Abstract
Sulphate-rich wastewaters can be generated due to (i) use of saline water as secondary-quality water for sanitation in urban environments (e.g. toilet flushing), (ii) discharge of industrial effluents, (iii) sea and brackish water infiltration into the sewage and (iv) use of chemicals, which contain sulphate, in drinking water production. In the presence of an electron donor and absence of oxygen or nitrate, sulphate can be reduced to sulphide. Sulphide can inhibit microbial processes in biological wastewater treatment systems. The objective of the present study was to assess the effects of sulphide concentration on the anaerobic and aerobic physiology of polyphosphate-accumulating organisms (PAOs). For this purpose, a PAO culture, dominated by Candidatus Accumulibacter phosphatis clade I (PAO I), was enriched in a sequencing batch reactor (SBR) fed with acetate and propionate. To assess the direct inhibition effects and their reversibility, a series of batch activity tests were conducted during and after the exposure of a PAO I culture to different sulphide concentrations. Sulphide affected each physiological process of PAO I in a different manner. At 189 mg TS-S/L, volatile fatty acid uptake was 55% slower and the phosphate release due to anaerobic maintenance increased from 8 to 18 mg PO4-P/g VSS/h. Up to 8 mg H2S-S/L, the decrease in aerobic phosphorus uptake rate was reversible (Ic60). At higher concentrations of sulphide, potassium (>16 mg H2S-S/L) and phosphate (>36 mg H2S-S/L) were released under aerobic conditions. Ammonia uptake, an indicator of microbial growth, was not observed at any sulphide concentration. This study provides new insights into the potential failure of enhanced biological phosphorus removal sewage plants receiving sulphate- or sulphide-rich wastewaters when sulphide concentrations exceed 8 mg H2S-S/L, as PAO I could be potentially inhibited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To reduce eutrophication in surface water bodies, phosphate needs to be removed from wastewater by biological or chemical means in wastewater treatment plants (Yeoman et al. 1988; Henze et al. 2008). The biological removal of phosphate is carried out by microorganisms broadly known as polyphosphate-accumulating organisms (PAOs) capable of storing phosphate beyond their biomass growth requirements as intracellular polyphosphate (poly-P) (Comeau et al. 1986; Mino et al. 1998). During anaerobic conditions, PAOs store volatile fatty acids (VFA) (e.g. propionate, acetate) as poly-hydroxy-β-alkanoates (PHA), which require additional energy and reduction equivalents. PAOs obtain most of the required energy (ATP) from the hydrolysis of intracellular poly-P, which results in the release of phosphate and cations (e.g. calcium, magnesium, potassium) (Comeau et al. 1986). Conversion of glycogen to PHA provides extra energy (ATP) and reducing equivalents (NADH) needed (Mino et al. 1998).
Thereafter, under aerobic or anoxic conditions (depending on the presence of oxygen, nitrate, and/or nitrite), PAOs consume the stored PHA to replenish their poly-P and glycogen storage pools, to grow and for maintenance purposes (Comeau et al. 1986; Wentzel et al. 1986). Thus, in order to support the development of PAOs and achieve enhanced biological phosphorus removal (EBPR) in a wastewater treatment plant (WWTP), mixed liquor activated sludge should be cycled through alternating anaerobic and aerobic/anoxic conditions and the influent should be directed to the anaerobic stage (Henze et al. 2008).
Sulphate-rich wastewaters (containing up to 500 mg SO4 2−/L) can be generated due to (i) discharge of sulphate into the WWTP by industrial effluents (Sears et al. 2004), (ii) use of sulphate-based chemicals in drinking water production (e.g. aluminium sulphate) (Bratby 2016), (iii) seawater and/or groundwater (rich in sulphate) intrusion (van den Brand et al. 2014), and (iv) use of seawater as secondary-quality water (e.g. cooling, toilet flushing) (Lee and Yu 1997). During sewage conveyance and in the anaerobic stages of a wastewater treatment (e.g. anaerobic sewerage and/or reactors), sulphate could be reduced to sulphide (H2S/HS−) and inhibit different organisms (Comeau et al. 1986; Koster et al. 1986). Sulphide might cause microbial inhibition due to either direct inhibition of the unionized form of sulphide (dihydrogen sulphide, H2S, which is able to pass through the cell membrane and reduce the intracellular pH) (Comeau et al. 1986; Koster et al. 1986) or precipitation of key micro-nutrients with sulphide (like copper, cobalt or iron) decreasing their bioavailability to cover the microbial metabolic requirements (Bejarano Ortiz et al. 2013; Zhou et al. 2014).
The sulphide inhibition effects on certain microorganisms have been already assessed. Chen et al. (2008), working with an anaerobic suspended sludge bioreactor, reported 50% methanogenic inhibition at sulphide concentrations between 50 and 125 mg H2S-S/L, whereas Koster et al. (1986) observed 50% inhibition at 250 mg H2S-S/L in an anaerobic granular sludge fed with acetate. Jin et al. (2013) reported that 32 mg H2S-S/L caused a 50% decrease in Anammox activity; meanwhile, Bejarano Ortiz et al. (2013) observed that 2.6 ± 0.3 and 1.2 ± 0.2 mg H2S-S/L caused 50% inhibition of the ammonia and nitrite oxidation activities in nitrifying cultures, respectively. These observations suggest that the aerobic or anoxic metabolic activities appear to be more sensitive to the presence of sulphide than the anaerobic one.
So far, only a few studies have focused on the effects of sulphide on the anaerobic metabolism of PAOs. Comeau et al. (1986) observed that the addition of sulphide under anaerobic conditions led to an increased phosphate release, suggesting that phosphate was released to re-establish the intracellular pH after the disassociation of sulphide inside the cell. Similarly, Saad et al. (2013) reported that the anaerobic acetate uptake rate of PAOs decreased around 50% at 60 mg H2S-S/L and observed 55% higher anaerobic P release, potentially associated with a detoxification process. However, no studies have reported the effects of sulphide on the aerobic metabolism. Furthermore, it is not clear whether and to what extent the effects of sulphide on the metabolism of PAOs are reversible.
Thus, the main objective of the present research was to study the short-term effects of sulphide on the anaerobic/aerobic physiology of an enriched PAO culture (dominated by Candidatus Accumulibacter phosphatis clade I, hereafter PAO I). To assess the direct inhibition effects, batch tests were performed at different sulphide concentrations ranging from 48 to 189 mg TS-S/L (H2S + HS−) added at the start of the anaerobic stage. Once sulphide was depleted, in order to assess the reversibility of the inhibition effects, additional batch tests were performed with the same sludge immediately after the direct exposure tests. The findings will improve our understanding regarding the inhibiting effects of sulphide on PAOs and serve to develop strategies to overcome their deleterious effects on EBPR systems.
Materials and methods
Reactor operation
The biomass was enriched in a 3.0-L double-jacket Applikon reactor with a working volume of 2.5 L (Delft, The Netherlands). Five hundred millilitres of activated sludge from WWTP Nieuwe Waterweg (Hoek van Holland, The Netherlands) was used as inoculum. The reactor was operated in cycles of 6 h (2 h 15 min anaerobic phase, 2 h 15 min aerobic phase, 1 h settling time and 30 min effluent removal). At the start of each cycle, 1.25 L of synthetic media was fed to the reactor (5 min feeding), resulting in a hydraulic retention time (HRT) of 12 h. Through the wastage of 78 mL of mixed liquor at the end of each aerobic phase, the solids retention time (SRT) was controlled at 8 days. The pH was kept at 7.6 ± 0.1 through the addition of 0.1 M HCl and 0.4 M NaOH. The dissolved oxygen (DO) concentration was maintained at around 20% of the saturation concentration through the automatic supply of compressed air or nitrogen gas. Temperature was externally controlled at 20 ± 1 °C. The orthophosphate, VFA, mixed liquor suspended solid (MLSS), and mixed liquor volatile suspended solid (MLVSS) concentrations were measured twice per week at the start and end of each phase (anaerobic/aerobic). When no significant changes in these parameters were observed for at least three SRT, it was assumed that the system was under pseudo steady-state conditions.
Synthetic media
The media was concentrated ten times and separated in two bottles (carbon source and mineral solution). After dilution, the synthetic media fed to the reactor contained per litre 637 mg NaAc·3H2O (295 mg COD/L), 66.7 μL propionic acid (100 mg COD/L), 107 mg NH4Cl, 111 mg NaH2PO4·H2O (25 mg PO4-P/L), 90 mg MgSO4·7H2O, 14 mg CaCl2·2H2O, 36 mg KCl, 1 mg yeast extract, 20 mg N-allylthiourea (ATU) and 300 μL of trace element solution prepared according to Smolders et al. (1994a, b).
Batch activity tests
Batch tests were performed by duplicate in two jacketed reactors, each one with a working volume of 400 mL. When the biomass performance in the parent sequencing batch reactor (SBR) was under steady-state conditions, 200 mL of sludge was transferred from the parent SBR to each batch reactor. Prior to the execution of each batch test, the media was sparged with nitrogen gas and adjusted to pH 7.6. The length of each batch test was composed of 75 min anaerobic and 125 min aerobic conditions. To ensure anaerobic conditions, nitrogen gas was sparged at the bottom of the batch reactors during feeding and thereafter to their headspace during the rest of the anaerobic phase. In the aerobic stage, compressed air was sparged from the bottom. Both gases were controlled at 10 L/h. The pH was kept at 7.6 ± 0.1 through the automatic addition of HCl and NaOH. As described elsewhere (Lopez-Vazquez et al. 2008), the oxygen consumption rates (OUR) were determined in 2–3-min time intervals at DO concentrations higher than 2 mg/L in a separate 10-mL unit equipped with an OXi 340i DO probe (WTW, Germany).
Direct inhibition H2S batch tests
Direct inhibition batch tests were performed at different initial total sulphide (H2S + HS−) concentrations added at the start of the anaerobic phase. Sulphide concentrations ranged between 48 and 189 mg TS-S/L. Anaerobic synthetic media was diluted two times to keep an organic load to biomass ratio (F/M) similar to that in the parent reactor and the pH adjusted to 7.6. Before the addition of the media to the batch reactor, either 2.5, 5.0, 7.5 or 10 mL of concentrated sulphide (containing 3.2 g H2S + HS−/L) was added and the final volume adjusted to 200 mL. The pH of the media was adjusted to 7.6 prior to the addition.
Reversible inhibition H2S batch tests
Immediately after the completion of each direct inhibition test (once sulphide was no longer detected), the reversibility tests were conducted in the same reactor and with the same biomass previously exposed to a defined sulphide concentration. Thus, following an approach the same as that in the direct inhibition tests but excluding the addition of sulphide, anaerobic conditions were created through nitrogen gas addition, the synthetic media was added and thereafter the sequential anaerobic-aerobic stages were conducted.
Analyses
The samples were filtered through 0.45-μm-pore-size filters. Fifty microlitres of butyric acid as internal standard was added to the samples of acetate (HAc) and propionate (HPr) and stored in 1-mL sampling bottles. Iron (Fe2+, Fe+3), potassium (K+), magnesium (Mg2+) and calcium (Ca+) were stored in 0.5% nitric acid solution. Orthophosphate (PO4 3−-P), ammonia (NH4 +-N), acetate (HAc) and propionate (HPr) were analysed within 2 h after each batch test. Total sulphide (H2S + HS−) was measured immediately after sampling. Orthophosphate (PO4 3−-P), total sulphide (H2S + HS−), total suspended solids (TSS) and volatile suspended solids (VSS) were analysed as described in APHA et al. (2005). Ammonia (NH4 +-N) was measured according to NEN 6472 (1983). Acetate (HAc) and propionate (HPr) were measured using a Varian 430-GC gas chromatograph (GC) equipped with a split injector (200 °C) and a WCOT Fused Silica column (105 °C) and coupled to a FID detector (300 °C). Helium gas was used as carrier gas. Iron (Fe2+, Fe+3), potassium (K+), magnesium (Mg2+) and calcium (Ca+) were measured in an inductively coupled plasma mass spectroscopy (Thermo Scientific in Bremen, Germany).
Fluorescence in situ hybridization
To identify the dominant microbial communities in the sludge, fluorescence in situ hybridization (FISH) analyses were performed according to Amman (1995). To target all bacteria, the EUB MIX probe (mixture of EUB 338, EUB338 II and EUB 338 III probes) was applied (Amman 1995). PAOs were targeted with the PAOMIX probe (composed of probes PAO 462, PAO 651 and PAO846) (Crocetti et al. 2000). The presence of PAO clade I and clade II was estimated through the addition of probes Acc-1-444 (1A) and Acc-2-444 (2A, 2C, 2D) (Flowers et al. 2009). Candidatus Competibacter phosphatis was targeted with the GB probe (Kong et al. 2002). Defluvicoccus clusters 1 and 2 were identified with the TFO-DF215, TFO-DF618, DF988 and DF1020 probes (Wong et al. 2004; Meyer et al. 2006). Vectashield containing a DAPI concentration was used to amplify the fluorescence signal and stain all living organisms (Nielsen et al. 2009).
An estimation of the relative biomass fractions of the organisms of interest was performed by analysing 25 random FISH image fields taken with an Olympus BX5i microscope and analysed with the software Olympus Cell Dimensions 1.5 (Hamburg, Germany). The relative abundance of the organisms was estimated by expressing the relative surface area, which stained positive with the specific probes, with regard to the total surface area stained positive with DAPI (Flowers et al. 2009). The standard error of the mean was calculated as described elsewhere.
Stoichiometric and kinetic parameters of interest
The net P released per VFA consumption ratio (P-mmol/C-mmol) was calculated based on the total P released observed at the end of the anaerobic phase and the total VFA consumed. The ratios mmol-K+/P-mmol, mmol-Mg2+/P-mmol and mmol-Ca+/P-mmol were calculated based on their net differences at the start and end of the anaerobic phase (anaerobic ratios) and at the start and end of the aerobic phase (aerobic ratios). The poly-P content in the biomass was not measured but estimated based on the ash content, as described by Welles et al. (2015).
All kinetic rates were calculated by linear regression and expressed as milligrams compound per gram of volatile suspended solids (VSS) per hour (mg/(g VSS h)), as described in Smolders et al. (1995). The anaerobic rates of interest were:
-
(i)
\( {q}_{VFA}^{\max } \), maximum specific VFA consumption rate
-
(ii)
\( {q}_{PO_4,\mathrm{AN}}^{\max } \), maximum specific total phosphate release rate
-
(iii)
\( {q}_{PO_4,VFA} \), maximum specific phosphate release rate for VFA uptake, estimated as
-
(iv)
\( {m}_{PO_4,\mathrm{AN}} \), specific anaerobic phosphate release rate due to maintenance purposes (after VFA consumption)
-
(v)
\( {m}_{I{,PO}_4} \), specific anaerobic phosphate release rate for maintenance or detoxification purposes (after VFA consumption) associated with the presence of sulphide, estimated as the increase in the phosphate release rate between the \( {m}_{PO_4,\mathrm{AN}} \) observed in the control test (at 0 mg H2S-S/L) and in the different (direct and reversibility inhibition) tests exposed to sulphide (48 and 89 mg TS-S/L)
Similarly, the aerobic rates of interest were:
-
(i)
\( {q}_{PO_4,\mathrm{Ox}}^{Ini} \), maximum specific initial phosphate uptake rate, determined based on the phosphate uptake rate observed during the presence of sulphide (in the direct inhibition tests) or within the first 60 min of the aerobic phase (in the reversibility tests)
-
(ii)
\( {q}_{PO_4,\mathrm{Ox}}^{Res} \), residual phosphate uptake rate, estimated based on the phosphate uptake rates measured once sulphide was not detected in the aerobic phase
-
(iii)
\( {q}_{NH_x,\mathrm{Ox}} \), specific ammonia uptake rate
-
(iv)
\( {m}_{{\mathrm{O}}_2,\mathrm{Ox}} \), oxygen uptake rate measured at the end of the aerobic phase (associated with maintenance purposes) once phosphate uptake stopped and sulphide was not detected
Inhibitory sulphide expressions
Mathematical expressions were developed to describe the direct inhibition effects of total sulphide on the anaerobic and aerobic phosphate profiles of PAOs. Sulphide was modelled as total sulphide (HS− + H2S). The results from the direct inhibition tests, showing the effect of sulphide on the physiology of PAO I, were used to calibrate the model. Based on the phosphate profiles, three mathematical expressions were proposed and added to the model developed by Lopez-Vazquez et al. (2009). The anaerobic phosphate release rate due to detoxification caused by sulphide (\( {m}_{I{,PO}_4} \)) was described with the following expression (Eq. 2):
where
\( {m}_{I{,PO}_4}^s \)corresponds to the increase in the anaerobic P release rate (presumably associated with the detoxification process) caused by sulphide, in milligrams PO4-P/litre.
\( {m}_{I{,PO}_4}^{\max } \)is the maximum increase in the anaerobic P release rate caused by sulphide, in milligrams PO4-P/gram VSS/hour.
\( {K}_{{\mathrm{H}}_x\mathrm{S},\mathrm{AN}} \)is the half-saturation inhibition constant affecting the increased P release rate associated with the presence of sulphide, in milligrams S/litre.
\( {S}_{{\mathrm{H}}_x\mathrm{S}} \)is the sulphide concentration in the liquid phase, in milligrams S/litre.
X PAOis the fraction of PAO biomass, in grams VSS.
For the description of the aerobic physiology, two different phases were considered: (i) the first one under the presence of sulphide, hereafter identified as directly inhibited activity, and (ii) the second one once sulphide was no longer detected, henceforward referred to as residual activity. To describe the directly inhibited activity, the aerobic P uptake rate was affected by the inclusion of an inhibition expression (Eq. 3). For the description of the residual activity, which takes into consideration that the inhibition may be partially reversible, an empirical inhibiting expression for the aerobic P uptake rate was proposed (Eq. 4).
where
K I , Oxis the half-saturation inhibition constant of sulphide on the aerobic metabolism of PAOs, in milligrams S/litre.
\( {S}_{{\mathrm{H}}_x\mathrm{S}}^{ref} \)is a reference sulphide concentration at which an irreversible inhibition of the aerobic phosphate uptake rate can occur, in milligrams S/litre.
\( {S}_{{\mathrm{H}}_x\mathrm{S}}^{Ini} \)is the initial sulphide concentration at the start of the aerobic phase, in milligrams S/litre.
Aquasim was used to estimate the kinetic parameters used in the proposed expressions and to simulate the phosphate concentration profiles in the different experiments (Reichert 1998). The percent error was calculated through the normalized root mean square deviation (NRMSD), as described by Oehmen et al. (2010).
Results
Sludge performance and microbial community in the parent reactor
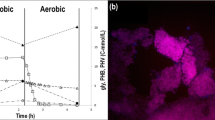
Prior to the execution of the batch inhibition tests, the parent reactor was operated for more than 200 days, showing a stable pseudo steady-state performance with a VSS/TSS ratio of 0.59 g VSS/g TSS and complete phosphate removal at the end of the aerobic phase (Fig. 1, i). All VFA was consumed within 10 min of the anaerobic phase at 534 mg COD/g VSS/h, releasing phosphate at a rate of 377 mg PO4-P/g VSS/h and reaching a P to VFA ratio of 0.76 P-mmol/C-mmol. In the presence of oxygen, phosphate was taken up at an initial rate of 57.9 mg PO4-P/g VSS/h, consuming 0.54 O2-mmol/P-mmol taken up. FISH analyses were performed on day 217, showing that the biomass was composed of 97 ± 4% Candidatus Accumulibacter phosphatis (with regard to DAPI-stained biomass), from which around 99% belonged to Candidatus Accumulibacter phosphatis clade I (Fig. 1, ii).
Sludge characterization in the parent SBR displaying i the present profiles of carbon (blue circles), phosphate (orange triangles) and ammonia (as NH4-N) (yellow diamonds) observed in a typical operating cycle under steady-state conditions and ii microbial identification by fluorescence in situ hybridization (FISH) showing the microbial community in the sludge (the bar indicates 20 μm): A all living organisms (DAPI) in green, B GAO (Cy5) in blue, C PAOs (Cy3) in red and D PAO I (Fluos) in yellow
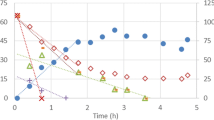
A control test was performed under the same conditions as the direct inhibition test (without the addition of sulphide). In the control test, all VFA (measured as COD) were consumed within 15 min, at a rate of 510 mg COD/g VSS/h. During the anaerobic stage, 76 mg PO4-P/L was released at a rate of 399 mg PO4-P/g VSS/h (\( {q}_{PO_4,\mathrm{AN}}^{\max } \)). The stoichiometric P to VFA ratio was 0.78 P-mmol/C-mmol. In the aerobic stage, phosphate was taken up with a maximum specific P uptake rate of 68 mg PO4-P/g VSS/h (\( {q}_{PO_4,\mathrm{Ox}}^{Ini} \)), consuming 0.55 O2-mmol per P-mmol taken up (Fig. 2). The anaerobic/aerobic stoichiometry and kinetics of the control test were in the same range as those observed in the cycle of the parent reactor.
Direct sulphide inhibition effects on the anaerobic and aerobic metabolism of PAO I
Under anaerobic conditions, the sulphide concentrations added in the beginning of each test were rather stable. Figure 3 shows the phosphate, VFA (as COD), ammonia, and sulphide profiles during and after a short-term exposure to the different concentrations of sulphide assessed that increased from 0 to 42 mg TS-S/L (8 mg H2S-S/L), 86 mg TS-S/L (16 mg H2S-S/L), 115 mg TS-S/L (22 mg H2S-S/L) and 189 mg TS-S/L (36 mg H2S-S/L). In the different anaerobic stages studied, the VFA uptake rates decreased progressively (Fig. 4) from 510 to 227 mg COD/g VSS/h as the sulphide concentration increased. Nevertheless, in all cases, VFA was taken up within the length of the anaerobic phases. The maximum phosphate release rate (\( {q}_{PO_4,\mathrm{AN}}^{\max } \)) showed a similar trend decreasing from 399 mg PO4-P/g VSS/h to 119 mg PO4-P/g VSS/h. On the contrary, the anaerobic maintenance P release rates (\( {m}_{PO_4,\mathrm{AN}} \)) increased from 8 to 18 mg PO4-P/g VSS/h as the sulphide concentration reached 189 mg TS-S/L (36 mg H2S-S/L).
Phosphate (PO4-P), VFA (as COD), ammonia (as NH4-N) and total sulphide (H2S + HS− as S) profiles during the direct inhibition and subsequent reversibility tests executed at a 42 mg TS-S/L (8 mg H2S-S/L), b 86 mg TS-S/L (16 mg H2S-S/L), c 115 mg TS-S/L (22 mg H2S-S/L) and d 189 mg TS-S/L (36 mg H2S-S/L)
Regardless of the sulphide concentration, in the first 60 min of the aeration phase, sulphide dropped below detection limits (Fig. 3). When sulphide was present, the direct exposure severely affected the initial phosphate uptake rates. At 42 mg TS-S/L, the initial phosphate uptake rate decreased 81% compared to the rate measured at 0 mg TS-S/L (13 versus 68 mg PO4-P/g VSS/h, respectively). It decreased even further, and at 115 mg TS-S/L, no phosphate uptake was observed. Furthermore, at 189 mg TS-S/L, phosphate was not taken up but released at a rate of 12 mg PO4-P/g VSS/h (Fig. 4).
After sulphide was no longer detected (around 60 min), the aerobic P uptake activities resumed (hereafter identified as residual phosphate uptake rate, \( {q}_{PO_4,\mathrm{Ox}}^{Res} \)), reaching 28, 23 and 12 mg PO4-P/g VSS/h at 48, 86 and 115 mg TS-S/L, respectively. Despite these uptake rates, suggesting that the sulphide inhibition effects were reversible, at 189 mg TS-S/L, phosphate was released but at a slower rate (9 mg PO4-P/g VSS/h) than when sulphide was present (Fig. 4).
Interestingly, in all direct inhibition tests, ammonia consumption was not observed. Possibly, due to the chemical oxidation of sulphide, the oxygen uptake rate (OUR) could not be determined when sulphide was present. Only at the end of the tests, when sulphide was not observed, was the residual OUR determined. The OUR measured at 48, 86 and 115 mg TS-S/L were similar (19, 22, 19 mg O2/g VSS/h, respectively) but at 189 mg TS-S/L was considerably lower (8 mg O2/g VSS/h) (Fig. 4), indicating that PAOs suffered a potential lethal effect.
Reversible effect of sulphide on the metabolism of PAO I
In order to assess to which extent the sulphide effects were reversible, fresh substrate (without sulphide) was added to the biomass previously exposed to sulphide. In these reversibility tests, full carbon removal was only observed on the biomass initially exposed to 42 mg TS-S/L (8 mg H2S-S/L) but at a rate 57% lower than that observed in the control test (510 and 221 mg COD/g VSS/h, respectively) (Fig. 4). In the rest of the reversibility tests, acetate leaked into the aerobic phase (Fig. 3) which was reflected in maximum VFA consumption rates that dropped to 112 and 105 mg COD/g VSS/h at 86 and 115 mg TS-S/L. A more pronounced effect by sulphide was observed at the reversibility test performed with 189 mg TS-S/L where almost all propionate and acetate leaked into the aerobic phase (24 and 77 mg COD/L, respectively). The maximum anaerobic phosphate release rate (\( {q}_{PO_4,\mathrm{AN}}^{\max } \)) was also inhibited. It dropped from 399 to 144, 88, 81 and 25 mg PO4-P/g VSS/h as the sulphide concentrations in the direct inhibition test increased (conducted with 0, 42, 86, 115 and 189 mg TS-S/L) (Fig. 4).
Moreover, the initial phosphate uptake rate (\( {q}_{PO_4,\mathrm{Ox}}^{Ini} \)) decreased from 68, 45 and 42 to 11 mg PO4-P/g VSS/h in the reversibility tests with 0, 48, 86 and 115 mg TS-S/L (Fig. 4). Similar to the observations in the aerobic phase of the direct inhibition test of 189 mg TS-S/L, in the reversibility test, phosphate was not taken up but released at a rate of 19 mg PO4-P/g VSS/h.
Since in the reversibility test part of the carbon (VFA) leaked into the aerobic phase, it was not possible to compare the initial OUR. Nevertheless, the \( {m}_{{\mathrm{O}}_2,\mathrm{Ox}} \) measured in the reversibility test of 42, 86 and 115 mg TS-S/L were not considerably different from each other (17, 18 and 17 mg O2/g VSS/h). Furthermore, similar to the findings of the direct inhibition test performed with 189 mg TS-S/L, in the reversibility test, the \( {m}_{{\mathrm{O}}_2,\mathrm{Ox}} \) dropped to 8 mg O2/g VSS/h. Like in the direct inhibition tests, in the reversibility tests, ammonia consumption was not observed.
Ions transport across the cell membrane in the direct and reversible inhibition batch tests
Phosphate is transported over the membrane together with counter ions like potassium, magnesium and calcium (Comeau et al. 1986). To assess if sulphide had an effect on the transport processes associated with P release and uptake, the ratios between the phosphate and counter ion concentrations were assessed (Table 1). In the anaerobic phase, no considerable difference in the ratios of interest was observed. However, the ratios measured in the aerobic phase were more sensitive. At 86 and 115 mg TS-S/L, 0.06 and 0.89 mol-K+/P-mol, respectively, were released instead of stored (Table 1). The aerobic potassium to phosphate ratio was marginally restored in the reversible tests. In addition, above 86 mg TS-S/L, a higher magnesium concentration was taken up together with phosphate (0.40 mol Mg2+/P-mol at 115 mg TS-S/L) but this ratio was fully restored to typical ratios in the reversible tests. In contrast, it was not possible to observe any transport of calcium in either the anaerobic or the aerobic phase of the batch test.
Mathematical description of the sulphide effects on PAOs
The direct inhibition effects of sulphide on the anaerobic/aerobic phosphate profiles were satisfactorily described with the proposed expressions (NRMSD of 0.095 ± 0.006) (Fig. 5). The increase in the net P release was described as an increase in the anaerobic maintenance activity due to sulphide presence. The inhibition constant of sulphide (K I , AN) for the anaerobic P release was 20.7 mg TS-S/L, and the maintenance coefficient (\( {m}_{I{,PO}_4}^{\max } \)) determined was 10 mg PO4-P/g VSS/h (Eq. 2).
To describe the sulphide effects on the aerobic physiology of PAOs, the mathematical expressions considered that the effects were dependant on the actual concentrations of sulphide at the start of the aerobic phase (\( {S}_{{\mathrm{H}}_x\mathrm{S}}^{Ini} \)). In addition, 118 mg TS-S/L was used as the reference concentration at which a complete irreversible inhibition (\( {S}_{{\mathrm{H}}_x\mathrm{S}}^{ref} \) ) occurred (Eq. 4). In these expressions, an aerobic half-saturation inhibition constant (K I , Ox) of 3.3 mg TS-S/L was used.
Discussion
Direct inhibition and reversibility effects of sulphide on the anaerobic metabolism of PAO I
At the direct inhibition test performed at 115 mg TS-S/L (22 mg H2S-S/L), the VFA uptake rate decreased 50% compared to the one observed in the control test. This H2S concentration (which corresponds to about 22 mg H2S-S/L) is lower than the 50% inhibition concentration reported by Saad et al. (2013) of 60 mg H2S-S/L. However, the experiments in this research were carried out at a pH of 7.6; on the contrary, Saad et al. (2013) performed their experiments within a pH range of 6.5 to 7.8. Thus, the difference in the sulphide inhibition observed in the past studies could be because of the pH used, as the external pH affects the transport of acetate and proton motor force (pmf) of PAO (Comeau et al. 1986; Smolders et al. 1994b).
In addition, the increase in the net P released per VFA uptake from 0.78 to 0.91 P-mmol/C-mmol at 0 and 189 mg TS-S/L, respectively, could be related to an increase in the anaerobic maintenance requirements (\( {m}_{PO_4,\mathrm{AN}} \)) (Fig. 4), which is from 8 to 18 mg PO4-P/g VSS/h. These observations are in agreement with Comeau et al. (1986). They observed a similar increase and suggested that sulphide could disassociate inside the cell, reducing the internal pH (affecting the pH gradient of the cell) and increasing the P release to stabilize the pH gradient across the cell membrane.
In the direct inhibition tests performed at 42, 86 and 115 mg TS-S/L, similar inhibition effects were observed on the maximum VFA uptake rate (\( {q}_{VFA}^{\max } \)) and its associated P release rate (\( {q}_{PO_4,VFA} \)) (of 85 and 87%, 58 and 57%, 50 and 54%, at the corresponding sulphide concentrations tested). Thus, this suggests that up to 115 mg TS-S/L, enough ATP (used for maintenance and acetate uptake) can be generated by poly-P hydrolysis. On the contrary, at 189 mg TS-S/L, the maximum VFA uptake rate (\( {q}_{VFA}^{\max } \)) and its associated P release rate (\( {q}_{PO_4,VFA} \)) were 66 and 75% lower than the rates observed in the control test. Hence, it seems that at 189 mg TS-S/L, either (i) the ATP generated by poly-P hydrolysis is not enough, which could suggest a higher glycogen consumption, or (ii) PAO has a lower ATP demand, which could be associated with a lethal effect of sulphide. Nevertheless, as glycogen was not measured, it is not possible to assess this hypothesis in this study.
The effect of sulphide on the VFA consumption seems to be more severely affected in the following reversible test as even at 86 mg TS-S/L an incomplete VFA consumption was observed. However, during the direct inhibition test, not all the phosphorus previously released was aerobically taken up. Thus, the estimated initial poly-P content in each reversibility test decreased progressively from 0.13 to 0.12, 0.11 and 0.07 mg PO4-P/mg VSS at the tests performed at 42, 86, 115 and 189 mg TS-S/L, respectively. Welles et al. (2015) demonstrated that the carbon uptake rate is affected by the poly-P content, which can also explain the slower carbon uptake rates seen in the reversibility tests.
Sulphide effects on the aerobic metabolism of PAO I
Based on the phosphorus profile, the aerobic metabolism seems to be more drastically affected than the anaerobic metabolism of PAO I (Fig. 3). This agrees with past studies where the effect of different toxic compounds (e.g. free nitrous acid (FNA) or copper) inhibited more severely the aerobic metabolism of PAOs (Wu and Rodgers 2010; Chen et al. 2012; Fang et al. 2012).
The initial P uptake (\( {q}_{PO_4,\mathrm{Ox}}^{Ini} \)) and residual P uptake (\( {q}_{PO_4,\mathrm{Ox}}^{res} \)) rates decreased proportionally to the increase in the sulphide concentrations (Fig. 4). The presence of sulphide could create stress conditions for PAO I, increasing the maintenance requirements and leaving less energy available for poly-P formation. Furthermore, in the direct inhibition test performed at 189 mg TS-S/L, the energy (ATP) provided by the oxidation of PHA seems to have become limiting and the observed aerobic P release was likely a consequence of the aerobic hydrolysis of poly-P for ATP generation. This statement is in agreement with Wu and Rodgers (2010), Zhou et al. (2012) and Welles et al. (2015) who also observed aerobic P release during the presence of FNA, copper or sodium chloride in EBPR cultures.
Furthermore, in the reversibility tests conducted at 48 and 86 mg TS-S/L, the initial phosphorus uptake rate was higher than the residual phosphate uptake rate observed in the direct inhibition tests (45 and 28 mg PO4-P/g VSS/h and 42 and 23 mg PO4-P/g VSS/h, respectively), suggesting that the residual phosphate uptake rate (\( {q}_{PO_4,\mathrm{Ox}}^{res} \)) was only partially reversible up to 86 mg TS-S/L (Fig. 4).
Ammonia uptake, which is usually associated with microbial growth, was not observed in either the direct inhibition or the reversibility test. This observation is in agreement with Pijuan et al. (2010) and Welles et al. (2015) who observed that ammonia uptake was the most inhibited aerobic metabolic process by FNA and salinity, respectively. The lack of iron (which can precipitate with sulphide as FeS) has been suggested to affect the growth of aerobic microorganisms (Isa et al. 1986) and can be considered to be another potential inhibition mechanism. However, in these experiments, iron was always present above the concentration of 0.4 mg Fe/L, which makes it unlikely that the microbial growth (ammonia uptake) was inhibited due to iron limitation. Possibly, the inhibition of ammonia uptake might have been caused by the higher ATP growth requirements compared to the energy needs of other aerobic metabolic processes (e.g. 1.6 ATP/C-mol for growth and 1.26 ATP/P-mol for PO4-P uptake).
Due to the likely oxidation of sulphide in the direct inhibition tests and the VFA leakage in the aerobic stages of the reversibility tests, it was not possible to determine the OUR. Nevertheless, during the last minutes of the aerobic phases (around 2 h), the OUR remained stable and it was assumed to correspond to certain aerobic maintenance or residual OUR (\( {m}_{{\mathrm{O}}_2,\mathrm{Ox}} \)). In Fig. 4, the \( {m}_{{\mathrm{O}}_2,\mathrm{Ox}} \) in the direct inhibition test increased from 13 to 22 mg O2/g VSS/h at 0 and 86 mg TS-S/L, respectively, and thereafter decreased to 8 mg O2/g VSS/h at 189 mg TS-S/L. Welles et al. (2015) observed a similar increasing effect on the aerobic maintenance energy requirements up to 2% salinity followed by a decrease at 3% salinity. Pijuan et al. (2010) determined that the anabolic processes of PAO (such as growth, glycogen replenishment and poly-P storage) were completely inhibited at 6 · 10−3 mg HNO2-N/L and the catabolic processes (maintenance) 40 to 50% inhibited at 2 to 10 · 10−3 mg HNO2-N/L.
Sulphide effects on the transport of cations across the cell membrane
Similar to the transport of phosphate, the aerobic transport of cations (e.g. calcium, magnesium and potassium) across the cell membrane was more affected than the anaerobic transport (Table 1). In agreement with Pattarkine and Randall (1999), it seems that calcium was not used for the transport of phosphate across the cell membrane. Sulphide affected marginally the Mg2+/P ratio at the sulphide concentrations tested. However, in the direct inhibition test, potassium was released above 86 mg TS-S/L. As suggested elsewhere, both potassium and magnesium are essential for P uptake, and the absence of one of them can result in the failure of the EBPR system once poly-P is depleted (Rickard and Mcclintock 1992; Brdjanovic et al. 1996; Pattarkine and Randall 1999; Barat et al. 2005). Nevertheless, since in the reversibility tests potassium was taken up together with phosphate (Table 1), a shock of sulphide (up to 16 mg H2S-S/L) would not likely lead to EBPR failure due to poly-P depletion.
Furthermore, as K+ and Mg2+ are co-transported across the cell membrane together with phosphate, the K release could have implied that phosphate was also released. However, excluding the direct inhibition and reversibility tests performed at 189 mg TS-S/L, aerobic P release was not observed. As suggested by Comeau et al. (1986), potassium and magnesium were likely released to re-establish the pH gradient across the cell membrane, which can be supported by the higher mol-K+/P-mol ratio that increased from 0.29 to 0.36 mol-K+/P-mol in the direct inhibition test performed at 0 and 115 mg TS-S/L.
Limitations and applications of the mathematical description of the effects of sulphide on PAO I
The equations used to model the phosphate profile of these experiments are based on the assumption that sulphide affects the metabolism of PAO I due to diffusion into the cell membrane. The diffusion of sulphide into the cell membrane can cause an increase in the energy requirements, which results in a higher anaerobic maintenance and slower phosphate uptake rate. Hence, the mathematical expression proposed could be used to try to predict the total anaerobic phosphate release and phosphate uptake rate of an enriched culture of PAO I up to 189 mg TS-S/L. For example, the operator of a wastewater treatment plant (WWTP) could measure the concentration of sulphide at the start of the anaerobic and aerobic tanks, and use these mathematical expressions to increase the aerobic retention time or to identify the chemical dose that would need to be added to meet the phosphate effluent standard. Nevertheless, these mathematical equations do not include pH, which affects the speciation of sulphide (HS− + H2S). Moreover, the presence of other microorganisms capable of oxidizing sulphide might reduce the inhibition effect of sulphide on PAO I.
Sulphide effects on full-scale EBPR systems
These experiments focus on the short-term (hours) effects during and after the exposure of PAO to sulphide. Such conditions can occur not only in sewage treatment plants that regularly receive saline wastewater but also in WWTP exposed to the sudden discharge of industrial effluents rich in sulphate or WWTP in coastal zones subject to saline intrusion into the sewer due to tidal events or poor conditions of sewage pipes. Such conditions may lead to process upsets and deterioration if the sulphide concentrations exceed 8 mg H2S-S/L, as potentially the growth of PAO could be inhibited. However, the long-term exposure to sulphide could lead to biomass acclimatization or selection of sulphide-tolerant PAO species or sulphide-oxidizing organisms (Schulz et al. 1999; Brock et al. 2012; Ginestet et al. 2015). These organisms could reduce the deleterious effects of sulphide on EBPR systems. Further research is needed to assess the long-term effects of sulphide on EBPR systems.
References
Amman RI (1995) In situ identification of micro-organisms by whole cell hybridization with rRNA-targeted nucleic acid probes. In: Ackkermans A, van Elsas J, de Bruijn F (eds) Molecular microbial ecology manual. Klower Academy Publications, Dordrecht, Holland
APHA, AWWA, WEF (2005) Standard methods for the examination of water and wastewater, 22th edn. American Water Works Assn
Barat R, Montoya T, Seco A, Ferrer J (2005) The role of potassium, magnesium and calcium in the Enhanced Biological Phosphorus Removal treatment plants. Env tech 26(9):983–992
Bejarano Ortiz DI, Thalasso F, Cuervo López FDM, Texier AC (2013) Inhibitory effect of sulfide on the nitrifying respiratory process. Journal of Chem Tech and Bio 88:1344–1349 (October 2012)
Bratby J (2016) Coagulation and flocculation in water and wastewater treatment, 3rd edn. IWA publishing, London
Brdjanovic D, Hooijmans CM, van Loosdrecht MCM, Alaerts GJ, Heijnen JJ (1996) The dynamic effects of potassium limitation on biological phosphorus removal. WR 30(10):2323–2328
Brock J, Rhiel E, Beutler M, Salman V, Schulz-Vogt HN (2012) Unusual polyphosphate inclusions observed in a marine Beggiatoa strain. Antonie van Leeuwenhoek International Journal of Gen and Molecular Microbiology 101(2):347–357
Chen Y, Chen H, Zheng X, Mu H (2012) The impacts of silver nanoparticles and silver ions on wastewater biological phosphorous removal and the mechanisms. Journal of HM 239-240:88–94
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Biores Technol 99(10):4044–4064
Comeau Y, Hall K, Hancock R, Oldham W (1986) Biochemical model for enhanced biological phosphorus removal. WR. 20(12):1511–1521
Crocetti GR, Hugenholtz P, Bond PL, Schuler A, Keller J, Jenkins D, Blackall LL (2000) Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. AEM 66(3):1175–1182
Fang J, Sun PD, Xu SJ, Luo T, Lou JQ, Han JY, Song YQ (2012) Impact of Cr(VI) on P removal performance in enhanced biological phosphorus removal (EBPR) system based on the anaerobic and aerobic metabolism. BT 121:379–385
Flowers JJ, He S, Yilmaz S, Noguera DR, McMahon KD (2009) Denitrification capabilities of two biological phosphorus removal sludges dominated by different “Candidatus Accumulibacter” clades. EMr 1(6):583–588
Ginestet P, Nicol R, Holst T, Lebossé X, (2015) Evidence for sulfide associated autotrophic biological phosphorus removal in a full scale wastewater treatment plant. IWA Nutrient Removal and Recovery 2015: moving innovation into practice
Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (2008) Biological wastewater treatment—principles, modelling and design, 1st edn. IWA publishing, London
Isa Z, Grusenmeyer S, Verstraete W (1986) Sulfate reduction relative to methane production in high-rate anaerobic digestion: microbiological aspects. AEM 51(3):580–587
Jin RC, Yang GF, Zhang QQ, Ma C, Yu JJ, Xing BS (2013) The effect of sulfide inhibition on the ANAMMOX process. Water Res 47(3):1459–1469
Kong Y, Ong S, Ng W, Liu W (2002) Diversity and distribution of a deeply branched novel proteobacterial group found in anaerobic–aerobic activated sludge processes. Environ Microbiol 4(11):753–757
Koster I, Rinzema A, Devegt A, Lettinga G (1986) Sulfide inhibition of the methanogenic activity of granular sludge at various pH-levels. WR
Kuba T, Murnleitner E, van Loosdrecht MC, Heijnen JJ (1996) A metabolic model for biological phosphorus removal by denitrifying organisms. Biotechnol Bioeng 52(6):685–695
Kulakovskaya TV, Lichko LP, Ryazanova LP (2014) Diversity of phosphorus reserves in microorganisms. Biochemistry (Mosc) 79(13):1602–1614
Lee, C., Yu, C., 1997. Conservation of water resources—use of sea water for flushing in Hong Kong. Aqua-Journal of WS.
Lopez-Vazquez CM, Oehmen A, Hooijmans CM, Brdjanovic D, Gijzen HJ, Yuan Z, van Loosdrecht MCM (2009) Modeling the PAO-GAO competition: effects of carbon source, pH and temperature. WR 43(2):450–462
Lopez-Vazquez CM, Song YI, Hooijmans CM, Brdjanovic D, Moussa MS, Gijzen HJ, van Loosdrecht MCM (2008) Temperature effects on the aerobic metabolism of glycogen-accumulating organisms. Biotechnol Bioeng 101(2):295–306
Meyer RL, Saunders AM, Blackall LL (2006) Putative glycogen-accumulating organisms belonging to the Alphaproteobacteria identified through rRNA-based stable isotope probing. Micro. 152(2):419–429
Mino T, Loosdrecht MV, Heijnen J (1998) Microbiology and biochemistry of the enhanced biological phosphate removal process. WR 32(11)
NEN 6472 (1983) Water Forometrische bepaling van het gehalte aan ammonium
Nielsen PH, Daims H, Lemmer H, Arslan-Alaton I, Olmez-Hanci T (2009) FISH handbook for biological wastewater treatment. IWA Publishing, London
Oehmen A, Lopez-Vazquez CM, Carvalho G, Reis MAM, van Loosdrecht MCM (2010) Modelling the population dynamics and metabolic diversity of organisms relevant in anaerobic/anoxic/aerobic enhanced biological phosphorus removal processes. WR 44(15):4473–4486
Pattarkine VM, Randall CW (1999) The requirement of metal cations for EBPR removal by activated sludge. WST 40(2):159–165
Pijuan M, Ye L, Yuan Z (2010) Free nitrous acid inhibition on the aerobic metabolism of poly-phosphate accumulating organisms. WR 44(20):6063–6072
Reichert P (1998) Computer program for the identification and simulation of aquatic systems
Rickard LF, Mcclintock SA (1992) Potassium and magnesium requirements for enhanced biological phosphorus removal from wastewater. WST 26(9):2203–2206
Saad S, Welles L, Lopez-Vazquez CM, Brdjanovic D (2013) Sulfide effects on the anaerobic kinetics of phosphorus-accumulating organisms
Schulz HN, Brinkhoff T, Ferdelman TG, Mariné MH, Teske A, Jorgensen BB (1999) Dense populations of a giant sulfur bacterium in Namibian shelf sediments. Science (New York, NY) 284(1999):493–495
Sears K, Alleman JE, Barnard JL, Oleszkiewicz JA (2004) Impacts of reduced sulfur components on active and resting ammonia oxidizers. Journal of IMB 31:369–378
Smolders GJ, van der Meij J, van Loosdrecht MC, Heijnen JJ (1994a) Stoichiometric model of the aerobic metabolism of the biological phosphorus removal process. BB 44:837–848
Smolders GJF, Van Der Meij J, Van Loosdrecht MCM, Heijnen JJ (1994b) Model of the anaerobic metabolism of the biological phosphorus removal process: stoichiometry and pH influence. BB 43:461–470
Smolders GJF, van der Meij J, van Loosdrecht MCM, Heijnen JJ (1995) A structured metabolic model for anaerobic and aerobic stoichiometry and kinetics of the biological phosphorus removal process. BB 47(3):277–287
van den Brand TPH, Roest K, Brdjanovic D, Chen GH, van Loosdrecht MCM (2014) Temperature effect on acetate and propionate consumption by sulfate-reducing bacteria in saline wastewater. AMB 98(9):4245–4255
Welles L, Lopez-Vazquez CM, Hooijmans CM, van Loosdrecht MCM, Brdjanovic D (2015) Impact of salinity on the aerobic metabolism of phosphate-accumulating organisms. AMB 99(8):3659–3672
Wentzel M, Lötter L, Loewenthal R, Marais G (1986) Metabolic behaviour of Acinetobacter spp. in enhanced biological phosphorus removal—a biochemical model. WSA 12(4):7700
Wong M-T, Tan FM, Ng WJ, Liu WT (2004) Identification and occurrence of tetrad-forming Alphaproteobacteria in anaerobic-aerobic activated sludge processes. Micro 150(11):3741–3748
Wu G, Rodgers M (2010) Inhibitory effect of copper on enhanced biological phosphorus removal. WST 62(7):1464
Yeoman S, Stephenson T, Lester J, Perry R (1988) The removal of phosphorus during wastewater treatment: a review. Envir Poll 49
Zhou Y, Ganda L, Lim M, Yuan Z, Ng WJ (2012) Response of poly-phosphate accumulating organisms to free nitrous acid inhibition under anoxic and aerobic conditions. BT 116:340–347
Zhou Z, Xing C, An Y, Hu D, Qiao W, Wang L (2014) Inhibitory effects of sulfide on nitrifying biomass in the anaerobic-anoxic-aerobic wastewater treatment process. Journal of Che Tech and Biot 89:214–219
Acknowledgments
The authors would like to acknowledge the National Council for Science and Technology of Mexico (CONACYT, Mexico) which provided financial support (214775). The authors would like to thank the lab staff of UNESCO-IHE and TU-Delft for their support and help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Rubio-Rincón, F.J., Lopez-Vazquez, C.M., Welles, L. et al. Sulphide effects on the physiology of Candidatus Accumulibacter phosphatis type I. Appl Microbiol Biotechnol 101, 1661–1672 (2017). https://doi.org/10.1007/s00253-016-7946-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7946-1