Abstract
Carotenoids are a class of naturally occurring pigment, carrying out important biological functions in photosynthesis and involved in environmental responses including nutrition in organisms. Saproxanthin and myxol, which have monocyclic carotenoids with a γ-carotene skeleton, have been reported to show a stronger antioxidant activity than those with β-carotene and zeaxanthin. In this research, a yellow-orange bacterium of strain 11shimoA1 (JCM19538) was isolated from a seaweed collected at Nabeta Bay (Shizuoka, Japan). The 16S rRNA gene sequence of strain 11shimoA1 revealed more than 99.99 % similarity with those of Jejuia pallidilutea strains in the family Flavobacteriaceae. Strain 11shimoA1 synthesized two types of carotenoids. One of them was (3R, 3’R)-zeaxanthin with dicyclic structure and another was identified as (3R, 2’S)-2′-isopentenylsaproxanthin, a novel monocyclic carotenoid with pentenyl residue at C-2′ position of saproxanthin, using FAB-MS, 1H NMR, and CD analyses. Culturing strain 11shimoA1 in an alkaline medium at pH 9.2 resulted in a markedly increased in production of 2′-isopentenylsaproxanthin per dry cell weight, but a decreased in zeaxanthin production as compared to their respective production levels in medium with pH 7.0. These carotenoids are likely to play some roles in the adaptation of the bacterium to the environmental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids are a naturally occurring yellow, orange, and red pigments synthesized by photosynthetic organisms, such as higher plants, algae, and photosynthetic bacteria as well as some species of non-photosynthetic bacteria, yeasts, and fungi. To date, more than 750 different carotenoids have been isolated and identified from natural sources (Jackson et al. 2008). Carotenoids can be classified into three groups based on their structure, dicyclic, monocyclic, and acyclic. β-Carotene and lycopene are the representative of carotenoids with dicyclic and acyclic structures, respectively, which are abundant in vegetables and fruits. Monocyclic carotenoids are commonly distributed in cyanobacteria (Takaichi et al. 2001). Myxoxanthophyll (myxol glucoside) is a well-known example of a monocyclic carotenoid with a single β-ionone ring and sugar moiety which is only unique to the members of the phylum Cyanobacteria (Goodwin 1980; Takaichi et al. 2005; Takaichi and Mochimaru 2007). Aglycone and myxol have been found in marine bacteria, along with saproxanthin (Yokoyama et al. 1995; Shindo et al. 2007).
These structurally diverse carotenoids have a variety of biological functions in photosynthesis and photoprotection (Naguib 2000; Sommerburg et al. 1999), in stabilizing membrane fluidity (Yokoyama et al. 1995), and as precursors for hormones (Milborrow 2001). Some kinds of carotenoids have attracted greater attention because of their beneficial effects in the prevention of serious diseases such as cancer (Tanaka et al. 2012), cardiovascular disease (Pashkow et al. 2008), and metabolic syndrome (Miyashita et al. 2011). Recently, myxol and saproxanthin, which are rarely found in carotenoids in nature, have been reported to show stronger antioxidant activities than those of β-carotene and zeaxanthin against lipid peroxidation in the rat brain homogenate model (Shindo et al. 2007). In addition, both of these monocyclic carotenoids showed a neuroprotective effect (Shindo et al. 2007). These results suggest that monocyclic carotenoids including saproxanthin and myxol have potential to be used in nutraceutical and pharmaceutical industries. However, only few organisms produce monocyclic carotenoids compared to dicyclic and acyclic carotenoids. Therefore, it is important to identify the organisms that produce these monocyclic carotenoids and to investigate the biological functions of these compounds.
Since marine bacteria have shown large diversity in term of carotenoid biosynthesis (Britton et al. 2004; Misawa 2011), the present study attempted to isolate marine bacteria which produced a novel monocyclic carotenoids. In addition, culture conditions such as the carbon source and pH are also important factors in carotenoid biosynthesis in bacteria. Therefore, the effects of different pH and different carbon sources on cell growth and production of carotenoid were determined in this study. A detailed understanding of the factors that promote carotenoid biosynthesis will allow the development of an optimized fermentation medium for carotenoid production.
In the present study, a yellow-orange pigmented bacterium, strain 11shimoA1 (JCM19538), belonging to the species Jejuia pallidilutea in the family Flavobacteriaceae (Lee et al. 2009), was isolated. Strain 11shimoA1 synthesized a novel monocyclic carotenoid, 2′-isopentenylsaproxanthin, as well as zeaxanthin. The strain 11shimoA1 produced more 2′-isopentenylsaproxanthin in the alkaline medium (pH 9.2) than in medium with a pH of 7.0.
Materials and methods
Screening of pigment-producing marine bacteria
Sample of seaweeds and seawater was collected at Nabeta Bay in Shimoda (Shizuoka Prefecture of Japan) in June 2011. Artificial seawater-soaked seaweed and seawater were conventionally plated on Marine Agar 2216 (Difco). The agar plates were incubated for several days at 25 °C. After incubation, the pigmented colonies were isolated. Among pigmented colonies, the strain 11shimoA1 with yellow-orange pigment was selected. The strain 11shimoA1 (JCM19538) was then deposited in the Japan Collection of Microorganisms, RIKEN BioResource Center (Tsukuba, Japan).
Isolation and identification of carotenoids
The strain11shimoA1 was incubated for 2–3 days in a 300 ml Marine Broth 2216 (Difco) in a 500-ml Sakaguchi flasks on a rotary shaker at 140 rpm and 25 °C.
After cultivation, the cell pellet was collected by centrifugation at 12,000 rpm, and the total lipid was extracted from the pellet using the method previously described by Folch et al. (1957). The total lipid was then applied onto a preparative thin-layer chromatography (TLC) plate with silica gel 60 (1 mm; Merck, Germany) and developed with n-hexane/ethyl acetate (4:6, v/v). Yellow and orange fractions (Rf = 0.35 of yellow, 0.46 of dark orange) were scraped and pigments were eluted from silica gel with acetone. To purify carotenoids, each pigmented fraction obtained by TLC was subjected to high performance liquid chromatography (HPLC) with Develosil ODS column (20 × 250 mm, Nomura Chemical, Japan), eluting with methanol/acetonitrile (7:3, v/v) at a flow rate of 8.0 ml/min. Carotenoids were detected with UV–VIS detector (Hitach L-2400) at 450 nm.
Spectroscopic analysis
The proton nuclear magnetic resonance (1H NMR) spectra of the carotenoids in CDCl3 were measured at 500 MHz using a UNITY INOVA-500 NMR system (Varian, USA) with TMS as an internal control. The high-resolution fast atom bombardment-mass spectrometry (FAB-MS) spectra of the carotenoids were measured by a JMS-HX/HX110A mass spectrometer (JEOL, Japan) with m-nitrobenzyl alcohol as a matrix. The peak assignments of the 1H NMR spectra were made on the basis of 1H–1H COSY (correlated spectroscopy) and NOESY spectra and by comparison with previously reported data. The circular dichroism (CD) spectra were as recorded in ether at room temperature using a J-500C spectropolarimeter (Jasco, Japan).
Molecular phylogeny
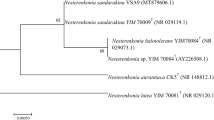
Bacterial genomic DNA of strain 11shimoA1 was purified using Wizard genomic DNA purification kit (Promega, Madison, WI) according to the manufacturer’s instructions. The 16S ribosomal RNA (rRNA) gene was amplified from the DNA using 24 F and 1509R primer set (Sawabe et al. 1998). PCR was performed using GoTaq Green Master mix (Promega) according to the protocol provided by the company. The single PCR product was purified using the Gel and PCR purification system (Promega), and the sequences were determined using the SolGent DNA sequence service (Leave a Nest Co., Ltd.). The sequences were aligned and analyzed using the ClustalX (Thompson et al. 1997) and MEGA version 5 programs (Tamura et al. 2011), respectively. In the phylogenetic analysis, the sequences of small-subunit rRNA gene retrieved from the GenBank/EMBL/DDBJ database were used. The domain used to construct the phylogenetic tree was from the region of the small-subunit rRNA gene at the position from 124 to 1,378 (Escherichia coli str. K12, AP012306 numbering, Fig. S1). The phylogenetic analyses were performed using three different methods, neighbor-joining (NJ), maximum-likelihood (ML), and maximum-parsimony (MP) implemented in MEGA version 5. The robustness of each topology was checked by NJ and 500 bootstrap replications. The tree was drawn by MEGA version 5.
Nucleotide sequence accession number
The 16S rRNA sequence of the strain 11shimoA1 (JCM19538) is available in GenBank/EMBL/DDBJ under the accession number of AB848003.
Cultivated conditions in carotenoid synthesis and cell growth
In order to investigate the cultivated conditions in carotenoid synthesis of strain 11shimoA1, 50 mM d-glucose or sucrose was added into Marine Broth 2216. The pH of the culture media (Marine Broth 2216) was adjusted by the addition of HCl or NaOH into Marine Broth 2216. After the cultivation of strain 11shimoA1 in 50 ml Marine Broth 2216 in 100-ml Erlenmeyer flask for 2–3 days at 140 rpm and at temperature of 25 °C, total lipid was extracted by the method previously described by Folch et al. (1957). As for the quantification analysis of carotenoids, the total lipid was subjected to HPLC equipped with Develosil ODS-UG-5 column (4.6 × 250 mm, Nomura Chemical, Japan) and was analyzed with diode array delivery detector L-7455 (Hitachi, Japan). Mobile phase of methanol/acetonitrile (7:3, v/v) was isocratically eluted at a flow rate of 1.0 ml/min. The temperature of the column was maintained at 30 °C using column oven L-7300 (Hitachi, Japan). Carotenoid content was calculated based on the calibration curves of integrated peak areas by HPLC analysis and weight of carotenoid standards. Carotenoid levels were expressed in specific cellular units (mg/g cell dry weight (CDW)) (Bhosale and Bernstein 2004) or content per 1 ml of culture medium (μg/ml culture medium). For cell dry weight estimation, the culture was centrifuged at 12,000 rpm and the cell pellet was then dried at 105 °C to weight.
Cell growth was monitored by measuring the optical density at 600 nm using a U-2800A spectrophotometer (Hitachi, Japan) during cultivation.
Statistical analysis
Data is expressed as means ± standard error of the mean (SEM). Statistical analysis was performed using one-way ANOVA with Dunnett’s test. A P value of less than 0.01 was considered to be a significant difference.
Results
Identification of pigments produced by strain 11shimoA1
Strain 11shimoA1 which was isolated from a type of seaweed (unidentified) produced a yellow-orange pigmented colonies on the agar plates. After culturing the bacterium in Marine Broth 2216, two major peaks were detected by HPLC analysis in their total lipid extract (Fig. 1).
HPLC chromatogram of pigments produced by strain 11shimoA1. Strain 11shimoA1 was cultivated in Marine Broth 2216 for 48 h at 25 °C and 140 rpm. Pigments in total lipid from the strain 11shimoA1 were analyzed by HPLC equipped with ODS column. Mobile phase was methanol/acetonitrile (7:3, v/v) and flow rate was 1.0 ml/min. Pigments were detected at 450 nm using UV detector
Peak 1 showed the same retention time (6.0 min) as that of the (all-trans) zeaxanthin authentic standard. Analyses using positive ion high-resolution fast atom bombardment mass spectrometry (FAB-MS) and 1H NMR (data not shown) confirmed the identification as zeaxanthin (Fig. 2). The CD spectrum was comparable to that of (3R, 3’R)-zeaxanthin as described in a previous report by Buchecker and Noack (1995).
Peak 2 was detected at 8.1 min of retention time from the HPLC analysis (Fig. 1). This carotenoid showed a maximum absorption at 472 and 502 nm, indicating the presence of a myxol (saproxanthin)-type chromophore (Britton et al. 1995). The molecular formula of this compound was determined to be C45H64O2 from positive ion high-resolution FAB-MS data (m/z 636.4901 [M+] C45H64O2 calc. 636.4907). This suggested that an isopentenyl group was attached to the carotenoid moiety. The 1H NMR spectrum revealed the presence of 36 methyl protons (12 methyl groups), 6 methylene protons, 1 oxy methine, 1 aliphatic methine, and 18 olefinic protons in the molecule. These signals were assigned by COSY and NOESY spectra and by comparisons with zeaxanthin, saproxanthin, and myxoxanthophyll. 1H NMR data is summarized in Table 1. The 1H NMR data of the carotenoid moiety were similar to those of saproxanthin, except for the H-2’ position (Englert 1995). The remaining part was assigned to an isopentenyl moiety as shown in Table 1. In the case of saproxanthin, the methylene proton at H-2 appears at 2.31 ppm as doublet. For the carotenoid produced by strain 11shimoA1, however, a methine proton at H-2′ (2.09 ppm) was multiplet. This indicated that an isopentenyl group was attached at C-2′ position of saproxanthin. COSY spectrum clearly indicated the connection between carotenoid moiety at H-2′ and isopentenyl moiety at H-1″. Therefore, the structure of this compound was determined to be 2′-(3-methylbut-2-enyl)-3′, 4′-didehydro-1′, 2′-dihydro-β, ψ-carotene-3, 1′-diol, which is a novel carotenoid with modified saproxanthin binding isopentenyl group at C-2′ position. Peak 2 is named 2′-isopentenylsaproxanthin (Fig. 2). CD of this compound showed [(EPA) nm (Δε) 225 (0), 232 (+2.0), 245 (0), 255 (−4.5), 285 (0), 307 (−3.2), 320 (0), 357 (+4.5), 378 (0), 420 (−2.3), 435 (0)], resembled those to myxoxanthophyll with having (3R, 2’S) chirality (Britton et al. 1995). Therefore, the (3R, 2’S) chirality was proposed for 2′-isopentenylsaproxanthin (Fig. 2).
Phylogenetic analysis of the strain 11ShimoA1
The phylogenetic analysis showed that strain 11shimoA1, which produces the novel carotenoid 2′-isopentenylsaproxanthin, belongs to the genus Jejuia in the family Flavobacteriaceae. In more details, the strain 11shimoA1 formed a robust clade with Jejuia pallidilutea type strains with more than 99.99 % sequence similarity (Fig. S1). The clade was distinctively separated from the other clades involved with Hyunsoonleella spp. and unidentified and/or environmental clones.
Effect of additional sugars on growth and carotenoid production
Strain 11shimoA1 produced 2’-isopentenylsaproxanthin and zeaxanthin at 0.5 and 1.6 mg/g CDW, respectively, in Marine Broth 2216 after 73 h of incubation at 140 rpm and at 25 °C. To examine the effects of additional sugars as carbon source on growth and carotenoid production, strain 11shimoA1 was cultured in the media supplemented with 50 mM d-glucose or 50 mM sucrose. As shown in Fig. 3a, the growth of strain 11shimoA1 did not change after the addition of d-glucose or sucrose. On the other hand, d-glucose supplementation showed a markedly decreased of zeaxanthin content to 0.6 mg/g CDW after 73 h of culture (Fig. 3b) and a slightly decreased of 2′-isopentenylsaproxanthin content to 0.4 mg/g CDW. Sucrose supplementation did not affect cell growth or carotenoid production (Fig. 3).
Effect of d-glucose or sucrose on cell growth (a) and carotenoid production (b). Growth was measured by monitoring optical density at 600 nm (a). Carotenoid production was measured after 73 h culture at 25 °C and 140 rpm. Carotenoid content is expressed as mg/g cell dry weight. Mean values are shown (n = 3) (b). *P < 0.01 versus zeaxanthin content in control. **P < 0.01 versus 2′-isopentenysaproxanthin content in control
Effect of pH of media on growth and production of carotenoid
In order to examine the effect of pH of the cultured medium on growth and production of carotenoid, strain 11shimoA1 was cultivated in Marine Broth 2216 and the pH was adjusted to 7.0, 8.8, or 9.2. The growth of strain 11shimoA1 was slower at pH 8.8 and 9.2 than at pH 7.0 (Fig. 4a). However, after 72 h of culture in the medium of pH 8.8, the cell density of 11shimoA1 reached the same level as that in the medium with pH 7.0. Cell growth was slightly slower in the medium with pH 9.2 than in the medium with pH 7.0 and 8.8.
Effect of culture medium pH on growth (a) and carotenoid production (b) of strain 11shimoA1. Growth was measured by optical density at 600 nm (a). Carotenoid production was measured after 138 h culture at 25 °C and 140 rpm. Mean values are shown (n = 3). Carotenoid content is expressed as mg/g cell dry weight (b). *P < 0.01 versus zeaxanthin content in cells grown in pH 7.0 medium. **P < 0.01 vs. 2′-isopentenysaproxanthin content in cells grown in pH 7.0 medium
It is worth to note that, in the production of carotenoid, 2’-isopentenylsaproxanthin content (mg/g CDW) is increased when in culture media with higher pH (Fig. 4b). In medium with pH 9.2, 2′-isopentenylsaproxanthin content was 1.1 mg/g CDW, as compared to 0.4 mg/g CDW in medium with pH 7.0. Since the final cell density was lower in medium with pH 9.2 than that in pH 7.0, the content of 2′-isopentenylsaproxanthin per volume of culture medium was compared. The 2’-isopentenylsaproxanthin content per milliliter of culture medium was higher in medium with pH 9.2 (1.2 μg/ml) than in the ones with pH 7.0 (0.6 μg/ml) after 138 h of culture. These data show that 2′-isopentenylsaproxanthin biosynthesis was enhanced in the strain 11shimoA1 under alkaline conditions. In contrast, zeaxanthin production was significantly decreased to 1.0 mg/g CDW at pH 9.2 compared to 1.3 mg/g CDW at pH 7.0. Thus, the pH of the culture medium is an important factor which affects the production of carotenoid 11shimoA1.
Discussion
In this study, 35 yellow-red pigmented bacteria were successfully isolated from seawater and seaweeds collected at Nabeta Bay (Shizuoka, Japan). Among those bacteria, strain 11shimoA1 was selected for further analysis, as its colony color was yellow-orange, which was distinct from the colony colors of the other strains.
Strain 11shimoA1 was identified to J. pallidilutea based on16S rRNA gene sequencing. J. pallidilutea EM39T was proposed to be a novel genus and species in the family Flavobacteriaceae (Lee et al. 2009). The strain EM39T showed an orange color of water insoluble pigmentation. However, the chemical structures of the components from the pigment have yet to be identified. This study first elucidated that the strain 11shimoA1 produced two types of carotenoid which chemical structures were determined by FAB-MS, 1H NMR, and CD analyses. One of the carotenoids was (3R, 3’R)-zeaxanthin, a yellow xanthophyll. Zeaxanthin has dicyclic structure and is synthesized by some species of bacteria including the Flavobacterium species (McDermott et al. 1974). Zeaxanthin and lutein are normally found in the human eye and prevent age-related macular degeneration and light-induced photoreceptor death (Beatty et al. 2001; Thomson et al. 2002). The nutraceutical applications of zeaxanthin have been identified and well researched in the scientific world.
On the other hand, the other carotenoid was identified as a novel monocyclic carotenoid, 2′-isopentenylsaproxanthin. It is of a unique carotenoid structure comprising saproxanthin (3′,4′-didehydro-1′,2′-dihydro-β, ψ-carotene-3,1′-diol) with a pentenyl residue at C-2′ position. Saproxanthin is synthesized by the marine bacterium strain 04OKA-13-27 (Shindo et al. 2007) and Saprospira grandis (Aasen and Liaaen-Jensen 1966). It has been reported to show a stronger antioxidative activity than those of β-carotene and zeaxanthin against lipid peroxidation in the rat brain homogenate (Shindo et al. 2007). In addition, another monocyclic carotenoid, myxol (3′,4′-didehydro-1′,2′-dihydro -β, ψ-carotene-3,1′,2′-triol) (Yokoyama and Miki 1995; Teramoto et al. 2004), which has a similar structure to that of saproxanthin, showed a neuroprotective effect against l-glutamate toxicity in the neuronal hybridoma cell line, N18-RE-105 (Shindo et al. 2007). Since the structure of 2′-isopentenylsaproxanthin identified in this study is similar to that of saproxanthin and myxol, it is likely to have a strong antioxidant and a neuroprotective effect.
The production of 2′-isopentenylsaproxanthin was affected by sugar supplementation and pH of the medium; a slight decrease using d-glucose supplementation and an increase in alkaline conditions. Zeaxanthin content (per CDW) decreased markedly and 2′-isopentenylsaproxanthin content (per CDW) showed a slightly decrease after 72 h of cultivation in Marine Broth 2216 added with 50 mM d-glucose. Nevertheless, the growth of strain 11shimoA1 was not affected by d-glucose supplementation. Sucrose supplementation did not show any effects on the production of carotenoid and the cell growth of strain 11shimoA1. Comparing to the study of the other type of Flavobacterium sp. in which the rapid growth and pigment production are supported by the supplementation of glucose, sucrose, and xylose (Dasek et al. 1973; Sheperd and Dasek 1974; Sheperd et al. 1974; Shoeher and Wis 1972), it is possible for us to determine the metabolic diversity in the production of carotenoid among Flavobacteria, which has recently been well studied especially in the genome survey (Fernández-Gómez et al. 2013).
In this study, the effect of pH on growth production of carotenoid of strain 11shimoA1 was also evaluated, since it was previously reported that J. pallidilutea EM39T grew at pH 6.9–9.0 (Lee et al. 2009). In alkaline medium at pH 9.2, 2′-isopentenylsaproxanthin content per CDW was more than twice of that produced in the medium with pH 7.0 after 138 h of cultivation, while growth rate of strain 11shimoA1 was slower and the final cell concentration was also slightly lower. Therefore, the amount of 2′-isopentenylsaproxanthin and zeaxanthin per volume of culture medium was compared at pH 9.2 and 7.0. Total carotenoid content per CDW significantly increased by the incubation at medium with pH 9.2. Especially, the content of 2′-isopentenylsaproxanthin content (1.2 μg/ml medium) was doubled that in the medium with pH 7.0 (0.6 μg/ml medium) after 138 h cultivation. These data show that 2′-isopentenylsaproxanthin synthesis in strain 11shimoA1 was promoted under alkaline conditions. In contrast, zeaxanthin content (per CDW and per ml culture medium) was lower in medium with pH 9.2 than in the medium with pH 7.0. Thus, cultivation in alkaline conditions markedly altered the composition of carotenoid in strain 11shimoA1 (Fig. 4b).
In several bacteria, a large number of specific stress proteins are induced in harmful environmental conditions such as pH, oxygen, and light (Storz G and Hengge-Aronis R 2000). The gene expressions are controlled by alternative sigma factors such as extracytoplasmic functions (ECF) subfamily. It has been reported that σW is one of the ECF subfamily and is induced by alkali shock in bacteria (Wiegert T et al. 2001). In addition, another ECF sigma factor, CarQ is also known to regulate carotenogenesis in Myxococcus xanthus (Gorham et al. 1996). Therefore, it is suggested that carotenoid synthesis in strain 11 shimoA1 is regulated by a sigma factor response to alkaline condition at pH 9.2. Long-chain C-50 bacterioruberin-type carotenoids have been isolated from several extremophilic bacteria and archaea, including species of Halobacterium, Haloferax (D’Souza et al. 1997), and psychrotrophic bacteria, Micrococcus roseus (Jagannadham et al. 1991) and Arthrobacter agilis (Fong et al. 2001). In these organisms, bacterioruberin-type carotenoids have been reported to play roles in adaptation and survival to extreme environments. Long-chain carotenoids may also reinforce the membrane bilayer to reduce O2 diffusion in the cytoplasm (Wisniewska and Subczynski 1998) and raise the hydrophobic barrier for polar molecules and ions. Since 2′-isopentenylsaproxanthin content in strain 11shimoA1 was higher in alkaline medium (pH 9.2) than in medium at pH 7.0, C-45 carbon chain 2′-isopentenylsaproxanthin, but not C-40 chain zeaxanthin, might contribute to the adaptation to this high-pH conditions.
Strain 11shimoA1 accumulated lycopene by the addition of nicotine, which inhibits β-end cyclization and hydration or C-1, 2 double bond saturation (Takaichi et al. 1997; Britton 1990), in the medium (Fig. S2). Further, chemical mutagenesis of the strain 11shimoA1 using ethyl methanesulfonate produced a red-colony mutant, 11shimoA1R. In the 11ShimoA1R, lycopene was detected as a major carotenoid (Fig. S3). These results suggest that the strain may have unique carotenoid synthetic pathways, which is converted to 2′-isopentenylsaproxanthin or zeaxanthin via lycopene. This also suggest that strain 11shimoA possesses a sensing system to regulate dicyclic or monocyclic carotenoids production depending on the pH of the medium. In Flavobacterium p99-3 producing a monocyclic carotenoid, myxol, which has similar structure with 2′–OH in the place of isopentenyl residue of 2′-isopentenylsaproxanthin, it has been reported that CrtC and CrtD which conduct desaturation and hydroxylation are conducted by CrtC and CrtD. We have just analyzed the draft genome sequence of the strain 11shimoA1. On the basis of the genome data, synthetic pathways of carotenoids and the gene regulations in strain 11shimoA1 could be further elucidated.
In conclusion, the marine bacterium strain 11shimoA1 isolated from seaweed was identified as a strain of J. pallidilutea in the family Flavobacteriaceae based on its 16S rRNA gene sequences. Interestingly, the strain 11shimoA1 produced a novel monocyclic carotenoid, 2′-isopentenylsaproxanthin, as well as zeaxanthin. Culturing strain 11shimoA1 in medium with a high pH enhanced 2′-isopentenylsaproxanthin synthesis. These results demonstrate that the bacterium may possess a unique physiological system in carotenoid production.
References
Aasen AJ, Liaaen-Jensen S (1966) The carotenoids of flexibacteria: II. A new xanthophyll from Saprospira grandis. Acta Chem Scand 20:811–881
Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME (2001) Macular pigment and risk for age-related macular degeneration in subjects from a Northern European Population. Invest Ophthalmol Vis Sci 42:439–446
Bhosale P, Bernstein PS (2004) β-Carotene production by Flavobacterium multivorum in the presence of inorganic salts and urea. J Ind Microbiol Biotechnol 31:565–571
Britton G (1990) Carotenoid biosynthesis—an overview. In: Krinsky NI, Mathews-Roth MM, Taylor RF (eds) Carotenoids: Chemistry and biology. Plenum Press, New York, pp 167–184
Britton G, Liaaen-Jensen S, Pfander H (1995) Carotenoids volume 1a: Isolation and analysis. Birkhäuser, Basel, Switzerland
Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids Handbook. Birkhauser Verlag, Basel, Switzerland
Buchecker R, Noack K (1995) Circular dichroism. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids, vol 1B. Birkhäuser, Basel, pp 63–116
D’Souza SE, Altekar W, D’Souza SF (1997) Adaptive response of Haloferax mediterranei to low concentrations of NaCl (<20 %) in the growth medium. Arch Microbiol 168:68–71
Dasek J, Sheperd D, Traelnes RK (1973) Proced de production de zeaxanthin. Belgium Patent 790289
Englert G (1995) NMR spectroscopy. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids, vol 1B. Birkhäuser, Basel, pp 147–160
Fernández-Gómez B, Richter M, Schüler M, Pinhassi J, Acinas SG, González JM, Pedrós-Alió C (2013) Ecology of marine Bacteroidetes: a comparative genomics approach. ISME J 7:1026–1037
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fong NJ, Burgess ML, Barrow KD, Glenn DR (2001) Carotenoid accumulation in the psychrotrophic bacterium Arthrobacter agilis in response to thermal and salt stress. Appl Microbiol Biotechnol 56:750–756
Goodwin TW (1980) The biochemistry of carotenoids, 1. Plants, 2nd edn. Chapman and Hall, London
Gorham HC, McGowan SJ, Robson PR, Hodgson DA (1996) Light-induced carotenogenesis in Myxococcus Xanthus: light-dependent membrane sequestration of ECF sigma factor CarQ by anti-sigma factor CarR. Mol Microbiol 19:171–186
Jackson H, Braun CL, Ernst H (2008) The chemistry of novel xanthophyll carotenoids. Am J Cardiol 101:50D–57D
Jagannadham MV, Rao VJ, Shivaji S (1991) The major carotenoid pigment of a psychrotrophic Micrococcus roseus strain: Purification, structure, and interaction with synthetic membranes. J Bacteriol 173:7911–7917
Lee DH, Kahng HY, Lee YS, Jung JS, Kim JM, Chung BS, Park SK, Jeon CO (2009) Jejuia pallidilutea gen. nov., sp. nov., a new member of the family Flavobacteriaceae isolated from seawater. Int J Syst Evol Microbiol 59:2148–2152
McDermott JCB, Brown DJ, Britton G, Goodwin TW (1974) Alternative pathway of zeaxanthin biosynthesis in a Flavobacterium species. Biochem J 144:231–243
Milborrow BV (2001) The pathway of biosynthesis of abscisic acid in vascular plants: a review of the present state of knowledge of ABA biosynthesis. J Exp Bot 52:1145–1164
Misawa N (2011) Carotenoid β-ring hydroxylase and ketolase from marine bacteria-promiscuous enzymes for synthesizing functional xanthophylls. Mar Drugs 9:757–771
Miyashita K, Nishikawa S, Beppu F, Tsukui T, Abe M, Hosokawa M (2011) The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J Sci Food Agric 91:1166–1174
Naguib YM (2000) Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem 48:1150–1154
Pashkow FJ, Watumull DG, Campbell CL (2008) Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol 101:58D–68D
Sawabe T, Sugimura I, Ohtsuka M, Nakano K, Tajima K, Ezura Y, Christen R (1998) Vibrio halioticoli sp. nov., a nonmotile alginolytic marine bacterium isolated from the gut of the abalone Haliotis discus hannai. Int J Syst Bacteriol 48:573–580
Sheperd D, Dasek J (1974) Proced de preparation de zeaxanthin. Belgium Patent 816876
Sheperd D, Dasek J, Carels M (1974) Proced de production de zeaxanthin. Belgium Patent 816767
Shindo K, Kikuta K, Suzuki A, Katsuta A, Kasai H, Yasumoto-Hirose M, Matsuo Y, Misawa N, Takaichi S (2007) Rare carotenoids, (3R)-saproxanthin and (3R,2’S)-myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. Appl Microbiol Biotechnol 74:1350–1357
Shoeher A, Wis O (1972) Proced pour la preparation de zeaxanthin. Belgium Patent 770744
Sommerburg OG, Siems WG, Hurst JS, Lewis JW, Kliger DS, van Kuijk FJ (1999) Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr Eye Res 19:491–495
Storz G, Hengge-Aronis R (2000) Bacterial stress responses. American Society for Microbiology, Washington, DC
Takaichi S, Mochimaru M (2007) Carotenoids and carotenogenesis in cyanobacteria: Unique ketocarotenoids and carotenoid glycosides. Cell Mol Life Sci 64:2607–2619
Takaichi S, Wang ZY, Umetsu M, Nozawa T, Shimada K, Madigan MT (1997) New carotenoids from the thermophilic green sulfur bacterium Chlorobium tepidum: 1′,2’-dihydro-gamma-carotene, 1′,2’-dihydrochlorobactene, and OH-chlorobactene glucoside ester, and the carotenoid composition of different strains. Arch Microbiol 168:270–276
Takaichi S, Maoka T, Masamoto K (2001) Myxoxanthophyll in Synechocystis sp. PCC 6803 is myxol 2’-dimethylfucoside, (3R, 2’S)-myxol 2’-(2,4-di-O-methyl-a-L-fucoside), not rhamnoside. Plant Cell Physiol 42:756–762
Takaichi S, Mochimaru M, Maoka T, Katoh H (2005) Myxol and 4-ketomyxol 2’-fucosides, not rhamnosides, from A nabaena sp. PCC 7120 and Nostoc punctiforme PCC 73102, and proposal for the biosynthetic pathway of carotenoids. Plant Cell Physiol 46:497–504
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tanaka T, Shnimizu M, Moriwaki H (2012) Cancer chemoprevention by carotenoids. Molecules 17:3202–3242
Teramoto M, Rählert N, Misawa N, Sandmann G (2004) 1-Hydroxy monocyclic carotenoid 3,4-dehydrogenase from a marine bacterium that produces myxol. FEBS Lett 570:184–188
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Thomson LR, Toyoda Y, Langner A, Delori FC, Garmett KM, Craft N, Nichols CR, Cheng KM, Dorey CK (2002) Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Invest Ophthalmol Vis Sci 43:3538–3549
Wiegert T, Homuth G, Versteeg S, Schumann W (2001) Alkaline shock induces the Bacillus subtillis sigma (W) regulon. Mol Microbiol 41:59–71
Wisniewska A, Subczynski WK (1998) Effects of polar carotenoids on the shape of the hydrophobic barrier of phospholipid bilayers. Biochim Biophys Acta 1368:235–246
Yokoyama A, Miki W (1995) Isolation of myxol from a marine bacterium Flvobacterium sp. associated with a marine sponge. Fish Sci 61:684–686
Yokoyama A, Sandmann G, Hoshino T, Adachi K, Sakai M, Shizuri Y (1995) Thermozeaxanthins, new carotenoid-glycoside-esters from thermophilic eubacterium Thermus thermophilus. Tetrahedron Lett 36:4901–4904
Acknowledgements
This work was supported by Grants-in Aid for Scientific Research from MEXT (Ministry of Education, Culture, Sports, Science, and Technology of Japan) (No. 23380120 to M.H.) and Strategic Japanese-Brazilian Cooperative Program (JST-CNPq).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 67 kb)
Rights and permissions
About this article
Cite this article
Takatani, N., Nishida, K., Sawabe, T. et al. Identification of a novel carotenoid, 2′-isopentenylsaproxanthin, by Jejuia pallidilutea strain 11shimoA1 and its increased production under alkaline condition. Appl Microbiol Biotechnol 98, 6633–6640 (2014). https://doi.org/10.1007/s00253-014-5702-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5702-y