Abstract
Prolyl endopeptidases (PEP) (EC 3.4.21.26), a family of serine proteases with the ability to hydrolyze the peptide bond on the carboxyl side of an internal proline residue, are able to degrade immunotoxic peptides responsible for celiac disease (CD), such as a 33-residue gluten peptide (33-mer). Oral administration of PEP has been suggested as a potential therapeutic approach for CD, although delivery of the enzyme to the small intestine requires intrinsic gastric stability or advanced formulation technologies. We have engineered two food-grade Lactobacillus casei strains to deliver PEP in an in vitro model of small intestine environment. One strain secretes PEP into the extracellular medium, whereas the other retains PEP in the intracellular environment. The strain that secretes PEP into the extracellular medium is the most effective to degrade the 33-mer and is resistant to simulated gastrointestinal stress. Our results suggest that in the future, after more studies and clinical trials, an engineered food-grade Lactobacillus strain may be useful as a vector for in situ production of PEP in the upper small intestine of CD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Celiac disease is a chronic inflammatory disorder of the small intestine caused by the ingestion of gluten proteins in genetically predisposed individuals. The disease is associated with a range of symptoms related to malabsorption, such as weight loss, fatigue, diarrhea, anemia, and osteoporosis (Green and Jabri 2003; Shan et al. 2002). Untreated disease is even associated with increased mortality (Corrao et al. 2001). Celiac disease (CD) occurs in most parts of the world with a high prevalence (1:100) (Gujral et al. 2012).
Upon ingestion of gluten, this dietary protein is broken down into numerous proline-rich peptides, which are resistant to further gastrointestinal digestion. These peptides persist in the gut lumen, where they can trigger inflammation (Shan et al. 2002). The 33-residue gluten peptide (33-mer) derived from α2-gliadin is a vivid example of a particularly immunogenic peptide that harbors six copies of inflammatory T cell epitopes (Qiao et al. 2004).
The only treatment currently available for CD is a strict long-life gluten-free diet. Not only is this diet expensive, but inadequate food labeling practices and cross-contamination during food preparation adversely impacts the health and quality of life of celiac patients. Therefore, alternative treatments for CD are urgently needed. Recent advances in our understanding of CD pathogenesis have opened the door to potential non-dietary therapies. One example is the detoxification of dietary gluten by enzymatic cleavage of gliadin fragments by prolyl endopeptidases (PEP) (EC 3.4.21.26). Several microbial PEP have been studied, including those from Flavobacterium meningosepticum (Yoshimoto et al. 1980, 1991), Sphingomonas capsulata (Kabashima et al. 1998), and Myxococcus xanthus (Gass et al. 2005). We have chosen the PEP of M. xanthus (Mx PEP) based on the comparative analysis between these three PEP realized by Shan et al. (2004). Mx PEP shows high capacity to degrade the 33-mer epitopes involved in celiac disease, relative resistance to pancreatic and brush border enzymes, and an optimum pH profile around pH 7.0. However, this enzyme is strongly susceptible to deactivation under gastric conditions (pepsin and low pH). We therefore sought to generate a system capable of delivering intact PEP into in an in vitro model of the small intestine environment. Our approach is complementary to that reported by Gass et al., who engineered an enteric coated formulation of the enzyme (Gass et al. 2005).
Lactic acid bacteria (LAB) are an attractive vehicle for mucosal delivery of bioactive molecules (del Rio et al. 2008, 2010; Wells and Mercenier 2008). They are extensively used in fermented foods, and many of them have been recognized as Generally Regarded as Safe (GRAS) microorganisms by the US Food and Drug Administration (FDA) and as Qualified Presumption of Safety (QPS) by the European Food Safety Authority. Among LAB, the genus Lactobacillus has several advantages for this application because many strains are members of the human intestinal microbiota (Carr et al. 2002; Turroni et al. 2013). They can survive passage through the stomach and remain viable in the gastrointestinal tract (GIT) for some period of time (Axelsson 1998; Daniel et al. 2011). Moreover, they can be engineered to express heterologous genes keeping their food-grade status (Martin et al. 2000).
In this work, we generated two Lactobacillus casei strains with a food-grade integrative cloning system (Martin et al. 2000), which produce intracellular and extracellular PEP to be delivered to the simulated intestinal lumen. We demonstrated that the strain producing extracellular PEP is more efficient in that it resists inactivation under simulated gastric conditions and then continues to secrete PEP. Therefore, it could be tested in vivo to degrade immunotoxic gluten peptides such as the 33-mer peptide derived from α2 gliadin.
Materials and methods
Bacterial strains, plasmid, primers, and culture conditions
The bacterial strains, plasmids, and primers used in this study are listed in Table 1. Escherichia coli S11 was grown with standard media and growth conditions (Sambrook et al. 1989). L. casei BL23 (Mazé et al. 2010) was cultivated at 37 °C without aeration in MRS medium (Oxoid, Basingstoke, Hampshire, England). When necessary, the medium was supplemented with the appropriate antibiotics: 100 μg ml−1 ampicillin (Ap), 50 μg ml−1 kanamycin (Km), 0.1 mM IPTG, and 80 μg ml−1 X-gal for E. coli, and 2.5 μg ml−1 erythromycin (Em), 5 μg ml−1 chloramphenicol (Cm), and 0.4 μg ml−1 novobiocin for L. casei (all from Sigma, Madrid, Spain).
In some cases, the Lactobacillus strains were grown in carbon-free basal medium (CFB) containing bacteriological meat extract, 8 g l−1; yeast extract, 4 g l−1; dipotassium phosphate, 2 g l−1; diammonium citrate, 2 g l−1; sodium acetate, 5 g l−1; magnesium sulfate, 0.2 g l−1; manganese sulfate, 0.04 g l−1; and Tween 80, 1 ml l−1. The carbon source was 0.5 % glucose, lactose, or maltose (all from Sigma, Madrid, Spain).
For preparation of cell suspensions for gastrointestinal simulation assay, cells were grown overnight in MRS medium, harvested by centrifugation, 3,000 × g for 15 min at room temperature, washed with 0.85 % NaCl, and resuspended in one volume of 10 % reconstituted skim milk powder (Oxoid, Basingstoke, UK).
To study the effect of pH on extracellular PEP activity, the strains were cultivated in a Sixfors bioreactor (Infors AG, Bottmingen, Switzerland). The program for data logging and control of the bioreactor was Iris NT5 (Infors). Sterile stirred fermentation vessels were set up and aseptically filled with 400 ml sterile CFB medium (0.5 % glucose). No headspace gas was used. Each vessel was inoculated with 1 % cell suspensions. Fermentations were carried out at different pH values: pH 5.0, 6.0, and 7.0-controlled by the automatic addition of 2 N NaOH- and uncontrolled pH. The temperature was maintained at 37 °C and the agitation speed was 30 rpm. Batch cultures were grown for 24 h, and 10 ml samples was obtained from each vessel at 10, 12, 14, 16, 18, 20, and 22 h for PEP activity assay, and at 22 h for 33-mer peptide degradation and secreted protein precipitation.
Codon usage optimization of the pep gene sequence of M. xanthus
The gene encoding Mx PEP (available from the NCBI database with the accession number AAD31004) (Shan et al. 2004) was synthesized based on the preferred codon usage of Lactobacillus to enhance the translational efficiency (accession number HG321354). The pep gene was synthesized by Biomedal S. L. (Sevilla, Spain).
DNA manipulations
L. casei genomic DNA was prepared using 2× Kirby lytic mix (Parish 1986). Plasmid isolation was achieved as before (Scheirlinck et al. 1989). Isolation of plasmid DNA from E. coli was performed by the alkaline lysis method (Sambrook et al. 1989). Restriction endonuclease digestions, alkaline phosphatase treatments, ligations, and others manipulations were performed according to standard procedures (Sambrook et al. 1989).
Electroporation of E. coli was undertaken in a Bio-Rad pulser apparatus following the manufacturer’s recommendations. L. casei was electroporated as described by Wei et al. (1995). The correct integration of the vectors into the L. casei genome was analyzed by PCR amplification using the previously described oligonucleotide primers att1 and att7 (Alvarez et al. 1998) (Table 1) in a Mini Cycler™ (MJ Research, Watertown, MA, USA). Site-specific resolution was confirmed by Southern blot. DNA hybridization was performed using the non-radioactive DNA Labeling and Detection Kit (Roche Molecular, Mannheim, Germany) using protocols provided by the supplier.
Generation of two integrative plasmids carrying the pep gene of M. xanthus
The aggregation-promoting factor (apf) gene codifies a cell surface protein which is hypothesized to be involved in the maintenance of cell shape (Jankovic et al. 2003; Ventura et al. 2002). The APF protein was selected as a vector molecule due to its high secretion level in the supernatant (Jankovic et al. 2003; Marcotte et al. 2004; Ventura et al. 2002). Thus, two expression systems based on different elements of the Lactobacillus crispatus apf gene were constructed for intracellular or secreted production of Mx PEP (Martin et al. 2011). Plasmid pEM182 (Martin et al. 2011) was used as a template for amplification of three different DNA fragments of the apf gene. The sequences of the primers used for amplification are shown in Table 1. The promoter region of apf gene was amplified using the primers Papf-BglIIF and Papf-NcoIR (fragment 1). The promoter region plus the signal peptide of the apf gene was amplified using the primers Papf-FglIIF and PapfSP-NcoIR (fragment 2). The apf terminator region was amplified using the primers term-XbaIF and term-EcoRIR (fragment 3). Fragments 1 and 2 were cloned in BglII and NcoI sites of pUK21 vector generating pIPLA1253 and pIPLA1214, respectively. Fragment 3 was cloned in XbaI and EcoRI sites of pIJ2925 (Janssen and Bibb 1993) plasmid generating pIPLA1215. Plasmid pIPLA1253 and pIPLA1214 were digested with SpeI-XbaI and ligated to XbaI-digested pIPLA1215 yielding pIPLA1254 and pIPLA1262, respectively. Plasmid pIPLA1258 and pIPLA1265 were generated by cloning the 984-bp and the 1,108-bp BglII fragments from pIPLA1254 and pIPLA1262 into BamHI-digested pEM76 (Martin et al. 2000), an integrative vector based on the integration machinery of bacteriophage A2. The pIPLA1258 and pIPLA1265 plasmids were used as vectors to integrate in the chromosome of L. casei a synthetic codon-optimized gene encoding the Mx PEP (pep). The pep gene was codon-optimized based on the tRNA pool and the codon usage preference of Lactobacillus genus to enhance the translational efficiency. The codon-optimized pep was cloned in a pUCE57 plasmid yielding pUC57-pep.
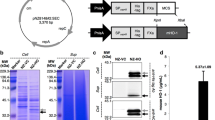
For the construction of the integrative plasmids carrying pep, the pUC57-pep plasmid was digested with XbaI and NcoI and the resultant 2.1-kb fragment corresponding to pep gene was cloned into the multicloning site of pUK21 (Vieira and Messing 1991) to generate pIPLA1500. The NcoI-XbaI insert of pIPLA1500 was then cloned into the NcoI-XbaI site of the integrative pIPLA1258 and pIPLA1265 vectors, yielding pIPLA1503 and pIPLA1506, respectively (Fig. 1). Because pIPLA1503 and pIPLA1506 are derived from pEM76, they contain its depuration system. Following integration, the non-food-grade DNA of the vector (antibiotic resistance genes and E. coli DNA) remains flanked by two direct repeat six sites, thus allowing its deletion by the action of a β-recombinase (Martin et al. 2000).
Physical map of plasmids generated in this work. The physical map and relevant features of plasmids pIPLA1503 (a) and pIPLA1506 (b) are shown. Indicated are the ampicillin (Ap) and erythromycin (Em) resistance genes; the integration region of bacteriophage A2 (int-attP); the β-recombinase binding sites (six); the promoter, terminator and signal peptide of the aggregation-promoting factor gene of L. crispatus (Papf, ter, and SPapf) and the prolyl endopeptidase gene of M. xanthus (pep). Relevant restriction sites are also shown
Enzymatic assays
PEP activity was determined using a synthetic substrate, Succinyl-Ala-Pro-pNA (NA, nitroanilide) (Bachem, Bubendorf, Switzerland). The assay mixture contained 625 μl of 50 mM phosphate buffer (pH 7.5), 0.2 M NaCl, 125 μl substrate (1.2 mM), and 250 μl of cell extracts. The reaction was stopped by adding 500 μl of 20 % trichloroacetic acid. Then it was centrifuged (10,000 × g for 10 min) and the release of the pNA was spectrophotometrically detected at a wavelength of 410 nm in a U-2800 Digilab Hitachi spectrophotometer (Hitachi High-Technologies Corporation, Tokyo, Japan).
Cell extracts of L. casei IPLA1503 were assayed because the activity is intracellular; however, for L. casei IPLA1506, which secretes PEP, the supernatant of the cultures was assayed. For preparation of cell extracts, bacterial cultures (20 ml) were harvested by centrifugation (8,000 × g for 10 min), washed twice, and resuspended in 2 ml of 50 mM phosphate buffer, 0.2 M NaCl (pH 7.5). Then, the samples were disrupted using a French Press operating at 2.3 kbar (Constant Cell Disruption Systems [Low March, Daventry, Northents, UK]). Cell debris was removed by centrifugation (10,000 × g for 30 min at 4 °C), and the supernatant was used for the activity assays.
One activity unit was defined as the amount of enzyme required to release 1 μmol of p-NA per min under the assay conditions. The enzyme activities assays were performed in triplicate. Specific enzyme activity was expressed as milliunits per milligram protein. The protein concentration was measured using a Pierce BCA Assay Kit (Thermo Fisher scientific).
SDS-PAGE of secreted proteins
L. casei strains were grown in CFB (0.5 % glucose) at pH 7.0 for 22 h. One hundred milliliters aliquots of the cultures were centrifuged and the supernatant proteins were precipitated as previously described (Sánchez et al. 2009). The pellets were re-solubilized in 200 μl of Laemmli buffer (Laemmli 1970). Proteins were analyzed by SDS-PAGE in 8 % (w/v) polyacrylamide gels and then visualized by standard silver staining.
Determination of 33-mer peptide degradation by RP-HPLC and LC/MS
The capacity of the strain to degrade the highly immunogenic 33-mer peptide derived from α2 gliadin (LQLQPFPQPQLPYPQPQLPYPQPQLPYPQPQPF) was assayed chromatographically (Sealey-Voyksner et al. 2010; Shan et al. 2002). The 33-mer was chemically synthesized at a purity of 95 % (Immunosteps S.L., Salamanca, Spain). The peptide was dissolved at 10 mM concentration in PBS, pH 7.4. Proteolysis reactions were performed at 37 °C. The mixture contained the 33-mer (25.5 μM, which corresponds to 100 ppm) and the cellular suspension of each individual strain previously grown at pH 7.0 and glucose 0.5 % (109 cfu ml−1). After each time interval, 150 μl aliquots were removed and boiled (95 °C for 10 min) to inactivate enzyme activity. The sample aliquots were filtered using a low binding protein filter (0.2 μm) (HT Tuffryn Membrane, Pall, VWR, Spain) and stored at −20 °C. The decrease in substrate concentration, as well as concomitant intermediate and product build-up were assessed with RP-HPLC and LC/MS.
The HPLC chromatographic system consisted of separation module Alliance 2795, PDA photodiode detector 2996, and data acquisition software Empower (Waters, Milford, MA). The column used in these analyses was an XTerra MS C18 5 μm, 4.6 × 150 mm with a precolumn Nova-Pak C18 4 μm, 3.9 × 20 mm (Waters), thermostated to 30 °C. The elution phases consisted of (A) water with 0.1 % (v/v) trifluoroacetic acid and (B) acetonitrile with 0.1 % (v/v) trifluoroacetic acid. The gradient program was started with 100 % of solvent A and 0 % of solvent B, and changed linearly to reach 62.6 % of solvent A and 37.4 % of solvent B in 34 min. The column was cleaned with 100 % of solvent B (5 min) and equilibrated with the initial conditions for 10 min. UV absorbance was recorded at 215 and 280 nm.
LC/MS was performed on an Agilent 1260 Infinity liquid chromatograph directly interfaced to an Agilent 6520 Accurate Mass Q-TOF mass spectrometer (Agilent Technologies, Santa Clara, CA). Ten microliters aliquots of each sample were injected via the autosampler onto a 5-μm 100 × 2.0 mm Gemini-NX C18 column (Phenomenex, Torrance, CA). After a 2-min hold at 97 % A, compounds were eluted with a linear gradient of 3 to 95 % B over 30 min, where A was water with 0.1 % (v/v) formic acid and B was acetonitrile with 0.1 % (v/v) formic acid. The flow rate was 0.4 ml min−1. Between each run, the column was washed with 95 % B for 10 min and equilibrated with starting conditions for 7 min.
Mass spectrometric detection was achieved in the electrospray positive ion (ESI+) mode. The source parameters were as follows: 4,000 V capillary voltage, 150 V fragmentor voltage, 65 V skimmer voltage, 350 °C drying gas at a flow rate of 11 l min−1, and 35 psig nebulizer pressure. TOF spectra were acquired in high resolution mode over m/z 100 to 1,700.
Data analysis was performed using the Agilent MassHunter Qualitative Analysis Software Package. The find compounds by formula option were used to extract ion chromatograms corresponding to the molecular formula of the 33-mer (C190H274N43O47) and the expected Mx PEP degradation products (Shan et al. 2004): YPQPQPF (C43H58N9O11), QPQPF (C49H42N7O8), QPQLP (C26H44N7O8), LQLQP (C27H48N7O8), FPQP (C24H34N5O6), and YPQP (C24H34N5O7). The software was set to allow H+, K+, Na+, and NH4 + as charge carriers, and an option allowing for discovery of ions resulting from the neutral loss of water [M-H2O]+z was selected. The permitted charge states were z = +1 to +4. Additionally, a list of all possible 33-mer degradation products was generated by systematically cleaving the 33-mer after each proline residue, generating 74 unique peptides with 68 unique molecular masses. The ion chromatograms were then screened for the presence of peptides from this list.
In vitro gastrointestinal stress tolerance assay
The survival of the strain during the passage through the GIT was studied in an in vitro model described previously (Fernández de Palencia et al. 2008) with some modifications. This model simulates the GIT normal conditions. The presence of 0.1 % lysozyme mimics the human saliva. The gastric (G) stress is simulated with the addition of 0.3 % pepsin and by decreasing the pH value every 20 min (pH 5.0, pH 4.1, pH 3.0, pH 2.1, and pH 1.8) with 1 M HCl (Marteau et al. 1997). The gastrointestinal (GI) stress was assayed after G stress at pH 5.0, pH 4.1, or pH 3.0 by adding bile salts and pancreatic enzymes and adjusting to pH 6.5 with 1 M NaHCO3. Samples were obtained from intestinal conditions at 20 and 120 min.
To simulate small intestinal digestion, pancreatic enzymes were used as indicated before (Shan et al. 2004). A cocktail of 1 mg ml−1 amylase, 1 mg ml−1 trypsin, and 1 mg ml−1 chymotrypsin was added in a final concentration of 5 μg ml−1. The bile salts were added to a concentration of 0.3 %.
Bacterial counts were determined at each sampling point. After serial dilutions of samples in 0.85 % NaCl solution, samples were plated on MRS media with 2 % agar and colonies were counted after incubation for 48 h at 37 °C.
To verify PEP production, samples recovered from intestinal conditions were inoculated in 5 ml of new CFB medium for 4 h, and PEP activity was assayed in the supernatant.
Results
Generation of food-grade L. casei strains with a synthetic gene encoding Mx PEP integrated into the chromosome
Two strains of L. casei were constructed using a previously described food-grade cloning system (Martin et al. 2000, 2011). Two integrative plasmids carrying the pep gene of M. xanthus were constructed for intracellular (pIPLA1503) or secreted (pIPLA1506) production of Mx PEP (Fig. 1). They contain a depuration system to remove the non-food-grade DNA of the vector. Thus, L. casei BL23 (Mazé et al. 2010) was electroporated with both, pIPLA1503 and pIPLA1506 plasmids. The resulting strains were transformed with the replicative plasmid pEM94 (Martin et al. 2004) which contains the gene encoding the β-recombinase, with the aim of removing by site-specific recombination, the non-food grade DNA located between two six sites. To eliminate pEM94, which carries a temperature-sensitive origin of replication, the strains were cultured at 37 °C. The resulting strains were designated as L. casei IPLA1503 and L. casei IPLA1506, respectively. Proper integration of pIPLA1503 and pIPLA1506, as well as elimination of the non-food-grade DNA and pEM94, was verified by PCR analysis through att1 and att7 primers (Table 1) and by Southern blotting (data not shown).
Effect of different carbon sources in PEP production
To optimize the culture medium for maximal enzyme production, the effect of different carbon sources on the production of PEP enzyme was analyzed. The strains L. casei IPLA1503 and L. casei IPLA1506 were grown at 37 °C in CFB with glucose, lactose, or maltose. Growth (OD600) and pH were evaluated at time intervals of 1 h. Because the growth of both strains was similar, Fig. 2a shows only the data from L. casei IPLA1506 culture.
Effect of different carbon sources on the growth and PEP production of L. casei IPLA1503 and L. casei IPLA1506. L. casei IPLA1506 was grown at 37 °C in CFB media with different carbon sources: glucose, lactose, and maltose. Panel (a) shows measurements of OD (600 nm) and pH values at a time intervals of 1 h under each carbon source. Discontinuous lines show the points were the PEP activity was measured. Panel (b) shows the PEP production by L. casei IPLA1503 (left) and L. casei IPLA1506 (right) at three different points of the growth curve (5, 13, and 24 h) in three different carbon sources (glucose, lactose, and maltose). The enzyme activities assays were performed in triplicate
Samples were collected from media with different carbon sources (glucose, lactose, and maltose) and in different phases of growth in the above experiments (i.e., at 5, 13, and 24 h; Fig. 2a), and the PEP activity was measured in cell extracts (L. casei IPLA1503) or the culture medium (L. casei IPLA1506). As seen in Fig. 2b, PEP activity was significantly higher in the samples corresponding to L. casei IPLA1503 (from 1.7 to 1.9 mU mg−1 depending on the carbon source used) compared to L. casei IPLA1506 (0.006 to 0.1 mU mg−1). Moreover, although better growth was observed in the presence of glucose, PEP activity of L. casei IPLA1506 decreased throughout the growth curve, suggesting a potential effect of media acidification on enzyme activity, enzyme stability, and/or enzyme production rate.
Effect of the medium pH on extracellular PEP activity
As shown previously, the optimal pH of Mx PEP activity is near 7.0 (Yoshimoto et al. 1991). This is not a drawback for intracellular PEP production due to the fact that LAB are able to maintain a neutral cytoplasmic pH even when the pH of the external medium varies (Nyanga-Koumou et al. 2012). Nevertheless, acidification of the culture medium may be responsible for the observed drop in extracellular PEP activity assay. We therefore grew L. casei IPLA1506 at a controlled pH in a bioreactor, and tested PEP activity of the supernatant in CFB (0.5 % glucose) at controlled pH values in the 5–7 range (Fig. 3). PEP activity was not detected at pH 5.0. Consistent with this finding, activity disappeared completely after 14 h when cells were grown without pH control. However, at pH 6.0 and pH 7.0, PEP activity increased over time. At pH 6.0, PEP activity in the culture medium increased up to 0.7 mU mg−1, four times more than the maximum value observed in the absence of pH control. Similarly, the PEP activity at pH 7.0 increased more sharply, with maximum activity of 2.5 mU mg−1 after 22 h (i.e., higher than the intracellular activity from L. casei IPLA1503; Fig. 2b).
To verify that PEP was properly processed during secretion and is intact in the culture medium of L. casei IPLA 1506, the supernatant proteins were analyzed by SDS-PAGE (Fig. 4). The secreted protein profile was similar with that of the host strain (L. casei BL23), but showed an extra band whose size corresponds to that of PEP.
Proteolysis of the immunotoxic gliadin peptide (33-mer)
The α2-gliadin 33-mer is both resistant to human digestive enzymes and also highly immunogenic, thus rendering it as a major trigger of the inflammatory response to gluten in CD (Shan et al. 2002). The capacity of purified Mx PEP to degrade the 33-mer has been described previously (Gass et al. 2005; Shan et al. 2004). We therefore investigated the ability of L. casei IPLA1503 and L. casei IPLA1506 to degrade the 33-mer compared to the control strain L. casei BL23, which does not produce PEP. A cellular suspension of each strain was incubated with the 33-mer peptide at 37 °C during 12 h. At 0, 4, 8, and 12 h, samples were taken out and peptide hydrolysis was determined as the decrease on the peptide peak area, quantified by HPLC (Fig. 5). The HPLC conditions used allowed the elution of the intact peptide at 31.7 min. Cultures of L. casei IPLA1503 showed the ability to degrade the 33-mer at 12 h but not at 8 h, with the concomitant appearance of a new fragment eluting at 31 min. In contrast, when the 33-mer was incubated with L. casei IPLA1506, more gradual disappearance of the 33-mer was observed. The strain was able to break down the 63 % of the 33-mer after 8 h of incubation and it was almost completely degraded after 12 h, without the appearance of new concomitant fragments in the chromatogram.
Hydrolysis of the immunotoxic 33-mer peptide. The strains L. casei BL23 (a), L. casei IPLA1503 (b), and L. casei IPLA1506 (c) were incubated with the 33-mer (25.5 μM) for 12 h. Every 4 h, aliquots were removed and the substrate concentration was monitored by RP-HPLC analysis. Chromatograms show the hydrolysis time course of 33-mer peptide by the strains. The complete chromatogram appears in a box with the substrate arrowed
These results indicated that L. casei IPLA1506 secretes the protein correctly, has higher activity at neutral pH, and shows increased degradation of the 33-mer peptide. Beside of the good results obtained under the experimental conditions tested, the ability to secret this activity could be an important tool to eliminate the toxic gluten peptides from different environments (matrix food, intestinal lumen). In order to identify degradation products of the 33-mer, the samples from L. casei BL23 and IPLA1506 were also analyzed by LC/MS. In this case, the proteolytic activity was monitored over 0–16 h. After 8 h of incubation the L. casei IPLA1506 strain degraded the 73 % of the peptide and it was completely hydrolyzed at end time. The data agree with the results found by HPLC: the 33-mer was totally degraded by the L. casei IPLA1506 strain but not by L. casei BL23 (Fig. 6), and no degradation intermediates or products were detectable.
LC-MS analysis of 33-mer proteolysis products. The strains L. casei BL23 and L. casei IPLA1506 were incubated with the 33-mer peptide (25.5 μM) for 16 h at 37 °C. Aliquots were taken at the times shown and the analyzed by LC-MS. After 16 h of incubation with the IPLA1506 strain, the 33-mer is below the instrument limit of quantification. The 33-mer peak is highlighted in gray, and labels represent absolute ion counts
Resistance to simulated gastrointestinal conditions
To check if L. casei IPLA1506 is able to survive the gastrointestinal stress and maintain the ability to produce the active PEP in the intestinal lumen—where the 33 mer toxic peptide is accumulated and triggers inflammation—GIT conditions were simulated in vitro (Fernández de Palencia et al. 2008). To evaluate relevant G and the GI stresses, we considered the effect of lysozyme, pepsin, pH, bile salts, and pancreatic enzymes on bacterial viability, while also simulating the gastric emptying process. Figure 7a shows strain survival in response to G and GI stresses at different pH values. Neither lysozyme nor pepsin in the pH 3–5 range affected strain viability. At a pH of 2.1, the bacterial count decreased 1.3 log units, whereas at pH 1.8 the colony count had dropped by 3.4 log units.
Cell survival (a) and prolyl endopeptidase production (b) after gastric and gastrointestinal stresses of L. casei IPLA1506. The L. casei strain IPLA1506 was resuspended in milk and submitted to simulated gastrointestinal conditions to determine the cell survival. To simulate the gastric stress (G), samples were incubated with lysozyme and pepsin in a decreasing pH values. To simulate the gastrointestinal stress, samples taken from pH 5.0, pH 4.1, and pH 3.0 where further incubated with bile salts and pancreatic enzymes during 20 min (GIa) and 120 min (GIb). Samples from GIa and GIb (asterisk) were used as pre-inoculum in a new basal medium. After 4 h of growth, PEP activity was assayed in the supernatant of cell culture
To simulate the GI stress, samples taken from incubations at pH 5.0, pH 4.1, and pH 3.0 (G stress) were further incubated with 0.3 % bile salts and 0.05 μg ml−1 trypsin, chymotrypsin, and amylase for 20 or 120 min (GI20 min and GI120 min, respectively in Fig. 7). Intestinal juice caused a slight reduction in viability (ca. 1 log unit). This different behavior could be correlated with the degree of severity of the previous gastric incubation of the cell suspensions. Samples from GIa and GIb were used as inoculum in a CFB medium. PEP activity was assayed after 4 h, verifying that the strain maintained the ability to secrete active PEP.
Discussion
In recent years, oral enzyme therapy has emerged as a promising treatment of CD (Bethune and Khosla 2012). Microbial PEP enzymes are particularly interesting therapeutic candidates (Gass et al. 2005; Hausch et al. 2002; Marti et al. 2005; Piper et al. 2004; Pyle et al. 2005; Shan et al. 2002, 2004). We chose the Mx PEP for our study, based on the comparative analysis of three PEP enzymes by Shan et al. (2004). However, Mx PEP is susceptible to acidic pH and pepsin, and is most suitable for gluten detoxification in the luminal environment of the duodenum (Shan et al. 2004). Thus, a system that delivers the intact enzyme into the duodenum is needed. Here we have supported that L. casei is particularly well suited for this purpose. It was capable to survive GIT conditions and to maintain the ability to produce PEP. In addition, it avoids the previous steps of PEP synthesis and purification, thus lowering treatment costs.
LAB have been proposed as the ideal microorganisms to be used as live vectors for mucosal delivery of therapeutic proteins (Wells and Mercenier 2008). We have engineered L. casei, an acid-tolerant and probiotic LAB that can colonize the gut, to produce Mx PEP at the site of action. To achieve this goal, we had to integrate the pep gene stably into the chromosome, keep the food-grade status of the strain, produce the enzyme in sufficient quantity constitutively, and produce the enzyme both in the cytoplasm and in the extracellular media. The two first challenges were overcome via the food-grade integrative system of Martin et al. (2000, 2004, 2011). This system was used to deliver a codon-optimized gene encoding Mx PEP under the control of the constitutive apf promoter of L. crispatus (Martin et al. 2011), and using the apf signal peptide, it was possible to secrete the 76.8-kDa protein outside the cell.
The engineered strain totally degraded the immunogenic 33-mer peptide. Previously, it was shown that the Mx PEP specifically cleaves the 33-mer post-proline into 4–7 amino acid peptides (Shan et al. 2004); however, these degradation products were not detected by LC/MS. This suggests that initial degradation of the 33-mer by Mx PEP may be rate limiting. Subsequently, peptidases of the host strain may then quickly further degrade the Mx PEP peptide products into their constituent amino acids. LAB are known to possess oligopeptide transporters as well as a variety of intracellular peptidases, and it is likely that the 33-mer is too large to be imported before its degradation by Mx PEP (Mazé et al. 2010; Savijoki et al. 2006).
These features have potential implications for the use of the Mx PEP-secreting L. casei strain in the detoxification of dietary gluten of CD patients. The engineered strain was incubated with 100 ppm of the immunogenic 33-mer peptide and it was totally degraded in 12 h. As it was described, the 33-mer represents approximately the 2.5 % of the gliadin (Comino et al. 2012) and this one is the 50 % of the gluten content. Therefore, 100 ppm of the 33-mer peptide would be roughly equivalent to 8,000 ppm of whole gluten. Considering that the daily gluten intake of a study population in the Netherlands was 13.1 g (van Overbeek et al. 1997), this strain could be a suitable therapy to hydrolyze the daily gluten traces in the foods during digestion, because as we have supported this strain survived under simulated GIT conditions, and retained the ability to secrete PEP (Fig. 7), However, future studies to assess the required dose of the PEP-producing strain to degrade the inmunotoxic peptides before they trigger inflammation are necessary. Notably, milk plays an important role in cell survival; in preliminary studies with the strain resuspended in culture media or in saline solution, survival was significantly lower (data not shown). Several studies have shown that the ability of LAB to survive GIT conditions is considerably enhanced by milk (Conway et al. 1987; Charteris et al. 1998; Wang et al. 2012).
It is well known that LAB are members of the normal gastrointestinal microbiota (Axelsson 1998). Moreover, many LAB strains are used as probiotics and are beneficial to the health of their host (Tuohy et al. 2003). More recently, Lactobacillus species have been used to treat inflammatory bowel diseases (Clarke et al. 2012). Our studies suggest that L. casei IPLA1506 could be a good candidate for in vitro assays such as the analysis of the 33-mer peptide hydrolysis under intestinal conditions simulation and in vivo assays, in celiac models, and for clinical trials in the future. Different species have been used as animal model of CD including dogs, horses, monkeys, rats, rabbits, and mice, even if none mimics all the features of the disease (Marietta and Murray 2012; Schuppan et al. 2009; Stoven et al. 2013). Nevertheless for our purpose, the mice are good candidates due to their shorter lifespan, the advances in their genetic manipulation, and the ability to radically alter their intestinal microbiota. Furthermore, a T cell adjuvant effect of L. casei has been demonstrated in a mouse model of gluten sensitivity (D'Arienzo et al. 2008), and the potential of L. casei to reduce gliadin-specific enteropathy has been observed in this same mouse model (D'Arienzo et al. 2011). In fact, administration of L. casei reduces villous atrophy and the abnormal homeostasis of the gut mucosa. These results support the use of live L. casei to deliver PEP in the intestinal lumen and motivate additional studies to check the ability of the developed strain to degrade the 33-mer in vivo.
In summary, we have engineered a food-grade strain of L. casei to deliver Mx PEP activity into the gut environment. The strain has stably integrated into the chromosome a codon-optimized gene encoding Mx PEP, which is constitutively expressed and whose product is secreted. The secreted enzyme degrades the immunotoxic 33-mer peptide that plays a pivotal role in CD pathogenesis. This strain survives simulated gastrointestinal transit while maintaining the ability to produce and secrete Mx PEP, and could be a good system to deliver Mx PEP to the duodenum of celiac patients.
References
Alvarez MA, Herrero M, Suárez JE (1998) The site-specific recombination system of the Lactobacillus species bacteriophage A2 integrates in gram-positive and gram-negative bacteria. Virology 250(1):185–193. doi:10.1006/viro.1998.9353
Axelsson L (1998) Lactic acid bacteria: Classification and physiology. In: Salminen SVWA (ed) Lactic acid bacteria: Microbiological and functional aspects, 2nd edn. CRC, Boca Raton, pp 1–66
Bethune MT, Khosla C (2012) Oral enzyme therapy for celiac sprue. Methods Enzymol 502:241–271
Carr FJ, Chill D, Maida N (2002) The lactic acid bacteria: a literature survey. Crit Rev Microbiol 28(4):281–370. doi:10.1080/1040-840291046759
Charteris, Kelly, Morelli, Collins (1998) Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol 84(5):759–768. doi:10.1046/j.1365-2672.1998.00407.x
Clarke G, Cryan JF, Dinan TG, Quigley EM (2012) Review article: probiotics for the treatment of irritable bowel syndrome—focus on lactic acid bacteria. Aliment Pharmacol Ther 35(4):403–413. doi:10.1111/j.1365-2036.2011.04965.x
Comino I, Real A, Vivas S, Síglez MÁ, Caminero A, Nistal E, Casqueiro J, Rodríguez-Herrera A, Cebolla Á, Sousa C (2012) Monitoring of gluten-free diet compliance in celiac patients by assessment of gliadin 33-mer equivalent epitopes in feces. Am J Clin Nutr 95(3):670–677. doi:10.3945/ajcn.111.026708
Conway PL, Gorbach SL, Goldin BR (1987) Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci 70(1):1–12. doi:10.3168/jds.S0022-0302(87)79974-3
Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, Sategna Guidetti C, Usai P, Cesari P, Pelli MA, Loperfido S, Volta U, Calabro A, Certo M (2001) Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet 358(9279):356–361. doi:http://www.ncbi.nlm.nih.gov/pubmed/11502314
Daniel C, Roussel Y, Kleerebezem M, Pot B (2011) Recombinant lactic acid bacteria as mucosal biotherapeutic agents. Trends Biotechnol 29(10):499–508. doi:10.1016/j.tibtech.2011.05.002
D'Arienzo R, Maurano F, Luongo D, Mazzarella G, Stefanile R, Troncone R, Auricchio S, Ricca E, David C, Rossi M (2008) Adjuvant effect of Lactobacillus casei in a mouse model of gluten sensitivity. Immunol Lett 119(1–2):78–83 doi:10.1016/j.imlet.200804.006
D'Arienzo R, Stefanile R, Maurano F, Mazzarella G, Ricca E, Troncone R, Auricchio S, Rossi M (2011) Immunomodulatory effects of Lactobacillus casei administration in a mouse model of gliadin-sensitive enteropathy. Scand J Immunol 74(4):335–341. doi:10.1111/j.1365-3083.2011.02582.x
del Rio B, Dattwyler RJ, Aroso M, Neves V, Meirelles L, Seegers JFML, Gomes-Solecki M (2008) Oral immunization with recombinant Lactobacillus plantarum induces a protective immune response in mice with Lyme disease. Clin Vaccine Immunol 15(9):1429–1435. doi:10.1128/cvi.00169-08
del Rio B, Fuente JL, Neves V, Dattwyler R, Seegers JFML, Gomes-Solecki M (2010) Platform technology to deliver prophylactic molecules orally: an example using the class A select agent Yersinia pestis. Vaccine 28(41):6714–6722. doi:10.1016/j.vaccine.2010.07.084
Fernández de Palencia P, López P, Corbí AL, Peláez C, Requena T (2008) Probiotic strains: survival under simulated gastrointestinal conditions, in vitro adhesion to Caco-2 cells and effect on cytokine secretion. Eur Food Res Technol 227(5):1475–1484. doi:10.1007/s00217-008-0870-6
Gass J, Ehren J, Strohmeier G, Isaacs I, Khosla C (2005) Fermentation, purification, formulation, and pharmacological evaluation of a prolyl endopeptidase from Myxococcus xanthus: implications for Celiac Sprue therapy. Biotechnol Bioeng 92(6):674–684. doi:10.1002/bit.20643
Green PH, Jabri B (2003) Coeliac disease. Lancet 362(9381):383–391
Gujral N, Freeman HJ, Thomson AB (2012) Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol 18(42):6036–6059. doi:10.3748/wjg.v18.i42.6036
Hausch F, Shan L, Santiago NA, Gray GM, Khosla C (2002) Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol 283(4):G996–G1003. doi:10.1152/ajpgi.00136.2002
Jankovic I, Ventura M, Meylan V, Rouvet M, Elli M, Zink R (2003) Contribution of aggregation-promoting factor to maintenance of cell shape in Lactobacillus gasseri 4B2. J Bacteriol 185(11):3288–3296. doi:10.1128/jb.185.11.3288-3296.2003
Janssen GR, Bibb MJ (1993) Derivatives of pUC18 that have BgfII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene 124(1):133–134. doi:10.1016/0378-1119(93)90774-W
Kabashima T, Fujii M, Meng Y, Ito K, Yoshimoto T (1998) Prolyl endopeptidase from Sphingomonas capsulata: isolation and characterization of the enzyme and nucleotide sequence of the gene. Arch Biochem Biophys 358(1):141–148. doi:10.1006/abbi.1998.0836
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Marcotte H, Ferrari S, Cesena C, Hammarström L, Morelli L, Pozzi G, Oggioni MR (2004) The aggregation-promoting factor of Lactobacillus crispatus M247 and its genetic locus. J Appl Microbiol 97(4):749–756. doi:10.1111/j.1365-2672.2004.02364.x
Marietta EV, Murray JA (2012) Animal models to study gluten sensitivity. Semin Immunopathol 34(4):497–511. doi:10.1007/s00281-012-0315-y
Marteau P, Minekus M, Havenaar R, Huisin’t Veld JHJ (1997) Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J Dairy Sci 80(6):1031–1037. doi:10.3168/jds.S0022-0302(97)76027-2
Marti T, Molberg O, Li Q, Gray GM, Khosla C, Sollid LM (2005) Prolyl endopeptidase-mediated destruction of T cell epitopes in whole gluten: chemical and immunological characterization. J Pharmacol Exp Ther 312(1):19–26. doi:10.1124/jpet.104.073312
Martin MC, Alonso JC, Suarez JE, Alvarez MA (2000) Generation of food-grade recombinant lactic acid bacterium strains by site-specific recombination. Appl Environ Microbiol 66(6):2599–2604
Martin MC, Fernandez M, Martin-Alonso JM, Parra F, Boga JA, Alvarez MA (2004) Nisin-controlled expression of Norwalk virus VP60 protein in Lactobacillus casei. FEMS Microbiol Lett 237(2):385–391. doi:10.1016/j.femsle.2004.07.002
Martin MC, Pant N, Ladero V, Gunaydin G, Andersen KK, Alvarez B, Martinez N, Alvarez MA, Hammarstrom L, Marcotte H (2011) Integrative expression system for delivery of antibody fragments by lactobacilli. Appl Environ Microbiol 77(6):2174–2179. doi:10.1128/AEM.02690-10
Mazé A, Boël G, Zúñiga M, Bourand A, Loux V, Yebra MJ, Monedero V, Correia K, Jacques N, Beaufils S, Poncet S, Joyet P, Milohanic E, Casarégola S, Auffray Y, Pérez-Martínez G, Gibrat J-F, Zagorec M, Francke C, Hartke A, Deutscher J (2010) Complete genome sequence of the probiotic Lactobacillus casei strain BL23. J Bacteriol 192(10):2647–2648. doi:10.1128/jb.00076-10
Nyanga-Koumou AP, Ouoba LII, Kobawila SC, Louembe D (2012) Response mechanisms of lactic acid bacteria to alkaline environments: a review. Crit Rev Microbiol 38(3):185–190. doi:10.3109/1040841X.2011.640978
Parish JH (1986) Genetic manipulation of Streptomyces—a laboratory manual: by D A Hopwood, M J Bibb, K F Chatter; T Kieser CJ Bruton, H M Kieser, D J Lydiate, C P Smith, J M Ward and H Schrempf. pp 356. The John Innes Foundation, Norwich, UK and Cold Spring Harbour Laboratory. 1985. ISBN 0-7084-0336-0. Biochem Educ 14(4):196–196
Piper JL, Gray GM, Khosla C (2004) Effect of prolyl endopeptidase on digestive-resistant gliadin peptides in vivo. J Pharmacol Exp Ther 311(1):213–219. doi:10.1124/jpet.104.068429
Pyle GG, Paaso B, Anderson BE, Allen DD, Marti T, Li Q, Siegel M, Khosla C, Gray GM (2005) Effect of pretreatment of food gluten with prolyl endopeptidase on gluten-induced malabsorption in celiac sprue. Clin Gastroenterol Hepatol 3(7):687–694
Qiao SW, Bergseng E, Molberg O, Xia J, Fleckenstein B, Khosla C, Sollid LM (2004) Antigen presentation to celiac lesion-derived T cells of a 33-mer gliadin peptide naturally formed by gastrointestinal digestion. J Immunol 173(3):1757–1762
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sánchez B, Schmitter JM, Urdaci MC (2009) Identification of novel proteins secreted by Lactobacillus rhamnosus GG grown in de Mann–Rogosa–Sharpe broth. Lett Appl Microbiol 48(5):618–622. doi:10.1111/j.1472-765X.2009.02579.x
Savijoki K, Ingmer H, Varmanen P (2006) Proteolytic systems of lactic acid bacteria. Appl Microbiol Biotechnol 71(4):394–406. doi:10.1007/s00253-006-0427-1
Scheirlinck T, Mahillon J, Joos H, Dhaese P, Michiels F (1989) Integration and expression of alpha-amylase and endoglucanase genes in the Lactobacillus plantarum chromosome. Appl Environ Microbiol 55(9):2130–2137
Schuppan D, Junker Y, Barisani D (2009) Celiac disease: from pathogenesis to novel therapies. Gastroenterology 137(6):1912–1933. doi:10.1053/j.gastro.2009.09.008
Sealey-Voyksner JA, Khosla C, Voyksner RD, Jorgenson JW (2010) Novel aspects of quantitation of immunogenic wheat gluten peptides by liquid chromatography–mass spectrometry/mass spectrometry. J Chromatogr A 1217(25):4167–4183. doi:10.1016/j.chroma.2010.01.067
Shan L, Molberg O, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C (2002) Structural basis for gluten intolerance in celiac sprue. Science 297(5590):2275–2279. doi:10.1126/science.1074129
Shan L, Marti T, Sollid LM, Gray GM, Khosla C (2004) Comparative biochemical analysis of three bacterial prolyl endopeptidases: implications for coeliac sprue. Biochem J 383(Pt 2):311–318. doi:10.1042/BJ20040907
Stoven S, Murray JA, Marietta EV (2013) Latest in vitro and in vivo models of celiac disease. Expert Opin Drug Discov 8(4):445–457. doi:10.1517/17460441.2013.761203
Tuohy KM, Probert HM, Smejkal CW, Gibson GR (2003) Using probiotics and prebiotics to improve gut health. Drug Discov Today 8(15):692–700. doi:10.1016/S1359-6446(03)02746-6
Turroni F, Ventura M, Buttó LF, Duranti S, O'Toole PW, Motherway MOC, Sinderen D (2013) Molecular dialogue between the human gut microbiota and the host: a Lactobacillus and Bifidobacterium perspective. Cell Mol Life Sci 1–21. doi: 10.1007/s00018-013-1318-0
van Overbeek FM, Uil-Dieterman IG, Mol IW, Kohler-Brands L, Heymans HS, Mulder CJ (1997) The daily gluten intake in relatives of patients with coeliac disease compared with that of the general Dutch population. Eur J Gastroenterol Hepatol 9(11):1097–1099
Ventura M, Jankovic I, Walker DC, Pridmore RD, Zink R (2002) Identification and characterization of novel surface proteins in Lactobacillus johnsonii and Lactobacillus gasseri. Appl Environ Microbiol 68(12):6172–6181. doi:10.1128/aem.68.12.6172-6181.2002
Vieira J, Messing J (1991) New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene 100:189–194
Wang J, Zhong Z, Zhang W, Bao Q, Wei A, Meng H, Zhang H (2012) Comparative analysis of the gene expression profile of probiotic Lactobacillus casei Zhang with and without fermented milk as a vehicle during transit in a simulated gastrointestinal tract. Res Microbiol 163(5):357–365. doi:10.1016/j.resmic.2012.04.002
Wei M-Q, Rush CM, Norman JM, Hafner LM, Epping RJ, Timms P (1995) An improved method for the transformation of Lactobacillus strains using electroporation. J Microbiol Methods 21(1):97–109. doi:10.1016/0167-7012(94)00038-9
Wells J, Mercenier A (2008) Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol 6(5):349–362. doi:10.1038/nrmicro1040
Yoshimoto T, Walter R, Tsuru D (1980) Proline-specific endopeptidase from Flavobacterium. Purification and properties. J Biol Chem 255(10):4786–4792
Yoshimoto T, Kanatani A, Shimoda T, Inaoka T, Kokubo T, Tsuru D (1991) Prolyl endopeptidase from Flavobacterium meningosepticum: cloning and sequencing of the enzyme gene. J Biochem 110(6):873–878
Acknowledgments
The authors are grateful to Isabel Cuesta and Jorge R. Álvarez-Buylla for their technical assistance. This research was supported by project 201370E094 from the CSIC. P.A. is the recipient of a fellowship from FICYT (BP09093) and B.D.R. is a beneficiary of a JAE-DOC contract (CSIC). C.K. is supported by a grant from the NIH (R01 DK 063158).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alvarez-Sieiro, P., Martin, M.C., Redruello, B. et al. Generation of food-grade recombinant Lactobacillus casei delivering Myxococcus xanthus prolyl endopeptidase. Appl Microbiol Biotechnol 98, 6689–6700 (2014). https://doi.org/10.1007/s00253-014-5730-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5730-7