Abstract
The hydrolytic activity of a recombinant β-glycosidase from Dictyoglomus turgidum that specifically hydrolyzed the xylose at the C-6 position and the glucose in protopanaxatriol (PPT)-type ginsenosides followed the order Rf > Rg1 > Re > R1 > Rh1 > R2. The production of aglycone protopanaxatriol (APPT) from ginsenoside Rf was optimal at pH 6.0, 80 °C, 1 mg ml−1 Rf, and 10.6 U ml−1 enzyme. Under these conditions, D. turgidum β-glycosidase converted ginsenoside R1 to APPT with a molar conversion yield of 75.6 % and a productivity of 15 mg l−1 h−1 after 24 h by the transformation pathway of R1 → R2 → Rh1 → APPT, whereas the complete conversion of ginsenosides Rf and Rg1 to APPT was achieved with a productivity of 1,515 mg l−1 h−1 after 6.6 h by the pathways of Rf → Rh1 → APPT and Rg1 → Rh1 → APPT, respectively. In addition, D. turgidum β-glycosidase produced 0.54 mg ml−1 APPT from 2.29 mg ml−1 PPT-type ginsenosides of Panax ginseng root extract after 24 h, with a molar conversion yield of 43.2 % and a productivity of 23 mg l−1 h−1, and 0.62 mg ml−1 APPT from 1.35 mg ml−1 PPT-type ginsenosides of Panax notoginseng root extract after 20 h, with a molar conversion yield of 81.2 % and a productivity of 31 mg l−1 h−1. This is the first report on the APPT production from ginseng root extract. Moreover, the concentrations, yields, and productivities of APPT achieved in the present study are the highest reported to date.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For over several thousand years, ginseng (the roots of Panax ginseng C. A. Meyer) has been used as a traditional medicine in Asian countries to strengthen immunity, supply nutrition, and decrease fatigue. Triterpene saponin ginsenosides found in ginseng have important biological and pharmaceutical activities, including anticancer (Chen et al. 2013), antineoplastic, antistress (Attele et al. 1999), antioxidant (Li et al. 2010), antiallergic (Choo et al. 2003), antidiabetic (Ni et al. 2010), and antiaging effects (Zhang et al. 1994).
Deglycosylated ginsenosides are more readily absorbed into the bloodstream and have better pharmacological properties than glycosylated ginsenosides (Kim et al. 2005). Deglycosylated ginsenosides, including F1, F2, Rg3, Rh1, Rh2, compound Y, compound Mc, compound K, aglycone protopanaxdiol (APPD), and aglycone protopanaxatriol (APPT), can be produced by hydrolyzing the sugar moieties of the major glycosylated ginsenosides, such as Rb1, Rb2, Rc, Rd, Rf, Re, R1, and Rg1 (Park et al. 2010). In ginseng, deglycosylated ginsenosides exist at a low concentration or are absent. Therefore, many studies have focused on the hydrolysis of sugars in the major glycosylated ginsenosides.
APPT is absent in ginseng but can be produced from the glycosylated protopanaxatriol (PPT)-type ginsenosides such as Rf and Rh1 by intestinal bacteria. APPT is a very promising drug candidate because of its powerful pharmacological effects such as improvement of memory, increase in hippocampal excitability (Wang et al. 2009), mediation of antitumor effects (Hasegawa et al. 2002), and decrease in the proliferation of leukemia cells (Popovich and Kitts 2002). APPT can be converted from PPT-type ginsenosides by bacteria and β-glycosidases. Bacteroides JY-6 converted Re to APPT(Bae et al. 2005); other human intestinal microflora (Wang et al. 2001) and a β-glucosidase from Actinosynnema mirum (Cui et al. 2013) converted Rg1 to APPT; naringinase from Penicillium decumbens (Ko et al. 2003) and ginsenosidase type IV from Aspergillus sp. 39g (Wang et al. 2012) converted Rf and Rg2 to APPT; and β-glucosidases from Aspergillus niger (Liu et al. 2010) and Penicillium aculeatum (Lee et al. 2013) converted Rf to APPT. However, the APPT production from R1 and ginseng root extract has not been reported.
In this study, we investigated the hydrolytic activity of a recombinant β-glycosidase from Dictyoglomus turgidum that specifically hydrolyzed the xylose at the C-6 position and the glucose in PPT-type ginsenosides. The enzymatic reaction conditions, such as pH, temperature, and the concentrations of enzyme and substrate, were optimized for APPT production. Under the optimized conditions, D. turgidum β-glycosidase produced APPT from the reagent-grade ginsenosides R1, Rf, and Rg1, and P. ginseng and Panax notoginseng root extracts.
Materials and methods
Materials
The ginsenoside standards R1, R2, Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh1, and APPT were purchased from Sigma (St. Louis, MO, USA), BTGin (Daejeon, Korea), and Ambo Laboratories (Daejeon, Korea).
Preparation of ginseng root extract
To extract ginsenosides from ginseng roots, 50 g of P. ginseng and P. notoginseng powdered dry roots were incubated with 500 ml of methanol/water mixture (4:1, v/v) at 80 °C for 12 h. After cooling, the extract was filtered through a 0.45-μm filter, the methanol was removed by evaporation, and then the residue was dissolved in 500 ml of distilled water. The solution was loaded onto a glass column (length × diameter: 500 mm × 12 mm) packed with Diaion HP-20 resin, which was then rinsed with 300 ml of distilled water to remove the unbound sugars and other hydrophilic compounds leaving ginsenosides attached to the resin. The adsorbed ginsenosides were eluted at a flow rate of 0.5 ml min−1 with the methanol/water mixture (4:1, v/v); methanol in the eluted product was removed by evaporation, and then the residue was dissolved in 500 ml of distilled water (Noh et al. 2009). The weights of the final extracted residues from P. ginseng and P. notoginseng were 4,914 and 3,627 mg, respectively.
Bacterial strains, plasmid, and gene cloning
D. turgidum DSM 6724 (DSMZ, Brauschweig, Germany), Escherichia coli ER2566 (New England Biolabs, Herfordshire, UK), and plasmid pET-24a (+) (Novagen, Darmstadt, Germany) were used as the sources of β-glycosidase gene, host cells, and expression vector, respectively. D. turgidum genomic DNA was extracted using a genomic DNA buffer set (Qiagen, Hilden, Germany). The β-glycosidase gene (2,247 bp) was amplified by PCR using D. turgidum genomic DNA as a template. The oligonucleotide primers used for gene cloning were based on the sequence of glycosyl hydrolase (GH) 3 family domain protein from D. turgidum (GenBank accession number YP_002352162). Forward (5′-GCTAGCATGAGTGTGGATATAAAAAAGCTCA-3′) and reverse (5′-CTCGAGTTAGCTATTAAGTTCTCTCAGTAGGT-3′) primers, designed to introduce the underlined NheI and XhoI restriction sites, were synthesized by Bioneer (Daejeon, Korea). The β-glycosidase gene was amplified by DNA by PCR using Pfu DNA polymerase (Solgent, Daejeon, Korea), purified using a gel extraction kit (Promega, Madison, WI, USA), and cloned into the pGEM-T Easy vector (Promega). The NheI-XhoI fragment from the T-vector harboring the β-glycosidase gene was subcloned into the same sites of pET-24a (+), and the resulting plasmid was transformed into E. coli ER2566. A kanamycin-resistant colony was selected on LB agar containing 20 μg ml−1 kanamycin, and the plasmid DNA was isolated using a plasmid purification kit (Promega). DNA sequencing was performed at the Macrogen facility (Seoul, Korea).
Enzyme expression and enzyme purification
For enzyme expression, recombinant E. coli cells were cultivated in a 2-l flask containing 500 ml of Luria–Bertani (LB) medium supplemented with 20 μg ml−1 kanamycin at 37 °C with shaking at 200 rpm. When the optical density of the bacteria reached 0.6 at a wavelength of 600 nm, enzyme expression was induced by the addition of isopropyl-β-d-thiogalactopyranoside to a final concentration of 0.1 mM, after which the culture was incubated at 16 °C with shaking at 150 rpm for 16 h.
The recombinant cells were harvested from culture broth by centrifugation at 6,000×g for 30 min at 4 °C, washed twice with 0.85 % NaCl, resuspended in 50 mM phosphate buffer (pH 8.0) containing 300 mM NaCl and 1 mg ml−1 lysozyme, and disrupted on ice using a sonicator (Fisher Scientific Model 100, Pittsburg, PA, USA). The cell debris was removed by centrifugation at 13,000×g for 20 min at 4 °C, and the supernatant was filtered through a 0.45-μm filter. The filtrate was applied to a His-Trap affinity chromatography column (GE Healthcare, Piscataway, NJ, USA) equilibrated with 50 mM phosphate buffer (pH 8.0) containing 300 mM NaCl. The column was then washed extensively with the same buffer, and the bound protein was eluted with a linear gradient of 10 to 250 mM imidazole at a flow rate of 1 ml min−1. The purification step using the column was carried out in a cold room at 4 °C using a fast protein liquid chromatography system (Bio-Rad Laboratories, Hercules, CA, USA). The active fractions were collected, dialyzed against 50 mM phosphate/citrate buffer (pH 6.0), and used as the purified enzyme.
Hydrolytic activity
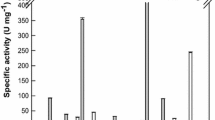
One unit (U) of the β-glycosidase activity was defined as the amount of enzyme required to release 1 μmol p-nitrophenol (pNP) from pNP-β-d-glucopyranoside (pNPG) per minute at 80 °C in 50 mM phosphate/citrate buffer (pH 6.0). The hydrolytic reaction toward pNPG was performed at 80 °C for 5 min in 50 mM phosphate/citrate buffer (pH 6.0) containing 1 mM pNPG and 0.04 U ml−1 enzyme, and the release of pNP was assessed by the increase in absorbance at 450 nm. The hydrolytic activity toward the glycosylated PPT-type ginsenoside was measured from the increase in the concentration of the product ginsenoside. The substrate specificity was determined after incubation at 80 °C for 10 min in 50 mM phosphate/citrate buffer (pH 6.0) containing 0.4 mg ml−1 R1, R2, Re, Rf, Rg1, Rg2, or Rh1, enzyme, and 4 % (v/v) dimethyl sulfoxide (DMSO). The enzyme concentrations were 0.26 U ml−1 for R1, Re, Rg1, and Rg2; 10.24 U ml−1 for R2; 0.05 U ml−1 for Rf; and 5.12 U ml−1 for Rh1.
Optimization of reaction conditions for APPT production
The optimal pH and temperature for APPT production from Rf using D. turgidum β-glycosidase were determined in the temperature range from 70 to 90 °C at a constant pH of 5.5, and the pH range from 5.0 to 7.0 at a constant temperature of 80 °C. The reactions were performed in 50 mM citrate/phosphate buffer containing 0.4 mg ml−1 Rf, 2.56 U ml−1 enzyme, and 4 % (v/v) DMSO for 40 min. Thermal inactivation of D. turgidum β-glycosidase was evaluated by incubation at the temperatures from 70 to 85 °C for various times. A sample was withdrawn at each time point and assayed in 50 mM citrate/phosphate buffer (pH 6.0) containing 0.4 mg ml−1 Rf, 2.56 U ml−1 enzyme, and 4 % (v/v) DMSO, at 80 °C for 40 min.
To determine the optimal concentrations of enzyme and substrate, enzyme concentrations from 2.65 to 26.5 U ml−1 (at 1 mg ml−1 Rf) and substrate concentrations from 0.4 to 2 mg ml−1 (at 10.6 U ml−1 enzyme) were evaluated. The reactions were performed at 80 °C in 50 mM citrate/phosphate buffer (pH 6.0) containing enzyme, ginsenoside Rf, and 4 % (v/v) DMSO for 40 min. The time course reactions of APPT production were performed at 80 °C in 50 mM citrate/phosphate buffer (pH 6.0) containing 1 mg ml−1 ginsenoside or 10 % (w/v) ginseng root extract, 10.6 U ml−1 enzyme, and 4 % (v/v) DMSO. The reaction times were 6.6 h for Rf and Rg1, and 24 h for R1 and ginseng root extracts.
Analytical methods
A reaction mixture containing digoxin as an internal standard was extracted with an equal volume of n-butanol. The n-butanol fraction was then evaporated to dryness, and methanol was added (Huang et al. 2006). Ginsenosides were analyzed by HPLC using an Agilent 1100 system (Santa Clara, CA, USA) equipped with a C18 column and a UV detector. The ginsenosides were eluted at 37 °C with a linear gradient of acetonitrile/water from 20:80 to 100:0 (v/v) for 80 min at a flow rate of 1 ml min−1 and assayed at 203 nm.
Results
Substrate specificity of D. turgidum β-glycosidase
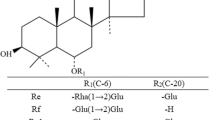
The substrate specificity of D. turgidum β-glycosidase toward PPT-type ginsenosides as substrates is shown in Table 1. The specific activity followed the order Rf > Rg1 > Re > R1 > Rh1 > R2, and the reaction products were Rh1, Rh1, Rg2, R2, APPT, and Rh1, respectively. No activity was observed for Rg2. Thus, the glycosylated PPT-type ginsenosides were transformed by D. turgidum β-glycosidase via the pathways of R1 → R2 → Rh1 → APPT, Rf → Rh1 → APPT, Rg1 → Rh1 → APPT, and Re → Rg2 (Fig. 1).
Optimization of the reaction conditions for APPT production by D. turgidum β-glycosidase
No APPT was formed when the reactions were performed under the experimental conditions without enzyme or with grown cells of E. coli ER2566, which did not contain the β-glycosidase gene from D. turgidum. In experiments on the optimization of APPT production, Rf was used as a substrate because D. turgidum β-glycosidase showed the highest substrate specificity for Rf among the PPT-type ginsenosides. APPT production was examined over a temperature range from 70 to 90 °C at pH 5.5 (Fig. 2a), and maximum activity was observed at 80 °C. At 70 and 85 °C, the activity was approximately 70 % of the maximum. The effect of pH on APPT production from Rf was investigated in a pH range from 5.0 to 7.0 at 80 °C, and maximum activity was recorded at pH 6.0 (Fig. 2b). The production of APPT from R1 and Rg1 was also optimal at pH 6.0 and 80 °C. Thermal stability of D. turgidum β-glycosidase was assessed by measuring the residual activity after incubation at 70, 75, 80, and 85 °C (Fig. 3). Thermal inactivation of the enzyme followed first-order kinetics, with half-lives for Rf of 267, 99, 27, and 0.32 h at 70, 75, 80, and 85 °C, respectively. Due to the instability of the enzyme at 85 °C, the optimum temperature for APPT production was determined to be 80 °C.
Effects of pH and temperature on the activity of D. turgidum β-glycosidase for Rf. a pH effect. The reactions were performed in 50 mM phosphate/citrate buffer containing 0.4 mg ml−1 Rf, 2.65 U ml−1, and 4 % (v/v) DMSO enzyme at 80 °C for 40 min by varying the pH from 5.0 to 7.5. b Temperature effect. The reactions were performed in 50 mM phosphate/citrate buffer (pH 5.5) containing 0.4 mg ml−1 Rf, 2.65 U ml−1, and 4 % (v/v) DMSO enzyme for 40 min by varying the temperature from 70 to 90 °C. Data represent the means of three experiments, and error bars represent the standard deviation
Thermal inactivation of the activity of D. turgidum β-glycosidase in the reactions with Rf. The enzyme was incubated at 70 °C (open triangle), 75 °C (filled triangle), 80 °C (open circle), and 85 °C (filled circle) in 50 mM phosphate/citrate buffer (pH 6.0) for various times. A sample was withdrawn at each time point and assayed in 50 mM citrate/phosphate buffer (pH 6.0) containing 0.4 mg ml−1 Rf, 2.65 U ml−1 enzyme, and 4 % (v/v) DMSO at 80 °C for 40 min. Data represent the means of three experiments, and error bars represent the standard deviation
APPT production was investigated at enzyme concentrations ranging from 2.65 to 26.5 U ml−1 at 1 mg ml−1 Rf as a substrate for 40 min (Fig. 4a). APPT production from Rf increased proportionally with enzyme concentrations up to 10.6 U ml−1 and reached a plateau at concentrations above 10.6 U ml−1 as a substrate Rf of 1 mg ml−1. Therefore, the optimal enzyme concentration was determined to be 10.6 U ml−1. APPT production was tested at 10.6 U ml−1 enzyme with Rf concentrations ranging from 0.4 to 2 mg ml−1 for 40 min (Fig. 4b). APPT production increased with increasing APPT concentrations up to 1 mg ml−1 Rf and reached a plateau above 1 mg ml−1 Rf. Thus, the optimal substrate concentration was determined to be 1 mg ml−1. The effect of enzyme concentration on APPT production was also investigated with 2 mg ml−1 Rf for 90 min, and the optimal enzyme concentration was 10.6 U ml−1 (data not shown). The time course reactions of APPT production were performed with 2 mg ml−1 Rf and 10.6 U ml−1 enzyme for 7 h (data not shown). The productivity of APPT with 2 mg ml−1 Rf was higher than that with 1 mg ml−1 Rf. The molar conversion yield of Rf to APPT with 2 mg ml−1 Rf was 75 % after 7 h whereas that with 1 mg ml−1 Rf was 100 % after 6.6 h. Rf was determined to be 1 mg ml−1 due to the low conversion yield at 2 mg ml−1.
Effect of the concentrations of substrate and enzyme on APPT production from Rf using D. turgidum β-glycosidase. a Effect of enzyme concentration. The reactions were performed in 50 mM citrate/phosphate buffer (pH 6.0) containing 1 mg ml−1 Rf, enzyme, and 4 % (v/v) DMSO at 80 °C for 40 min. b Effect of substrate concentration. The reactions were performed in 50 mM citrate/phosphate buffer (pH 6.0) containing Rf, 10.6 U ml−1 enzyme, and 4 % (v/v) DMSO at 80 °C for 40 min. Data represent the means of three experiments, and error bars represent the standard deviation
APPT production from ginsenosides R1, Rf, and Rg1 by D. turgidum β-glycosidase
The optimal reaction conditions for APPT production from Rf using D. turgidum β-glycosidase were pH 6.0, 80 °C, 10.6 U ml−1 enzyme, and 1 mg ml−1 ginsenoside. Under these conditions, the time course reactions of APPT production were performed from R1 for 24 h and from Rf and Rg1 for 6.6 h. D. turgidum β-glycosidase converted R1 to APPT by the transformation pathway of R1 → R2 → Rh1 → APPT, with a molar conversion yield of 75.6 % and a productivity of 15 mg l−1 h−1 after 24 h (Fig. 5a). The enzyme completely converted Rf and Rg1 to APPT via Rh1 as an intermediate, with a productivity of 1,515 mg l−1 h−1 after 6.6 h (Fig. 5b, c).
APPT production from R1, Rf, and Rg1 using D. turgidum β-glycosidase. a APPT production (filled circle) from R1 (open diamond) via R2 (open diamond, x-hair) and Rh1 (semi-filled circle). b APPT production (filled circle) from Rf (open circle) via Rh1 (semi-filled circle). c APPT production (filled circle) from Rg1 (open square) via Rh1 (semi-filled circle). The reaction was performed at 80 °C in 50 mM phosphate/citrate buffer (pH 6.0) containing 1 mg ml−1 R1, Rf, or Rg1, 10.6 U ml−1 enzyme, and 4 % (v/v) DMSO. Data represent the means of three experiments, and error bars represent the standard deviation
APPT production from P. ginseng and P. notoginseng root extracts by D. turgidum β-glycosidase
P. ginseng and P. notoginseng root extracts contained the PPT-type ginsenosides R1, Re, Rf, Rg1, Rg2, and Rh1 and the protopanaxadiol (PPD)-type ginsenosides Rb1, Rb2, Rc, Rd, and Rg3 (Table 2). The total ginsenoside contents in 10 % (w/v) P. ginseng and P. notoginseng root extracts were 3.51 and 2.34 mg ml−1, respectively. The content of specific PPT-type ginsenoside in P. ginseng root extract for the total PPT-type ginsenosides followed the order Rg1 (43.7 %) > Re (33.6 %) > Rf (19.2 %) > Rg2 (2.2 %) > Rh1 (1.3 %), whereas that in P. notoginseng root extract followed the order Rg1 (74.1 %) > R1 (16.3 %) > Re (8.9 %) > Rh1 (0.7 %). Under the optimal conditions (pH 6.0, 80 °C, and 10.6 U ml−1 enzyme), the time course reactions of APPT production by D. turgidum β-glycosidase were performed from 10 % (w/v) P. ginseng and P. notoginseng root extracts for 24 h. The enzyme produced 0.54 mg ml−1 APPT from 2.29 mg ml−1 PPT-type ginsenosides of P. ginseng root extract after 24 h, with a molar conversion yield of 43.2 % and a productivity of 23 mg l−1 h−1 (Fig. 6a) and 0.62 mg ml−1 APPT from 1.35 mg ml−1 PPT-type ginsenosides of P. notoginseng root extract after 20 h, with a molar conversion yield of 81.2 % and a productivity of 31 mg l−1 h−1 (Fig. 6a).
APPT production from ginseng root extract using D. turgidum β-glycosidase. a APPT production (filled circle) from Rf (open circle) and Rg1 (open square) in P. ginseng root extract via Rh1 (semi-filled circle). Re (open triangle) in P. ginseng root extract was converted to Rg2 (close triangle). b APPT production (filled circle) from ginsenosides R1 (open diamond) and Rg1 (open square) via R2 (open diamond, x-hair) and Rh1 (semi-filled circle) in P. notoginseng root extract. Re (open triangle) in P. notoginseng root extract was converted to Rg2 (close triangle). The reactions were performed at 80 °C in 50 mM phosphate/citrate buffer (pH 6.0) containing 10 % (w/v) ginseng root extract, 10.6 U ml−1 enzyme, and 4 % (v/v) DMSO. Data represent the means of three experiments, and error bars represent the standard deviation
Discussion
D. turgidum β-glycosidase has been used for the production of PPD-type ginsenosides, including compound Mc, compound Y, and APPD (Lee et al. 2012). However, this enzyme has not been applied for the production of PPT-type ginsenosides. In the present study, we investigated the hydrolytic activity of D. turgidum β-glycosidase in the conversion of PPT-type ginsenosides to APPD. PPT-type ginsenosides contain different sugar moieties at the C-6 and C-20 positions. The sugars at the C-6 position are β-d-glucopyranose, β-d-glucopyranosyl-(1→2)-β-d-glucopyranose, α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranose, and β-d-xylopyranoside-(1→2)-β-d-glucopyranose, whereas the sugar at the C-20 position is β-d-glucopyranose. D. turgidum β-glycosidase hydrolyzes the outer β-d-glucopyranose at the C-6 position to convert Rf to Rh1 and β-d-xylopyranoside at the C-6 position to convert R2 to Rh1, whereas it cleaves β-d-glucopyranose at the C-20 position to convert R1, Re, and Rg1 to R2, Rg2, and Rh1, respectively. However, the enzyme cannot hydrolyze α-l-rhamnopyranoside at the C-6 position of Rg2 (Table 1). Therefore, the enzyme specifically hydrolyzes the xylose at the C-6 position and the glucose in PPT-type ginsenosides.
β-Glucosidases from Sanguibacter keddieii (Kim et al. 2012) and Penicillium sclerotiorum (Wei et al. 2011) hydrolyze β-d-glucopyranose at the C-6 position to Rg1 convert to F1, but does not hydrolyze β-d-glucopyranose at the C-20 position. Ginsenosidase type IV from Aspergillus sp. 39g (Wang et al. 2012; Lee et al. 2013) and ginsenoside-α-l-rhamnosidase from Absidia sp. 39 (Yu et al. 2002) hydrolyze α-l-rhamnopyranoside at the C-6 position to convert Rg2 to Rh1. Bacteroides JY6 (Bae et al. 2005), a crude enzyme from A. niger (Chi and Ji 2005), naringinase from P. decumbens (Ko et al. 2003) hydrolyze α-l-rhamnopyranoside at the C-6 position to convert Re to Rg1. These results indicate that D. turgidum β-glycosidase has a novel specificity for PPT-type ginsenosides different from those of other enzymes that hydrolyze PPT-type ginsenosides.
The biotransformation pathways of the glycosylated PPT-type ginsenosides to APPT are summarized in Table 3. D. turgidum β-glycosidase utilizes three transformation pathways of the glycosylated PPT-type ginsenosides to APPT: R1 → R2 → Rh1 → APPT, Rf → Rh1 → APPT, and Rg1 → Rh1 → APPT. The transformation pathway of R1 → R2 → Rh1 → APPT is first reported in the present study. The pathway of Rf → Rh1 → APPT has been reported for the crude β-glucosidase from A. niger (Liu et al. 2010), ginsenosidase type IV from Aspergillus sp. 39g (Wang et al. 2012), β-glucosidase from P. aculeatum (Lee et al. 2013), and naringinase from P. decumbens (Ko et al. 2003). The pathway of Rg1 → Rh1 → APPT has been reported in intestinal microflora (Wang et al. 2001), including Bacteroides JY-6 (Bae et al. 2005), and β-glucosidase from A. mirum (Cui et al. 2013). The pathway of Rg2 → Rh1 → APPT has been demonstrated for ginsenosidase type IV from Aspergillus sp. 39g (Wang et al. 2012) and naringinase from P. decumbens (Ko et al. 2003). However, D. turgidum β-glycosidase does not use this pathway because it lacks l-rhamnosidase activity (Table 1). These results indicate that D. turgidum β-glycosidase exhibits hydrolytic activity for PPT-type ginsenosides different from those of other enzymes.
This is the first report of the conversion of R1 to APPT (Fig. 5a). A. niger β-glucosidase converted Rf to APPT with the highest previously reported molar conversion yield and productivity of 90.4 % and 5.4 mg l−1 h−1, respectively (Liu et al. 2010). However, D. turgidum β-glycosidase converted Rf to APPT with a molar conversion yield of 100 % and a productivity of 1,515 mg l−1 h−1 (Fig. 5b), which are 9.6 % and 280-fold higher, respectively, than those of A. niger β-glucosidase. Thus, D. turgidum β-glycosidase produces APPT from Rf with the highest conversion yield and productivity reported to date. D. turgidum β-glycosidase also completely converted Rg1 to APPT after 6.6 h, with a productivity of 1,515 mg l−1 h−1 (Fig. 5c). This is the first report of the quantitative APPT production from Rg1.
Ginseng root extract has been used for the production of PPD-type ginsenosides, including compound K and APPD, using microorganisms and enzymes. Compound K was produced from ginseng root extract by the fungi Paecilomyces bainier (Zhou et al. 2008) and Fusarium sacchari (Han et al. 2007), a pectinase from A. niger (Kim et al. 2006), and β-glycosidases from Sulfolobus solfataricus (Noh et al. 2009), Sulfolobus acidocaldarius (Noh and Oh 2009), and Pyrococcus furiosus (Yoo et al. 2011). The highest conversion yield of compound K from ginseng root extract was reported for P. bainier, whereas the highest productivity was shown for β-glycosidase from P. furiosus. APPD production from ginseng root extract using β-glycosidase from P. furiosus has been reported. However, ginseng root extract has not been used for the production of PPT-type ginsenosides.
D. turgidum β-glycosidase utilized the transformation pathways of R1 → R2 → Rh1 → APPT, Rf → Rh1 → APPT, Rg1 → Rh1 → APPT, and Re → Rg2 (Fig. 1), but did not convert Re and Rg2 to APPT (Table 1). The contents of the two ginsenosides Re and Rg2 in P. ginseng and P. notoginseng root extracts for total PPT-type ginsenosides were 35.8 and 8.9 % (w/w), respectively (Table 2). P. notoginseng root extract is a better substrate for APPT production using D. turgidum β-glycosidase because of its low content of Re and Rg2. The enzyme produced 0.62 mg ml−1 APPT from 1.35 mg ml−1 PPT-type ginsenosides of P. notoginseng root extract after 20 h, with a molar conversion yield of 81.2 % and a productivity of 31 mg l−1 h−1 (Fig. 6a), which were 35 % and 1.4-fold higher, respectively, than those of P. ginseng root extract (Fig. 6b). This is the first report of the production of PPT-type ginsenoside from ginseng root extract.
In conclusion, APPT was produced from R1, Rf, Rg1, and ginseng root extract using D. turgidum β-glycosidase that specifically hydrolyzed the xylose at the C-6 position and the glucose in PPT-type ginsenosides. This is the first report on APPT production from R1 and ginseng root extract. Moreover, the concentrations, yields, and productivities of APPT achieved in the present study are the highest reported to date. Our results should contribute to an improvement in the industrial production of APPT by biotransformation. β-Glycosidase with a broader specificity is required for the conversion of Re and Rg2 to APPT in order to increase the APPT production from ginseng root extract.
References
Attele AS, Wu JA, Yuan CS (1999) Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol 58:1685–1693
Bae EH, Shin JE, Kim DH (2005) Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Bio Pharm Bull 28:1903–1908
Chen Y, Xu YJ, Zhu Y, Li XL (2013) Anti-cancer effects of ginsenoside compound K on pediatric acute myeloid leukemia cells. Cancer Cell Int 13:24
Chi H, Ji GE (2005) Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol Lett 27:765–771
Choo MK, Park EK, Han MJ, Kim DH (2003) Antiallergic activity of ginseng and its ginsenosides. Planta Med 69:518–522
Cui CH, Kim SC, Im WT (2013) Characterization of the ginsenoside transforming recombinant β-glucosidase from Actinosynnema mirum and bioconversion of major ginsenosides into minor ginsenosides. Appl Microbiol Biotechnol 97:649–659
Han Y, Sun B, Hu X, Zhang H, Jiang B, Spranger MI, Zhao Y (2007) Transformation of bioactive compounds by Fusarium sacchari fungus isolated from the soil cultivated ginseng. J Agric Food Chem 55:9373–9379
Hasegawa H, Suzuki R, Nagaoka T, Tezuka Y, Kadota S, Saiki I (2002) Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid conjugate of protopanaxatriol. Bio Pharm Bull 25:861–866
Huang CR, Wang GJ, Li H, Xie HT, Sun HG, Lv H, Lv T (2006) Sensitive and selective liquid chromatography electrospray ionisation mass spectrometry analysis of astragaloside-IV in rat plasma. J Pharm Biomed Anal 40:788–793
Kim MK, Lee JW, Lee KY, Yang DC (2005) Microbial conversion of major ginsenoside Rb1 to pharmaceutically active minor ginsenoside Rd. J Microbiol 43:456–462
Kim BH, Lee SY, Cho HJ, You SN, Kim YJ, Park YM, Lee JK, Baik MY, Park CS, Ahn SC (2006) Biotransformation of Korean Panax ginseng by Pectinex. Biol Pharm Bull 29:2472–2478
Kim JK, Cui CH, Yoon MH, Kim SC, Im WT (2012) Bioconversion of major ginsenosides Rg1 to minor ginsenoside F1 using novel recombinant ginsenoside hydrolyzing glycosidase cloned from Sanguibacter keddieii and enzyme characterization. J Biotechnol 161:294–301
Ko SR, Choi KJ, Suzuki K, Suzuki Y (2003) Enzymatic preparation of ginsenosides Rg2, Rh1, and F1. Chem Pharm Bull 51:404–408
Lee GW, Kim KR, Oh DK (2012) Production of rare ginsenosides (compound Mc, compound Y and aglycon protopanaxadiol) by β-glucosidase from Dictyoglomus turgidum that hydrolyzes beta-linked, but not α-linked, sugars in ginsenosides. Biotechnol Lett 34:1679–1686
Lee GW, Yoo MH, Shin KC, Kim KR, Kim YS, Lee KW, Oh DK (2013) β-Glucosidase from Penicillium aculeatum hydrolyzes exo-, 3-O-, and 6-O-β-glucosides but not 20-O-β-glucoside and other glycosides of ginsenosides. Appl Microbiol Biotechnol 97:6315–6324
Li BH, Wang CZ, He TC, Yuan CS, Du W (2010) Antioxidants potentiate American ginseng-induced killing of colorectal cancer cells. Cancer Lett 289:62–70
Liu L, Gu LJ, Zhang DL, Wang Z, Wang CY, Li Z, Sung CK (2010) Microbial conversion of rare ginsenoside Rf to 20(S)-protopanaxatriol by Aspergillus niger. Biosci Biotechnol Biochem 74:96–100
Ni HX, Yu NJ, Yang XH (2010) The study of ginsenoside on PPARgamma expression of mononuclear macrophage in type 2 diabetes. Mol Biol Rep 37:2975–2979
Noh KH, Oh DK (2009) Production of the rare ginsenosides compound K, compound Y, and compound Mc by a thermostable β-glycosidase from Sulfolobus acidocaldarius. Bio Pharm Bull 32:1830–1835
Noh KH, Son JW, Kim HJ, Oh DK (2009) Ginsenoside compound K production from ginseng root extract by a thermostable β-glycosidase from Sulfolobus solfataricus. Biosci Biotechnol Biochem 73:316–321
Park CS, Yoo MH, Noh KH, Oh DK (2010) Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol 87:9–19
Popovich DG, Kitts DD (2002) Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch Biochem Biophys 406:1–8
Wang Y, Liu TH, Wang W, Wang BX (2001) Research on the transformation of ginsenoside Rg1 by intestinal flora. China J Chin Mater Med 26:188–190
Wang YZ, Chen J, Chu SF, Wang YS, Wang XY, Chen NH, Zhang JT (2009) Improvement of memory in mice and increase of hippocampal excitability in rats by ginsenoside Rg1's metabolites ginsenoside Rh1 and protopanaxatriol. J Pharmacol Sci 109:504–510
Wang DM, Yu HS, Song JG, Xu YF, Jin FX (2012) Enzyme kinetics of ginsenosidase type IV hydrolyzing 6-O-multi-glycosides of protopanaxatriol type ginsenosides. Process Biochem 47:133–138
Wei Y, Zhao W, Zhang Q, Zhao Y, Zhang Y (2011) Purification and characterization of a novel and unique ginsenoside Rg1-hydrolyzing β-D-glucosidase from Penicillium sclerotiorum. Acta Biochim Biophys Sin 43:226–231
Yoo MH, Yeom SJ, Park CS, Lee KW, Oh DK (2011) Production of aglycon protopanaxadiol via compound K by a thermostable β-glycosidase from Pyrococcus furiosus. Appl Microbiol Biotechnol 89:1019–1028
Yu HS, Gong J, Zhang C, Jin FX (2002) Purification and characterization of ginsenoside-β-L-rhamnosidase. Chem Pharm Bull 50:175–178
Zhang Y, Takashina K, Saito H, Nishiyama N (1994) Anti-aging effect of DX-9386 in senescence accelerated mouse. Biol Pharm Bull 17:866–868
Zhou W, Yan Q, Li JY, Zhang XC, Zhou P (2008) Biotransformation of Panax notoginseng saponins into ginsenoside compound K production by Paecilomyces bainier sp 229. J Appl Microbiol 104:699–706
Acknowledgments
This work was supported by the Basic Research Lab program (no. 2010–0019306), the National Research Foundation, the Ministry of Education, Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, HJ., Shin, KC., Lee, GW. et al. Production of aglycone protopanaxatriol from ginseng root extract using Dictyoglomus turgidum β-glycosidase that specifically hydrolyzes the xylose at the C-6 position and the glucose in protopanaxatriol-type ginsenosides. Appl Microbiol Biotechnol 98, 3659–3667 (2014). https://doi.org/10.1007/s00253-013-5302-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5302-2