Abstract
The protein 3D8 single-chain variable fragment (3D8 scFv) has potential anti-viral activity due to its ability to penetrate into cells and hydrolyze nucleic acids. Probiotic Lactobacillus paracasei engineered to secrete 3D8 scFv for oral administration was used to test the anti-viral effects of 3D8 scFv against gastrointestinal virus infections. We found that injection of 3D8 scFv into the intestinal lumen resulted in the penetration of 3D8 scFv into the intestinal villi and lamina propria. 3D8 scFv secreted from engineered L. paracasei retained its cell-penetrating and nucleic acid-hydrolyzing activities, which were previously shown with 3D8 scFv expressed in Escherichia coli. Pretreatment of RAW264.7 cells with 3D8 scFv purified from L. paracasei prevented apoptosis induction by murine norovirus infection and decreased messenger RNA (mRNA) expression of the viral capsid protein VP1. In a mouse model, oral administration of the engineered L. paracasei prior to murine norovirus infection reduced the expression level of mRNA encoding viral polymerase. Taken together, these results suggest that L. paracasei secreting 3D8 scFv provides a basis for the development of ingestible anti-viral probiotics active against gastrointestinal viral infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Outbreaks of human norovirus, a gastrointestinal virus, are continuous and worldwide; however, there are no prescribed vaccines or specific treatments for infected patients (Arias et al. 2013). At present, research on human norovirus is limited due to its incompatibility with in vitro culture conditions (Duizer et al. 2004). Murine norovirus (MNV) strain 1 shares many common characteristics with human norovirus and thus has acted as a surrogate for studies on mechanisms of human norovirus infection as well as its prevention and treatment (Karst et al. 2003; Wobus et al. 2006).
With respect to developing new methods for treating gastrointestinal viruses such as norovirus, the possibility of using live probiotics for the delivery of therapeutic proteins to the intestinal mucosal surface is based on the fact that probiotics constitute the normal bacterial population of the intestine and thus have been proven to be safe and beneficial for human health (Pagnini et al. 2010; Wells and Mercenier 2008). Indeed, identifying approaches to deliver therapeutic agents to the mucosal surface has been a primary theme of various lines of research employing probiotics as a delivery vehicle. Several probiotics have been engineered to deliver anti-viral proteins, including Escherichia coli Nissle 1917, secreting an HIV fusion inhibitor peptide (Rao et al. 2005), Lactobacillus jensenii, secreting RANTES and a CCR5 antagonist against HIV infection (Vangelista et al. 2010), and Lactobacillus casei, expressing a heavy-chain antibody fragment against rotavirus (Pant et al. 2006). Because of the ease of administration, the oral route is considered to be the most practical way to deliver drugs to the intestinal mucosa. However, the harsh acidic pH and enzymatic barrier of the stomach and intestinal environment are major considerations influencing the retention time and activity of anti-viral protein drugs (Morishita and Peppas 2006; Hamman et al. 2005).
The 3D8 single-chain variable fragment (3D8 scFv) is an anti-nucleic acid antibody that can bind and hydrolyze nucleic acid without sequence specificity (Kim et al. 2006). Previous studies have shown that 3D8 scFv can penetrate into cells via a caveolae-lipid raft pathway (Jang et al. 2009). The ability of 3D8 scFv to hydrolyze nucleic acid and penetrate into cells has prompted its use in various anti-viral applications. For example, treatment of porcine kidney cells with 3D8 scFv confers resistance against classical swine fever virus infection (Jun et al. 2010).
In this study, we investigated the possibility of using Lactobacillus paracasei (L. paracasei) as a delivery system for 3D8 scFv in order to utilize its anti-viral effects against gastrointestinal virus infection, using murine norovirus (MNV) as a model. Specifically, we engineered L. paracasei secreting 3D8 scFv to facilitate the delivery of 3D8 scFv into the intestinal cells. The mouse-originated 3D8 scFv gene sequence was modified to eliminate rare codons and achieve a suitable guanine-cytosine (GC) content for optimal expression in L. paracasei (Nakamura et al. 2000). 3D8 scFv expressed by L. paracasei retained its nucleic acid-hydrolyzing and cell penetration ability. Pretreatment of murine macrophage RAW264.7 cells with 3D8 scFv before MNV infection prevented apoptosis induction by MNV and reduced the expression level of messenger RNA (mRNA) encoding viral capsid protein VP1. We also showed that 3D8 scFv injected into the intestinal lumen was able to penetrate into the intestinal villi and lamina propria. Finally, mice given three oral administrations of L. paracasei secreting 3D8 scFv before infection with MNV exhibited lower expression of mRNA encoding viral polymerase.
Material and method
Bacterial culture conditions
E. coli DH5α was cultured aerobically in LB media at 37 °C. Transformed E. coli was selected on LB plates containing 5 μg/ml chloramphenicol. L. paracasei ATCC 334 was kindly provided from Dr. Jos Seegers (Falcobio, Netherlands). Wild-type L. paracasei was cultured in antibiotic-free de Man, Rogosa, and Sharpe (MRS) media anaerobically. Expression vectors were transformed into L. paracasei by electroporation using Bio-Rad Gene Pulser Xcell electroporator (Bio-Rad Laboratories, Hercules, CA, USA) as previously described (Natori et al. 1990). Transformed L. paracasei was cultured anaerobically in MRS media containing 3 μg/ml chloramphenicol.
Cloning the 3D8 scFv gene into an L. paracasei pSLP-LDH expression vector
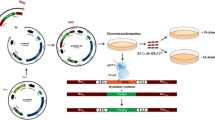
A pSLP111.3 expression vector provided by Dr. Jos Seegers (Falcobio, Netherland) was modified to replace the xylose-inducible promoter with a lactate dehydrogenate (LDH) constitutive promoter (Oozeer et al. 2005). Codon-optimized 3D8 scFv was chemically synthesized (IDT, Coralville, IA, USA). An original or codon-optimized 3D8 scFv gene (975 bp) was cloned into pSLP-LDH vector to express secreted or cell wall-anchored 3D8 scFv in L. paracasei. To construct a secreted form of 3D8 scFv, the 3D8 scFv gene fused with a protein A tag was placed downstream of a SlpA secretion signal using NcoI and AscI restriction enzyme sites. In addition, a stop codon (TAA) was added after the protein A tag to prevent fusion of the 3D8 scFv with a cell wall-anchoring domain of PrtP (anchor sequence). Conversely, a cell wall-anchored form of 3D8 scFv was generated by inserting the 3D8 scFv gene using NcoI and AscI restriction enzyme sites upstream of the anchor sequence (Fig. S1 and Fig. 1a).
Comparison of 3D8 scFv expression between L. paracasei expressing codon-optimized and original sequence 3D8 scFv. a Expression cassette designed to express 3D8 scFv as a secreted form (left) or anchored on the L. paracasei cell wall (right). P LDH lactate dehydrogenase promoter, Ss SlpA secretion signal, Pro A protein A tag, As anchor sequence. b Coomassie staining of 5 μg original 3D8 scFv, codon-optimized 3D8 scFv purified from L. paracasei, and 3D8 scFv purified from E. coli. Ori scFv original 3D8 scFv, Opt scFv codon-optimized 3D8 scFv. c Western blot analysis of eluted fraction from 3D8 scFv-secreting L. paracasei in comparison with 3D8 scFv purified from E. coli. One microgram of 3D8 scFv purified from E. coli and 20 μl from eluted fraction of codon-optimized or original sequence 3D8 scFv were subjected to SDS-PAGE and Western blot with anti-3D8 scFv antibody or anti-protein A antibody. d Relative intensity of Western blot signals was quantified by Western blot densitometry. Each column represented the intensity of the above correlated Western blot band. e Flow cytometry analysis of L. paracasei expressing cell wall-anchored form of codon-optimized 3D8 scFv (red line) or original 3D8 scFv (black line). Wild-type L. paracasei was illustrated by gray line
Flow cytometry
L . paracasei transformed with pSLP-LDH-3D8 scFv (codon-optimized and original 3D8 scFv gene sequence) designed to be anchored to the cell wall or wild-type L. paracasei was incubated with anti-3D8 scFv antibody (AbFrontier, Seoul, Korea) followed by tetramethylrhodamine (TRITC)-conjugated anti-rabbit antibody (Abcam, Cambridge, MA, USA). Flow cytometry was performed using a GUAVA instrument according to the manufacturer’s instructions (Millipore, Bedford, MA, USA). Results were analyzed using GUAVA software.
Bacterial expression, purification, and Western blotting of 3D8 scFv secreted from E. coli BL21(DE3)pLysE and L. paracasei
The expression and purification of 3D8 scFv in the supernatant of E. coli BL21(DE3)pLysE containing pIg20-3D8 scFv were perform as previously described (Kim et al. 2006). In order to purify 3D8 scFv from L. paracasei containing pSLP-LDH-3D8 scFv secret form (codon-optimized and original sequence), supernatant of cultured L. paracasei until OD600 = 2 was collected. Supernatant was then purified by IgG-sepharose affinity chromatography. Eluted fractions were concentrated using a 10-kDa filter cutoff Centricon (Millipore). Purified protein was analyzed by 12 % SDS-PAGE and transferred to PVDF membrane (GE Healthcare, Piscataway, NJ, USA) by semidry transfer. The membrane was incubated with anti-protein A antibody (Sigma-Aldrich, St. Louis, MO, USA) or anti-3D8 scFv antibody (AbFrontier) overnight at 4 °C. Then, the membrane was incubated with HRP-conjugated anti-mouse or anti-rabbit antibody (Cell Signaling Technology, Inc., Danver, MA, USA), respectively. Western blot signals were quantified by Western blot densitometry software, ImageJ (Schneider et al. 2012)
Virus and cells
RAW264.7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) media (Hyclone, Logan, UT, USA) supplemented with 10 % (v/v) heat-inactivated fetal bovine serum (Life Technologies, Gibco, Carlsbad, CA, USA), 100 units/ml of penicillin, 100 μg/ml streptomycin, 10 mM HEPES, and 2 mM glutamine at 37 °C in a humidified 5 % CO2 incubator. Murine norovirus MNV-1.CW1 (MNV) was generated and maintained by infection to the RAW264.7 cell line (McCartney et al. 2008).
Immunocytochemistry
RAW264.7 cells were seeded at 5 × 105 cells/well in six-well plates with coverslips and incubated overnight. Cells were then treated for 4 h with 10 μM 3D8 scFv purified from L. paracasei, corresponding to 700 μg 3D8 scFv in 2-ml DMEM media. After treatment, the cells were processed with fixation buffer (2 % paraformaldehyde in phosphate-buffered saline (PBS)) for 10 min at room temperature and followed by treatment with permeabilization buffers (1 % BSA, 0.1 % saponine, 0.1 % sodium azide in PBS) for 10 min. The cells were then incubated sequentially with anti-3D8 scFv and a TRITC-conjugated anti-rabbit antibody. Finally, the cells were mounted, stained with DAPI (Vector Laboratories, Burlingame, CA, USA), and visualized by confocal microscopy.
Quantification of MNV titer
The 50 % tissue culture infectious dose (TCID50) assay was performed as previously described (Thackray et al. 2007). Briefly, 3 × 104 RAW264.7 cells/well were seeded in 96-well plates, to which 100 μl of 10-fold serial dilutions of MNV in DMEM media was added. After 1 week of incubation at 37 °C, 5 % CO2, cytopathic effects were evaluated by microscopy. The TCID50 value was calculated according to the Reed-Muench equation.
MTT assay
RAW264.7 cells were seeded at 5 × 103 cells/well in a 96-well plate and cultured overnight. The cells were then treated for 4 h with 5 μM 3D8 scFv purified from L. paracasei, corresponding to 350 μg 3D8 scFv in 2-ml DMEM media; controls cells were not treated. Next, the cells were infected with MNV (multiplicity of infection (MOI) = 2) and incubated for 24 h. Cell viability was determined by colorimetric [3-(4,5-dimethylthiazol-2-yl)-2,5-zolium bromide] (MTT) (Sigma) absorbance at 540 nm.
Oral administration of bacteria and virus to ICR mice
Four-week-old, specific pathogen-free and MNV-seronegative female imprinting control region (ICR) (CD-1®) outbred mice (Koatech, Korea) were housed under standard laboratory conditions. All experiments were carried out in compliance with the protocols approved by the Ethics Committee for Animal Experiments of Sungkyunkwan University. Mice were assigned to four experimental groups, with three mice per group. For maintaining expression vectors in L. paracasei and partially removing of native bacteria in intestine, all mice were given drinking water containing 3 μg/ml chloramphenicol for 24 h. Food and water were withdrawn 18 h before mice received any treatment. The treatment scheme for administration of bacteria and virus infection is illustrated in Fig. S3. In the experimental group, mice were fed 108 CFU of L. paracasei secreting 3D8 scFv or L. paracasei expressing cell wall-anchored 3D8 scFv once every 2 days for 6 days, followed by two times infection with 3 × 104 PFU of MNV every 24 h. In the MNV-positive group, mice were infected with MNV without any pretreatment. After feeding, food and water containing 3 μg/ml chloramphenicol were returned to the cages. In the negative group, mice were given PBS once every 2 days for 6 days without MNV infection. Stainless steel feeding needles (20 gauge) were used for all oral gavage procedures. Total intestinal tissue was sampled 24 h after infection. Tissue was homogenized in liquid nitrogen for preparation of total RNA. Complementary DNAs (cDNAs) synthesized from RNA were subjected to quantitative real-time PCR (qRT-PCR) with primers specific for viral polymerase or cytokines. The statistics of this experiment was calculated based on three independent experiments.
RNA quantification
Total RNA from cells or intestinal tissue was extracted with TRI reagent (Molecular Research Center, Inc., Cincinnati, OH, USA). cDNA was then synthesized from 5 μg of total RNA using random hexamers and Moloney murine leukemia virus (MMLV) reverse transcriptase (Bioneer, Daejeon, Korea). The levels of expression of MNV major capsid protein (VP1), the anti-apoptosis protein survivin (Bok et al. 2009), MNV polymerase (Hsu et al. 2005), and other cytokines were determined by qRT-PCR (Yeom et al. 2009). Each sample was analyzed in triplicate. The specific primers used for qRT-PCR are listed in Table S1.
Synthesis of RNA encoding MNV polymerase
The MNV polymerase gene was amplified from cDNA of RAW264.7 cells infected with MNV. The MNV polymerase gene was then cloned into the pGEM-Teasy vector (Promega, Madison, WI, USA), yielding the pGEM-MNV polymerase vector. RNA for MNV polymerase was generated using a T7 High Yield RNA Synthesis kit (New England BioLabs, Ipswich, MA, USA). MNV polymerase RNA was incubated with 3D8 scFv protein for 15-min intervals. Samples were run on a 1 % agarose gel to visualize the hydrolysis pattern.
Immunohistochemistry
Mice were anesthetized and dissected to access their peritoneal cavities. Then, 100 μg of purified 3D8 scFv was injected into the intestinal lumen and incubated for 4 h. The section of intestine downstream of the injection site was collected, fixed in 10 % PFA for 1 h, and immersed in 4 % sucrose overnight. Tissue samples were embedded in freezing medium and sectioned using a cryomicrotome (Leica CM3050S, Wetzlar, Germany). Tissue samples were sectioned at a thickness of 4 μm and mounted on silane-coated slides. After incubation with an anti-3D8 scFv antibody, tissue sections were visualized using a TRITC-conjugated anti-rabbit antibody with a fluorescent microscope (Nikon Eclipse 80i).
Statistical analysis
GraphPad Prism (GraphPad software version 5) was used to analyze experimental results. One-way ANOVA t test was used for statistical analyses. Data are presented as the mean ± SEM.
Results
Codon optimization of 3D8 scFv gene for expression in L. paracasei
Based on the ability of 3D8 scFv to penetrate into cells and hydrolyze viral nucleic acid, we engineered L. paracasei to secrete 3D8 scFv for delivery into the intestinal lumen and subsequent penetration into the epithelial layer and lamina propria for prevention of MNV infection. We also developed a form of 3D8 scFv anchored on the L. paracasei cell wall as a control for our hypothesis. Original 3D8 scFv sequence (accession number AF232220 for variable heavy-chain sequence and AF232221 for variable light-chain sequence) contains rare codons for L. paracasei expression distributing throughout its gene sequence (Fig. S2) and has a GC content of 52 %, which is much higher than the 40 % GC observed for most L. paracasei genes (Nakamura et al. 2000). These unique features of 3D8 scFv compelled us to perform codon optimization in order to eliminate rare codons and tune the GC content for optimized expression in L. paracasei. In the codon-optimized 3D8 scFv sequence (accession number KJ939261), the rare codons were eliminated and GC content was turned to 41 %. Using the original and codon-optimized gene sequences, we generated four constructs, consisting of the original and codon-optimized forms of the 3D8 scFv gene as secreted or cell wall-anchored forms (Fig. 1a). These gene constructs were then used to transform into L. paracasei by electroporation. The expression of 3D8 scFv in secreted and anchored forms was evaluated to determine the effect of codon optimization on 3D8 scFv expression.
In order to compare the effect of codon optimization on the amount of 3D8 scFv secreted by L. paracasei, we purified 3D8 scFv in 1-l culture supernatant of L. paracasei containing original 3D8 scFv or codon-optimized 3D8 scFv and collect 10 ml of elution fraction. Coomassie blue staining of 5 μg purified 3D8 scFv from L. paracasei or from E. coli was performed to visualize the purity of 3D8 scFv after purification (Fig. 1b). Twenty microliters of elution fraction from original or codon-optimized 3D8 scFv together with 1 μg of 3D8 scFv purified from E. coli was subjected to Western blot analysis with anti-protein A antibody and anti-3D8 scFv antibody (Fig. 1c). The Western blot signals were quantified comparatively by Western blot densitometry (Fig. 1d). Based on the Western blot densitometry analysis, we calculated that about 410 μg of codon-optimized 3D8 scFv and about 105 μg of original 3D8 scFv were purified from 1 l of culture supernatant. This result suggested that codon optimization enhances the expression of 3D8 scFv in L. paracasei by around 4-fold. Consistent with these observations, flow cytometric analysis of the codon-optimized form of 3D8 scFv anchored on the L. paracasei cell wall revealed a significant shift in fluorescence whereas original form of 3D8 scFv anchored on L. paracasei cell wall just showed a slight shift in comparison with wild-type L. paracasei (Fig. 1e). Because codon optimization improved the expression of 3D8 scFv in L. paracasei, L. paracasei containing the codon-optimized form of 3D8 scFv was used to evaluate the anti-viral effects in vitro and in vivo challenging experiment.
Characterization of 3D8 scFv secreted from L. paracasei
RNA-hydrolyzing analysis was performed to confirm that the 3D8 scFv secreted by L. paracasei retained its intrinsic biochemical characteristics. In the RNA-hydrolyzing assay, mRNA of the MNV polymerase gene was mixed with 3D8 scFv purified from supernatant of L. paracasei secreting 3D8 scFv or with 3D8 scFv purified from E. coli or with PBS in the presence of MgCl2 at 37 °C. While the mixture of mRNA with PBS showed a slight degradation of mRNA caused by prolonged incubation period, mixture of mRNA with 3D8 scFv purified from L. paracasei or E. coli leads to an obvious time-dependent hydrolysis as shown by a smear mRNA pattern on 1 % agarose gel (Fig. 2a).
Characteristics of 3D8 scFv secreted from L. paracasei. a 3D8 scFv purified from supernatant of L. paracasei/pSLP-LDH-3D8 scFv hydrolyzes mRNA encoding MNV polymerase. Five-microgram mRNA was incubated with 5 μg purified 3D8 scFv from L. paracasei or with 3D8 scFv purified from E. coli or with PBS at 37 °C in 15-min intervals for 2 h. Samples were then subjected to electrophoresis on a 1 % agarose gel. b Penetration of 3D8 scFv into RAW264.7 cells. RAW264.7 cells were treated with 10 μM of 3D8 scFv purified from L. paracasei supernatant for 4 h, stained with anti-3D8 scFv antibody followed by TRITC-conjugated anti-rabbit antibody. Cells were mounted using DAPI and visualized under confocal microcopy, 100× magnification
In order to test the ability of 3D8 scFv purified from L. paracasei to penetrate into cells, RAW264.7 cells were pretreated with 10 μM 3D8 scFv (corresponds to 700 μg 3D8 scFv in 2-ml DMEM media) for 4 h before staining with an anti-3D8 scFv antibody. Confocal microscopy analysis of 3D8 scFv-treated RAW264.7 cells revealed 3D8 scFv signal in the cytosol, indicating the ability of 3D8 scFv secreted by L. paracasei to penetrate into RAW264.7 cells (Fig. 2b). The RNA hydrolysis assay and the cell penetration test proved that the 3D8 scFv secreted by L. paracasei retained its original characteristics.
3D8 scFv secreted from L. paracasei protects RAW264.7 cells from MNV infection
RAW264.7 cells were treated with 5 μM 3D8 scFv (corresponds to 350 μg 3D8 scFv in 2-ml DMEM media) for 4 h before MNV infection (MOI = 2). Cells were collected 24 h postinfection for RNA analysis, while cytopathic effects were observed 36 h postinfection. MNV-infected RAW264.7 cells exhibited cytopathic effects, such as rounding of cells, cell membrane rupture, and cell death triggered by apoptosis. Conversely, RAW264.7 cells treated with 3D8 scFv prior to infection with MNV exhibited normal cell morphology (Fig. 3a). We also used an MTT assay to evaluate cell viability and qRT-PCR to determine the expression of the MNV coating protein, VP1, and the anti-apoptosis protein, survivin. MNV triggers apoptosis in infected cells, and thus, the cell viability and mRNA expression of survivin were decreased by MNV infection while the mRNA expression of survivin in cells treated with 3D8 scFv was similar to that of mock-infected cells (Fig. 3b, c). Moreover, compared with cells infected with MNV without 3D8 scFv pretreatment, the expression of the mRNA encoding the viral capsid protein VP1 in 3D8 scFv-treated cells was significantly lower (Fig. 3d). Together with the biochemical activity of 3D8 scFv, especially its RNA-hydrolyzing activity, these results suggest that 3D8 scFv protected RAW264.7 cell against MNV infection.
3D8 scFv purified from L. paracasei protects RAW264.7 cells from MNV infection. a Morphology of uninfected or MNV-infected RAW264.7 cells (MOI = 2) at 36 h postinfection that pretreated with or without 3D8 scFv. b, c Measurement of cell viability by MTT assay and relative mRNA expression level of the anti-apoptosis factor survivin by qRT-PCR at 24 h postinfection. d Relative expression level of the viral capsid protein VP1 quantified by qRT-PCR at 24 h postinfection. mRNA levels were normalized to GAPDH mRNA, and relative expression was determined by the delta delta CT method. Data are presented as the mean ± SE. *p < 0.05, **p < 0.01, statistical significant from RAW 264.7 pretreated with 3D8 scFv, one-way ANOVA t test
Penetration of 3D8 scFv into intestinal epithelial cells
In order to evaluate the possibility that 3D8 scFv penetrates into the intestinal tissue, 100 μg of purified 3D8 scFv was injected into the intestinal lumen of mice, incubated for 4 h, and subjected to immunohistochemistry. Intestinal sections were incubated with anti-3D8 scFv antibody, followed by staining with a TRITC-conjugated anti-rabbit antibody. Under fluorescence microscopy, the signal of 3D8 scFv is apparent at the villi, lamina propria, and muscular propria (Fig. 4).
Penetration of 3D8 scFv into the gastrointestinal tract. Mouse intestines with 3D8 scFv injected into intestinal lumen or without any treatment were sectioned, stained with an anti-3D8 scFv antibody followed by TRITC-conjugated anti-rabbit antibody. Tissues were mounted in DAPI and visualized using fluorescence microscopy at a 100× and b 400× magnification
3D8 scFv secreted from L. paracasei protects mice from MNV infection
In order to confirm that oral administration of L. paracasei secreting 3D8 scFv could protect mice from MNV infection, L. paracasei containing pSLP-LDH vector only or containing a secreted or cell wall-anchored form of 3D8 scFv was fed to mice three times prior to virus infection. In comparison with mice that did not receive any pretreatment before MNV infection, mice pretreated with L. paracasei expressing an anchored form of 3D8 scFv or L. paracasei transformed with pSLP-LDH vector showed 3.0- and 1.9-fold reduction in mRNA expression level of the MNV polymerase gene, respectively. In addition, mice pretreated with L. paracasei expressing secret form of 3D8 scFv showed 20.1-fold reduction in mRNA expression level of the MNV polymerase gene than mock-treated mice before MNV infection (Fig. 5). This result showed that oral administration of L. paracasei secreting 3D8 scFv reduced MNV infection in the mouse intestine. Additionally, in order to determine whether L. paracasei stimulated an immune response that contributed to the anti-viral effect, we performed qRT-PCR on tissues in the intestine to determine expression of various cytokines. There was no significant difference in mRNA expression levels of IFN-β, iNOS, TNF-α, or IL-6 among the different treatment groups (Fig. 6).
Expression of MNV polymerase in intestine of MNV-infected mice without any pretreatment or pretreated with L. paracasei expressing secreted or cell wall-anchored 3D8 scFv or L. paracasei containing pSLP-LDH. Relative mRNA expression of MNV polymerase quantified by qRT-PCR. Mice were fed L. paracasei expressing a secreted or cell wall-anchored form of 3D8 scFv or L. paracasei containing pSLP-LDH vector or without treatment three times in 6 days before twice MNV challenge. The relative mRNA level of MNV polymerase was normalized to the GAPDH mRNA level and calculated using the delta delta CT method. Experiments were performed in triplicate with three mice in each group. qRT-PCR data are presented as the mean ± SE. *p < 0.05, ***p < 0.001, statistical significant of MNV polymerase relative mRNA level of MNV from mock-treated mice group that was infected with MNV compared with other groups, one-way ANOVA t test
Cytokine profiles of MNV-infected mice without any pretreatment or pretreated with L. paracasei expressing secreted or cell wall-anchored 3D8 scFv or L. paracasei containing pSLP-LDH. Mice were fed L. paracasei expressing a secreted or cell wall-anchored form of 3D8 scFv or with L. paracasei containing pSLP-LDH vector or without any pretreatment three times in 6 days before two times of MNV challenge. At 24 h after virus challenge, intestines were collected and total mRNA was prepared. cDNA generated from the collected mRNA was subjected to qRT-PCR for the indicated cytokines using specific primers. The relative mRNA level of cytokines was normalized to the GAPDH mRNA level and calculated using the delta delta CT method. Experiments were performed in triplicate with three mice in each group. qRT-PCR data are presented as the mean ± SE. Statistical data were analyzed by one-way ANOVA t test
Discussion
Over the last two decades, the application of probiotics in the anti-viral field has consisted primarily of anchoring viral antigens on the cell wall of probiotics in order to trigger an immune response (Seegers. 2002; Lee et al. 2006) or using probiotic-secreting proteins that act on cell surfaces to interrupt fusion between cellular and viral membranes (Rao et al. 2005; Vangelista et al. 2010). In a different approach, we investigated the possibility of using 3D8 scFv secreted by L. paracasei to penetrate into intestinal cells to hydrolyze viral nucleic acids in order to achieve an anti-viral activity.
Together with 3D8 scFv, numerous antibodies have been discovered with various catalytic activities, including DNase, RNase, peptidase, and amidase activities (Shuster et al. 1992; Hifumi et al. 2013). Exogenous treatment with anti-DNA antibodies results in nuclear localization, nuclear damage, and apoptosis (Ruiz-Argüelles et al. 2003; Kozyr et al. 2002). Unlike other cytotoxic anti-DNA antibodies, 3D8 scFv only penetrates into the cytosol and does not induce cytotoxicity when administrated at low concentrations (Jang et al. 2009). In this study, while a 3D8 scFv signal in intestines of mice fed L. paracasei secreting 3D8 scFv could not be detected by immunohistochemistry (data not shown), the anti-viral effect against MNV infection conferred by feeding L. paracasei secreting 3D8 scFv was evident (Fig. 5). By injection of the purified 3D8 scFv into intestinal lumen, 3D8 scFv was clearly detected not only at intestinal epithelial layers but also at the lamina propria (Fig. 4), which could be a tentative replication site of MNV (Mumphrey et al. 2007). This result could be another evidence for the anti-viral effect provided by feeding L. paracasei secreting 3D8 scFv. The observed anti-viral activity was mainly attributed to the activity of 3D8 scFv rather than to an innate immune response triggered by L. paracasei because the levels of cytokines were not significantly different between mice that did not receive any treatment and mice fed L. paracasei containing different vectors (Fig. 6). Although other studies have observed induction of certain cytokines by oral administration of probiotics over long feeding periods (Perdigón et al. 2002; Maassen et al. 2000), the lack of an immune boost by L. paracasei in our study may be related to the short feeding period (three times in 6 days) or to the low retention rate of L. paracasei in the mouse intestine (Pant et al. 2006). In addition, a reduction in mRNA level of MNV polymerase was observed in mice treated with L. paracasei containing empty vector pSLP-LDH or pSLP-LDH-optimized 3D8 scFv anchor in comparison with mice that did not receive L. paracasei before MNV infection (Fig. 5). Besides immunomodulation mechanism, probiotics were reported to prevent viral infection by binding directly to the virus and inhibit virus attachment to the host cell receptor. It was reported that specific strains of lactobacilli and bifidobacteria are able to bind and inactivate rotavirus (Salminen et al. 2010) and vesicular stomatitis virus (Botić et al. 2007). In the case of norovirus, there was a recent research about the binding of L. casei BL23 with the protruding (P) domain from norovirus VP1 capsid protein, noroviral P-particles (Rubio-del-Campo et al. 2014). However, this noroviral P-particle model does not allow concluding whether the binding of L. casei BL23 with noroviral capsid protein resulted in an anti-viral effect or not. In further study, the mechanism by which L. paracasei itself helps in interacting and reducing murine norovirus infection should be studied more.
Because the MNV-1 (CW1) used in this study was reported to have peak of replication at 1 day postinfection and been cleared from intestinal tissue after 3 days postinfection (Karst et al. 2003), we demonstrated the preventive effect of feeding L. paracasei expressing 3D8 scFv against MNV infection after 24 h postinfection. To investigate the anti-viral effect during longer period, other MNV strains with long-term shedding in the mouse intestine such as MNV-1 (CW3) or MNV-3 should be used for further study (Thackray et al. 2007).
Previous studies have indicated that, at low concentrations, treatment with 3D8 scFv produces anti-viral effects against certain DNA viruses in vivo and in vitro (Jun et al. 2010; Lee et al. 2014); however, this had not been previously confirmed against RNA viruses in vivo. In this study, the anti-viral effect against MNV, which has a RNA genome, demonstrated the ability of 3D8 scFv to confer resistance against RNA viruses in vivo. The most important requirement for the success of our approach was the high level of expression of 3D8 scFv provided by L. paracasei. Although there are cases where codon optimization does not enhance protein expression, such as RANTES cytokine expression in L. jensenii with similar high expression level in both original and codon-optimized genes (about 0.5 mg/l), the reasons for which are unknown (Vangelista et al. 2010). On the other hand, other studies have observed significantly improved protein expression following codon optimization, including expression of Mycobacterium avium ssp. paratuberculosis antigen in Lactobacillus salivarius (Johnston et al. 2013) and expression of fluorescent proteins in gram-positive bacteria (Sastalla et al. 2009). In our study, codon optimization improved the expression of 3D8 scFv L. paracasei in both the secreted (Fig. 1c, d) and cell wall-anchored forms (Fig. 1e). Additional studies utilizing promoter engineering to achieve even better protein expression during the late exponential or stationary phase are another possibility for enhancing protein expression.
In conclusion, our results demonstrated that expression of 3D8 scFv was improved by codon optimization compared with the original sequence. Further, 3D8 scFv encoded by the codon-optimized sequence retained its characteristics and protected RAW264.7 cells from MNV infection. In vivo mouse studies revealed that injection of 3D8 scFv into the intestinal lumen resulted in its penetration into the intestinal epithelial lining of villi as well as the other part of the intestine including lamina propria and submucosa. Finally, oral administration of L. paracasei secreting 3D8 scFv reduced the relative expression level of mRNA encoding MNV polymerase while maintaining expression of intestinal cytokines at a level similar to that of mice that received no treatment or were fed L. paracasei expressing cell wall-anchored 3D8 scFv or L. paracasei containing pSLP-LDH vector. Although L. paracasei is generally regarded as safe, there remains the possibility that the antibiotic resistance marker in the expression vector could transfer to other bacteria in the environment (Steidler et al. 2003). Thus, in order to be approved as a food-grade recombinant probiotic secreting 3D8 scFv for anti-viral applications, the expression cassette of 3D8 scFv should be integrated into the genome of L. paracasei (Martín et al. 2000).
References
Arias A, Emmott E, Vashist S, Goodfellow I (2013) Progress towards the prevention and treatment of norovirus infections. Future Microbiol 8:1475–1487
Bok K, Prikhodko VG, Green KY, Sosnovtsev SV (2009) Apoptosis in murine norovirus-infected RAW264.7 cells is associated with downregulation of survivin. J Virol 83:3647
Botić T, Klingberg TD, Weingartl H, Cencič A (2007) A novel eukaryotic cell culture model to study antiviral activity of potential probiotic bacteria. Int J Food Microbiol 115:227–234
Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MP, Estes MK (2004) Laboratory efforts to cultivate noroviruses. J Gen Virol 85:79–87
Hamman JH, Enslin GM, Kotzé AF (2005) Oral delivery of peptide drugs: barriers and developments. BioDrugs 19:165–177
Hifumi E, Fujimoto N, Arakawa M, Saito E, Matsumoto S, Kobayashi N, Uda T (2013) Biochemical features of a catalytic antibody light chain, 22F6, prepared from human lymphocytes. J Biol Chem 288:19558–19568
Hsu CC, Wobus CE, Steffen EK, Riley LK, Livingston RS (2005) Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin Diagn Lab Immunol 12:1145–1151
Jang JY, Jeong JG, Jun HR, Lee SC, Kim JS, Kim YS, Kwon MH (2009) A nucleic acid-hydrolyzing antibody penetrates into cells via caveolae-mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell Mol Life Sci 66:1985–1997
Johnston C, Douarre PE, Soulimane T, Pletzer D, Weingart H, MacSharry J, Coffey A, Sleator RD, O'Mahony J (2013) Codon optimisation to improve expression of a Mycobacterium avium ssp. paratuberculosis-specific membrane-associated antigen by Lactobacillus salivarius. Pathog Dis 68:27–38
Jun HR, Pham CD, Lim SI, Lee SC, Kim YS, Park S, Kwon MH (2010) An RNA-hydrolyzing recombinant antibody exhibits an antiviral activity against classical swine fever virus. Biochem Biophys Res Commun 395:484–489
Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW (2003) STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578
Kim YR, Kim JS, Lee SH, Lee WR, Sohn JN, Chung YC, Shim HK, Lee SC, Kwon MH, Kim YS (2006) Heavy and light chain variable single domains of an anti-DNA binding antibody hydrolyze both double- and single-stranded DNAs without sequence specificity. J Biol Chem 281:15287–15295
Kozyr AV, Sashchenko LP, Kolesnikov AV, Zelenova NA, Khaidukov SV, Ignatova AN, Bobik TV, Gabibov AG, Alekberova ZS, Suchkov SV, Gnuchev NV (2002) Anti-DNA autoantibodies reveal toxicity to tumor cell lines. Immunol Lett 80:41–47
Lee JS, Poo H, Han DP, Hong SP, Kim K, Cho MW, Kim E, Sung MH, Kim CJ (2006) Mucosal immunization with surface-displayed severe acute respiratory syndrome coronavirus spike protein on Lactobacillus casei induces neutralizing antibodies in mice. J Virol 80:4079–4087
Lee G, Yu J, Cho S, Byun SJ, Kim DH, Lee TK, Kwon MH, Lee S (2014) A nucleic-acid hydrolyzing single chain antibody confers resistance to DNA virus infection in hela cells and C57BL/6 mice. PLoS Pathog 10(6):e1004208
Maassen CB, van Holten-Neelen C, Balk F, den Bak-Glashouwer MJ, Leer RJ, Laman JD, Boersma WJ, Claassen E (2000) Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 18:2613–2623
Martín MC, Alonso JC, Suárez JE, Alvarez MA (2000) Generation of food-grade recombinant lactic acid bacterium strains by site-specific recombination. Appl Environ Microbiol 66:2599–2604
McCartney SA, Thackray LB, Gitlin L, Gilfillan S, Virgin HW, Colonna M (2008) MDA-5 recognition of a MNV-1. PLoS Pathog 4:e1000108
Morishita M, Peppas NA (2006) Is the oral route possible for peptide and protein drugs delivery? Drug Discov Today 11:905–910
Mumphrey SM, Changotra H, Moore TN, Heimann-Nichols ER, Wobus CE, Reilly MJ, Moghadamfalahi M, Shukla D, Karst SM (2007) Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J Virol 81:3251–3263
Nakamura Y, Gojobori T, Ikemura T (2000) Codon usage tabulated from the international DNA sequence databases: status for the year 2000. Nucl Acids Res 28:292
Natori Y, Kano Y, Imamoto F (1990) Genetic transformation of Lactobacillus casei by electroporation. Biochimie 72:265–269
Oozeer R, Furet JP, Goupil-Feuillerat N, Anba J, Mengaud J, Corthier G (2005) Differential activities of four Lactobacillus casei promoters during bacterial transit through the gastrointestinal tracts of human-microbiota-associated mice. Appl Environ Microbiol 71:1356–1363
Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F (2010) Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A 107:454–459
Pant N, Hultberg A, Zhao Y, Svensson L, Pan-Hammarstrom Q, Johansen K, Pouwels PH, Ruggeri FM, Hermans P, Frenken L, Boren T, Marcotte H, Hammarstrom L (2006) Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lactobodies) confer protection against rotavirus-induced diarrhea. J Infect Dis 194:1580–1588
Perdigón G, Maldonado Galdeano C, Valdez JC, Medici M (2002) Interaction of lactic acid bacteria with the gut immune system. Eur J Clin Nutr 56(Suppl 4):S21–S26
Rao S, Hu S, McHugh L, Lueders K, Henry K, Zhao Q, Fekete RA, Kar S, Adhya S, Hamer DH (2005) Toward a live microbial microbicide for HIV: commensal bacteria secreting an HIV fusion inhibitor peptide. Proc Natl Acad Sci U S A 102:11993–11998
Rubio-del-Campo A, Coll-Marqués JM, Yebra MJ, Buesa J, Pérez-Martínez G, Monedero V, Rodríguez-Díaz J (2014) Noroviral p-particles as an in vitro model to assess the interactions of noroviruses with probiotics. PLoS One 9(2):e89586
Ruiz-Argüelles A, Rivadeneyra-Espinoza L, Alarcón-Segovia D (2003) Antibody penetration into living cells: pathogenic, preventive and immuno-therapeutic implications. Curr Pharm Des 9:1881–1887
Salminen S, Nybom S, Meriluoto J, Carmen Collado M, Vesterlund S, El-Nezami H (2010) Interaction of probiotics and pathogens-benefits to human health? Curr Opin Biotechnol 21:157–167
Sastalla I, Chim K, Cheung GY, Pomerantsev AP, Leppla SH (2009) Codon-optimized fluorescent proteins designed for expression in low-GC gram-positive bacteria. Appl Environ Microbiol 75:2099–2110
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Seegers JF (2002) Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol 20:508–515
Shuster AM, Gololobov GV, Kvashuk OA, Bogomolova AE, Smirnov IV, Gabibov AG (1992) DNA hydrolyzing autoantibodies. Science 256:665–667
Steidler L, Neirynck S, Huyghebaert N, Snoeck V, Vermeire A, Goddeeris B, Cox E, Remon JP, Remaut E (2003) Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol 21:785–789
Thackray LB, Wobus CE, Chachu KA, Liu B, Alegre ER, Henderson KS, Kelley ST, Virgin HW 4th (2007) Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J Virol 81:10460–10473
Vangelista L, Secchi M, Liu X, Bachi A, Jia L, Xu Q, Lusso P (2010) Engineering of Lactobacillus jensenii to secrete RANTES and a CCR5 antagonist analogue as live HIV-1 blockers. Antimicrob Agents Chemother 54:2994–3001
Wells JM, Mercenier A (2008) Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol 6:349–362
Wobus CE, Thackray LB, Virgin HW (2006) Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol 80:5104–5112
Yeom CH, Lee G, Park JH, Yu J, Park S, Yi SY, Lee HR, Hong YS, Yang J, Lee SC (2009) High dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J Transl Med 7:70
Acknowledgments
This study was supported by a grant from the Agenda (No. PJ0102012014) from the Rural Development Administration (RDA) of Korea and a grant from the Korea Institute of Ocean Science and Technology project (No. PE99154). The authors wish to thank Dr. Jos Seegers (Falcobio, Netherlands) for providing the Lactobacillus casei strain and expression vector, as well as for providing valuable advice. We also wish to thank Dr. Jai Myung Yang for providing RAW264.7 cells and murine norovirus.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 558 kb)
Rights and permissions
About this article
Cite this article
Hoang, P.M., Cho, S., Kim, K.E. et al. Development of Lactobacillus paracasei harboring nucleic acid-hydrolyzing 3D8 scFv as a preventive probiotic against murine norovirus infection. Appl Microbiol Biotechnol 99, 2793–2803 (2015). https://doi.org/10.1007/s00253-014-6257-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6257-7