Abstract
This study investigated the production of triacylglycerols in cells of the wild type of Escherichia coli and of a strain with a deleted diacylglycerol kinase gene (dgkA). By overexpression of atfA from Acinetobacter baylyi ADP1 and fadD from E. coli in the dgkA deletion mutant, cellular contents of up to 4.9 % (w/w) triacylglycerols could be achieved in batch cultivation. Furthermore, heterologous expression of atfA relieves the negative effects of dgkA deletion on growth. Process optimization and fed-batch fermentation resulted in the production of 530 mg l−1 triacylglycerols and a maximal content of 8.5 % (w/w) triacylglycerols of the cell dry mass. This clearly exceeded all previous results concerning triacylglycerol production in E. coli. Furthermore, the production of extracellular free fatty acids and fatty acid ethyl esters was investigated. Like triacylglycerols, both products are potential biofuels, and we show their continuous production in a repeated batch process, with recovery of the production cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, much attention has been paid towards the microbial production of biofuels and of compounds that can easily be transformed into biofuels (Lennen and Pfleger 2013; Zhang et al. 2011). The main goal was and still is the production of biofuels from resources that are renewable, not used as food and that do not compete with the food production by the use of agricultural area.
The two biofuels that are already produced in large amounts are bioethanol and biodiesel (Uthoff et al. 2009). Whereas the first is a product of fermentations of yeast on glucose obtained from maize or sucrose from sugar cane or sugar beet as carbon sources, while the latter is produced by the transesterification of fatty acids from plant oils with methanol, yielding fatty acid methyl esters. Current research aims at the microbial production of fatty acids, oils, biodiesel, or other biofuels from sugars (Kalscheuer et al. 2006; Kurosawa et al. 2010; Liu et al. 2012; Zhang et al. 2012) and from renewable or residual resources like cellulose, hemicellulose, carbon dioxide, molasses, and others (Bokinsky et al. 2011; Ruffing and Jones 2012; Steen et al. 2010).
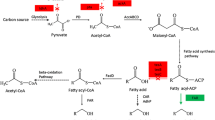
The use of Escherichia coli for microbial biofuel production offers the advantages of a fast growing and well known bacterium. However, besides small amounts of ethanol, E. coli does not produce biofuel compounds, and thus suitable metabolic pathways have to be designed or engineered in this bacterium. A good possibility is the use of the fatty acid biosynthesis pathway for example by overexpression of an acyl-ACP thioesterase. By the thioesterase activity, free fatty acids are released from the acyl carrier protein and can only be used for the membrane biosynthesis if they are activated with coenzyme A (Ray and Cronan 1975). This ATP-dependent reaction is catalyzed by FadD and usually, but not necessarily, coupled with the transport of exogenous fatty acids across the inner membrane. Acyl-CoA that is not used for the membrane synthesis can be degraded via the β-oxidation pathway. Overexpression of only a thioesterase gene is sufficient for the accumulation of high amounts of fatty acids in both the cytosol and the culture medium (Cho and Cronan 1995; Zheng et al. 2012). Additional deletion of the fadD gene or an otherwise downregulated β-oxidation pathway further improve fatty acid yields (Liu et al. 2012; Steen et al. 2010).
Despite the fact that free fatty acid production has so far yielded good results, a problem is that high concentrations exert membrane damages in E. coli and other bacteria (Ruffing and Jones 2012; Desbois and Smith 2010; Lennen et al. 2011). Therefore, it may be advantageous to produce the fatty acids in an inert form, for example as triacylglycerols (TAG) inside the cell or as fatty acid ethyl esters (FAEE) upon transesterification, because E. coli cells tolerate both in high amounts (Steen et al. 2010).
The production of FAEE by E. coli has been established in 2006 by coexpression of the pyruvate decarboxylase and alcohol dehydrogenase B from Zymomonas mobilis for ethanol production together with the wax ester synthase/acyl-CoA:diacylglycerol acyltransferase (WS/DGAT or AtfA) of Acinetobacter baylyi ADP1 to catalyze the esterification of ethanol with acyl-CoA (Kalscheuer et al. 2006). A limitation in the first study was the need for externally fed fatty acids, which could be overcome by the coexpression of a thioesterase gene and a fatty acyl-CoA ligase, and the deletion of the fatty acyl-CoA dehydrogenase to provide higher amounts of fatty acyl-CoA (Steen et al. 2010).
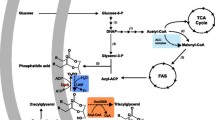
Pathways that lead to TAG synthesis, in E. coli, are summarized in Fig. 1. As substrates, diacylglycerol and fatty acyl-CoA are needed, which can be esterified to TAG plus free CoA by the activity of AtfA or any other diacylglycerol acyltransferase. The production of fatty acyl-CoA can be achieved as described above for FAEE production. The second substrate can be provided by overexpression of a phosphatidylglycerol phosphate phosphatase gene, since the respective enzyme also exhibits activity towards the dephosphorylation of diacylglycerol-3-phosphate (Touze et al. 2008). To date there are two studies published that deal with TAG production by overexpression of a phosphatase and a diacylglycerol acyltransferase gene (Comba et al. 2013; Rucker et al. 2013). In the study of Rucker and coworkers, the content of TAG was determined to be 0.5 % of the cell dry weight.
Pathway for TAG synthesis in engineered E. coli. Apart from AtfA, all proteins are native to E. coli; however, some of the respective genes were deleted or overexpressed in this study, as detailed in the text. The lipase proteins, responsible for the degradation of diacylglycerol and monoacylglycerol, are yet unidentified. Further, it is not clear weather triacylglycerol accumulates within the leaflets of the cytoplasmic membrane, or as a distinct granule, as in bacteria that naturally accumulate triacylglycerols
An alternative strategy to achieve high intracellular concentrations of DAG is the deletion of dgkA, which codes for a diacylglycerol kinase. DgkA recycles diacylglycerol that is formed during the synthesis of membrane-derived oligosaccharides (Raetz and Newman 1978; Raetz and Newman 1979). The membrane-derived oligosaccharides are formed in the periplasm of E. coli and reduce the osmotic pressure on the inner membrane in hypotonic media (Kennedy 1982). Raetz and Newman (1978) showed that deletion of dgkA leads to a strong decrease of the growth rate, when cells of such an E. coli mutant were transferred to a medium with reduced osmotic pressure. For the production of TAG, only the expression of a diacylglycerol acyltransferase gene is necessary in a dgkA deletion mutant (Arabolaza et al. 2008). However, the amount of TAG, produced in the latter study had not been determined. In a very recent study, this strategy was applied and under optimized conditions, TAGs of nearly 5 % of the cell dry weight were produced (Lin et al. 2013).
The objective of this study was to compare the two strategies for TAG synthesis and to further improve the TAG production by heterologous overexpression of atfA, fadD, plsB, and pgpB in several combinations. Following to optimizations of the cultivation conditions, the best strains were compared by fed-batch cultivation in 2 l scale.
Material and methods
Strains, plasmids, and media
All strains and plasmids that were used in this study are summarized in Table 1. Cells were cultivated in LB broth (1 %, w/v, NaCl; 1 %, w/v, tryptone; and 0.5 %, w/v, yeast extract) at 37 °C for cloning procedures and in LB broth or mineral medium, both with 1 % (w/v) lactose × H2O at 30 °C for growth experiments and fermentations. The composition of the mineral medium was as follows: KH2PO4 0.4 % (w/v), (NH4)2HPO4 0.2 % (w/v), trace metal solution 1 % (v/v), MgSO4 × 7 H2O 0.12 % (w/v), lactose x H2O 1 % (w/v). Magnesium sulfate and lactose were autoclaved as one separate solution and added to the phosphate and trace metal solution afterwards. The trace metal solution contained ammonium-Fe(III)-citrate 0.6 % (w/v), MnCl2 × 4 H2O 0.15 % (w/v), ZnSO4 × 7 H2O 0.125 % (w/v), H3BO3 0.03 % (w/v), Na2MoO4 × 2 H2O 0.025 % (w/v), CoCl2 × 6 H2O 0.025 % (w/v), CuCl2 × 2 H2O 0.015 % (w/v), EDTA 0.84 % (w/v). For preparation of solid media, 1.5 % (w/v) agar was added. If necessary, antibiotics were added in the following concentrations: kanamycin 50 mg l−1, ampicillin 75 mg l−1, gentamycin 10 mg l−1, and tetracycline 12.5 mg l−1.
Batch cultivations in Erlenmeyer flasks
Batch cultivations were inoculated from overnight cultures to an optical density of 0.05 and were performed in baffled 300 ml Erlenmeyer flasks with 10–20 % (v/v) medium at 130 rpm and 30 °C. Growth was monitored for 24 h, by measuring the optical density at 600 nm, with a GENESYS 20 spectrophotometer (Thermo Fischer Scientific, Schwerte, Germany). After the cultivation, the cells were harvested by centrifugation and freeze dried for determination of the cell density, lipid content, and lipid profile. Data shown for every batch experiment represents the mean of three independent cultivations.
Fed-batch cultivations in 2 l bioreactors
Fed-batch cultivations were done in 2 l Biostat B plus bioreactors (Sartorius, Göttingen, Germany). The pH was adjusted to 6.8 and maintained by addition of 2 M NaOH, or 7 % NH4OH (w/v) if an additional feed of nitrogen was intended. The temperature was kept at 30 °C and the dissolved oxygen concentration at 15 % by automatic control of the speed of agitation. Aeration was applied with a flow of 1.5 l min−1. The carbon source was fed from a 20 % (w/v) stock solution of lactose × H2O. If necessary, 1 ml of a 50 % (v/v) solution of struktol SB 2121 (Schill + Seilacher, Hamburg, Germany) was added to prevent excessive foam formation. Samples were withdrawn regularly to determine the optical density at 600 nm, the cell density, ammonium, and lactose concentrations, as well as the fatty acid profile and content.
Repeated batch cultivations
The repeated batch cultivation was performed in Erlenmeyer flasks with LB medium and with mineral medium, supplied with 10 g l−1 lactose. For both media, three different ratios of the medium volume and the flask volume were applied: (a) 15 ml medium in a 100-ml, baffled, flask; (b) 20 ml medium in a 100-ml flask without baffles; and (c) 50 ml medium in a 100-ml flask without baffles. After inoculation from overnight grown precultures, to an initial optical density of 0.05, the flasks were incubated at 30 °C and 130 rpm.
After 24 h, the optical density was determined, the cultures were harvested, the cells were suspended in fresh medium, and the cultivation was continued. The supernatant was used for determination of fatty acid, FAEE, and ethanol concentrations. After 48, 72, 96, 120, and 144 h, this procedure was repeated. The LB cultures were always suspended in fresh LB medium. On the contrary, the cultures that were started in mineral medium were suspended in fresh mineral medium after 24 h and in a nitrogen limiting solution at the following times. The nitrogen limiting solution consisted of KH2PO4 0.4 % (w/v), (NH4)2HPO4 0.04 % (w/v), Na2HPO4 × 2H2O 0.22 % (w/v), trace metal solution 1 % (v/v), MgSO4 × 7 H2O 0.12 % (w/v), and lactose × H2O 1 % (w/v).
General molecular biological techniques
Standard molecular biological techniques were conducted according to Sambrook et al. (1989). Enzymes for cloning procedures were purchased from Thermo Fischer Scientific GmbH (St. Leon-Rot, Germany). Polymerase chain reaction (PCR) was performed according to the guidelines of the manufacturer (Invitrogen, Darmstadt, Germany), using Pfx-Polymerase for cloning and sequencing, and Taq-Polymerase for analytical PCR reactions.
Deletion of the dgkA gene
The E. coli ΔdgkAΩGenR deletion mutant was constructed by use of the “E. coli Gene Deletion Kit” (Gene Bridges GmbH, Dresden, Germany). As the first step, the flanking regions up- and downstream of the dgkA gene of E. coli K12 MG1655 were amplified with the primers 1–4 (Table 2). In a second PCR, both flanking regions were fused, amplified with the primers 1 and 4, and ligated into the vector pJet1.2/blunt. The gentamycin resistance cassette was amplified with the primers 5 and 6 and ligated in the XmaI restriction site of pJet::ΔdgkA to gain plasmid pJet::ΔdgkAΩgenR. Using primers 1 and 4, the deletion construct was amplified and, by electroporation, transferred into the strain E. coli K12 MG1655 pRedET, which was always cultivated at 30 °C. Upon induction of the plasmid pRedET, the linear deletion construct was inserted into the genome of E. coli MG1655 by homologous recombination. Loss of plasmid pRedET was triggered by cultivation at 37 °C. The genotype of the resulting strain E. coli K12 MG1655 ΔdgkAΩgenR was verified by PCR with the primers pairs 7–8 and 9–10, and by sequencing of the fragment, amplified with primers 7 and 8.
Construction of the expression plasmids
The genes fadD, pgpB, and plsB were amplified by PCR from genomic DNA of E. coli MG1655, using the primer pairs 11 and 12, 13 and 14, and 15 and 16, respectively. After digestion with suitable restriction enzymes, they were ligated successively into the vectors pBBR1MCS-2::atfA or pBBR1MCS-3, collinear to the lacZ promoter. The correct orientation and sequence of all genes was verified by restriction analysis and sequencing. Plasmid pBBR1MCS-2::atfA/placZ/atfA/fadD was constructed by ligation of the EcoRI restriction fragment of pMicrodiesel into the EcoRI-digested plasmid pBBR1MCS-2::atfA/fadD. The EcoRI restriction fragment contained a lacZ promoter downstream of atfA.
Analysis of the cellular fatty acid contents
Fatty acid contents were quantified by transesterification to fatty acid methyl esters and subsequent gas chromatographic (GC) analysis. The lipid profile was analyzed by thin layer chromatography (TLC), and TAG spots on the TLC plate were scraped out for quantification of fatty acids, corresponding to TAG. Both methods were performed as described elsewhere (Brandl et al. 1988; Wältermann et al. 2000) and were performed with a stock solution of triolein as control.
Analysis of extracellular fatty acids and FAEE
For the analysis of extracellular lipids, 10 ml of supernatant was extracted with 4 ml chloroform, by vortexing for 1 min. The organic phase was withdrawn and concentrated for determination of FAEE by gas chromatography, as described previously (Wältermann et al. 2000). The residual organic phase was subjected to transesterification (Brandl et al. 1988), followed by a second analysis by gas chromatography. This step gave the total fatty acid concentration in the culture medium.
Quantification of lactose and ethanol
The carbon source and main metabolites were assayed in cell free supernatants, by high performance liquid chromatography (HPLC) with 4.5 mM sulfuric acid as mobile phase. A Lachrom Elite HPLC system (VWR-Hitachi, Darmstadt, Germany), equipped with a Metacarb 67H column (300 × 6.5 mm, VWR-Varian, Darmstadt, Germany) and a refractive index detector (type 2490, VWR, Darmstadt, Germany), was used. The column was kept at 75 °C and a flow rate of 0.8 ml min−1 was applied.
Analysis of the ammonium concentration
The concentration of ammonium in the culture broth was determined after the appropriate dilution of cell free supernatants in 0.1 M NaOH with a gas sensitive ammonium electrode (type 152303000, Mettler Toledo, Gießen, Germany).
Differential interference contrast microscopy
The strains were grown on LB agar plates, over night at 30 °C, and a small amount of culture material was diluted in a 0.9 % (w/v) NaCl solution. Microscopy was performed using a Zeiss Axio Imager M1 upright wide-field fluorescence microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany) and pictures were recorded with an AxioCam MRm 16 bit digital monochrome charge-coupled device camera (Carl Zeiss MicroImaging GmbH).
Results
TAG production in the wild type
Table 3 summarizes the total fatty acid contents and the TAG contents of the strains used in this study. Expression of solely atfA led to no significant changes in the fatty acid profile and content of the E. coli wild-type cells (Fig. 2). Furthermore, the growth behavior of E. coli strains K12 p2 and K12 p2A was identical (Table 3). Contrary, the expression of pgpB, atfA, and fadD was sufficient for TAG synthesis. However, TAG was synthesized to only about 0.3 % of the cell dry mass in cells of the recombinant strain, and according to the results of TLC, the lipid profile was the same as in wild-type cells.
Deletion of dgkA
Deletion of dgkA led to a drastic change in the fatty acid profile, as well as in the growth behavior. DAG, MAG, and free fatty acids (FFA) form clearly visible spots on the TLC plate (Fig. 2). Additionally, a small TAG spot is visible on the TLC plate and a content of 0.3 % of the cell dry mass was determined. Interestingly, no accumulation of free fatty acids occurred until the end of the exponential growth phase, while MAG and DAG were accumulated to the same degree in every growth phase. Overexpression of atfA in the deletion mutant led to an enhanced formation of TAG, i.e., to nearly 3 % of the cellular dry weight at the end of the exponential growth phase and 4.5 % at the end of the cultivation. According to the TLC analysis, the contents of FFA, DAG, and MAG were comparable to those of the deletion mutant.
Concerning growth, a clear negative effect of the dgkA deletion was observed (Fig. 3). Inoculation of the main culture was followed by a slight decrease of turbidity and a lag phase; however, the growth rate in the exponential phase was only slightly lower than in the wild type (0.89 h−1 compared to 0.95 h−1). The transition to the stationary phase was accompanied by a loss of turbidity and with a second lag phase following. After 8 h, the turbidity was again slowly increasing. These growth impairments were mainly relieved upon overexpression of atfA. In a batch culture of strain Δp2A, the lag phase was lacking, and after the exponential phase (μ = 0.82 h−1) the turbidity increased for several hours. Contrary, the turbidity in cultures of the strains K12 p2 and K12 p2A did not change during the stationary phase.
Strain improvement by metabolic engineering
From the TLC analysis (Fig. 2), it became obvious that strain Δp2A still accumulated significant amounts of DAG and fatty acids. Additional expression of certain genes was investigated to improve the conversion of DAG and fatty acids to TAG. By co-expression of atfA and fadD, the content of free fatty acids could be reduced, but the TAG content was also a bit lower as without fadD expression (Table 3). Concerning the cellular phenotype a clear elongation of the cell length was observed, ranging from 5 μm up to 20 μm, while cells of strains Δp2 and Δp2A exhibited the normal size (Fig. 4).
Microscopic pictures of E. coli strains with significant phenotype. Pictures were recorded after overnight cultivation at 30 °C on LB agar plates. a Picture of strain K12 p2A as an example for the unaltered morphology. Strains K12 p2, K12 p2PAF, Δp2, and Δp2 showed the same morphology. b Picture of strain Δp2BAF, which exhibited the same morphology as strain Δp2A p3B. c Picture of strain Δp2AAF. d Picture of strain Δp2AAF
Co-expression of atfA and plsB was investigated to enhance the activity for glycerol-3-phosphate acyltransferase. In the resulting strain, the contents of free fatty acids and TAG were reduced. Additional co-expression of fadD reduced the TAG content clearly, to less than 50 % of the TAG content of strain Δp2A. However, the proportion of free fatty acids was enhanced and was equal to those of strains Δp2 and Δp2A. In strains Δp2A p2B and Δp2BAF, only few cells were slightly longer than the wild-type cells.
The highest contents of TAG and total fatty acids were achieved by co-expression of fadD together with two copies of atfA. In comparison to strain Δp2AF, the striking cell elongation was clearly reduced. Anyway, cells of strain Δp2AAF exhibited lengths of 2–5 μm and were therefore longer than cells of the E. coli wild type and of all other investigated strains with the exception of cells of Δp2AF (Fig. 4).
Optimization of the culture conditions
Due to the observed cell elongation of strains Δp2AF and Δp2AAF, the latter strain was cultivated in baffled flasks containing LB medium to 15 % of the total volume, and in flasks without baffles, filled with LB medium to 50 %. It was found that the cultures in the baffled flasks, with a higher air to medium ratio reached about 70 % higher optical densities after 24 h cultivation, with comparable contents of total fatty acids and TAG (data not shown).
Cultivations were performed routinely at 30 °C. Cultivation of strain Δp2A in LB medium at 37 °C resulted in a clearly increased growth rate in the exponential growth phase. However, the final optical density was reduced to 70 % when compared to the cultivation at 30 °C (Table 4). Furthermore, also the total fatty acid and TAG contents were reduced.
Due to the role of membrane derived oligosaccharides in the resistance towards media with low osmolarity, cultivations in LB medium with altered salt concentrations were performed. We expected that differences in the synthesis of membrane derived oligosaccharides lead to differences in the formation of DAG, FFA and TAG. In the presence of 14 and 10 g l−1 NaCl, the growth behavior and final OD of strain Δp2A cultures were nearly identical. Reduction of the NaCl concentration to 6 and 2 g l−1 resulted in a reduction of the final optical density to 60 % and to 34 %, respectively. However, the initial growth rate was identical at all tested NaCl concentrations. The cellular contents of total fatty acids and TAG were significantly lower after cultivation in presence of 14 g l−1 NaCl, than in the presence of only 10 g l−1 (Table 4). Both contents were slightly increased with further reduced NaCl concentrations. In addition, TLC analysis revealed a clear increase in FFA, DAG and MAG contents after cultivation in presence of 6 or 2 g l−1 NaCl.
Production of extracellular FFA and FAEE
Two, in the light of biofuel production, interesting by-products that were detected by TLC were free fatty acids and fatty acid ethyl ester (FAEE). After harvesting of the cells, both substances were found in the cell pellet as well as in the supernatant. With the aim to investigate the possibility of a continuous, extracellular FFA and FAEE production, we performed repeated batch cultivations of strains Δp2 and Δp2AAF. While the cells were recovered and used for the following batch cultivation, every 24 h, the supernatant was used for extraction and analysis of FFA and FAEE. Cultivations were performed in LB and in mineral medium.
Under the applied conditions, the extracellular concentrations of both products never exceeded concentrations of 15 mg l−1. Owing to a continuously increasing cell density, the specific productivity (product concentration divided by the cell density) decreased over the entire 144 h duration of all batch cultivations (Fig. 5). Concerning the two media that were compared, the specific fatty acid productivity of strain Δp2 was highest in mineral medium, independent of the medium to air ratio. In LB medium, fatty acid productivity was enhanced with an increasing medium to air ratio. Productivity of FAEE synthesis, by strain Δp2AAF, is best in mineral medium with a low medium to air ratio, and also good in LB medium with a high medium to air ratio. In LB medium with the lowest medium to air ratio, as well as in mineral medium with a high medium to air ratio, nearly no FAEE were produced. By HPLC ethanol of less than 1 g l−1 was detected in the cultures that produced significant amounts of FAEE, while in the other cultures, no ethanol could be detected.
Extracellular production of FFA and FAEE by strains Δp2 and Δp2AAF. The productivity was determined every 24 h by division of the product concentration by the optical density. Afterwards, the cells were cultivated in fresh medium, as described in the materials and methods section. a Productivity of FFA formation by strain Δp2. b Productivity of FAEE formation by strain Δp2AAF. The bars represent different cultivation conditions; from dark to light: 15 ml LB medium in 100 ml baffled flasks; 20 ml LB medium in 100 ml flasks without baffles; 50 ml LB medium in 100 ml flasks without baffles; 15 ml mineral medium in 100 ml baffled flasks; 20 ml mineral medium in 100 ml flasks without baffles; and 50 ml mineral medium in 100 ml flasks without baffles. All media were supplied with 10 g l−1 lactose
Analysis of the cell dry mass after 144 h cultivation revealed that the FAEE concentration of the cellular fraction of strain Δp2AAF is roughly the same, as the extracellular concentration. FFA concentrations after cultivation of strain Δ p2A were roughly ten times higher in the cellular fraction, than in the culture medium.
TAG production in fed-batch fermentations
Fed-batch fermentations were performed in LB medium containing a reduced NaCl concentration (4 g l−1) and at 30 °C. The growth of the three strains with the highest TAG content in batch cultures was very similar, and a final OD of at least 9 was reached (Table 5). The total fatty acid content (13.4 % of the cell dry mass), as well as the total fatty acid concentration of the whole culture medium (700 mg l−1) was highest in cells of strain Δp2A. However, strain Δp2AAF produced the highest TAG content (6.1 % of the cell dry mass).
The latter strain was also cultivated in mineral medium. After 32 h, one culture was supplied with an excess of nitrogen and consumed 0.038 g NH4 + per hour. The second culture was continuously fed with only 0.015 g NH4 + per hour, which was consumed directly. The nitrogen limitation had a slightly negative effect on the growth of the strain (Table 5). While the total fatty acid content was nearly the same, a slightly higher proportion was obviously converted into TAG under nitrogen limited conditions.
Fermentation in mineral medium resulted in a reduced growth rate of 0.17 h−1 in the first 20 h; compared with 0.39 h−1 in the first 8 h during fermentation in LB medium. In return, the final optical density and accordingly the cell density were higher, when the fermentation was done in mineral medium. In addition, the TAG content of the cells was enhanced by at least 25 %.
Discussion
This study compares two approaches for TAG production in E. coli. Our results concerning the overexpression of atfA, pgpB, and fadD in wild-type cells were in accordance with previous studies (Comba et al. 2013; Rucker et al. 2013). However, in our study, the TAG content of the cells reached only 0.3 % of the cellular dry mass, while a content of 0.5 % was reported in the study of Rucker and coworkers, for overexpression of atfA and pgpB. Apart from the fact that a different strain of E. coli was used, it may be that coexpression of fadD in our study causes the slightly lower TAG content. In any case, the comparison with the study of Rucker et al. (2013) shows that fadD coexpression does not lead to an enhanced TAG synthesis when compared to the wild type with atfA and pgpB expression.
On the TLC plates (Fig. 2), the spots for MAG, DAG, and free fatty acids were not or only slightly enhanced. In contrast, we showed a clear accumulation of these substances in the dgkA deletion mutant, and it seems reasonable that the higher concentrations of DAG and fatty acids were the reason for enhanced TAG formation upon atfA expression. Interestingly, a small amount of TAG was synthesized in the deletion mutant without atfA expression, which shows that E. coli has some diacylglycerol acyltransferase activity. This may be due to a lipase or unspecific activity of one of the acyltransferases that is involved in the membrane lipid biosynthesis.
Expression of atfA in the dgkA deletion mutant led to the formation of TAG up to 4.5 % and of total fatty acids up to 10.1 % of the cell dry weight. These data are comparable to the recent study of Lin and coworkers (2013), who detected TAG (4 % of the cell dry weight) and total fatty acids (8 % of the cell dry weight) in a dgkA deletion mutant of E. coli BL Star DE3, upon expression of WS1 (wax ester synthase 1) of Marinobacter hydrocarbonoclasticus. Lin and coworkers reported TAG contents of 0–4 % for expression of different wax ester synthase and diacylglycerol acyltransferase coding genes, so the small difference in TAG content compared with our study is likely due to the use of different enzymes.
Accumulation of DAG in the dgkA deletion mutant has been observed previously (Raetz and Newman 1978). Here we show the additional accumulation of free fatty acids and MAG and conclude that DAG is subject to degradation by phospholipase A, but not with an appropriate rate. Phospholipase A1 and A2 activity in the cytosol and inner membrane fractions of E. coli was described previously (Albright et al. 1973).
Besides the altered lipid profile, we noticed significant growth impairment of the deletion mutant, in the lag-phase and upon the transition from the exponential to the stationary growth phase, and even a loss of cell density for several hours. The exponential phase of the deletion mutant lasted only for about four cell divisions, and a final optical density of only 25 % of the final OD of wild-type cells was reached (Fig. 3). Since free fatty acids were not accumulated during the exponential phase, the early stop of cell division was probably caused by the elevated concentrations of DAG and MAG. The loss of cell density after transition to the stationary phase can be explained by changes of the E. coli fatty acid profile and head groups of the membrane lipids, in order to acquire survival of bad growth conditions (Zhang and Rock 2008). If this adaption involves the recycling of DAG, further enhanced concentrations of free fatty acids and DAG would be formed in the deletion mutant. In addition, it was found that free fatty acids are released from the membrane during the stationary phase (Kol et al. 2004; Pech-Canul et al. 2011), and in the dgkA deletion mutant, their rate of synthesis seems to exceed the rate of degradation. Both, DAG and free fatty acids, have been shown to negatively affect the bacterial membrane (Raetz and Newman 1978; Ruffing and Jones 2012; Desbois and Smith 2010; Lennen et al. 2011). If DAG and free fatty acids are still synthesized in the stationary growth phase, the inability to degrade them fast enough due to the dgkA deletion, may explain the observed growth impairment of the deletion mutant.
Our experiments showed that expression of atfA is a suitable tool to diminish the negative effect of the dgkA deletion during the lag- and stationary growth phase of E. coli. It seems reasonable that this was caused by the conversion of free fatty acids and DAG to neutral lipids, which represents an alternative to their accumulation and degradation. According to our TLC analysis and gas chromatographic data, the cellular contents of free fatty acids and DAG were not reduced by atfA overexpression, although a significant amount of TAG was formed. Thus, we conclude that the consumption of FFA and DAG offered the possibility for the prolonged exponential growth phase that is obvious from Fig. 3.
To improve growth and the productivity of the cells in TAG synthesis, we combined atfA expression in the dgkA deletion mutant with expression of an acyl-CoA ligase and a glycerol-3-phosphate acyltransferase. Both co-expressions did not alter the TAG content significantly. However, in both cases the pool of free fatty acids that was formed in the stationary phase was clearly reduced. Additionally, we noticed a significant cell elongation in the deletion mutant upon atfA and fadD co-expression (Fig. 4), which matched observations of Heath and coworkers (Heath et al. 1994) upon homologous overexpression of plsB. In the previous study E. coli cells were deregulated by plsB overexpression, and membrane synthesis by incorporation of acyl-ACP continued, when no cell division was possible. Since PlsB has a similar affinity to acyl-CoA esters as to acyl-ACP esters (Ray and Cronan 1975), an enhanced acyl-CoA pool may overcome the inhibition of PlsB during stationary phase, which provides a good explanation for the long cells of strain Δp2AF. In contrast, cells of strain Δp2A p3B are only slightly elongated. Probably, in this strain the fatty acid degradation proceeds with its maximum efficiency, and fatty acyl-CoA are only accumulated if fadD is in addition overexpressed.
Co-expression of atfA, fadD, and plsB in the deletion mutant was expected to complete the recycling of DAG and fatty acids as membrane lipids and TAG. However, strain Δp2BAF exhibited a lower final OD and TAG content than all other strains based on the deletion mutant, except strain Δp2. Further investigations are necessary to unravel the reasons and to achieve an improved DAG and fatty acid recycling.
Since co-expression of atfA and fadD resulted in an enhanced synthesis of membrane lipids but not in higher TAG contents, it was concluded that the AtfA activity was limiting. In fact, introduction of a second copy of atfA enhanced the TAG content in batch and fed-batch cultivations. The content of free fatty acids was decreased when compared with strain Δp2, and the cells became considerably longer than cells of strain Δp2A. We conclude that FadD activity was high enough to activate most of the intracellular free fatty acids, but the following steps of the β-oxidation were limiting. As a result, more acyl-CoA could be used for reactions catalyzed by PlsB and AtfA.
Concerning the dependency between TAG formation and the osmotic pressure of the medium, we have shown that cultivation of strain Δp2A under reduced salt concentrations gave slightly increased contents of DAG, TAG and total fatty acids. This is in agreement with the role of MDO in osmotolerance (Kennedy 1982). However, it proves again that enough fatty acids and DAG are formed also without a reduction of the osmotic pressure. The main problem to overcome is the efficient synthesis of TAG.
Based on the results of the batch cultivations, fed-batch fermentations were performed at 30 °C at a reduced osmotic pressure. However, due to feeding and pH control, the osmotic pressure should increase during the cultivation. The three compared strains exhibited a very similar growth behavior in LB medium. However, contrary to the batch cultures, clear differences in TAG storage were detected. Strain Δp2AAF accumulated the highest TAG content, but the lowest total fatty acid content. By fed-batch fermentation in mineral medium, the TAG content and cell density could be further enhanced (Table 5), and we could produce TAG to a final concentration of 530 mg l−1. Contrary to our results, in the study of Lin et al. (2013), batch cultivations in M9 minimal medium resulted in 60 % lower amounts of TAG in comparison to cultivations in LB. Our study now shows that rather inexpensive mineral media can indeed be suitable for TAG production with genetically engineered E. coli.
In previous studies, a relationship between neutral lipid accumulation and limitation of at least one macroelement has been described for many bacterial species, like Cupriavidus necator, Pseudomonas oleovorans, Rhodococcus opacus, A. baylyi, and others (Schlegel et al. 1961; Lageveen et al. 1988; Alvarez et al. 1996; Fixter and McCormack 1976). In our study, a limited feed of nitrogen during the stationary phase was tested. Nitrogen limitation diminished the growth slightly whereas the TAG content was enhanced, so that the final concentration of TAG was nearly the same. A strict nitrogen limitation may be tested, but it remains also to be investigated, if strains with dgkA deletion produce enough fatty acids for an efficient TAG production under nitrogen starving conditions. In wild-type cells of E. coli, the fatty acid biosynthesis would be downregulated. Furthermore, a negative effect on the expression of the plasmid encoded genes must be prevented.
In batch cultures, we detected external FFA and FAEE as by-products. It is known that E. coli synthesizes a variety of fermentative products, such as ethanol, lactate or acetate, when oxygen is limiting. In combination with the enhanced production of fatty acyl-CoA and heterologous atfA expression, the pathway for FAEE biosynthesis is complete. FAEE synthesis was not reported in the previous studies on TAG production in E. coli, although Lin et al. (2013) detected unknown substances upon expression of WS2 (wax ester synthase 2) of M. hydrocarbonoclasticus in their dgkA deletion mutant. The authors suggested the synthesis of wax esters to occur, since WS2 has a high activity for their biosynthesis (Holtzapple and Schmidt-Dannert 2007). However, WS2 is also very active with ethanol and palmitoyl-CoA as substrates (Shi et al. 2012), and hence it is possible that FAEE are the unknown substances in the study of Lin et al. (2013).
This is of interest since FAEE and free fatty acids, which are formed by the deletion mutant, are potential biofuels that can be produced outside of the cells. Despite the fact that the amounts of extracellular FAEE and FFA that were obtained in this study were very low, this still offers a great potential. Production of extracellular biofuels is a requirement for continuous fermentations or repeated batch cultivations, by which much higher productivities than by fed-batch fermentations can be achieved. Of the two products, FAEE have the advantage to be non-toxic for E. coli cells, even at high concentrations (Steen et al. 2010). On the other hand, FFA production can be done, using a marker free dgkA mutant, without the need for further genetic engineering.
In this study, we demonstrated the possibility to produce FFA and FAEE, repeatedly, with recovery of the cells. However, it must be stated that the productivity decreases with each production cycle. To reach high productivities, further process optimization and strain improvement needs to be done. For example, the export of free fatty acids should be optimized, and the re-import prevented by deletion of fadL.
Overall, this study provides a detailed investigation of TAG production, using E. coli wild-type cells and a dgkA deletion mutant. Fed-batch fermentation led to the production of more than 500 mg l−1 of TAG. Furthermore, the developed strains offer potential for the continuous production of free fatty acids or FAEE.
References
Albright FR, White DA, Lennarz WJ (1973) Studies on enzymes involved in the catabolism of phospholipids in Escherichia coli. J Biol Chem 248:3968–3977
Alvarez HM, Mayer F, Fabritius D, Steinbüchel A (1996) Formation of intracytoplasmic lipid inclusions by Rhodococcus opacus strain PD630. Arch Microbiol 165:377–386
Arabolaza A, Rodriguez E, Altabe S, Alvarez H, Gramajo H (2008) Multiple pathways for triacylglycerol biosynthesis in Streptomyces coelicolor. Appl Environ Microbiol 74:2573–2582
Bokinsky G, Peralta-Yahya PP, George A, Holmes BM, Steen EJ, Dietrich J, Lee TS, Tullman-Ercek D, Voigt CA, Simmons BA, Keasling JD (2011) Synthesis of three advanced biofuels from ionic liquid-pretreated switchgrass using engineered Escherichia coli. Proc Natl Acad Sci U S A 108:19949–19954
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly(beta-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol 54:1977–1982
Cho H, Cronan JE Jr (1995) Defective export of a periplasmic enzyme disrupts regulation of fatty acid synthesis. J Biol Chem 270:4216–4219
Comba S, Menendez-Bravo S, Arabolaza A, Gramajo H (2013) Identification and physiological characterization of phosphatidic acid phosphatase enzymes involved in triacylglycerol biosynthesis in Streptomyces coelicolor. Microb Cell Fact 12:9
Desbois AP, Smith VJ (2010) Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol 85:1629–1642
Fixter LM, McCormack JG (1976) The effect of growth conditions on the wax content of various strains of Acinetobacter. Biochem Soc Trans 4:504–505
Heath RJ, Jackowski S, Rock CO (1994) Guanosine tetraphosphate inhibition of fatty acid and phospholipid synthesis in Escherichia coli is relieved by overexpression of glycerol-3-phosphate acyltransferase (plsB). J Biol Chem 269:26584–26590
Holtzapple E, Schmidt-Dannert C (2007) Biosynthesis of isoprenoid wax ester in Marinobacter hydrocarbonoclasticus DSM 8798: Identification and characterization of isoprenoid coenzyme A synthetase and wax ester synthases. J Bacteriol 189:3804–3812
Kalscheuer R, Steinbüchel A (2003) A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J Biol Chem 278:8075–8082
Kalscheuer R, Stölting T, Steinbüchel A (2006) Microdiesel: Escherichia coli engineered for fuel production. Microbiology (SGM) 152:2529–2536
Kennedy EP (1982) Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A 79:1092–1095
Kol MA, Kuster DW, Boumann HA, de Cock H, Heck AJ, de Kruijff B, de Kroon AI (2004) Uptake and remodeling of exogenous phosphatidylethanolamine in E. coli. Biochim Biophys Acta 1636:205–212
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM II, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
Kurosawa K, Boccazzi P, de Almeida NM, Sinskey AJ (2010) High-cell-density batch fermentation of Rhodococcus opacus PD630 using a high glucose concentration for triacylglycerol production. J Biotechnol 147:212–218
Lageveen RG, Huisman GW, Preusting H, Ketelaar P, Eggink G, Witholt B (1988) Formation of polyesters by Pseudomonas oleovorans: Effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol 54:2924–2932
Lennen RM, Pfleger BF (2013) Microbial production of fatty acid-derived fuels and chemicals. Curr Opin Biotechnol 21:1–10
Lennen RM, Kruziki MA, Kumar K, Zinkel RA, Burnum KE, Lipton MS, Hoover SW, Ranatunga DR, Wittkopp TM, Marner WD II, Pfleger BF (2011) Membrane stresses induced by overproduction of free fatty acids in Escherichia coli. Appl Environ Microbiol 77:8114–8128
Lin F, Chen Y, Levine R, Lee K, Yuan Y, Lin XN (2013) Improving fatty acid availability for bio-hydrocarbon production in Escherichia coli by metabolic engineering. PLOS ONE 8:e78595
Liu H, Yu C, Feng D, Cheng T, Meng X, Liu W, Zou H, Xian M (2012) Production of extracellular fatty acid using engineered Escherichia coli. Microb Cell Fact 11:41
Pech-Canul A, Nogales J, Miranda-Molina A, Alvarez L, Geiger O, Soto MJ, Lopez-Lara IM (2011) FadD is required for utilization of endogenous fatty acids released from membrane lipids. J Bacteriol 193:6295–6304
Raetz CR, Newman KF (1978) Neutral lipid accumulation in the membranes of Escherichia coli mutants lacking diglyceride kinase. J Biol Chem 253:3882–3887
Raetz CR, Newman KF (1979) Diglyceride kinase mutants of Escherichia coli: inner membrane association of 1,2-diglyceride and its relation to synthesis of membrane-derived oligosaccharides. J Bacteriol 137:860–868
Ray TK, Cronan JE Jr (1975) Acylation of sn-glycerol 3-phosphate in Escherichia coli. Study of reaction with native palmitoyl-acyl carrier protein. J Biol Chem 250:8422–8427
Rucker J, Paul J, Pfeifer BA, Lee K (2013) Engineering E. coli for triglyceride accumulation through native and heterologous metabolic reactions. Appl Microbiol Biotechnol 97:2753–2759
Ruffing AM, Jones HD (2012) Physiological effects of free fatty acid production in genetically engineered Synechococcus elongatus PCC 7942. Biotechnol Bioeng 109:2190–2199
Sambrook J, Fritsch EF, Maniatisch T (1989) Molecular cloning a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schlegel HG, Gottschalk G, von Bartha R (1961) Formation and utilization of poly-beta-hydroxybutyric acid by Knallgas bacteria (Hydrogenomonas). Nature 191:463–465
Shi S, Valle-Rodríguez JO, Khoomrung S, Siewers V, Nielsen J (2012) Functional expression and characterization of five wax ester synthases in Saccharomyces cerevisiae and their utility for biodiesel production. Biotechnol Biofuels 5:7
Steen EJ, Kang Y, Bokinsky G, Hu Z, Schirmer A, McClure A, Del Cardayre SB, Keasling JD (2010) Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463:559–562
Touze T, Blanot D, Mengin-Lecreulx D (2008) Substrate specificity and membrane topology of Escherichia coli PgpB, an undecaprenyl pyrophosphate phosphatase. J Biol Chem 283:16573–16583
Uthoff S, Bröker D, Steinbüchel A (2009) Current state and perspectives of producing biodiesel-like compounds by biotechnology. Microb Biotechnol 2:551–565
Wältermann M, Luftmann H, Baumeister D, Kalscheuer R, Steinbüchel A (2000) Rhodococcus opacus strain PD630 as a new source of high-value single-cell oil? Isolation and characterization of triacylglycerols and other storage lipids. Microbiology (SGM) 146:1143–1149
Zhang YM, Rock CO (2008) Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233
Zhang F, Rodriguez S, Keasling JD (2011) Metabolic engineering of microbial pathways for advanced biofuels production. Curr Opin Biotechnol 22:775–783
Zhang F, Carothers JM, Keasling JD (2012) Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat Biotechnol 30:354–359
Zheng Y, Li L, Liu Q, Qin W, Yang J, Cao Y, Jiang X, Zhao G, Xian M (2012) Boosting the free fatty acid synthesis of Escherichia coli by expression of a cytosolic Acinetobacter baylyi thioesterase. Biotechnol Biofuels 5:76-6834-5-76
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janßen, H.J., Steinbüchel, A. Production of triacylglycerols in Escherichia coli by deletion of the diacylglycerol kinase gene and heterologous overexpression of atfA from Acinetobacter baylyi ADP1. Appl Microbiol Biotechnol 98, 1913–1924 (2014). https://doi.org/10.1007/s00253-013-5460-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5460-2