Abstract
Metarhizium species have recently been found to be plant rhizosphere associates as well as insect pathogens. Because of their abundance, rhizospheric Metarhizium could have enormous environmental impact, with co-evolutionary implications. Here, we tested the hypothesis that some Metarhizium spp. are multifactorial plant growth promoters. In two consecutive years, corn seeds were treated with entomopathogenic Metarhizium spp. and field tested at the Beltsville Facility in Maryland. Seed treatments included application of green fluorescent protein (GFP)-tagged strains of Metarhizium brunneum, Metarhizium anisopliae, Metarhizium robertsii, and M. robertsii gene disruption mutants that were either avirulent (Δmcl1), unable to adhere to plant roots (Δmad2), or poorly utilized root exudates (Δmrt). Relative to seeds treated with heat-killed conidia, M. brunneum, M. anisopliae, and M. robertsii significantly increased leaf collar formation (by 15, 14, and 13 %), stalk length (by 16, 10, and 10 %), average ear biomass (by 61, 56, and 36 %), and average stalk and foliage biomass (by 46, 36, and 33 %). Their major impact on corn yield was during early vegetative growth by allowing the plants to establish earlier and thereby potentially outpacing ambient biotic and abiotic stressors. Δmcl1 colonized roots and promoted plant growth to a similar extent as the parent wild type, showing that Metarhizium populations are plant growth promoters irrespective of their role as insect pathogens. In contrast, rhizospheric populations and growth promotion by Δmrt were significantly reduced, and Δmad2 failed to colonize roots or impact plant growth, suggesting that colonization of the root is a prerequisite for most, if not all, of the beneficial effects of Metarhizium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rhizosphere is a narrow zone of soil directly influenced by root secretions. It is the site of complex interactions between plants, bacteria, fungi, protists, nematodes, and insects (Bais et al. 2006). Such plant-microbes associations are important for nutrient cycling, ecosystem functioning, and carbon sequestration (Singh et al. 2004). Fungi, in particular, are crucial to plant growth and health as nutrient solubilizers, phytase producers, and antagonists of plant pathogens and insects (Bridge and Spooner 2001; Harman and Shoresh 2007; Hu and St. Leger 2002; Marx 2004). Species of the hypocrealean-ascomycete genus Metarhizium are among the most abundant fungi isolated from soils with titers reaching 106 conidia per gram in grasslands (Lomer et al. 2001). They have been known for decades as agents that control insect pests, but their use has expanded recently from the simple concept of biocontrol agents that can kill insect pests to their more recently established role as plant rhizosphere associates (Vega et al. 2009). The ability of Metarhizium robertsii (formerly known as Metarhizium anisopliae var. anisopliae; Bischoff et al. 2009) to colonize roots was first demonstrated with a green fluorescent protein (GFP)-expressing strain applied to a field of cabbages (Hu and St. Leger 2002) and has since been extended to many plant systems. For example, populations of Metarhizium increase over time within the rhizospheres of spruce trees and very effectively control target pests (Bruck and Donahue 2007; Fisher et al. 2011). Such studies place sharp focus on the soil/root interphase as a site where plants, insects, and pathogens will interact to determine fungal efficacy, cycling, and survival.
In previous field studies, treatment with conventional insecticides or M. anisopliae significantly increased yields of corn, while corn treated with both insecticides and M. anisopliae had the highest yields (Kabaluk and Ericsson 2007). A field study with onions showed a 29 % increase in yield when M. anisopliae was applied to control onion thrips versus when the insecticide dimethoate was used alone (Maniania et al. 2003). The fact that Metarhizium increases plant growth in the presence of insecticides implies that Metarhizium confers more than just protection from insects, though we know very little about the mechanisms that support these additional benefits. Entomopathogenicity may not be the principal lifestyle of Metarhizium spp., as their distribution and evolutionary divergence depends on their adaptations to specific soils and plant types rather than their pathogenicity to insects (Bidochka and Hajek 1998; Wyrebek and Bidochka 2013). A field trial on turf showed that M. robertsii populations are dependent on plant roots rather than insect hosts for cycling (Wang et al. 2011). Metarhizium spp. thereby provide an unusually versatile model system for studying interactions between fungi, insect, and plants, with potential benefits for agricultural production.
In a pilot laboratory study, diverse Metarhizium strains from different species were screened for rhizosphere competency and ability to boost growth of winter wheat in insect-free microcosms (Online Resource, Fig. S1). M. robertsii 2575, Metarhizium brunneum 3738, and M. anisopliae 8248 were selected based on rhizosphere competence and ability to boost seedling growth. To determine if microcosm studies predict impact in the field, GFP-expressing M. robertsii 2575, M. brunneum 3738, and M. anisopliae 8248 were applied to corn as seed treatments. Recent studies on Metarhizium-plant interactions have revealed some of the molecular mechanisms by which M. robertsii colonizes roots and the rhizosphere. M. robertsii adheres to roots using an adhesin MAD2 (Wang and St. Leger 2007) and employs a raffinose transporter MRT and an invertase MrINV to utilize plant-derived carbon sources (Fang and St. Leger 2010; Liao et al. 2013). Mutations in these genes prevent colonization of roots (Δmad2) or severely reduce rhizosphere competency (Δmrt and ΔMrInv). Therefore, we field tested Δmad2 and Δmrt on corn seeds to determine whether populations adhering to roots or utilizing plant nutrients are required to boost plant growth. We also determined whether survivorship on roots and plant growth promotion are dependent on recycling through insects by testing Δmcl1, an immune-compromised strain with greatly reduced virulence (Wang and St. Leger 2006). We report here that the three wild-type strains and Δmcl1 increase leaf collar formation, shoot growth, and grain yield of the field corn. However, in spite of retaining virulence to insects, the poorly root competent Δmrt was less efficient in boosting plant growth although it increased corn yield, whereas Δmad2 had no measurable effect on corn plant growth.
Materials and methods
Fungal strains and plant material
Seeds of the organic sweet corn (Zea mays subsp. mays) used in this field trial were kindly provided by Prof. Galen P. Dively. M. robertsii ARSEF 2575 (Mr2575); M. brunneum ARSEF 3738 (Mb3738); M. anisopliae ARSEF 8248 (Ma8248) wild-type strains (USDA/ARS Collection, Ithaca, USA); and three M. robertsii 2575 mutant strains Δmad2, Δmcl1, and Δmrt were grown and maintained on potato dextrose agar (PDA; Fluka, Buchs, Switzerland) plates at 27 °C. All strains were transformed with the GFP marker gene by Agrobacterium tumefaciens-mediated transformation (Fang et al. 2006; Liao et al. 2008) to monitor population levels and distribution in field conditions.
Fungal release
The field site was located in the University of Maryland Beltsville Facility (Beltsville, MD, USA). It is a frequently cultivated (tilled) site, and the soil is a Monmouth fine sandy loam. The rectangular 400-m2 field site was designed to allow for efficient maintenance and the detection of any dispersal of GFP-tagged Metarhizium outside the confines of the plot. The plot consisted of three 100-m2 subplots for replicates, each consisting of seven rows of corn (seven treatments) spaced at 1 m. A barren, plant-free zone surrounded the plot, and a low-maintenance fallow zone outside the plot was also monitored for the GFP-tagged fungi throughout the field tests. The field trials were conducted for two consecutive years (2011 and 2012) in neighboring sites, and similar results were obtained. The data provided in this manuscript are from the 2012 trial, with comparative information from the 2011 trial in Supplementary materials (See Online Resource). No fertilizers, fungicides, or herbicides were applied during the trials.
Conidia of each strain harvested from 14-day-old PDA plates were suspended in 0.05 % Tween 80 (Fisher Scientific, Pittsburgh, USA) + 10 % gum arabic (Sigma-Aldrich, St. Louis, USA) solution to produce a conidial density of 5 × 108 spores mL−1. The corn seeds were coated with conidia by loading spore suspensions (20 μL) on each side of seed surface, and the seeds were air-dried. The conidial load was estimated at 2 × 107 spores per seed. Control seeds were treated with an equal amount of heat-killed (autoclaved) M. robertsii 2575 conidia. The seeds were hand-planted by poking a 5-cm deep hole in the soil, placing the seed at the bottom, and covering the hole with soil followed by light firming. Twenty seeds each spaced at 20 cm were planted in a single row (one replicate), and three replicates of each treatment were individually planted in each subplot. The planting dates were 2 June 2011 and 10 June 2012. Water was supplied by spray irrigation as needed to avoid growth limitation, and environmental temperatures were recorded every day throughout the 3-month field trial. The treatment plots were maintained free of weeds by hand hoeing. Harvesting occurred on 1 September 2011 and 10 September 2012.
Rhizosphere competence and root colonization assays
Soil populations of Metarhizium strains were monitored as described by Liao et al. (2014) and Hu and St. Leger (2002). Briefly, before the start of the experiment and then weekly (first 1 month) and at monthly intervals thereafter, 0.5 g of each soil sample was taken at defined depths using a 5-cm soil core sampler from 50, 20, and 15 evenly spaced locations within the application zones, buffer zone, and fallow zone, respectively. Soil samples from each row of the application areas were taken at 4 to 5 cm from seminal corn roots as well as alongside roots (0–1 cm) to check for uneven distribution and persistence of spores close to the rhizosphere. Because corn has a fibrous-type root system, the soil collected alongside the root (0–1 cm) contained a high density of nodal roots, meaning that the sampled Metarhizium would be existing in overlapping rhizospheres. Soil suspensions were prepared by adding 5 mL 0.05 % Tween 80 solution and vortexing vigorously. Aliquots (100 μL) were spread onto rose bengal selective agar plates, and colony forming units (CFUs) were determined after 10 days at 27 °C. Nine replicates were sampled from each treatment. Rhizosphere competence was recorded as CFUs per gram of soil.
Root colonization was assayed according to a modification of the method of Viterbo et al. (2005). Corn roots were collected from plants 1 week and 3 months after planting. The nodal roots from nine replicates of each Metarhizium treatment were weighed and vortexed three times in 0.05 % Tween 80 solution before grinding in a mortar and pestle in 2-mL 0.05 % Tween 80 solution. Serial dilutions were assayed for CFU on rose bengal selective agar plates as described above, and CFUs were calculated individually by CFUs/root weight.
Plant growth and corn yield measurements
To assess the effects of Metarhizium on vegetative growth of field corn, we monitored seed germination rate, foliage growth, leaf collar formation, leaf chlorophyll content, and shoot growth. Seed germination rate was determined by counting the number of emerged buds 5 days post planting. Starting 7 days post planting, the number of leaves was recorded daily (first 2 weeks) and at two weekly intervals thereafter. Foliar growth was estimated by the number and size of emerging leaves. Leaf blades of <1, 1–2, and >3 cm long were designated as short, medium, and long, respectively. To approximately quantify leaf size, these designations were translated into +0 (short), +0.35 (medium), and +0.7 (long) for data analysis. At two weekly intervals post planting, leaf collar formation was determined by counting the number of emerging collars (the collar is the “band” located at the base of an exposed leaf blade). Leaf chlorophyll content was read weekly using a SPAD 502 Plus chlorophyll meter (Konica Minolta Sensing, Inc., Japan). Shoot growth was determined at weekly intervals by measuring the stalk length. Corn plants were harvested by cutting at ground level using pruning shears. The corn yield was determined from the average weight of each stalk, foliage (gram per fresh corn plant), and each corncob (gram per dry cob). For all experiments, data were statistically analyzed in the SPSS 19 program (IBM Corporation, Armonk, USA) with Tukey’s HSD test and Pearson correlation coefficient.
Results
Effect of Metarhizium treatment on vegetative growth and yield of field corn
The corn seed germination rate was the same in all treatments and controls (Online Resource, Fig. S2), suggesting that the Metarhizium strains used in this study have no impact on seed germination in the field. Overall, three wild-type Metarhizium strains and the avirulent Δmcl1 significantly increased metrics of corn plant growth and corn yield compared to the control. The poorly root competent Δmrt was less efficient in boosting corn plant growth but increased corncob yield by the same level as the wild type. However, Δmad2 had no measurable effect on corn plant growth.
Only M. brunneum 3738 enhanced foliar growth with a maximal increase of 16 % on the ninth day post planting relative to the control and other treatments (Fig. 1, P < 0.05). Metarhizium treatments had no impact on corn leaf chlorophyll formation (Online Resource, Fig. S3). Leaf collar formation was enhanced by all three wild-type Metarhizium spp., whereas the avirulent Δmcl1 and poor root-colonizing mutants (Δmrt and Δmad2) have no effect. Relative to controls, the numbers of collars on plants treated with M. robertsii 2575, M. brunneum 3738, and M. anisopliae 8248 were increased by 13, 15, and 14 %, respectively, 1 month post planting (Fig. 2, P < 0.05). Only Δmad2 failed to increase shoot growth during the course of the trial. Only M. brunneum 3738 significantly increased shoot growth (by 28 %, P < 0.05) 1 week after planting; thereafter, the three wild-type strains and Δmcl1 increased shoot growth at the same rate. The average increase in stalk length was 10, 16, and 10 % in plants treated with M. robertsii 2575, M. brunneum 3738, and M. anisopliae 8248 relative to controls (Table 1, P < 0.05).
There were no significant differences in the number of corncobs between controls and Metarhizium treatments. The average dry weight of each corncob was increased by 36 % (M. robertsii 2575), 56 % (M. anisopliae 8248), 61 % (M. brunneum 3738), 32 % (Δmcl1), and 27 % (Δmrt), respectively, compared to the controls (Table 2, P < 0.05). Δmad2 had no impact on the weight of corncobs. The yield of field corn was also determined as stalk and foliar fresh weight. Only wild-type Metarhizium treatments significantly increased the biomass of corn stalks relative to controls. The average fresh weight of each stalk was increased by 33 % (M. robertsii 2575), 36 % (M. anisopliae 8248), and 46 % (M. brunneum 3738), respectively, compared to the controls (Table 2, P < 0.05).
Rhizosphere competency of different Metarhizium strains and their correlations with plant growth and yield of field corn
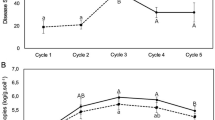
M. brunneum 3738 and M. anisopliae 8248 grew faster than M. robertsii 2575 on PDA plates at various temperatures (Online Resource, Table S1), implying that they might be able to colonize roots more rapidly. One-week-old seedlings from each treatment were harvested to measure early colonization of roots by Metarhizium. Rhizospheric soils were washed off with sterile distilled water and used to assay rhizospheric Metarhizium populations. One-centimeter sections of roots, shoots, and leaves were plated onto rose bengal selective agar medium. Metarhizium was recovered exclusively from the root sections (Online Resource, Fig. S4), confirming that seed treatment is a convenient way of introducing Metarhizium and that Metarhizium strains do not colonize above ground plant parts. Only the root adhesin-deficient Δmad2 failed to colonize corn roots during the 3-month field trial (Fig. 3, P < 0.01).
Root colonization of corn by different Metarhizium strains. Corn roots were collected from plants 1 week and 3 months after planting. Values for each strain are means of nine replicates ± standard error. Uppercase letters indicate means statistically different at the 0.01 level (n = 9). Data shown are from the 2012 trial
Over 3 months, rhizospheric populations of all Metarhizium strains progressively decreased in the field (Fig. 4). CFU counts of the three wild-type Metarhizium strains and Δmcl1 declined at the same rate, consistent with similar rhizosphere competency. Compared to these strains, rhizospheric populations of the non-adherent Δmad2 declined more precipitously immediately after inoculation. Average CFU counts of Δmad2 were still 25 % less than the parental M. robertsii 2575 strain 2 months post inoculation (Fig. 4, P < 0.05). Rhizospheric populations of the raffinose transporter mutant Δmrt declined at the same rate as wild-type strains during the first month post inoculation but declined more sharply thereafter. Average CFU counts of Δmrt were 40 % less than M. robertsii 2575 2 months post inoculation (Fig. 4, P < 0.05). At the end of the field trial (i.e., 3 months post inoculation), rhizospheric populations of wild-type Metarhizium strains, Δmad2 and Δmcl1, were at similar levels but CFU counts of Δmrt averaged 53 % less (P < 0.05) than the other strains. The average populations of Metarhizium strains in soil samples collected 4–5 cm from the roots were 66 % (1 month), 74 % (2 months), and 59 % (3 months) less than those in rhizospheric soils (Fig. 4, 0–1 cm from root), indicating that these fungi were not dispersing from roots. GFP-tagged Metarhizium was very rare 10–15 cm from roots, and could have been associated with laterally extended fibrous roots. Our results suggest that all the introduced Metarhizium strains stayed localized in the rhizosphere.
Populations of introduced Metarhizium strains were measured by counting GFP-tagged Metarhizium CFUs in soils 0–1 cm (top) or 4–5 cm (bottom) from the roots. Values for each strain are means of nine replicates ± standard error. Letters indicate means statistically different at the 0.05 level (n = 9). Data shown are from the 2012 trial
Both Δmad2 and Δmrt were greatly reduced in rhizosphere competency, and Δmrt was less efficient at boosting plant growth, whereas Δmad2 had no impact at all. We therefore determined if Metarhizium rhizospheric populations correlated with plant growth enhancement. Correlation coefficients obtained between the measured traits averaged across the four rhizospheric populations of M. robertsii 2575, Δmad2, Δmcl1, and Δmrt indicated a positive association with corn ear weight (r = 0.798, P = 0.032), stalk weight (r = 0.891, P = 0.007), and stalk height (r = 0.931, P = 0.002) (Table 3).
A comparison of results in 2011 and 2012
Population trends of Metarhizium strains and their impacts on corn plant growth and yield were very similar in 2011 and 2012 (Online Resource, Fig. S5, Tables S2–S4). In 2011, the population of wild-type 2575 was 34 % higher than Δmad2 2 months post inoculation, and Δmad2 had no impact on growth (Online Resource, Fig. S5). Likewise, Δmrt and its impact on plant growth were significantly less than wild type, while the effect of Δmcl1 was similar to the wild type. In contrast to 2012, in 2011, Δmcl1 enhanced leaf collar formation to the same extent as the wild type (Online Resource, Table S2). Overall, there were no significant differences between 2011 and 2012 in rhizospheric populations and their impact on corn plants.
Discussion
Species of Metarhizium are among the most commonly isolated soil fungi. This work presents evidence that they are general plant growth promoters. Corn seed germination was not affected by Metarhizium treatments. Likewise, in laboratory experiments, M. anisopliae strain F52 did not increase corn seed germination (Kabaluk and Ericsson 2007), and Trichoderma harzianum strain T22 only accelerated germination of aged tomato seeds (Mastouri et al. 2010). However, vegetative development during the early growth stage of corn is correlated with its ultimate yield (Mourtzinis et al. 2013). In this study, leaf collar counts was one of the several methods used to measure corn plant growth and estimate the impacts of different Metarhizium strains on foliar growth. The promotion of leaf collar development by the three wild-type strains mostly occurred during the first month of post planting. This would allow the plant to establish earlier and thereby potentially outpace ambient biotic and abiotic stressors (Mastouri et al. 2010).
A surprise of the study is that in 2011 and 2012, the non-pathogenic mutant Δmcl1 benefited corn plant growth and yield at wild-type levels. Given the well-known entomopathogenicity of Metarhizium spp., it would be expected that seed treatment would increase crop yield by killing or repelling armyworms and corn borers (Bruck 2010; Villani et al. 1994; Wang et al. 2008). St. Leger (2008) has suggested that M. robertsii in the rhizosphere could provide a repellent barrier around roots that would offer effective protection to the plant from root-feeding insect pests, as there is an inevitable time lag following fungal infection and cessation of feeding. The nature of fungal repellency has not been determined, but as Δmcl1 is deficient in a gene that is only expressed in the host hemolymph, it is not likely that it would be less repellant than the wild-type M. robertsii 2575 (Wang and St. Leger 2006). In addition, we observed little insect damage on corn plants in either 2011 or 2012, and regular screening of soils and plants detected few corn pests and only a few Metarhizium-infected insects (none infected by Δmcl1). This low level of pest insects may be due to residuals left behind from years of insecticide use or more likely because many of the surrounding fields at the test station were planted with experimental Bt-corn strains producing multiple toxins. Perhaps in a different scenario, with a greater pest burden, failure to infect insects would seriously undermine the beneficial effect of Δmcl1.
A previous field trial on a preferred habitat (turf) with many insects showed that M. robertsii populations are dependent on plant roots rather than insect hosts for cycling (Wang et al. 2011). The current data show that root associations of Metarhizium are also important for maintaining populations in a managed field site. Thus, the root adhesion-deficient mutant Δmad2 is as virulent as the wild type (Wang and St. Leger 2007), but its population declined and it was unable to promote corn growth. Following seed treatment, root colonization is initiated by the introduced microbes attaching to the emerging root tip (Weert and Bloemberg 2006). Δmad2 root colonization likely fails due to its initial inability to attach to the plant root, leading to subsequent attenuation of rhizosphere competency. A 25 % reduction in rhizospheric populations of Δmad2 translated to a total failure to boost plant growth, suggesting that colonization is a prerequisite for stimulating growth. Unlike Δmad2, Δmrt was able to colonize roots, but rhizospheric populations of Δmrt were gradually reduced, due to its inability to take up raffinose in root exudates (Fang and St. Leger 2010). This only had a small impact on growth stimulation by Δmrt consistent with colonization of the root being more important than a large rhizospheric population. We recently reported that disruption of an invertase gene (MrInv) greatly reduces rhizosphere competence of M. robertsii while significantly increasing colonization of roots, showing that they are distinct phenomena (Liao et al. 2013). These results suggest that physical colonization of the root is a prerequisite for most, if not all, of the beneficial effects of Metarhizium, aside perhaps for killing insects.
The three Metarhizium spp. used in this study accelerated growth of winter wheat seedlings in insect-free microcosms (Online Resource, Fig. S1), confirming and providing a model for insect-independent plant-growth-promoting properties. The mechanisms by which Metarhizium boosts plant growth are likely to be multifactorial, as reported for species of Trichoderma where the plant-growth-promoting effects include antibiosis, parasitism, induction of host plant resistance, and competition (Mukherjee et al. 2013). All Metarhizium strains used in this study produce plant-growth-promoting auxins on roots (X. Liao, W. Fang, and R. J. St. Leger, submitted for publication). M. robertsii produces a clearing zone in media containing insoluble phosphate, suggesting a possible role as a biofertilizer (O’Brien 2009). Furthermore, as shown by their antagonism to plant pathogenic fungi (Kang et al. 1996; Ownley et al. 2010) and pathogenicity of M. robertsii 2575 to soil amoeba (Bidochka et al. 2010), at least some Metarhizium isolates have unpredicted flexibility in their trophic capabilities that we are just beginning to appreciate.
There is evidence that plant host associations play an important role in evolutionary divergence within the genus Metarhizium. Thus, Wyrebek and Bidochka (2013) found that Mad2 sequences have diverged among Metarhizium lineages in a manner which suggests that plant relationships, rather than insect host, have driven the divergence of Metarhizium species. Consistent with this, different Metarhizium spp. associate with different groups of plants (Fisher et al. 2011). Wyrebek et al. (2011) showed that M. brunneum associates with the rhizosphere of shrubs and trees whereas M. robertsii preferentially colonizes grasslands. In this study, we found that M. brunneum 3738 and M. anisopliae 8248 colonize corn roots just as well as M. robertsii 2575, indicating that the natural distribution of these fungi does not necessarily predict the beneficial consequences of artificial introductions, at least in the short term. However, there is still a long way to go to unravel the beneficial effects of Metarhizium populations in the soil and their short- and long-term interactions with many other organisms. Understanding these interactions will reveal ecological links and molecular cross-talk that can be exploited to benefit plant productivity in natural and managed environments.
References
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57(1):233–266
Bidochka MJ, Hajek AE (1998) A nonpermissive entomophthoralean fungal infection increases activation of insect prophenoloxidase. J Invertebr Pathol 72(3):231–238
Bidochka MJ, Clark DC, Lewis MW, Keyhani NO (2010) Could insect phagocytic avoidance by entomogenous fungi have evolved via selection against soil amoeboid predators? Microbiology 156(7):2164–2171
Bischoff JF, Rehner SA, Humber RA (2009) A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 101(4):512–530
Bridge P, Spooner B (2001) Soil fungi: diversity and detection. Plant Soil 232(1–2):147–154
Bruck D (2010) Fungal entomopathogens in the rhizosphere. BioControl 55(1):103–112
Bruck DJ, Donahue KM (2007) Persistence of Metarhizium anisopliae incorporated into soilless potting media for control of the black vine weevil, Otiorhynchus sulcatus in container-grown ornamentals. J Invertebr Pathol 95(2):146–150
Fang W, St. Leger RJ (2010) Mrt, a gene unique to fungi, encodes an oligosaccharide transporter and facilitates rhizosphere competency in Metarhizium robertsii. Plant Physiol 154(3):1549–1557
Fang W, Pei Y, Bidochka MJ (2006) Transformation of Metarhizium anisopliae mediated by Agrobacterium tumefaciens. Can J Microbiol 52(7):623–626
Fisher JJ, Rehner SA, Bruck DJ (2011) Diversity of rhizosphere associated entomopathogenic fungi of perennial herbs, shrubs and coniferous trees. J Invertebr Pathol 106(2):289–295
Harman G, Shoresh M (2007) The mechanisms and applications of symbiotic opportunistic plant symbionts. In: Vurro M, Gressel J (eds) Novel biotechnologies for biocontrol agent enhancement and management. NATO Security through Science Series. Springer, Dordrecht, The Netherlands, pp 131–155
Hu G, St. Leger RJ (2002) Field studies using a recombinant mycoinsecticide (Metarhizium anisopliae) reveal that it is rhizosphere competent. Appl Environ Microbiol 68(12):6383–6387
Kabaluk JT, Ericsson JD (2007) Seed treatment increases yield of field corn when applied for wireworm control. Agron J 99(5):1377–1381
Kang S-C, Bark Y-G, Lee D-G, Kim Y-H (1996) Antifungal activities of Metarhizium anisopliae against Fusarium oxysporum, Botrytis cinerea, and Alternaria solani. Korean J Mycol 24(1):49–55
Liao X-G, Fang W-G, Zhang Y-J, Fan Y-H, Wu X-W, Zhou Q, Pei Y (2008) Characterization of a highly active promoter, PBbgpd, in Beauveria bassiana. Curr Microbiol 57(2):121–126
Liao X, Fang W, Lin L, Lu H-L, St. Leger RJ (2013) Metarhizium robertsii produces an extracellular invertase (MrINV) that plays a pivotal role in rhizospheric interactions and root colonization. PLoS ONE 8(10):e78118
Liao X, Lu H-L, Fang W, St. Leger RJ (2014) Overexpression of a Metarhizium robertsii HSP25 gene increases thermotolerance and survival in soil. Appl Microbiol Biotechnol 98(2):777–783
Lomer CJ, Bateman RP, Johnson DL, Langewald J, Thomas M (2001) Biological control of locusts and grasshoppers. Annu Rev Entomol 46(1):667–702
Maniania NK, Sithanantham S, Ekesi S, Ampong-Nyarko K, Baumgärtner J, Löhr B, Matoka CM (2003) A field trial of the entomogenous fungus Metarhizium anisopliae for control of onion thrips, Thrips tabaci. Crop Prot 22(3):553–559
Marx J (2004) The roots of plant-microbe collaborations. Science 304(5668):234–236
Mastouri F, Björkman T, Harman GE (2010) Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology 100(11):1213–1221
Mourtzinis S, Arriaga FJ, Balkcom KS, Ortiz BV (2013) Corn grain and stover yield prediction at R1 growth stage. Agron J 105(4):1045–1050
Mukherjee PK, Horwitz BA, Herrera-Estrella A, Schmoll M, Kenerley CM (2013) Trichoderma research in the genome era. Annu Rev Phytopathol 51(1):105–129
O’Brien T (2009) The saprophytic life of Metarhizium anisopliae. Dissertation, University of Maryland, College Park, MD
Ownley B, Gwinn K, Vega F (2010) Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. BioControl 55(1):113–128
Singh BK, Millard P, Whiteley AS, Murrell JC (2004) Unravelling rhizosphere-microbial interactions: opportunities and limitations. Trends Microbiol 12(8):386–393
St. Leger RJ (2008) Studies on adaptations of Metarhizium anisopliae to life in the soil. J Invertebr Pathol 98(3):271–276
Vega FE, Goettel MS, Blackwell M, Chandler D, Jackson MA, Keller S, Koike M, Maniania NK, Monzón A, Ownley BH, Pell JK, Rangel DEN, Roy HE (2009) Fungal entomopathogens: new insights on their ecology. Fungal Ecol 2(4):149–159
Villani MG, Krueger SR, Schroeder PC, Consolie F, Console NH, Preston-Wisey LM, Roberts DW (1994) Soil application effects of Metarhizium anisopliae on Japanese beetle (Coleoptera: Scarabaeidae) behavior and survival in turfgrass microcosms. Environ Entomol 23:502–513
Viterbo A, Harel M, Horwitz BA, Chet I, Mukherjee PK (2005) Trichoderma mitogen-activated protein kinase signaling is involved in induction of plant systemic resistance. Appl Environ Microbiol 71(10):6241–6246
Wang C, St. Leger RJ (2006) A collagenous protective coat enables Metarhizium anisopliae to evade insect immune responses. Proc Natl Acad Sci U S A 103(17):6647–6652
Wang C, St. Leger RJ (2007) The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot Cell 6(5):808–816
Wang C, Duan Z, St. Leger RJ (2008) MOS1 osmosensor of Metarhizium anisopliae is required for adaptation to insect host hemolymph. Eukaryot Cell 7(2):302–309
Wang S, O’Brien TR, Pava-Ripoll M, St. Leger RJ (2011) Local adaptation of an introduced transgenic insect fungal pathogen due to new beneficial mutations. Proc Natl Acad Sci U S A 108(51):20449–20454
Weert S, Bloemberg G (2006) Rhizosphere competence and the role of root colonization in biocontrol. In: Gnanamanickam S (ed) Plant-associated bacteria. Springer, Dordrecht, The Netherlands, pp 317–333
Wyrebek M, Bidochka MJ (2013) Variability in the insect and plant adhesins, Mad1 and Mad2, within the fungal genus Metarhizium suggest plant adaptation as an evolutionary force. PLoS ONE 8(3):e59357
Wyrebek M, Huber C, Sasan RK, Bidochka MJ (2011) Three sympatrically occurring species of Metarhizium show plant rhizosphere specificity. Microbiology 157(10):2904–2911
Acknowledgments
This work was supported by the Biotechnology Risk Assessment Grant Program competitive grant no. 2011-33522-30742 from the USDA National Institute of Food and Agriculture. We thank Prof. Galen P. Dively, Department of Entomology, University of Maryland, for his comprehensive technical assistance in planting and maintaining the field site. Many undergraduate students from the University of Maryland participated in collecting and analyzing samples, and we are particularly grateful to Bernard L. Stopak, Peter Nguyen, Tae Kim, and Vikas Mishra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 676 kb)
Rights and permissions
About this article
Cite this article
Liao, X., O’Brien, T.R., Fang, W. et al. The plant beneficial effects of Metarhizium species correlate with their association with roots. Appl Microbiol Biotechnol 98, 7089–7096 (2014). https://doi.org/10.1007/s00253-014-5788-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5788-2