Abstract
The use of saline water as secondary quality water in urban environments for sanitation is a promising alternative towards mitigating fresh water scarcity. However, this alternative will increase the salinity in the wastewater generated that may affect the biological wastewater treatment processes, such as biological phosphorus removal. In addition to the production of saline wastewater by the direct use of saline water in urban environments, saline wastewater is also generated by some industries. Intrusion of saline water into the sewers is another source of salinity entering the wastewater treatment plant. In this study, the short-term effects of salinity on the anaerobic metabolism of phosphate-accumulating organisms (PAO) and glycogen-accumulating organisms (GAO) were investigated to assess the impact of salinity on enhanced biological phosphorus removal. Hereto, PAO and GAO cultures enriched at a relatively low salinity level (0.02 % W/V) were exposed to salinity concentrations of up to 6 % (as NaCl) in anaerobic batch tests. It was demonstrated that both PAO and GAO are affected by higher salinity levels, with PAO being the more sensitive organisms to the increasing salinity. The maximum acetate uptake rate of PAO decreased by 71 % when the salinity increased from 0 to 1 %, while that of GAO decreased by 41 % for the same salinity increase. Regarding the stoichiometry of PAO, a decrease in the P-release/HAc uptake ratio accompanied with an increase in the glycogen consumption/HAc uptake ratio was observed for PAO when the salinity increased from 0 to 2 % salinity, indicating a metabolic shift from a poly-P-dependent to a glycogen-dependent metabolism. The anaerobic maintenance requirements of PAO and GAO increased as the salinity concentrations risen up to 4 % salinity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The benefits of using saline (sea and brackish) water as secondary quality water when compared to other water production and treatment applications (including a practically infinite availability of water in the oceans) make it a promising, cost-effective, and environmentally friendly alternative to alleviate fresh water stress in urban areas located in coastal zones and inland areas with brackish ground water (Tang et al. 2006; WSD 2009; Leung et al. 2012). From the water consumption perspective, up to 30 % of the fresh water use can be replaced by saline water for certain household activities such as toilet flushing. This practice leads to the generation of saline wastewater which may affect the biological processes employed at sewage treatment plants. In addition to the direct use of saline water in urban environments, saline wastewater (containing high phosphorus concentrations) is also generated by some industries such as food production and processing (dairy, fish processing, pickled vegetables, and meat canning) and tanneries (Gonzalez et al. 1983; Orhon et al. 1999; Fahim et al. 2000; Lefebvre and Moletta 2006). Due to tightening regulations to protect the surface water bodies against eutrophication in combination with the potential use of saline water as secondary quality water and the global growing industrialization (particularly in developing and emerging economies), the removal of nitrogen (N) and phosphorus (P) from saline wastewaters begins to attract an increasing attention.
While most of the research on saline wastewater has focused so far on the efficiency of the biological removal of organic matter and, to a lesser extent, on nitrogen removal, only a few studies have focused on the enhanced biological phosphorus removal (EBPR) process. The findings of these studies are inconsistent possibly because phosphate-accumulating organisms (PAO) were not the prevailing microorganisms and the operating conditions were not optimal to sustain the EBPR process. For instance, in certain cases, P-removal did not occur due to EBPR but through biosynthesis (Uygur and Kargi 2004; Kargi and Uygur 2005; Uygur 2006), while in other studies glycogen-accumulating organisms (GAO) appeared to be the prevailing microorganisms instead of PAO (Panswad and Anan (1999). Furthermore, in some studies, there was a lack of the appropriate carbon source (volatile fatty acids, VFA) in the influent (Hong et al. 2007; Wu et al. 2008) or the EBPR removal was affected by nitrite (NO2 −-N) and nitrate (NO3 −-N) intrusion into the anaerobic phase of the system (Intrasungkha et al. 1999). Moreover, in most of the studies, information needed to assess the impact of salinity on the metabolism of PAO (like the specific anaerobic P-release and anaerobic carbon-uptake rates) are not reported (Panswad and Anan 1999; Intrasungkha et al. 1999; Uygur and Kargi 2004; Kargi and Uygur 2005; Uygur 2006; Hong et al. 2007; Cui et al. 2009). In most cases, only the phosphorus removal efficiency was measured and just a few studies aimed to determine the impact of salinity on the process kinetic rates (Uygur 2006; Wu et al. 2008). Furthermore, no studies have been undertaken yet on enriched cultures of PAO and GAO (the competing microorganisms in an EBPR system). Overall, it still remains unclear how salinity affects the EBPR process and the microbial populations involved in or related to the process (like PAO and GAO, respectively).
To get a better understanding of the impact of salinity on EBPR, we investigated the effects of salinity on the anaerobic metabolism (stoichiometry and kinetics) of enriched PAO and GAO cultures, as the competition between PAO and GAO occurs in the anaerobic stage of the process. Enriched PAO and GAO cultures were cultivated in sequencing batch reactors (SBR). Through the execution of short-term (hours) anaerobic batch tests, the effects of salinity on the anaerobic physiology of PAO and GAO are studied with the aim to assess the competition between these two groups of microorganisms under saline conditions and consequently assess the feasibility to apply the EBPR process for the treatment of saline wastewaters.
Materials and methods
Enrichment of PAO and GAO cultures
Operation of SBR
PAO and GAO cultures were enriched in two similar double-jacketed laboratory SBR. The reactors were operated and controlled automatically in a sequential mode (SBR) by an Applikon ADI controller. Online operating data (e.g., pH and O2) were stored using the BioXpert software (Applikon, The Netherlands, Schiedam). Each SBR had a working volume of 2.5 L. Activated sludge from a municipal wastewater treatment plant with a five-stage Bardenpho configuration (Hoek van Holland, The Netherlands) was used as inoculum for the enrichment of the PAO culture, while enriched sludge from the PAO SBR (PAO-SBR) was used as inoculum for the GAO SBR (GAO-SBR).
The SBRs were operated in cycles of 6 h (2.25 h anaerobic, 2.25 aerobic, and 1.5 settling phase) following similar operating conditions used in previous studies (Smolders et al. 1994a; Brdjanovic et al. 1997). pH was maintained at 7.2 ± 0.05 (PAO-SBR) and 7.0 ± 0.05 (GAO-SBR) by dosing 0.4 M HCl and 0.4 M NaOH. Temperature was controlled in both reactors at 20 ± 1 °C.
The cycle started with nitrogen gas sparging for 5 min at a flow rate of 30 L/h to remove any oxygen remaining from the previous cycle and feed the substrate to the SBR under truly anaerobic conditions. After the first 5 min, 1.25 L of synthetic substrate was fed to the SBR over a period of 5 min. In the aerobic phase, compressed air was introduced to the SBR at a flow rate of 60 L/h. The resulting DO concentrations in the PAO-SBR were ranging between 6.4 and 8.2 mg O2/L and in the GAO-SBR between 2.7 and 8.2 mg O2/L.
The PAO-SBR and GAO-SBR were controlled at a nominal sludge retention time (SRT) of 8 and 10 days, respectively. For the estimation of the applied SRT, any potential loss of biomass, through the effluent or from the removal of the biofilms stuck to the walls of the reactors during the maintenance of the systems, was not considered. At the end of the settling period, supernatant was pumped out from the reactors, leaving 1.25 L of mixed liquor in the reactor resulting in a total hydraulic retention time (HRT) of 12 h. The mixed liquor was mixed by impellers operated at 500 and 300 rpm for the PAO-SBR and GAO-SBR, respectively, except during settling and decant phases when mixing was switched off.
Synthetic substrate
The main difference between the synthetic substrate supplied to the PAO-SBR and GAO-SBR was the phosphorus content. Namely, the influent of PAO-SBR contained 20 mg PO4 3−-P/L (0.65 P-mmol/L), leading to an influent P/C ratio of 0.05 (P-mol/C-mol), while the P content in GAO-SBR influent was limited to 2.2 mg PO4 3−-P/L (0.07 P-mmol/L) (Liu et al. 1997), resulting in an influent P/C ratio of 0.005 (P-mol/C-mol).
The concentrated medium was prepared in two separate solutions with demineralized water. The first solution contained only acetate (HAc) as carbon source, while the other contained all other nutrients, minerals, and trace elements required for biomass cultivation. After mixing the two solutions (prior to addition to the respective SBR), the influent of the reactors contained per liter: 860 mg NaAc·3H2O (12.6 C-mmol/L, 405 mg COD/L), 107 mg NH4Cl (2 N-mmol/L), 140 mg CaCl2.2H2O, 2 mg/L of N-allylthiourea (ATU) to inhibit nitrification, 0.3 mL/L trace elements solution, and 89 mg NaH2PO4·H2O (0.65 P-mmol/L, 20 mg PO4 3−-P/L) for the PAO-SBR, whereas the GAO-SBR contained 9.8 mg NaH2PO4·H2O, (2.2 mg PO4 3−-P/L, 0.07 P-mmol/L), 120 mg MgSO4·7H2O for PAO-SBR and 90 mg MgSO4·7H2O for GAO-SBR, 480 mg KCl for PAO-SBR, and 360 mg KCl for GAO-SBR. The trace element solution was prepared as described by Smolders et al. (1994a). Prior to use, both concentrated solutions were autoclaved at 110 °C for 1 h.
The performance of PAO-SBR and GAO-SBR was regularly monitored by measuring orthophosphate (PO4 3−-P), acetate (Ac-C), mixed liquor suspended solids (MLSS), and mixed liquor volatile suspended solids (MLVSS). The pseudo steady-state conditions of the reactors were confirmed based on daily observations of the aforementioned parameters as well as online pH and DO profiles recorded with the BioXpert software. When no significant changes of these parameters were observed on the online profiles for a time interval of at least three SRT, the condition was considered to be in pseudo steady-state.
Cycle measurements were carried out to determine the biomass activity when both SBR reached steady-state conditions. In the cycle measurements, polyhydroxyalkanoate (PHA) and glycogen were also measured in addition to PO4 3−-P, Ac-C, MLSS, and MLVSS. Estimation of the degree of enrichment of the bacterial populations of interest (PAO and GAO) was undertaken via fluorescence in situ hybridization (FISH) analysis.
Anaerobic batch tests
After the biomass activity reached pseudo steady-state conditions in the SBR, anaerobic batch experiments at different salt concentrations were performed in two double-jacketed laboratory reactors with a maximal operating volume of 0.5 L. In order to conduct anaerobic short-term salinity tests, a defined volume of enriched PAO and GAO sludge was withdrawn at the end of the aerobic phase from the PAO-SBR and GAO-SBR, respectively, and transferred to the 0.5-L batch reactor. After each sludge transfer, the wastage of sludge was adjusted to compensate for the sludge withdrawal due to batch tests and keep a stable SRT in the parent SBR. Batch tests were performed at controlled temperature and pH (20 ± 0.5 °C and 7.0 ± 0.05, respectively). pH was automatically maintained by dosing of 0.1 M HCl and 0.1 M NaOH. During the anaerobic batch experiments, the sludge was constantly stirred at 300 rpm.
The batch tests carried out for the determination of the anaerobic kinetic rates of PAO and GAO were executed after the addition of the same synthetic media used for their cultivation but, in addition, with NaCl added at certain defined concentrations. Prior to addition of the synthetic medium in the batch reactor, there was no settling and effluent removal phase, which normally took place in the SBR before adding the influent in the beginning of the next cycle. To execute the anaerobic batch tests with the same acetate-to-biomass ratio as in the parent PAO-SBR and GAO-SBR, the concentration of HAc was reduced by 50 %. To suppress any potential foam formation, a drop of silicon antifoam (1 % solution) was also added. All batch tests were carried out for 3 h. During this period, samples for the determination of MLVSS, MLSS, glycogen, PO4, and acetate samples were taken. The salinity concentrations studied were chosen in a step-wise manner from 0 to 4 % salinity (W/V) to cover the whole inhibition range of the microorganisms (from 0 to 100 % relative inhibition). The salinity concentrations assessed for PAO were 0.02, 0.27, 0.52, 0.60, 0.81, 1.02, 2.02, and 4.02 % (W/V) NaCl, and for GAO, they were 0.02, 0.14, 0.27, 0.39, 0.52, 0.77, 1.02, 1.52, 2.02, 3.02, and 4.02 % (W/V) NaCl.
Anaerobic maintenance tests
Anaerobic batch tests, similar to those described previously, but without acetate addition, were conducted for the determination of the anaerobic maintenance requirements of PAO and GAO following a procedure described elsewhere (Brdjanovic et al. 1997; Lopez-Vazquez et al. 2007).
N2 gas was sparged at a flow rate of 6 L/h into the mixed liquor for 5 min before the beginning and during the first 15 min of the batch tests to create anaerobic conditions. Thereafter, N2 was flushed through the headspace of the reactor to prevent oxygen intrusion. Influent was also sparged with N2 for 15 min to remove any potential dissolved oxygen present. After influent addition, the activity of PAO was followed for 3 h, and that of GAO for 8 h. During this period, samples for the determination of the orthophosphate concentrations were collected for PAO, while in the case of GAO, samples for glycogen analysis were collected. For PAO, two additional anaerobic maintenance tests were conducted to check if a potential shift from poly-P to glycogen consumption occurred. The tests were similar to those described for GAO. The salinity concentrations studied in the PAO and GAO anaerobic maintenance tests were in the range of 0 to 6 % NaCl (W/V) for both organisms. Similar to the HAc uptake tests, these salinity concentrations were selected to cover the whole inhibition range, which was higher in the maintenance tests (up to 6 % NaCl) than in the HAc uptake tests (up to 4 % NaCl). The salinity concentrations assessed for PAO were 0.02, 0.27, 0.52, 1.02, 1.52, 2.02, 2.52, 3.02, 3.52, 4.02, and 6.02 % (W/V) NaCl, and for GAO, they were 0.02, 1.02, 2.02, 3.02, 4.02, 5.02, and 6.02 % (W/V) NaCl.
Anaerobic kinetic and stoichiometric parameters
PAO and GAO kinetics
All rates were expressed as maximum active biomass specific rates based on the PO4 and HAc profiles observed in the tests as described by Smolders et al. (1994b), Zeng et al. (2003), Brdjanovic et al. (1997), and Lopez-Vazquez et al. (2007). For PAO, the HAc uptake rates were corrected for the presence of GAO by subtracting from the total observed HAc uptake rates the HAc uptake rate of GAO and dividing this value by the PAO fraction in the sludge from the PAO-SBR. The anaerobic P-release rates of PAO were also corrected for the presence of GAO by dividing the total P-release rates by the PAO fraction, determined by quantitative FISH analysis, and for the anaerobic maintenance activity by subtracting the maintenance P-release from the total anaerobic P-release.
In anaerobic tests executed without HAc, the effect of salinity on the maintenance activity was assessed by measuring the P-release and glycogen consumption of PAO and GAO. For PAO, the maintenance activity was corrected for the presence of GAO in the PAO-SBR sludge. The PAO maintenance activity was determined by dividing the observed P-release rates in the batch tests by the fraction of PAO in the sludge. The anaerobic specific ATP maintenance coefficients of PAO and GAO were determined by linear regression of the glycogen consumption and P-release profiles as described by Smolders et al. (1994a) and Zeng et al. (2003), respectively. However, when the PAO and GAO glycogen consumption profiles were leveling off over time due to a progressive inhibition at high salinity concentrations, only the initial glycogen consumption observed during the first 2 h of the tests were considered for the determination of the anaerobic maintenance coefficient.
PAO and GAO stoichiometry
Total P-release rate per HAc uptake rate and corrected P-release rate per HAc uptake rate and total glycogen consumption per HAc uptake were the stoichiometric parameters assessed for PAO. For GAO, the total glycogen consumption per HAc uptake and the corrected glycogen consumption per HAc uptake were the stoichiometric parameters evaluated. The corrected glycogen consumption of PAO and GAO was determined by subtracting the estimated glycogen consumption due to maintenance from the total glycogen consumption observed in the batch tests with HAc.
Analyses
PO4 3−-P (determined by the ascorbic acid method), MLSS, and MLVSS determination were performed in accordance with Standard Methods (A.P.H.A. 1995). HAc was determined using a Varian 430-GC Gas Chromatograph (GC), equipped with a split injector (split ratio 1:10), a WCOT Fused Silica column with a FFAP-CB coating (25 m × 0.53 mm × 1 μm), and coupled to a FID detector. Helium gas was used as carrier gas. Temperature of the injector, column, and detector were 200, 105, and 300 °C, respectively. PHA content (as PHB and PHV) of freeze dried biomass was determined according to the method described by Smolders et al. (1994a). Glycogen determination was also executed according to the method described by Smolders et al. (1994b) but with a digestion phase extended to 5 h.
To determine the microbial population distribution of the enriched PAO and GAO culture, FISH was performed according to the procedures described in Amman (1995). In order to target the entire bacterial population, the EUBMIX probe (mixture of probes EUB 338, EUB338-II and EUB338-III) was used. Accumulibacter was targeted by a PAOMIX probe (mixture of probes PAO462, PAO651, and PAO846) (Crocetti et al. 2000), whereas a GAOMIX probe (mixture of probes GAOQ431 and GAOQ989) (Crocetti et al. 2002) was used to target Competibacter. In order to distinguish the different PAO clades, PAO I (clade 1A and other type 1 clades) and PAO II (clade 2A, 2C, and 2D) were targeted by the probes Acc-1-444 and Acc-2-444 (Flowers et al. 2009), respectively. The FISH samples were hybridized under the conditions described by Crocetti et al. (2000, 2002) and Flowers et al. (2009).
The quantification of the PAO and GAO fractions in the biomass from PAO-SBR was carried out using the free ImageJ software package (version1.47b, Wayne Rasband, National Institute of Health, USA). The 8-bit images for each of the color channels (red for PAO, Cy3; green for GAO, Fluos; blue for EUB, Cy5) were converted into binary format. Image coverage was computed by dividing the number of pixels corresponding to the object with the total number of pixels of the image. Fractions of PAO and GAO were calculated as the ratio between their image coverage and that of the entire bacterial population. Around 20 separate images were evaluated.
Parameter fitting and evaluation of models
A structured model was developed (Online resource 1–3), describing the salinity effects on the different metabolic processes (Eqs. 1–9), to obtain better insight in the salinity effects on the kinetic rates and stoichiometry and to propose a model for practical applications. A detailed explanation of the equations is given in the Online resource 1–3.
PAO and GAO anaerobic maintenance coefficient
Being,
- \( {m}_{\mathrm{ATP},{\mathrm{PAO}}_{\mathrm{poly}}-P}^{an}(S) \) :
-
PAO poly-P maintenance coefficient at different salinity concentrations
- m anATP,GAO (S):
-
GAO maintenance coefficient at different salinity concentrations
- S :
-
Salinity concentration
And fitted parameters,
- m 0 ATP :
-
Maintenance coefficient at 0 % salinity concentration
- a :
-
Linear proportional increase in maintenance requirements per increase in salinity
- bi 1 :
-
Impact factor, describing the magnitude of the inhibition effect
- Si 1 :
-
Salinity concentration at which 50 % inhibition occurs
PAO and GAO anaerobic kinetic rates
Being,
- q MAXSA,PAO_total (S):
-
Total maximum PAO acetate uptake rate at different salinity
- q MAXSA,GAO (S):
-
Maximum GAO acetate uptake rate at different salinity
- q MAXP,PAO _ HAc (S):
-
Maximum PAO PO4 release rate at different salinity
- S :
-
Salinity concentration
And fitted parameters,
- q MAX,0SA,PAO_PAM :
-
Maximum PAO acetate uptake rate facilitated by a PAM at 0 % salinity
- q MAX,0SA,PAO_GAM :
-
Maximum PAO acetate uptake rate facilitated by a GAM at 0 % salinity
- q MAX,0SA,GAO :
-
Maximum GAO acetate uptake rate at 0 % salinity
- q MAX,0 P,PAO _ HAc :
-
Maximum PAO PO4 release rate at 0 % salinity
- bi 2 :
-
Impact factor, describing the magnitude of the inhibition effect on the acetate uptake and P-release
- Si 2 :
-
Salinity concentration at which 50 % inhibition of the acetate uptake occurs
- ba 1 :
-
Impact factor (equal to bi 2), describing the magnitude of the activation effect on the acetate uptake
- Sa 1 :
-
Salinity concentration (equal to Si 2) at which 50 % activation of the PAO GAM acetate uptake occurs.
PAO and GAO anaerobic stoichiometry
being,
- f totalP/HAc,PAO :
-
Total P-release rate/HAc uptake rate at different salinity
- f HAcP/HAc,PAO :
-
P-release rate corrected for maintenance activity/HAc uptake rate at different salinity
- f totalΔgly/HAc,GAO :
-
Net glycogen consumption/net HAc uptake
- f HAcΔgly/ΔHAc,GAO :
-
Net glycogen consumption corrected for maintenance activity/net HAc uptake
- ∆HAc:
-
Net acetate consumption during the tests
- ∆t :
-
Time interval of batch test
- X GAO :
-
Active biomass concentration of GAO
- S :
-
Salinity concentration
Model parameters were fit with the experimental values of the kinetic rates and stoichiometry at different salinity concentrations using the method of least squares, and the model simulation was evaluated using the ordinary least squares regression model (Mesple et al. 1996).
Results
Enrichment of PAO
The PAO-SBR was continuously operated for over 400 days. When it reached pseudo steady-state conditions, the biomass exhibited complete acetate uptake in the anaerobic stage and complete P-removal in the aerobic phase. The MLSS and MLVSS concentrations in the reactor were 2,913 and 1,990 mg/L, respectively, leading to a MLVSS/MLSS ratio of 0.69. This indicates a high ash content in the cell, as usually observed in enriched PAO cultures as a consequence of the high amount of the stored polyphosphate. During the steady-state period, the average observed P-release/HAc uptake ratio was 0.33 (s.d. = 0.02, n = 18) P-mol/C-mol.
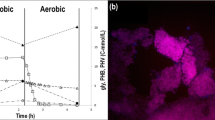
The change in carbon storage compounds and soluble phosphate in a steady-state SBR cycle is shown in Fig. 1a, and the composition of the microbial community by FISH is shown in Fig. 1b. The observed specific acetate uptake rate was 0.25 C-mol/(C-mol·h), the specific anaerobic phosphate release rate was 0.085 P-mol/(C-mol·h), and the specific aerobic phosphate uptake rate was 0.081 P-mol/(C-mol·h). FISH images show that the PAO-SBR sludge was indeed dominated by PAO, although a small fraction of GAO was present. Accumulibacter Type II was the dominant microorganism whereas Accumulibacter Type I was not observed. Quantitative FISH analysis indicated that PAO and GAO together comprised 98 % of the microbial community. The fractions of PAO and GAO were 94 % (s.d. = 4 %, n = 20) and 5 % (s.d. = 4 %, n = 20), respectively.
Enrichment of the PAO culture: a profile observed during a cycle under pseudo steady-state conditions in the PAO-SBR: acetate (black square), PO4 (white square), PHV (white circle), PHB (black circle), and glycogen (white triangle) concentrations. b FISH image obtained from PAO-SBR: PAO mix (purple), GAO mix (cyan green), EUB (blue)
Enrichment of GAO
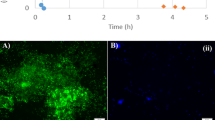
The GAO-SBR was continuously operated for more than 350 days. Once the biomass activity in the reactor was stable and reached (pseudo-) steady-state conditions, the MLSS and MLVSS concentrations in the GAO-SBR were 2,193 and 2,067 mg/L, respectively. The calculated MLVSS/MLSS ratio was 0.94, indicating that no significant polyphosphate accumulation occurred. Figure 2 displays a cycle illustrating the biomass activity observed in the GAO-SBR (Fig. 2a). The FISH analyses (Fig. 2b) confirmed that the sludge was dominated by GAO (Competibacter) and that only minor traces of PAO were present in the sludge. Since FISH quantification would not add significant additional information about the enrichment of the GAO sludge, it was not conducted. The specific acetate uptake rate was 0.18 C-mol/(C-mol·h), and the P-release/HAc uptake ratio was 0.012 P-mol/C-mol.
Enrichment of the GAO culture: a profile observed during a cycle under pseudo steady-state conditions in the GAO-SBR: acetate (black square), PO4 (white square), PHV (white circle), PHB (black circle), glycogen (white triangle). b Fish image obtained from GAO sludge: PAO mix (purple), GAO mix (cyan green), EUB (blue)
Impact of salinity on the anaerobic kinetics of PAO and GAO
PAO anaerobic maintenance requirements
At the different salinity concentrations, all anaerobic maintenance P-release profiles were linear, indicating that no progressive inhibition took place over time during the execution of each batch activity test (data not shown). On the basis of the P-release profiles, from 0 to 1.5 % salinity, a continuous increase in the anaerobic maintenance ATP coefficient of PAO was observed, whereas above 2 % salinity it decreased (Fig. 3a). To check if the observed decrease in P-release was compensated by glycogen consumption, two additional tests were carried out at 1 and 4 % salinity. In these tests, glycogen consumption was also observed. At 1 % salinity, the initial rate (determined during the first 2 h of the test) of maintenance energy production by glycogen conversion (m anATP,PAO _ gly ) was 1.6 times higher than the maintenance energy production rate by P-release (m anATP,PAO _ poly ‐ P ), while at 4 % the m anATP,PAO _ gly was 5.0 times higher than the m anATP,PAO _ poly ‐ P . This indicates that a shift in the energy generating mechanism for maintenance took place, from a poly-P- and glycogen-dependent mechanism to a mechanism mainly driven by glycogen. The total maintenance energy, produced by glycogen conversion and P-release (m anATP,PAO _ total ), at 1 and 4 % salinity were 0.0065 and 0.015 ATP-mol/(C-mol·h), respectively. Concerning the glycogen consumption profiles observed at 1 and 4 % salinity, it was linear at 1 %; however, at 4 % salinity it slowed down and leveled off during the test. This suggests that the anaerobic maintenance mechanism of PAO dependent on glycogen was progressively inhibited at 4 % salinity.
Effects of salinity on the anaerobic maintenance coefficients of a PAO: m anATP,PAO _ poly ‐ P (black square), m anATP,PAO _ gly (white triangle), m anATP,PAO _ total (black circle), simulation of m anATP,PAO _ poly ‐ P (solid line), and b GAO: m ATP (black circle), simulation of m anATP (solid line)
GAO anaerobic maintenance requirements
In all anaerobic maintenance tests, at increased salinity concentrations the anaerobic glycogen consumption increased, but full glycogen depletion was not observed within the test duration of 8 h (data not shown). Similar to PAO but at salinity concentrations above 2 %, the glycogen consumption profiles leveled off during the tests, implying that GAO were increasingly inhibited over time. In spite of this effect, the initial maintenance energy production rate by glycogen conversion (determined during the first 2 h of the test) of GAO (m anATP,GAO ) increased up to 4 % salinity (Fig. 3b), but above 4 % it decreased.
Modeling the anaerobic maintenance of PAO and GAO
The PAO m anATP,PAO _ poly ‐ P and GAO m anATP,GAO anaerobic maintenance activities at different salinity levels were successfully described by the empirical Eqs. 1 and 2. For PAO, both glycogen consumption and P-release should be considered for a complete description of the anaerobic maintenance coefficient m anATP,PAO _ total at different salinities, but the glycogen data at different salinities were insufficient.
Based on the development of Eqs. 1 and 2 (Online resource 1), the parameter values of these equations are shown in Table 1.
At 0 % salinity, the m 0ATP for GAO was 10-fold higher than that of PAO (m 0ATP ). Also, the increase in energy maintenance requirements per increase in salinity (a) was higher for GAO. Fifty percent inhibition of the m anATP,PAO _ poly ‐ P and m anATP,GAO of GAO occurred at 3.1 and 5.6 % salinity for PAO and GAO, respectively. However, the m anATP,PAO _ gly at 4 % salinity shows that the glycogen consumption is less inhibited and still occurs at higher salinity concentrations (Fig. 3a).
Maximum specific kinetic rates of PAO and GAO
The impact of salinity on the anaerobic acetate uptake of PAO was pronounced, and above 2 % salinity incomplete acetate uptake was observed within the 3 h duration of the tests (data not shown). Similarly, the anaerobic P-release decreased with increasing salinity (data not shown). The maximum specific acetate uptake and maximum specific effective P-release rates exhibited a drastic decrease (of about 71 and 81 %, respectively) when the salinity increased from 0 to 1 % (Fig. 4a). Above 2 % salinity, the concentrations of acetate taken up and P released were merely marginal.
Maximum specific kinetic rates of PAO and GAO: a PAO HAc uptake rate (black circle), and PAO PO4 release rate corrected for maintenance activity (white circle), during short-term tests. b GAO HAc uptake rate at different salinity concentrations (black circle). Continuous bold lines show the results of model simulations
For GAO, a gradual decrease in the acetate uptake rate occurred between 0 and 1.5 % salinity. At 1 % salinity, the maximum specific acetate uptake rate decreased by 41 %. Above 1.5 % salinity, complete acetate uptake was no longer observed within 3 h (data not shown). Fifty percent inhibition of the maximum specific acetate uptake rates occurred between 1 and 1.5 % salinity, further dropping by 85 % at 2 % salinity (Fig. 4b).
Modeling PAO and GAO kinetics
The impact of salinity on the maximum specific anaerobic HAc uptake and PO4 release rates of PAO were successfully described by Eqs. 2 and 4, respectively. Meanwhile, the impact of salinity on the maximum specific anaerobic HAc uptake rate of GAO was successfully described by Eq. 3. Based on the development of Eqs. 2, 3, and 4 (Online resource 2), the values of their different parameters are shown in Table 2.
According to the model parameters, the HAc uptake rate of PAO was 50 % inhibited at a salinity concentration of 0.6 %, while the HAc uptake rate of GAO at a concentration of 1.2 %. Furthermore, the impact factor “bi 2,” describing the magnitude of the inhibition effect of salinity on the HAc uptake rate, was higher for PAO than for GAO (3.7 versus 2.6, respectively) reflecting the higher sensitivity of PAO.
Impact of salinity on the anaerobic stoichiometry of PAO and GAO
PAO stoichiometry
When the P-release was corrected for the maintenance activity (to display the P-release/HAc uptake ratio caused by HAc uptake only), a significant decrease (of 45 %) in the P-release/HAc uptake ratio was observed when the salinity increased up to 2 % salinity (Fig. 5a). If no correction is made, the total P-release/HAc uptake ratio decreased up to 25 % at 1 % salinity, but at 2 % the total P-release/HAc uptake ratio increased due to a higher contribution of P-release for maintenance requirements. Due to insufficient data collected from the glycogen consumption profiles from the anaerobic maintenance tests, the corrected glycogen/HAc ratio could only be determined at 1 % salinity, which was 1.46 C-mol/C-mol. Nevertheless, the total glycogen/HAc ratio (Fig. 5a) increased from 1.2 to 2.3 C-mol/C-mol when the salinity concentration increased from 0 to 2 %.
Salinity effects on the anaerobic stoichiometry of a PAO: ratio of total P-release rate/HAc uptake rate corrected for GAO acetate uptake at different salinities (black square), ratio of P-release rate/HAc uptake rate corrected for maintenance P-release and GAO acetate uptake at different salinities (white square), and ratio of total glycogen consumption/HAc uptake corrected for GAO glycogen consumption at different salinities (black circle); b GAO: ratio of total glycogen consumption/HAc uptake at different salinity concentrations (black circle), ratio of glycogen consumption/HAc uptake corrected for maintenance glycogen consumption (white circle). Continuous bold lines show the results of model simulations
GAO stoichiometry
Through the execution of a series of batch tests with an enriched culture of GAO, the effects of salinity on the GAO stoichiometry were assessed. At higher salinity concentrations, the total glycogen consumption/HAc uptake ratio increased significantly (Fig. 5b). When the stoichiometry was corrected for the maintenance activity (determined under the absence of acetate), arguably, the glycogen consumption/HAc uptake ratio was insensitive to salinity.
Modeling PAO and GAO stoichiometry
The experimental values of the anaerobic P-release/HAc uptake stoichiometry of PAO and gly/HAc uptake stoichiometry of GAO were predicted (Fig. 5) with Eqs. 6 and 7 for PAO and Eqs. 8 and 9 for GAO developed in Online resource 3, using the same parameters as described in Tables 1 and 2 and experimentally determined fixed stoichiometric value of Eq. 9 (f HAcΔgly/ΔHAc,GAO ). However, the Eqs. 6 and 7 were not suitable to describe the PAO stoichiometry above 1 % (W/V). The main reason is that Eq. 7 cannot describe the P-release rate accurately above 1 % salinity, when P-release is almost fully inhibited. The fixed f HAcΔgly/ΔHAc,GAOc was determined as the average of the corrected glycogen/HAc uptake values observed at different salinities with a value of 0.77.
Discussion
Conversions of PAO and GAO enrichments
The activities of the enriched PAO and GAO cultures at fresh water conditions were compared against those from the literature. Concerning the activity of PAO (Table 3), the m anATP,PAO _ poly ‐ P , determined from the P-release profiles at 0 % salinity are lower than those reported by Smolders et al. (1994a) and Brdjanovic et al. (1997). In the original experimental design of this study, P-release was the only mechanism considered for energy production during anaerobic starvation. However, in the two additional tests at 1 and 4 % salinity, PAO were capable of using glycogen under anaerobic starvation conditions for maintenance purposes, which was also observed in previous studies by Lopez et al. (2006) and Lu et al. (2007). Potentially, in this study at 0 % salinity, glycogen was also used as another source of energy during anaerobic starvation. Meanwhile, the acetate uptake rates observed under fresh water conditions (Table 3) are in the range of those reported in previous studies (Lopez-Vazquez et al. 2007; Brdjanovic et al. 1997; Smolders et al. 1994a).
In the case of GAO (Table 4), the maximum acetate uptake rate of GAO obtained at 0 % salinity was close to the rates reported by other authors. However, the anaerobic maintenance requirements at 0 % salinity determined in this study were higher than those reported previously (Zeng et al. 2003; Filipe et al. 2001; Lopez-Vazquez et al. 2007). Considering that the enrichment conditions were similar in all the studies compared in Table 4, the reasons for the different maintenance requirements cannot be easily explained. As a consequence of the high anaerobic maintenance activity, the corrected glycogen/HAc stoichiometric value (corrected by excluding the glycogen consumption due to maintenance) observed in this study at fresh water conditions was lower when compared to values from previous studies (Zeng et al. 2003; Lopez-Vazquez et al. 2007; Liu et al. 1994), whereas it is similar to that observed by Filipe et al. (2001). Assuming a theoretical scenario in which the energy demand for HAc transport is zero, the energy demand for the biochemical conversion of acetate into PHA still requires about 1 C-mol glycogen/C-mol HAc or 0.88 C-mol Gly/C-mol HAc, depending on whether the model developed by Zeng et al. (2002) or Filipe et al. (2001) is used. However, the value observed in this study (0.67 C-mol Gly/C-mol HAc) is slightly below these theoretical values, indicating that the glycogen consumption estimated for maintenance requirements might be different during HAc uptake.
Effects of salinity on the kinetics of PAO and GAO
Both PAO and GAO are sensitive to short-term salinity exposure, PAO being comparatively more sensitive. The m anATP,PAO _ poly ‐ P and m anATP,GAO maintenance activities increased significantly when the salinity increased up to a threshold salinity concentration of 2.5 and 4 % for PAO and GAO, respectively. Above this concentration, the GAO m anATP,GAO and PAO m anATP,PAO _ poly ‐ P decreased while the m anATP,PAO _ gly for PAO continued to increase above 2.5 % salinity. Possibly, with the increasing salinity, certain salt ions leak through the cell membrane and the organisms needed additional energy to pump out the ions at the expense of energy (Castle et al. 1986), which could explain the increasing maintenance activity at higher salinity. The observed decrease in PAO m anATP,PAO _ poly ‐ P maintenance activity above the threshold concentration (2.5 % salinity) could be explained by the possible inhibition of the P-release pathway, forcing a shift in the metabolism from poly-P consumption to glycogen consumption, which can be supported by the additional test performed at 4 % salinity (Fig. 3). The non-linear glycogen consumption profiles at high salinity for both PAO and GAO and the decrease in the m anATP,GAO (above 4 % salinity) indicate that above certain threshold concentration the m anATP,GAO activity and m anATP,PAO _ gly were progressively inhibited. An explanation for this observation could be that at the threshold concentration, the organisms are not able to pump out the ions at the required rate to keep a zero salinity concentration within the intracellular environment. This phenomenon could be caused by the inhibition of the membrane proteins that are responsible for the generation of a proton motive force to pump out the ions or because the maximum rate to expel out the ions reaches a limit. Above this threshold concentration, possibly the ions will start to accumulate in the intracellular environment, affecting all metabolic activities, followed by a decrease in all metabolic rates as a consequence of the high intracellular salt concentration as observed in these tests (Fig. 3).
Concerning the salinity effects on the maximum acetate uptake of PAO and GAO, the salinity concentrations at which 50 % inhibition of the HAc uptake rates took place were 0.6 and 1.2 % salinity for PAO and GAO, respectively. When the salinity increased to 1 %, PAO showed a 71 % decrease in HAc uptake, whereas GAO showed only a 41 % decrease. Likely, the reduction in activity below 2 or 3 % salinity was mostly caused by the inhibition of the acetate uptake enzymes or, in the case of PAO, also due to the inhibition of the P-release enzymes. The decay or lyses of the cells could be discarded because the inhibition observed in the maintenance profiles occurred at higher concentrations.
The observation that the effective P-release rate is nearly zero at 2 % salinity, while HAc uptake continues, suggests that a switch in the metabolism occurs from a poly-P dependent HAc uptake (PAM) to a HAc uptake process that depends on glycogen as sole energy source (GAM). Once the salinity concentration is above the maximum threshold concentration (as reported in this study for the maintenance tests), salts are expected to accumulate in the intracellular environment, inhibiting all the intracellular metabolic activities, including all possible pathways for HAc uptake.
Effect of salinity on the stoichiometry of PAO
Between 0 and 2 % salinity, a significant decrease (45 %) in the corrected P-release/HAc uptake ratio was observed, which was accompanied by an increase in the total gly/HAc ratio and, at least at 1 % salinity, an increase in the corrected glycogen/HAc ratio. The corrected glycogen/HAc at 1 % salinity was 1.46 C-mol/C-mol, which was significantly higher than the uncorrected glycogen/HAc ratios between 0 and 0.5 % salinity (Table 3). As previously suggested, the increase in the corrected glycogen/HAc ratio at 1 % salinity and decrease in the corrected P/HAc ratio suggests that a shift from a PAM to a GAM occurred. Welles et al. (2014, submitted), Zhou et al. (2008), and Acevedo et al. (2012) demonstrated that PAO are able to switch from a PAM to a GAM when internal poly-P reserves become limited. Thus, the P-release could be completely substituted by glycogen consumption.
Effect of salinity on the stoichiometry of GAO
Although the total glycogen/HAc stoichiometry increased at higher salinity, the corrected glycogen/HAc stoichiometry, obtained after subtraction of the maintenance requirements, show that the stoichiometry for HAc uptake was not affected by salinity in case of GAO.
Modeling the kinetics and stoichiometry of PAO and GAO
The anaerobic maintenance energy requirements and kinetics of PAO and GAO were successfully described by the proposed model equations. This supports some of the mechanisms proposed in this study, such as the shift in the PAO metabolism from a PAM to a GAM for the uptake of HAc. Although the model could not describe the PAO stoichiometry above 1 % salinity, the GAO stoichiometry model described the GAO stoichiometry up to 2 %. The proposed model may be used to predict shock-load effects of salt in real wastewater treatment plants, but for such applications, the model should be validated using activated sludge from full scale wastewater treatment plants.
Possible effects of salinity on the PAO–GAO competition
When comparing the anaerobic maintenance requirements of PAO and GAO, the impact of salt on their metabolisms followed a similar trend, indicating that both organisms were affected in a similar way. Considering the effect of salinity on the acetate uptake rates of PAO and GAO, the effects on PAO were much more pronounced. Between 0 and 0.6 % salinity, PAO showed higher acetate uptake rates under the same operational conditions (pH = 7, T = 20 ºC), whereas GAO showed higher uptake rates above 0.6 % salinity. In theory, the organism with the fastest acetate uptake rate will have a competitive kinetic advantage in the system. Therefore, these results suggest that during short-term exposure to relatively low salinity levels in the range of 0 to 0.6 %, PAO may prevail in the system leading to successful EBPR. On the other hand, if the salinity concentrations are above 0.6 % salinity, GAO will likely prevail, leading to deterioration of the EBPR. These observations support the statement that it is feasible to remove phosphorus by biological means when treating saline wastewater with salinity concentrations in a range between 0 and 0.6 % (as NaCl).
In order to better understand the effect of salinity on the PAO and GAO cultures at short-term exposure, aerobic batch tests need to be conducted as well. If the aerobic metabolism suffers from serious deleterious effects, the aerobic phase will also play an important role in the occurrence of PAO and GAO as well as on their competition. Ultimately, the most affected part of the metabolism of these organisms (between the anaerobic or aerobic one) will determine the magnitude of their occurrence in the system and the reliability and success of EBPR when treating saline wastewaters. Finally, the assessment of the long-term (months) effects of saline wastewater (NaCl based) on the microbial community competition and physiology of enriched PAO and GAO cultures is necessary to understand the potential selection or adaptation of salt-tolerant strains and to achieve conclusive observations regarding the feasibility to biologically remove phosphorus from saline wastewaters.
References
Acevedo B, Oehmen A, Carvalho G, Seco A, Borras L, Barat R (2012) Metabolic shift of polyphosphate-accumulating organisms with different levels of poly-phosphate storage. Water Res 46:1889–1900
Amann RI (1995) In situ identification of microorganisms by whole cell hybridization with rRNA-targeted nucleic acid probes. In: Akkermans ADL, van Elsas JD, de Bruijn FJ (eds) Molecular microbial ecology manual. Kluwer Academic Publisher, London, pp 1–15
APHA (1995) Standard Methods for the Examination of Water and Wastewater, 19th edn. American Public Health Association, Washington, DC
Brdjanovic D, van Loosdrecht MCM, Hooijmans CM, Alaerts GJ, Heijnen JJ (1997) Temperature effects on physiology of biological phosphorus removal. J Environ Eng-ASCE 123(2):144–154
Castle AM, Macnab RM, Shulman RG (1986) Coupling between the sodium and proton gradients in respiring escherichia coli cells measured by 23Na and 31P nuclear magnetic resonance. J Biol Chem 261(17):7797–7806
Crocetti GR, Hugenholtz P, Bond PL, Schuler A, Keller J, Jenkins D, Blackall LL (2000) Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. Appl Environ Microbiol 66:1175–1182
Crocetti GR, Banfield JF, Keller J, Bond PL, Blackall LL (2002) Glycogen accumulating organisms in laboratory-scale and full-scale wastewater treatment processes. Microbiol 148:3353–3364
Cui Y, Peng C, Peng Y, Ye L (2009) Effects of salt on microbial populations and treatment performance in purifying saline sewage using the MUCT process. Clean-Soil Air Water 37(8):649–656
Fahim FA, Fleita DH, Ibrahim AM, El-Dars FMS (2000) Evaluation of some methods for fish canning wastewater treatment. Water Air Soil Pollut 127:205–226
Filipe CDM, Daigger GT, Grady CPL (2001) A metabolic model for acetate uptake under anaerobic conditions by glycogen-accumulating organisms: stoichiometry, kinetics and effect of pH. Biotechnol Bioeng 76(1):17–31
Flowers JJ, He S, Yilmaz S, Noguera DR, McMahon KD (2009) Denitrification capabilities of two biological phosphorus removal sludges dominated by different ‘Candidatus Accumulibacter’ clades. Environ Microbiol Rep 1(6):583–588
Gonzalez JF, Civit EM, Lupin HM (1983) Composition of fish filleting wastewater. Water SA 9(2):49–56
Hong CC, Chan SK, Shim H (2007) Effect of chloride on biological nutrient removal from wastewater. J Appl Sci Environ Sanit 2(3):85–92
Intrasungkha N, Keller J, Blackall LL (1999) Biological nutrient removal efficiency in treatment of saline wastewater. Water Sci Technol 39(6):183–190
Kargi F, Uygur A (2005) Improved nutrient removal from saline wastewater in an SBR by Halobacter supplemented activated sludge. Environ Eng Sci 22(2):170–176
Lefebvre O, Moletta R (2006) Treatment of organic pollution in industrial saline wastewater: a literature review. Water Res 40:3671–3682
Leung RWK, Li DCH, Yu WK, Chui HK, Lee TO, Van Loosdrecht MCM, Chen GH (2012) Integration of seawater and grey water reuse to maximize alternative water resource for coastal areas: the case of the Hong Kong International Airport. Water Sci Technol 65(3):410–417
Liu WT, Mino T, Nakamura K, Matsuo T (1994) Role of glycogen in acetate uptake and polyhydroxyalkanoate synthesis in anaerobic-aerobic activated sludge with a minimized polyphosphate content. J Ferment Bioeng 77(5):535–540
Liu WT, Nakamura K, Matsuo T, Mino T (1997) Internal energy-based competition between poly-phosphate- and glycogen-accumulating bacteria in biological phosphorus removal reactors-effect of P/C feeding ratio. Water Res 31(6):1430–1438
Lopez C, Pons MN, Morgenroth E (2006) Endogenous processes during long-term starvation in activated sludge performing enhanced biological phosphorus removal. Water Res 40(8):1519–1530
Lopez-Vazquez CM, Song YI, Hooijmans CM, Brdjanovic D, Moussa MS, Gijzen HJ, van Loosdrecht MCM (2007) Short-term temperature effect on the anaerobic metabolism of glycogen accumulating organisms. Biotechnol Bioeng 97(3):483–495
Lu H, Keller J, Yuan Z (2007) Endogenous metabolism of Candidatus Accumulibacter phosphatis under various starvation conditions. Water Res 41(20):4646–4656
Mesple F, Troussellier M, Casellas C, Legendre P (1996) Evaluation of simple statistical criteria to qualify a simulation. Ecol Model 88(1–3):9–18
Orhon D, Tasli R, Sozen S (1999) Experimental basis of activated sludge treatment for industrial wastewaters—the state of the art. Water Sci Technol 40(1):1–11
Panswad T, Anan C (1999) Impact of high chloride wastewater on an anaerobic/anoxic/aerobic process with and without inoculation of chloride acclimated seeds. Water Res 33(5):1165–1172
Smolders GJF, Van der Meij J, Van Loosdrecht MCM, Heijnen JJ (1994a) Model of the anaerobic metabolism of the biological phosphorus removal process: stoichiometry and pH influence. Biotechnol Bioeng 43:461–470
Smolders GJF, van der Meij J, Van Loosdrecht MCM, Heijnen JJ (1994b) Stoichiometric model of the aerobic biological phosphorus removal process. Biotechnol Bioeng 44:837–848
Tang SL, Yue DPT, Li XZ (2006) Comparison of engineering costs of raw freshwater, reclaimed water and seawater for toilet flushing in Hong Kong. Water Environ J 20(4):240–247
Uygur A (2006) Specific nutrient removal rates in saline wastewater treatment using sequencing batch reactor. Process Biochem 41(1):61–66
Uygur A, Kargi F (2004) Salt inhibition on biological nutrient removal from saline wastewater in a sequencing batch reactor. Enzym Microb Technol 34:313–318
WSD (2009) Annual report 2008/2009. Water Supply Department, Hong Kong SAR Government
Wu G, Guan Y, Zhan X (2008) Effect of salinity on the activity, settling and microbial community of activated sludge in a sequencing batch reactors treating synthetic saline wastewater. Water Sci Technol 58(2):351–358
Zeng RJ, Yuan ZG, Van Loosdrecht MCM, Keller J (2002) Proposed modifications to metabolic model for glycogen-accumulating organisms under anaerobic conditions. Biotechnol Bioeng 80(3):277–279
Zeng RJ, van Loosdrecht MCM, Yuan Z, Keller J (2003) Metabolic model for glycogen-accumulating organisms in anaerobic/aerobic activated sludge systems. Biotechnol Bioeng 81(1):92–105
Zhou Y, Pijuan M, Zeng RJ, Lu H, Yuan Z (2008) Could polyphosphate-accumulating organisms (PAO) be glyccogen-accumulating organisms (GAO)? Water Res 42:2361–2368
Acknowledgments
This research study was carried out as part of the SALINE project (http://www.salinesanitation.info) led by UNESCO-IHE Institute for Water Education and consortium partners KWR Watercycle Research Institute, Delft University of Technology, University of Cape Town, The Hong Kong University of Science and Technology, The Higher Polytechnic Institute “José Antonio Echeverría,” and Birzeit University. The SALINE project is financed by UNESCO-IHE internal research fund with a special generous contribution from Professor George Ekama from University of Cape Town, to whom the authors would like to gratefully thank. Special thanks to UNESCO-IHE laboratory staff, in particular to Don van Galen, for all their support during the research project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 60 kb)
Rights and permissions
About this article
Cite this article
Welles, L., Lopez-Vazquez, C.M., Hooijmans, C.M. et al. Impact of salinity on the anaerobic metabolism of phosphate-accumulating organisms (PAO) and glycogen-accumulating organisms (GAO). Appl Microbiol Biotechnol 98, 7609–7622 (2014). https://doi.org/10.1007/s00253-014-5778-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5778-4