Weissman JC (1987) Microalgae biotechnology. Trends Biotechnol 5(2):47–53
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14(2):557–577. doi:10.1016/j.rser.2009.10.009
Brown LM (1996) Uptake of carbon dioxide from flue gas by microalgae. Energy Convers Manag 37(6–8):1363–1367. doi:10.1016/0196-8904(95)00347-9
Camacho Rubio F, Fernández FGA, Pérez JAS, Camacho FG, Grima EM (1999) Prediction of dissolved oxygen and carbon dioxide concentration profiles in tubular photobioreactors for microalgal culture. Biotechnol Bioeng 62(1):71–86. doi:10.1002/(sici)1097-0290(19990105)62:1<71::aid-bit9>3.0.co;2-t
Carlsson AS (2009) Plant oils as feedstock alternatives to petroleum—a short survey of potential oil crop platforms. Biochimie 91(6):665–670. doi:10.1016/j.biochi.2009.03.021
Carmo AC Jr, de Souza LKC, da Costa CEF, Longo E, Zamian JR, da Rocha Filho GN (2009) Production of biodiesel by esterification of palmitic acid over mesoporous aluminosilicate Al-MCM-41. Fuel 88(3):461–468. doi:10.1016/j.fuel.2008.10.007
Cerff M, Morweiser M, Dillschneider R, Michel A, Menzel K, Posten C (2012) Harvesting fresh water and marine algae by magnetic separation: screening of separation parameters and high gradient magnetic filtration. Bioresour Technol 118:289–295
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306. doi:10.1016/j.biotechadv.2007.02.001
Chisti Y, Moo-Young M (1986) Disruption of microbial cells for intracellular products. Enzym Microb Technol 8(4):194–204. doi:10.1016/0141-0229(86)90087-6
Clarens AF, Resurreccion EP, White MA, Colosi LM (2010) Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol 44(5):1813–1819. doi:10.1021/es902838n
Dillschneider R, Steinweg C, Rosello-Sastre R, Posten C (2013) Biofuels from microalgae: photoconversion efficiency during lipid accumulation. Bioresour Technol 142:647–654
Doucha J, Lívanský K (2009) Outdoor open thin-layer microalgal photobioreactor: potential productivity. J Appl Phycol 21(1):111–117. doi:10.1007/s10811-008-9336-2
Fábregas J, Vázquez V, Cabezas B, Otero A (1993) Tris not only controls the pH in microalgal cultures, but also feeds bacteria. J Appl Phycol 5(5):543–545. doi:10.1007/bf02182514
Feron PHM, Hendriks CA (2005) Les différents procédés de capture du CO2 et leurs coûts. Oil Gas Sci Technol 60(3):451–459
Galloway RA, Gauch HG, Soeder CJ (1964) Effects of inhibitory levels of CO2 on Chlorella. Plant Physiol 39 (R8). http://www.nature.com/nature/journal/v206/n4979/abs/206035a0.html
García Sánchez JL, Berenguel M, Rodríguez F, Fernández Sevilla JM, Brindley Alias C, Acién Fernández FG (2003) Minimization of carbon losses in pilot-scale outdoor photobioreactors by model-based predictive control. Biotechnol Bioeng 84(5):533–543. doi:10.1002/bit.10819
Hassan M, Blanc PJ, Pareilleux A, Goma G (1994) Production of single-cell oil from prickly-pear juice fermentation by Cryptococcus curvatus grown in batch culture. World J Microbiol Biotechnol 10(5):534–537
Lee JS, Kim DK, Lee JP, Park SC, Koh JH, Cho HS, Kim SW (2002) Effects of SO2 and NO on growth of Chlorella sp. KR-1. Bioresour Technol 82(1):1–4
Liebert (1987) Final report on the safety assessment of oleic acid, lauric acid, palmitic acid, myristic acid and stearic acid. J Am Coll Toxicol 6(3):321–402
Ma FR, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70(1):1–15
Mann JE, Myers J (1968) On pigments, growth and photosynthesis of Phaeodactylum tricornutum. J Phycol 4(4):349–355. doi:10.1111/j.1529-8817.1968.tb04707.x
Matsunaga T, Takeyama H, Miura Y, Yamazaki T, Furuya H, Sode K (1995) Screening of marine cyanobacteria for high palmitoleic acid production. FEMS Microbiol Lett 133(1–2):137–141. doi:10.1016/0378-1097(95)00350-e
Meesters PAEP, Huijberts GNM, Eggink G (1996) High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl Microbiol Biotechnol 45(5):575–579. doi:10.1007/s002530050731
Moon NJ, Hammond EG (1978) Oil production by fermentation of lactose and effect of temperature on fatty acid composition. J Am Oil Chem Soc 55(10):683–688. doi:10.1007/bf02665361
Puanbut M, Leesing R (2012) Integrated cultivation technique for microbial lipid production by photosynthetic microalgae and locally oleaginous yeasts. World Acad Sci Eng Technol 64:975
Ratledge C, Cohen Z (2005) Single Cell Oils. Champaign, Illinois: AOCS Press
Ratledge C, Cohen Z (2008) Microbial and algal oils: do they have a future for biodiesel or as commodity oils? Lipid Technol 20(7):155–160. doi:10.1002/lite.200800044
Richardson JW, Outlaw JL, Allison M (2010) The economics of microalgae oil. AgBioforum 13(2):119–130
Schlagermann P, Göttlicher G, Dillschneider R, Rosello-Sastre R, Posten C (2012) Composition of algal oil and its potential as biofuel. J Combust 2012:14. doi:10.1155/2012/285185
Simmonds C (1919) Alcohol, its production, properties, chemistry and industrial applications
Singh A, Nigam PS, Murphy JD (2011) Renewable fuels from algae: an answer to debatable land based fuels. Bioresour Technol 102(1):10–16. doi:10.1016/j.biortech.2010.06.032
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101(2):87–96. doi:10.1263/jbb.101.87
Tredici MR (2010) Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biofuels 1(1):143–162. doi:10.4155/bfs.09.10
Varma A, Buscot F (eds) (2005) Microbial metabolism in soil. In: Microorganisms in soils: roles and genesis and functions, Springer pp 129–133
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329(5993):796–799. doi:10.1126/science.1189003
Yamori Y, Nara Y, Tsubouchi T, Sogawa Y, Ikeda K, Horie R (1986) Dietary prevention of stroke and its mechanisms in stroke-prone spontaneously hypertensive rats—preventive effect of dietary fiber and palmitoleic acid. J Hypertens 4(3):449–452
Yang ZH, Miyahara H, Hatanaka A (2011) Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-A(y) Mice with genetic type 2 diabetes. Lipids Health Dis 10(8):120. doi:10.1186/1476-511x-10-120
Yin P, Chen L, Wang Z, Qu R, Liu X, Xu Q, Ren S (2009) Biodiesel production from esterification of oleic acid over aminophosphonic acid resin D418. Fuel 102(0):499–505. doi:10.1016/j.fuel.2012.05.027
Yongmanitchai W, Ward OP (1991) Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Appl Environ Microbiol 57(2):419–425
Zhang J, Fang X, Zhu X-L, Li Y, Xu H-P, Zhao B-F, Chen L, Zhang X-D (2011) Microbial lipid production by the oleaginous yeast Cryptococcus curvatus O3 grown in fed-batch culture. Biomass Bioenergy 35(5):1906–1911. doi:10.1016/j.biombioe.2011.01.024
Zhao X, Hu CM, Wu SG, Shen HW, Zhao ZK (2011) Lipid production by Rhodosporidium toruloides Y4 using different substrate feeding strategies. J Ind Microbiol Biotechnol 38(5):627–632. doi:10.1007/s10295-010-0808-4
Acknowledgments
This work was funded by the “Bundesministerium für Wirtschaft und Technologie” within the ERA SME project BiCycle Integrated new concept(s) for the production of Single Cell Oils (SCO’s) on an economic scale in cooperation with the companies Evonik Industries AG, EnBW Energie Baden-Württemberg AG, Phytowelt Green Technology GmbH and B.R.A.I.N AG.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Robert Dillschneider and Ines Schulze contributted equally to this paper.
Rights and permissions
About this article
Cite this article
Dillschneider, R., Schulze, I., Neumann, A. et al. Combination of algae and yeast fermentation for an integrated process to produce single cell oils. Appl Microbiol Biotechnol 98, 7793–7802 (2014). https://doi.org/10.1007/s00253-014-5867-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5867-4