Abstract
Biodiesels are mostly produced from lipid transesterification of vegetable oils, including those from soybean, jatropha, palm, rapeseed, sunflower, and others. Unfortunately, transesterification of oil produces various unwanted side products, including steryl glucosides (SG), which precipitate and need to be removed to avoid clogging of filters and engine failures. So far, efficient and cost-effective methods to remove SGs from biodiesel are not available. Here we describe for the first time the identification, characterization and heterologous production of an enzyme capable of hydrolyzing SGs. A synthetic codon-optimized version of the lacS gene from Sulfolobus solfataricus was efficiently expressed and purified from Escherichia coli, and used to treat soybean derived biodiesel containing 100 ppm of SGs. After optimizing different variables, we found that at pH 5.5 and 87 °C, and in the presence of 0.9 % of the emulsifier polyglycerol polyricinoleate, 81 % of the total amount of SGs present in biodiesel were hydrolyzed by the enzyme. This remarkable reduction in SGs suggests a path for the removal of these contaminants from biodiesel on industrial scale using an environmentally friendly enzymatic process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an urgent demand for sustainable and affordable alternatives to petroleum-based fuels. Biodiesels are environmentally friendly alternative fuels, comprised of mono-alkyl esters of long-chain fatty acids derived from vegetable oils or animal fats. Mostly based on rapeseed oil when first developed in the 1990s, nowadays the production includes various feedstocks such as soybean oil, palm oil, coconut oil, sunflower oil, used frying oil, and animal fat, depending on the market prices (Lacoste et al. 2009). As such, they are a renewable and potentially limitless source of fuel. In particular, biodiesels are useful as fuel for vehicles in replacement or as a supplement to petroleum-based diesel fuels. They can be utilized by traditional fuel-burning engines, produce fewer particulates when burnt, have a higher flash point, and are less toxic than petroleum-based fuels. In 2012, biodiesel production in the United States alone was estimated to be nearly 1.1 billion gal according to the US Environmental Protection Agency.
The content of insoluble contaminants in biodiesel is a closely monitored quality parameter, since an excess of them might cause operational problems in vehicles due to clogging of the engine filters. Insoluble solids present in biodiesel should not exceed the specification limits determined by the norms of the American National Standards Institute and European Standard for Biodiesel (Pfalzgraf et al. 2007; Van Hoed et al. 2008). The formation of sediments in biodiesel is linked to the presence of steryl glucosides (SG) (Bondioli et al. 2007; Moreau et al. 2008; Pfalzgraf et al. 2007; Van Hoed et al. 2008). SG and acylated SG (ASG) are naturally present in plant tissues and vegetable oils, as derivatives of sterols that are the main unsaponifiable components (Sugawara and Miyazawa 1999). In ASG, the 6-position of the sugar is esterified with a long chain fatty acid. Under alkaline conditions, this ester bond between the glucose and the fatty acid is broken, and the ASGs are converted to SG. Such a side reaction occurs during transesterification, resulting in an increased SG concentration in biodiesel in comparison to their initial concentration in the feedstock oil (Van Hoed et al. 2008). Due to the loss of the fatty acid chain, SG have an increased polarity compared to ASG, which explains the formation of precipitates (Lacoste et al. 2009). If not removed from the biodiesel, SG can clog oil filters or cause engine failures. Particles of clumped SG molecules can also promote crystallization, aggregation, or precipitation of other compounds in the biodiesel. This further reduces biodiesel flowability and increases the likelihood of clogging. SGs typically have a high melting point, and thus cannot simply be heated to allow them to pass through an oil filter. As a consequence, they form crystals in the biodiesel, especially at low temperatures, which creates cold-flow problems and can cause blockages in fuel lines under cold conditions. Additionally, the formation of these precipitates may cause several problems during the biodiesel production process resulting in an increase in production costs (Menzella et al. 2012; Soe 2010).
The levels of SG in finished biodiesel may differ depending on the processing method for vegetable oil and the biodiesel production process (Lee et al. 2007). It was reported that the SG contents ranged from 25 to 270 ppm in soybean oil-based biodiesel, 8 to 22 ppm in cottonseed oil-based biodiesel, 480 ppm in corn oil-based biodiesel, and 140 ppm in palm oil-based biodiesel (Ringwald 2007). The only method capable of completely removing SGs from biodiesel is distillation, which is energy-intensive, and therefore reduces the cost efficiency and net energy gain of biodiesel production (Tang et al. 2010). Distillation also results in a significant decrease in the natural antioxidant content, reducing the oxidative stability of the biodiesel. Filtering, such as through diatomaceous earth, is expensive and not easily scalable to large quantities. Adding adsorbents requires an additional removal step, and is similarly expensive and time-consuming (Brask and Nielsen 2010).
An enzyme with steryl glycosidase activity may be used to digest SGs, producing a glycoside and a sterol. In this process, the resulting sterols would be soluble in biodiesel, and the sugar moiety could be further removed during the water-washing steps typically used in plants. Only a few examples of enzymatic hydrolysis of SG have been reported in literature, describing this activity in aqueous media (Kalinowska and Wojciechowski 1978; Kesselmeier et al. 1985; Nystrom 2008). However, to the best of our knowledge, the enzymes responsible for such activity have not been identified so far.
Here we describe for the first time the identification, characterization and heterologous production of an enzyme capable of hydrolyzing SGs, suggesting a path for the enzymatic removal of this contaminants from biodiesel on industrial scale.
Materials and methods
General
Enzymes were obtained from New England Biolabs (USA) and used as recommended. DH5α, BL21(DE3) and W3110 Escherichia coli strains were made chemically competent with a kit from Zymo Research (USA). DNA sequencing was performed on an ABI 3730 DNA analyzer (Applied Biosystems, USA) according to the manufacturer's recommended protocol. All other reagents were obtained from Sigma (USA).
Codon optimization, gene synthesis and cloning
A synthetic version of each gene used in this work was designed as previously described (Menzella 2011; Ravasi et al. 2012) where a codon randomization algorithm with the Optimizer software and a codon table containing a fractional preference for each codon equal to that found in the genome of E. coli W3110 was used. DNA sequences were designed according to the amino-acidic sequences of the following proteins: GH from Sulfolobus islandicus (YP_002830364.1), GH from Caldivirga maquilingensis IC-167 (YP_001540243.1), Bgal from Sulfolobus acidocaldarius DSM 639 (YP_256448.1), GH from Vulcanisaeta distributa DSM 14429 (YP_003902017.1), BgpA from Terrabacter ginsenosidimutans Gsoil 3082 (ACZ66247.2), LacS from Sulfolobus solfataricus P2 (NP_344331.1), BGal from Acidilobus saccharovorans 345–15 (YP_003816826.1), BGal from Thermoplasma volcanium GSS1 (NP_111204.1), GH from Ignisphaera aggregans DSM 17230 (YP_003859636.1), GH from Thermosphaera aggregans DSM 11486 (YP_003650331.1), BGal from Thermoproteus uzoniensis 768–20 (YP_004338090.1). DNA sequences were synthesized by Genscript (USA), cloned into pET28a at NdeI/EcoRI restriction sites, and verified by sequencing. In all the cases, E. coli DH5α was used for cloning. Codon optimized genes were deposited in GenBank under the accession numbers listed in Table 1.
Culture growth, gene expression and purification
For the expression of heterologous proteins, E. coli BL21 (DE3) strains harboring the corresponding plasmids were grown at 37 °C in shake flasks in LB medium in the presence of 50 mg/l kanamycin for plasmid maintenance. Overnight cultures were diluted 1:100 in fresh medium and grown to an OD600 of 0.5 to 0.8 before the addition of IPTG to a final concentration of 0.5 mM. Induction was allowed to proceed for 14 h at 22 °C. The cells were harvested, resuspended in 50 mM phospate buffer pH 6.8, 150 mM NaCl, and 10 % glycerol, and disrupted by sonication. After disruption, crude extracts were clarified by centrifugation during 30 min at 15,000 g. Enzymes were purified by affinity chromatography, using Ni-NTA Agarose resin (Invitrogen) according to the protocol supplied by the manufacturer. Elution fractions containing the corresponding recombinant enzyme were analyzed by SDS–PAGE (Laemmli 1970) to check for purity. Protein concentration was determined by the method of Bradford (1976), using BSA as standard. Coomassie Brilliant Blue staining was utilized to reveal SDS–PAGE.
SG hydrolysis in aqueous buffer/biodiesel emulsions
Polyglycerol polyricinoleate (PGPR; trade name: ADMUL WOL) and distilled biodiesel were mixed at different proportions to obtain a content of 0, 0.3 and 0.9 g of PGPR for 100 g of emulsion. The w/o emulsions were prepared with 15 ml of distilled biodiesel supplemented with PGPR and 100 ppm of SG, and 2.25 ml of 50 mM buffer with different pHs containing the enzyme. The reactions were incubated at different temperatures and agitated at 500 rpm in a VP 710 magnetic tumble stirrer (V&P Scientific). At different incubation times, 400-μl aliquots were taken for glucose quantification.
SG hydrolysis analysis
Three different methods were used to analyze the hydrolysis of SG under different reaction conditions.
In all cases, SG extent of hydrolysis was determined by the formation of glucose, one of its reaction products. Since the amount of glucose released from SGs is quantitatively partitioned in the water phase in water/biodiesel emulsions, this method could be used both in aqueous and water/biodiesel reactions. Glucose was specifically converted to gluconate 6-phosphate in a coupled enzymatic reaction (hexokinase, glucose 6-phosphate dehydrogenase), with formation of NADPH, which was determined fluorometrically at 340 nm in a Synergy 2 Multi-Mode Microplate Reader (BioTek) (Bondar and Mead 1974). Protocols were obtained from www.sigma.com. A calibration curve was obtained by measuring known amounts of glucose. The lowest limit of glucose quantitation using this method is equivalent to 3 ppm of SG.
In order to confirm the extent of SG hydrolysis in biodiesel, SG concentration was also measured by GC-FID. For this, 100 mg of homogenized biodiesel sample were placed in a 20 ml vial, and 100 μl of Internal Standard (tricaprin in pyridine; Sigma 44897-U) and 100 μl of N-Methyl-N-trimethylsilyltrifluoroacetamide (MSTFA; Sigma M7891) were added to the sample vials. The vials were hermetically sealed and shaken vigorously and incubated at room temperature for 15 min. Next, 8 ml heptane was added to each vial and 1 μl of the reaction mixture was automatically injected into an Agilent 7890A gas chromatograph equipped with a temperature programmable capillary injector and an FID. Peak separation was achieved with a 5-m pre-column linked to a capillary column, 15 m long, 0.32 mm internal diameter, and film thickness of 0.1 μm, with a 95 % dimethyl–5 % diphenyl-polysiloxane stationary phase (DB5-HT; J&W Scientific). The oven temperature was kept at 100 °C for 5 min, and then increased by 10 °C/min up to 370 °C. The final temperature was maintained for 15 min. The FID temperature was set to 370 °C and the hydrogen pressure was fixed at 100 kPa. SG quantification was based on internal standard and a ratio of 1:100 or higher in the peak areas was required.
The presence of free sterols, generated as a result of steryl glycoside hydrolysis after the enzymatic treatment in water was determined by thin layer chromatography (TLC). Sterols from the reaction were extracted three times with 0.6 volumes of ethyl acetate and evaporated to dryness under vacuum. Samples were re-dissolved in 10 μl of ethyl acetate and spotted onto silica gel G-60 F254 TLC plate (Merck). TLC plates were developed using hexane/methanol 85:15 as running buffer and compounds were visualized by spraying the plates with anisaldehyde/sulfuric acid/ethanol (1:1:9) and heating with a hot-air gun until color developed.
β-Glucosidase activity assay
β-Glucosidase activity was assayed using a modification of the assay described by Hang et al. (Hang and Woodams 1994). The reaction mixture (500 μl) contained 465 μl of sodium citrate buffer (25 mM, pH 5.5), 25 μl of 20 mM p-nitrophenyl-β-d-glucopyranoside (pNPG), and 10 μl of the appropriate dilution of enzyme-containing sample. After incubation at 80 °C for 5 min, the reaction was stopped by adding 1 ml of cold 200 mM sodium carbonate. The activity of LacS was estimated spectrophotometrically by reading the absorbance of the liberated p-nitrophenol at 405 nm (ε = 18,700). One unit (U) was defined as the amount of enzyme required for the hydrolysis of 1 μmol pNPG/min, under the assay conditions.
Results
Gene selection, design and expression vector construction
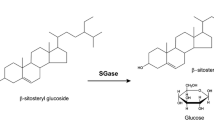
SGs are a family of compounds characterized by a sugar moiety attached to the 3 β-hydroxy group at the C3-atom of a sterol characterized by a planar sterol backbone made up of four condensed aliphatic rings and a hydrocarbon side chain at C17. Figure 1 shows the structure of β-sitosteryl glucoside, the most abundant SG in vegetable oil derived biodiesel along with campesteryl glucoside and stigmasteryl glucoside (Nystrom et al. 2012). In order to provide a novel enzymatic solution to address this problem, we decided to start our efforts investigating type III β-glycosidases; a family of enzymes that recognize the glucose moiety and display a relaxed specificity with regard to the aglycone portion of the molecule. Since SGs are mostly insoluble in biodiesel at concentrations above 25 ppm at room temperature, we additionally restricted our search to thermostable enzymes. Three enzymes meeting both criteria mentioned above; LacS from S. solfataricus, BgaS from S. acidocaldarius and BgpA from T. ginsenosidimutans have been previously reported to hydrolyze ginsenosides, a group of molecules with structural analogy to SGs (Fig. 1) (An et al. 2010; Noh and Oh 2009; Noh et al. 2009). Thus, the three genes encoding these enzymes and eight additional sequences encoding archaeal enzymes with a homology higher than 68 % related to LacS were selected for our initial screening (Table 1).
The 11 protein sequences were codon-optimized for expression in E. coli using the software Optimizer (Puigbo et al. 2007). The software accepts the amino acid sequences, which are reverse translated and modified using an strategy used for codon optimization consisting on randomly assigning a triplet for each amino acid using a preference table (http://www.kazusa.or.jp/codon/cgi-bin/showcodon.cgi?species=316407), with a probability based on the weight of each codon within the set encoding a given amino acid. The resulting sequences were synthesized adding an NdeI restriction site overlapping the ATG start codon and an EcoRI site downstrean the stop, and cloned into the same sites of the T7 based pET28a expression plasmid to obtain N-terminal fusion to a His6 tag.
Gene expression, enzyme purification and activity assays
Recombinant plasmids were transformed into the E. coli BL21(DE3) strain for expression tests. Cell cultures on LB medium were induced by the addition of IPTG when OD600 reached 0.5 and cells were harvested after 5 h. Analysis of soluble and insoluble fractions of cell lysates by SDS-PAGE showed that all the synthetic genes were successfully expressed, and that sufficient quantities of soluble protein was obtained to asses activity for all the enzymes under study.
Next, all individual proteins were prepared from 1-l culture and purified using a Ni2+-NTA affinity resin. The preparations were concentrated by ultrafiltration to obtain a final protein concentration of 1 mg/ml in ammonium acetate buffer pH5,5 and tested for steryl glucosidase (SGase) activity against a commercial mixture of SG containing β-sitosteryl glucoside, campesteryl glucoside and stigmasteryl glucoside (Matreya LLC; 98 % pure). After incubation at 70 °C for 6 h, TLC analysis revealed that eight of the 11 enzymes tested had the ability to hydrolyze SGs under the assay conditions. SGase activity was further confirmed by measuring the generated glucose through a coupled enzymatic assay as described in the Materials and methods section. It is worth mentioning that the presence of minor quantities of SG possessing sugars different from glucose (i.e., galactose) has been reported in some vegetable extracts (Grille et al. 2010). However, SG analysis by the glucose method used here strictly correlates with the total amount of SG determined by GC-FID, indicating that the amount of SG containing other sugars is negligible. Among the enzymes evaluated in the assay, LacS from S. solfataricus and BGal from Thermoproteus uzoniensis exhibited the highest efficiency, hydrolyzing 31 % and 18 % of the total SGs, respectively (Fig. 2a and b). Based on these results, these two enzymes were selected to be tested for SGase activity on biodiesel.
SGase activity analysis in aqueous buffer. Hydrolysis of SG was determined a qualitatively through detection of free sterols by TLC and b quantitatively through glucose measurement by the coupled enzymatic assay. Data correspond to mean values for duplicates of three independent experiments. Enzymes tested were: 1 GH from S. islandicus, 2 GH from C. maquilingensis, 3 BgaS from S. acidocaldarius, 4 GH from V. distributa, 5 BgpA from T. ginsenosidimutans, 6 LacS from S. solfataricus, 7 BGal from A. saccharovorans, 8 BGal from T. volcanium, 9 GH from I. aggregans, 10 GH from T. aggregans, 11 BGal from T. uzoniensis
Evaluation of steryl glucosidase activity in biodiesel
Non-aqueous solvents, like biodiesel, have a variety of effects on enzymes: they may bind specifically, compete with substrate binding, dissociate multimers, shift an equilibrium between two enzyme conformations, alter the amount of helix, react with the enzyme, stabilize or destabilize the enzyme, and affect the rate of the catalytic reaction in several different ways (Doukyu and Ogino 2010).
Both LacS and BGal, the enzymes selected from our screening, are multimeric enzymes that naturally work on aqueous environments (Aguilar et al. 1997). Thus, our next challenge was to evaluate different conditions to hydrolyze SGs in biodiesel. For this purpose, distilled soybean oil-derived biodiesel free of SGs was supplemented with 100 ppm of a mixture of β-sitosteryl glucoside, campesteryl glucoside and stigmasteryl glucoside (56:25:18), a typical quantity and composition of SGs found on industrial crude biodiesel (Bondioli et al. 2007), was used to test the enzymes.
For the initial test, different amounts of purified LacS or BGal in 2.25 ml of citrate buffer were added to 15 ml of biodiesel prepared as described above. The mixtures were incubated for up to 7 h at 80 °C and pH 5.5 with stirring, and the glucose generated from the hydrolysis of SGs was quantified using a coupled enzymatic assay. Figure 3a shows that about 8 % of the total content of SGs was hydrolyzed under the tested conditions using LacS, while no activity was detected for BGal or in the absence of the enzymes (data not shown). In all the experiments, SG content of the mixture was determined by GC-FID to confirm the hydrolysis by the loss of the corresponding quantities of this compound.
Effect of enzyme and emulsifier concentration on LacS catalyzed hydrolysis of SG in biodiesel. Time course of SG hydrolysis in biodiesel–water mixtures (87:13) at 80 °C and pH 5.5 using different amounts of LacS in the absence of PGPR (a), and supplemented with 0.3 % PGPR (b) or 0.9 % PGPR (c). Data correspond to mean values for duplicates of at least three independent experiments
We hypothesized that the inefficient hydrolysis of SGs in biodiesel compared to the aqueous assays could be due to mass-transfer limitations of this compound across the biodiesel/water interface. To address this issue, a new experiment was conducted by adding to the reaction mixture variable concentrations of PGPR, a powerful water-in-oil emulsifier. Its high emulsifying properties are attributed to the excellent water-binding capacity of the long hydrophilic polyglycerol chain, resulting in a large increase in interfacial area upon addition to the water–biodiesel biphasic system (Marquez et al. 2010). Figure 3b and c shows that the addition of PGPR significantly increased the hydrolysis by LacS in a concentration and time dependant manner, achieving 62 % of removal of the SGs present in the biodiesel treated.
Effect of pH and temperature on the activity of LacS in biodiesel
To further optimize the process, we next tested the influence of pH and temperature on the efficiency of LacS-mediated hydrolysis of SGs in biodiesel. Figure 4 illustrates the behavior of LacS at different pHs showing that the enzyme is active in the range 4.5–7, exhibiting a maximum performance between 5 and 6.5. Thus, no additional increase in SG hydrolysis could be achieved by modifying this variable.
The effect of temperature on the hydrolysis of SGs by LacS is shown in Fig. 5. The enzyme displays a maximum activity at 87 °C. Shifting the temperature from 80 °C to 87 °C raised the total amount of hydrolyzed SGs to 81 %. The amount of β-sitosteryl glucoside, campesteryl glucoside and stigmasteryl glucoside, the three types of SGs present in the samples, decreased at the same proportion, indicating no preference by LacS among these substrates (data not shown). It was also observed that the activity at 94 °C significantly decays over the time relative to the activity at 80 °C, suggesting that LacS might lose stability in water/biodiesel emulsions at temperatures above 80 °C. This assumption was supported by the results of an additional experiment, where we measured the β-glycosidase activity of LacS in the water fraction of samples taken from the experiments illustrated in Fig. 5. In this analysis, we found that the total amount of LacS added was recovered from the water fraction, and that at 94 °C the half-life of LacS is dramatically reduced (Fig. 6). We conclude from these experiments that: (1) LacS is completely located in the water fraction and (2) the enzyme is unstable in water/biodiesel emulsions supplemented with PGPR at temperatures above 87 °C.
Discussion
Steryl glucosides occur naturally in vegetable oils and fats both in the free or acylated form. In their acylated form, SGs are very soluble in vegetable oil. During the biodiesel conversion process, they are converted to nonacylated SGs. It has been found that the presence of SGs in biodiesel contribute to flowability problems in biodiesel and biodiesel blends, leading to filter clogging and causing engines fueled by biodiesel to stop. Accordingly, SGs removal from biodiesel is highly desirable but efficient and cost-effective methods are not available so far.
In this work we have identified for the first time an enzyme capable of hydrolyzing β-sitosteryl glucoside, campesteryl glucoside and stigmasteryl glucoside, the most common SGs derived from the vegetable oils used as a feedstock to produce biodiesel. SGase activity in aqueous media was previously reported in commercial enzymatic preparations from filamentous fungi such as Novozym 188 (Nystrom 2008), and in membrane fractions of Sinapis alba seedlings (Kalinowska and Wojciechowski 1978). Although in this latter example the enzyme was purified and characterized, its identity at primary structure level remains to be elucidated.
LacS, a thermostable water soluble enzyme which has been reported to hydrolyzed gingenosides, molecules with structural similarity to SGs, was successfully expressed at high levels in E. coli using a codon optimized gene, showing a path for its massive industrial production. Although it can degrade SGs in water, initial experiments showed that its efficiency in biodiesel–water mixtures was poor, presumably due to the amphipathic nature of SGs and its low diffusion to the water droplets where LacS is located. The problem was partially circumvented by the addition of PGPR, an emulsifier that increase the interfacial area in the used water–biodiesel system. Using this additive, a dramatic raise from 8 % to 62 % of the total hydrolysis was achieved. A further increase in the hydrolysis of SGs by LacS in our system was accomplished by shifting the reaction temperature from 80 °C to 87 °C. Such increase of activity of LacS is not observed in water nor for other substrates (Noh et al. 2009). We speculate that the obtained increase in the efficiency of SGs degradation might be attributed of a higher diffusion of SGs towards the aqueous phase, augmenting the effective concentration of the substrate and thus favoring the kinetics of the enzyme.
After optimizing all the tested variables, we found that at pH 5.5 and 87 °C, and in the presence of 0.9 % PGPR 81 % of the total amount of SGs present in biodiesel were hydrolyzed. This remarkable reduction in SGs content below 20 ppm may completely resolve the problem caused by these impurities and provide a biodiesel of superior quality. Interestingly, the enzyme works optimally at pH, temperature and water content compatible to those found in some stages of the biodiesel production process at industrial scale. For example, the enzymatic treatment may be introduced after the aqueous washing step following the transesterification process where the conditions meet those required for optimal activity of LacS.
The use of PGPR may be a problem, since part of this emulsifier may remain in biodiesel after the enzymatic treatment, separation of the water phase containing LacS and final drying. This problem may be circumvented by exploring alternative solutions which include immobilization of the enzyme on amphiphilic supports, which allows substrates and products to diffuse through a hydrophobic domain to a hydrophilic phase in which the enzyme is entrapped (Bruns and Tiller 2005). Conjugation of the enzyme to hydrophobic polymer groups to self-assemble at biodiesel/water interface could be another option, where it could catalyze interfacial reaction between SG dissolved in the oil and water molecules (Zhu and Wang 2004). These alternatives are currently being explored in our laboratory.
References
Aguilar CF, Sanderson I, Moracci M, Ciaramella M, Nucci R, Rossi M, Pearl LH (1997) Crystal structure of the beta-glycosidase from the hyperthermophilic archeon Sulfolobus solfataricus: resilience as a key factor in thermostability. J Mol Biol 271(5):789–802
An DS, Cui CH, Lee HG, Wang L, Kim SC, Lee ST, Jin F, Yu H, Chin YW, Lee HK, Im WT, Kim SG (2010) Identification and characterization of a novel Terrabacter ginsenosidimutans sp. nov. beta-glucosidase that transforms ginsenoside Rb1 into the rare gypenosides XVII and LXXV. Appl Environ Microbiol 76(17):5827–5836
Bondar RJ, Mead DC (1974) Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem 20(5):586–590
Bondioli P, Cortesi N, Mariani C (2007) Identification and quantification of steryl glucosides in biodiesel. Eur J Lipid Sci Technol 111:120–126
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Brask J, Nielsen R (2010) Enzymatic removal of steryl glycosides in fatty acid alkyl esters. WO2010102952 A1
Bruns N, Tiller JC (2005) Amphiphilic network as nanoreactor for enzymes in organic solvents. Nano Lett 5(1):45–48
Doukyu N, Ogino H (2010) Organic solvent-tolerant enzymes. Biochem Eng J 48(3):270–282
Grille S, Zaslawski A, Thiele S, Plat J, Warnecke D (2010) The functions of steryl glycosides come to those who wait: recent advances in plants, fungi, bacteria and animals. Prog Lipid Res 49(3):262–288
Hang YD, Woodams EE (1994) Apple pomace: a potential substrate for production of β-glucosidase by Aspergillus foetidus. Food Sci Technol 27:587–589
Kalinowska M, Wojciechowski ZA (1978) Purification and some properties of steryl beta-d-glucoside hydrolase from Sinapis alba seedlings. Phytochemistry 17(9):1533–1537
Kesselmeier J, Eichenberger W, Urban B (1985) High performance liquid chromatography of molecular species from free sterols and sterylglycosides isolated from oat leaves and seeds. Plant Cell Physiol 26:463–471
Lacoste F, Dejean F, Griffon H, Rouquette C (2009) Quantification of free and esterified steryl glucosides in vegetable oils and biodiesel. Eur J Lipid Sci Technol 111(8):822–828
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lee I, Pfalzgraf L, Poppe G, Powers E, Haines T (2007) The role of sterol glucosides on filter plugging. Biodiesel Mag 4:105–112
Marquez AL, Medrano A, Panizzolo LA, Wagner JR (2010) Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. J Colloid Interface Sci 341(1):101–108
Menzella HG (2011) Comparison of two codon optimization strategies to enhance recombinant protein production in Escherichia coli. Microb Cell Fact 10:15
Menzella H, Peiru S, Vetcher L (2012) Enzymatic removal of steryl glycosides. PCT/US2013/031769
Moreau RA, Scott KM, Haas MJ (2008) The identification and quantification of steryl glucosides in precipitates from commercial biodiesel. J Am Oil Chem Soc 85:761–770
Noh KH, Oh DK (2009) Production of the rare ginsenosides compound K, compound Y, and compound Mc by a thermostable beta-glycosidase from Sulfolobus acidocaldarius. Biol Pharm Bull 32(11):1830–1835
Noh KH, Son JW, Kim HJ, Oh DK (2009) Ginsenoside compound K production from ginseng root extract by a thermostable beta-glycosidase from Sulfolobus solfataricus. Biosci Biotechnol Biochem 73(2):316–321
Nystrom L (2008) Enzymatic hydrolisis of steryl ferulates and steryl glycosides. Eur Food Res Technol 227:727–733
Nystrom E, Schar A, Lampi AM (2012) Steryl glycosides and acylated steryl glycosides in plant foods reflect unique sterol patterns. Eur J Lipid Sci Technol 114(6):656–669
Pfalzgraf L, Lee I, Foster J, Poppe G (2007) The effect of minor components on cloud point and filterability. Inform Suppl Biorenewable Resour 4:17–21
Puigbo P, Guzman E, Romeu A, Garcia-Vallve S (2007) OPTIMIZER: a web server for optimizing the codon usage of DNA sequences. Nucleic Acids Res 35(Web Server issue):W126–W131
Ravasi P, Peiru S, Gramajo H, Menzella HG (2012) Design and testing of a synthetic biology framework for genetic engineering of Corynebacterium glutamicum. Microb Cell Fact 11:147
Ringwald SC (2007) Biodiesel characterization in the QC environment. The 98th AOCS Annual Meeting Abstracts. AOCS Press, Urbana 15
Soe JB (2010) Method. WO 2010004423 A2
Sugawara T, Miyazawa T (1999) Separation and determination of glycolipids from edible plant sources by high-performance liquid chromatography and evaporative light-scattering detection. Lipids 34(11):1231–1237
Tang H, De Guzman R, Salley S, Simon Ng KY (2010) Comparing process efficiency in reducing steryl glucosides in biodiesel. J Am Oil Chem Soc 87:337–345
Van Hoed V, Zyaykina N, de Greyt W, Maes J, Verhé R, Demeestere K (2008) Identification and occurrence of steryl glucosides in palm and soy biodiesel. J Am Oil Chem Soc 85:701–709
Zhu G, Wang P (2004) Polymer − enzyme conjugates can self-assemble at oil/water interfaces and effect interfacial biotransformations. J Am Chem Soc 126(36):11132–11133
Acknowledgments
This work was funded by Keclon SA and the grant from Agencia Nacional de Promocion Cientifica y Tecnologica PICT 2010–1157. The authors thank Raul Bernardi from Unitec Bio for providing biodiesel samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Andres Aguirre and Salvador Peiru contributed equally to this work
Rights and permissions
About this article
Cite this article
Aguirre, A., Peiru, S., Eberhardt, F. et al. Enzymatic hydrolysis of steryl glucosides, major contaminants of vegetable oil-derived biodiesel. Appl Microbiol Biotechnol 98, 4033–4040 (2014). https://doi.org/10.1007/s00253-013-5345-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5345-4