Abstract
The production of compound K and aglycon protopanaxadiol (APPD) from ginsenoside Rd and ginseng root extract was performed using a recombinant β-glycosidase from Pyrococcus furiosus. The activity for Rd was optimal at pH 5.5 and 95°C with a half-life of 68 h at 95°C. β-Glycosidase converted Rb1, Rb2, Rc, and Rd to APPD via compound K. With increases in the enzyme activity, the productivities of compound K and APPD increased. The substrate concentration was optimal at 4.0 mM Rd or 10% (w/v) ginseng root extract; 4 mM of Rd was converted to 3.3 mM compound K with a yield of 82.5% (mol/mol) and a productivity of 2,010 mg l−1 h−1 at 1 h and was hydrolyzed completely to APPD with 364 mg l−1 h−1 after 5 h. Rb1, Rb2, Rc, and Rd at 3.9 mM in 10% ginseng root extract were converted to 3.1 mM compound K with 79.5% and 1,610 mg l−1 h−1 at 1.2 h and were hydrolyzed completely to APPD with 300 mg l−1 h−1 after 6 h. The concentrations and productivities of compound K and APPD in the present study are the highest ever reported.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ginseng, the root of Panax ginseng C. A. Meyer, has been used as a traditional medicine, in China, Korea, Japan, and other countries in Asia. The ginsenosides in ginseng are the principal components responsible for these diverse biological and pharmaceutical activities (Wu et al. 1992; Yoshikawa et al. 2003). Pharmaceutically active ginsenosides exist as deglycosylated forms at low concentrations or are absent in ginseng (Tawab et al. 2003). The deglycosylated ginsenosides, including ginsenosides F1, F2, Rg3, Rh1, Rh2, compound Y, compound Mc, compound K, aglycon protopanaxadiol (APPD), and aglycon protopanaxatriol, can be produced via the hydrolysis of sugar moieties from the glycosylated major ginsenosides Rb1, Rb2, Rc, Rd, Re, and Rg1. In recent decades, many studies have focused on the production of the deglycosylated ginsenosides, as they evidence more profound pharmaceutical activity than the glycosylated major ginsenosides (Kim et al. 2005; Chen et al. 2008; Park et al. 2010).
The deglycosylated ginsenosides, compound K and APPD, are absent in ginseng root (Yu et al. 2007; Yan et al. 2008a; Noh et al. 2009). These compounds have been recognized as inducers of apoptosis in tumor cells (Suda et al. 2000) and inhibitors of allergy (Bae et al. 2002a), carcinogenesis (Chae et al. 2009), tumor metastasis (Hasegawa et al. 1997), and tumor cell proliferation (Wakabayashi et al. 1997; Kim et al. 2003). Compound K production from ginseng root extract has been achieved via enzymatic methods using the β-glycosidase of Aspergillus sp. (Yu et al. 2007), lactase from Penicillium sp. (Ko et al. 2007), β-galactosidase from Aspergillus oryzae (Ko et al. 2007), β-glucosidase from Fusobacterium sp. (Park et al. 2001), β-glucosidase from Paecilomyces bainier (Yan et al. 2008b), β-glucosidase from Terrabacter ginsenosidimutans (An et al. 2010), pectinase from Aspergillus niger (Kim et al. 2006), β-glycosidase from Sulfolobus solfataricus (Noh et al. 2009), and β-glycosidase from Sulfolobus acidocaldarius (Noh and Oh 2009). However, the production of compound K, thus far, has proven inefficient in terms of productivity. APPD has been produced by acid hydrolysis (Tanaka et al. 1972) and by fermentation using human intestinal bacteria (Bae et al. 2002b). These methods are poorly selective and evidence extremely low productivity. The enzymatic method has been proposed as the most promising for APPD production, owing to its high specificity, yield, and productivity. Although only one qualitatively enzymatic production of APPD has been reported (An et al. 2010), the quantitative study for the production of APPD via enzymatic methods has not been demonstrated.
In this study, the production of APPD via compound K from ginseng root extract and reagent-grade Rd was attempted using a thermostable β-glycosidase from Pyrococcus furiosus. To enhance the production of these ginsenosides, reaction conditions such as pH, temperature, enzyme and substrate concentrations, and reaction time were optimized. Under optimal conditions, the enhanced production of compound K and APPD via β-glycosidase from P. furiosus was achieved.
Materials and methods
Materials
Reagent-grade Rb1, Rb2, Rc, Rd, compound K, and APPD were purchased from Ambo Institute (Daejeon, Korea). Ginseng root extract was prepared via the method reported previously (Son et al. 2008).
Cloning and gene expression
The genomic DNA from P. furiosus DSMZ 3638 (Microbank, Daejeon, Korea), Escherichia coli ER2566 (New Englands Biolabs, Herfordshire, UK), and pET24a plasmid (Novagen, Madison, WI) were used as the source of the β-glycosidase gene, host cells, and expression vector. The gene encoding the β-glycosidase was amplified by polymerase chain reaction (PCR) using the genomic DNA isolated from P. furiosus as a template. The sequence of the oligonucleotide primers used for gene cloning was based on the DNA sequence of P. furiosus β-glycosidase (GenBank accession number No. AAC25555). Forward (5′-GGATCCATGAAGTTCTCCAAAAAAC-3′) and reverse primers (5′-GTCGACCTTTCTTGTAACAAATT-3′) were designed to introduce the BamHI and SalI restriction sites (underlined), respectively, and were synthesized by Bioneer (Daejon, Korea). The amplified DNA fragment obtained by PCR was purified and inserted into the pET24a(+) vector digested with the same restriction enzymes. E. coli ER2566 strain was transformed with the ligation mixture using an electroporator (MicroPulser, Bio-Rad, Hercules, CA) and plated on Luria–Bertani (LB) agar containing 25 μg ml−1 of kanamycin. A kanamycin-resistant colony was selected, and plasmid DNA from the transformant was isolated with a plasmid purification kit (Promega, Madison, WI). DNA sequencing was conducted using a DNA analyzer (ABI Prism 3730xl, Perkin-Elmer, Waltham, MA). Gene expression was evaluated by both SDS-PAGE and enzyme activity.
Enzyme preparation
E. coli cells containing the β-glycosidase/pET24a(+) gene from P. furiosus were grown in LB medium containing 25 μg ml−1 of kanamycin at 37°C with agitation at 200 rpm until an optical density at 600 nm of 1.0 was achieved. Isopropyl-β-d-thiogalactopyranoside was added to the culture medium at a final concentration of 0.1 mM, and incubation continued for 12 h with agitation at 150 rpm at 16°C as the previously reported method (Son et al. 2008; Noh and Oh 2009; Noh et al. 2009). The induced cells were subsequently harvested and disrupted via sonication in 50 mM citrate buffer (pH 5.5). Unbroken cells and cell debris were removed by centrifugation at 13,000×g for 20 min, and the supernatant was treated at 90°C for 10 min to remove E. coli-derived proteins. Following heat treatment, the suspension was centrifuged at 13,000×g for 20 min in order to remove the insoluble denatured proteins. The supernatant was used as the soluble enzyme.
Hydrolytic activity
The hydrolytic reactions of P. furiosus β-glycosidase were performed at 95°C in 50 mM citrate buffer (pH 5.5) containing p-nitrophenyl (pNP)-β-d-glucopyranoside, pNP-β-d-galactopyranoside, oNP-β-d-galactopyranoside, pNP-α-l-arabinopyranoside, or pNP-α-l-arabinofuranoside (Sigma, St. Louis, MO) for 5 min and in 50 mM citrate buffer (pH 5.5) containing 1 mM reagent-grade ginsenoside Rb1, Rb2, Rc, Rd, or compound K for 20 min. The nitrophenyl-glycoside activity was determined by the increase in absorbance at 420 nm due to the release of nitrophenol, and the ginsenoside activity was measured by the increase of the ginsenoside product. The enzyme amounts used for nitrophenyl-glycoside and ginsenoside were 0.2 and 0.02 U ml−1, respectively. One unit of enzyme activity used for ginsenosides or nitrophenyl-glycosides was defined as the amount of enzyme required to liberate 1 μmol of compound K from ginsenoside Rd as a substrate or to liberate 1 μmol of nitrophenol from nitrophenyl-glycoside per min at 95°C and pH 5.5, respectively. One unit of enzyme activity used for ginsenosides corresponded to 0.18 mg protein.
Effects of temperature and pH
To evaluate the effects of pH and temperature on β-glycosidase activity, the temperatures were varied from 70°C to 95°C at pH 5.5, and the pH values were varied from 4.5 to 6.5 using 50 mM citrate buffer (pH 4.5–6.0) and 50 mM phosphate buffer (pH 6.0–6.5) at 95°C. The effect of temperature on enzyme stability was monitored as a function of incubation time by applying the enzyme solution at five different temperatures (75°C, 80°C, 85°C, 90°C, and 95°C) in 50 mM citrate buffer (pH 5.5). Samples were withdrawn at time intervals and then assayed in 50 mM citrate buffer (pH 5.5) containing 1 mM Rd at 95°C for 20 min. The half-life of the enzyme was calculated using Sigma Plot 9.0 software (Systat Software, San Jose, CA).
Effects of enzyme and substrate concentrations on the production of compound K and APPD
In order to evaluate the effect of the amount of enzyme on the production of compound K and APPD, the reactions were performed at 95°C in 50 mM citrate buffer (pH 5.5) by varying the enzyme activity from 1.2 to 48 U ml−1 of enzyme with 4.0 mM Rd for 5 h and from 1.2 to 96 U ml−1 of enzyme with 10% (w/v) ginseng root extract for 6 h. The effect of substrate concentration was investigated at 95°C in 50 mM citrate buffer (pH 5.5) by varying the concentrations of Rd from 1 to 8 mM with 48 U ml−1 of enzyme for 15 h and by varying the concentrations of ginseng extract from 5% to 20% (w/v) with 96 U ml−1 of enzyme for 30 h. Samples were withdrawn at time intervals, after which the time required for the maximal production of compound K and APPD was determined and employed in order to calculate the productivities.
Time courses for the production of compound K and APPD
The time courses for the production of compound K and APPD from ginsenoside Rd and ginseng root extract by β-glycosidase from P. furiosus were investigated. The reactions were performed at 95°C in 50 mM citrate buffer (pH 5.5) containing 48 U ml−1 of enzyme and 4 mM reagent-grade Rd for 5 h, and 96 U ml−1 of enzyme and 10% (w/v) ginseng root extract for 6 h.
Analytical methods
Digoxin as an internal standard was added to the samples and then extracted with an identical volume of n-butanol for 1 min via vortexing. After centrifugation at 13,000×g for 5 min, the upper organic phase as a n-butanol fraction was transferred into a clean tube and evaporated to dryness in the centrifugal evaporator (Eyela CVE-3100, Tokyo, Japan). The residue was then reconstituted by methanol for HPLC analysis (Huang et al. 2006). Ginsenosides were assayed using an HPLC system (Agilent 1100, Santa Clara, CA) equipped with a UV detector at 203 nm using a C18 column (250 × 4.6 mm, YMC, Kyoto, Japan). The column was initially eluted with a mixture of acetonitrile and water of 20:80 (v/v) as the mobile phase, a gradient of 40:60 for 40 min, and then a gradient of 80:20 for 80 min. The flow rate was 1.0 ml min−1 and the column temperature was 37°C.
Results
Effects of temperature and pH on the activity of β-glycosidase from P. furiosus.
A gene encoding P. furiosus β-glycosidase, with the same sequence as that reported in GenBank (accession number AAC25555), was cloned and expressed in E. coli. The enzyme was purified from crude extract obtained from harvested cells as a soluble protein by heat treatment. There was little inclusion body in cell debris (Fig. 1). The molecular mass of the expressed protein analyzed by SDS-PAGE was about 55.5 kDa, consistent with the calculated value of 55,488 Da based on the 472 amino acid residues plus six histidine residues.
No compound K and APPD were formed when the reactions were carried out under the experimental conditions without enzyme or with grown cells of E. coli ER2566, which did not harbor the β-glycosidase gene from P. furiosus. In order to evaluate the effect of temperature on the hydrolytic activity of Rd to compound K by β-glycosidase from P. furiosus, the temperature was varied from 70°C to 95°C at pH 5.5. The enzyme activity increased with increases in temperature, evidencing maximal activity at 95°C (Fig. 2a). The effect of pH on the production of compound K from Rd was investigated at 95°C in a pH range of 4.5–6.5. Maximal production was observed at pH 5.5. At pH values of 5.0 and 6.0, the activity was approximately 80% of the maximum (Fig. 2b). Thus, the reaction pH was determined to be 5.5.
Effects of temperature and pH on the activity of β-glycosidase from P. furiosus. a temperature effect: the reactions were performed in 50 mM citrate buffer (pH 5.5) containing 1 mM Rd and 0.01 U ml−1 of enzyme for 20 min. b pH effect: the reactions were performed in 50 mM citrate buffer (filled circle, pH 4.5–6.0) or 50 mM phosphate buffer (empty circle, pH 6.0–6.5) containing 1 mM Rd and 0.01 U ml−1 of enzyme at 95°C for 20 min. Data are expressed as the means of three experiments and the error bars represent standard deviation
The thermal stability of P. furiosus β-glycosidase was examined by measuring the enzyme activity over time at pH 5.5 and at temperatures ranging from 75°C to 95°C (Fig. 3). Thermal inactivation of the enzyme followed first-order kinetics with half-lives of 572 h, 333 h, 172 h, 118 h, and 68 h at 75°C, 80°C, 85°C, 90°C, and 95°C, respectively. Due to the stability of the enzyme at 95°C, this temperature was selected as the reaction temperature for the production of compound K and APPD.
Thermal inactivation of the activity of β-glycosidase from P. furiosus. The enzymes were incubated at 75°C (filled circle), 80°C (empty circle), 85°C (filled triangle down), 90°C (empty triangle), and 95°C (filled square) for varying time periods. A sample was withdrawn at each time interval and the relative activity was determined. Data are expressed as the means of three experiments and the error bars represent standard deviation
Substrate specificity of β-glycosidase from P. furiosus
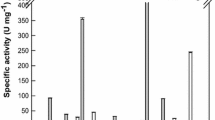
The substrate specificity of the enzyme was investigated using aryl-glycosides and ginsenosides (Table 1). The specific activity for the aryl-glycoside substrates followed the order pNP-β-d-glucopyranoside > oNP-β-d-glucopyranoside > pNP-β-d-galactopyranoside > oNP-β-d-galactopyranoside > pNP-α-l-arabinopyranoside > pNP-α-l-arabinofuranoside. The specific activity of the enzyme for pNP-β-d-glucopyranoside was approximately 60- or 200-fold higher than pNP-α-l-arabinopyranoside or pNP-α-l-arabinofuranoside, respectively.
The specific activity of the enzyme for the ginsenoside substrates followed the order Rd > Rb1 > Rb2 > Rc > compound K. Ginsenosides Rb1, Rb2, or Rc were converted to Rd, Rd was converted to compound K, and compound K was converted to APPD by β-glycosidase from P. furiosus, thereby indicating that the transformation pathway of ginsenosides is as follows: Rb1, Rb2, or Rc → Rd → compound K → APPD (Fig. 4).
Effect of enzyme activity on the production of compound K and APPD by β-glycosidase from P. furiosus
The effect of enzyme activity on the production of compound K and APPD was investigated at pH 5.5 and 95°C by varying enzyme activity from 1.2 to 48 U ml−1 of enzyme with 4.0 mM Rd for 5 h (Fig. 5a). The optimal enzyme activity for compound K production was 8 U ml−1 of enzyme after 5 h and the conversion yield and the productivity of compound K at this activity were 80.8% (mol/mol) and 399 mg l−1 h−1, respectively. Ginsenoside Rd was converted completely to APPD at 48 U ml−1 of enzyme after 5 h with a productivity of 364 mg l−1 h−1. When 48 U ml−1 of enzyme was used, 3.3 mM (2.1 mg ml−1) compound K was produced after 1 h, corresponding to a productivity of 2,010 mg l−1 h−1 and a conversion yield of 82.5% (mol/mol). With increasing enzyme activity, the productivities of compound K and APPD also increased, although the conversion yields remained almost constant.
Effect of enzyme amount on compound K and APPD production by β-glycosidase from P. furiosus. a Ginsenoside Rd as substrate: the reactions were performed at 95°C in 50 mM citrate buffer (pH 5.5) containing 4 mM Rd and 1.2–48 U ml−1 of enzyme for 5 h. b Root extract as substrate: the reactions were performed at 95°C in 50 mM citrate buffer (pH 5.5) containing 10% root extract and 1.2–96 U ml−1 of enzyme for 6 h. Rb1 (empty triangle), Rb2 (empty square), Rc (empty diamond), Rd (filled square), compound K (empty circle), and APPD (filled circle). Data are expressed as the means of three experiments and the error bars represent standard deviation
The effect of enzyme activity on compound K and APPD production was investigated by varying enzyme activity from 1.2 to 96 U ml−1 of enzyme with 10% (w/v) ginseng root extract for 6 h (Fig. 5b). At the optimal enzyme activity for compound K production of 16 U ml−1 of enzyme after 6 h, the productivity and conversion yield of compound K from ginsenosides Rb1, Rb2, Rc, and Rd of compound K were 322 mg l−1 h−1 and 79.5% (mol/mol), respectively. At 96 U ml−1 of enzyme, 3.9 mM ginsenosides of Rb1, Rb2, Rc, and Rd in 10% (w/v) ginseng root extract were converted completely to APPD after 6 h, corresponding to a productivity of 300 mg l−1 h−1. At this enzyme activity, 3.1 mM (2.0 mg ml−1) compound K was produced after 1.2 h, thereby indicating that the conversion yield of compound K from Rb1, Rb2, Rc, and Rd was 79.5% (mol/mol) and the productivity of compound K was 1,610 mg l−1 h−1.
Among the enzyme activities tested, APPD productivity was maximal at 48 U ml−1 of enzyme for 4.0 mM Rd and 96 U ml−1 of enzyme for 10% (w/v) ginseng root extract; these were, therefore, considered the optimal enzyme activities for the production of compound K and APPD. The productivities of compound K and APPD using ginseng root extract were decreased, and the amount of enzyme was increased, relative to those achieved using reagent-grade Rd. The retardation noted with the use of ginseng root extract may be attributable to the inhibition of the enzyme by other ginsenosides in ginseng root extract (Son et al. 2008).
Effect of substrate concentration on compound K and APPD production
The effect of substrate concentration on compound K and APPD production was investigated at pH 5.5 and 95°C by varying the concentrations of ginsenoside Rd from 1.0 to 8.0 mM with 48 U ml−1 of enzyme for 15 h (Fig. 6a). When Rd concentrations of 1.0, 2.0, 4.0, 6.0, and 8.0 mM were employed, the maximal production of compound K was 0.80 mM at 0.25 h, 1.6 mM at 0.50 h, 3.3 mM at 1.00 h, 4.7 mM at 1.75 h, and 6.3 mM at 2.67 h, respectively. The conversion yield of compound K from Rd was constant at approximately 80% (mol/mol). The productivity of compound K was constant within 4.0 mM Rd but was reduced at above 4.0 mM Rd. When Rd concentrations of 1.0, 2.0, 4.0, 6.0, and 8.0 mM were employed, the maximal production of APPD was 1.0 mM at 1.25 h, 2.0 mM at 2.5 h, 4.0 mM at 5.0 h, 4.6 mM at 8.8 h, and 4.8 mM at 13.4 h, respectively. With increasing concentrations of Rd, APPD productivity and yield were constant within 4.0 mM Rd. However, above that concentration, the productivity and yield were reduced even with the prolonged reaction time. Thus, the optimal Rd concentration was determined to be 4.0 mM.
Effect of substrate concentration on the production of compound K and APPD by β-glycosidase from P. furiosus. a Effect of Rd concentration: the reactions for the production of compound K and APPD were performed in 50 mM citrate buffer (pH 5.5) containing 1–8 mM Rd and 48 U ml−1 of enzyme at 95°C for 15 h. b Effect of root extract concentration: the reactions for the production of compound K and APPD were performed in 50 mM citrate buffer (pH 5.5) containing 5–20% (w/v) root extract and 96 U ml−1 of enzyme at 95°C for 30 h. A sample was withdrawn at the specified time interval and then the time for the maximal production of compound K, and APPD was determined and used to calculate the productivities. Productivity of compound K (empty circle), conversion yield of compound K (empty square), productivity of APPD (filled circle), and conversion yield of APPD (filled square). Data are expressed as the means of three experiments and the error bars represent standard deviation
The concentration of ginsenoside root extract was varied from 5% to 20% (w/v) with 96 U ml−1 of enzyme for a reaction time of 30 h (Fig. 6b). The maximal production of compound K was 1.6 mM at 0.6 h with 5% ginsenoside root extract, 3.1 mM at 1.2 h with 10% ginsenoside root extract, 4.7 mM at 2.4 h with 15% ginsenoside root extract, and 6.2 mM at 4.2 h with 20% (w/v) ginsenoside root extract. The conversion yield of compound K from Rb1, Rb2, Rc, and Rd in the ginseng root extract was constant at approximately 80% (mol/mol). The productivity of compound K was constant within a concentration of 10% (w/v) ginsenoside root extract but was reduce above this concentration owing to the retardation of the reaction time. The ginsenoside root extract within 5% (w/v) was hydrolyzed completely to APPD at 3 h and with 10% ginsenoside root extract at 6 h. However, 15% (w/v) ginsenoside root extract was converted to APPD with a yield of 60% (mol/mol) after 12 h, and 20% (w/v) ginsenoside root extract was converted to APPD with a yield of 43% (mol/mol) after 21 h. With increases in the concentration of ginsenoside root extract, the productivity and yield of APPD were constant within a concentration of 10% (w/v) ginsenoside root extract; however, at ginsenoside root extract concentration above 10% (w/v), the productivity and yield were reduced even with the prolonged reaction time. Thus, the optimal concentration of ginsenoside root extract was 10% (w/v).
Time courses for the production of compound K and APPD from ginsenoside Rd and ginseng root extract
The optimal conditions for the production of compound K and APPD were pH 5.5, 95°C, 4.0 mM Rd, and 48 U ml−1 of enzyme with the use of ginsenoside Rd and pH 5.5, 95°C, 10% (w/v) ginseng root extract, 96 U ml−1 of enzyme, and ginseng root extract. Under optimal conditions, the time courses of the production of compound K and APPD using β-glycosidase from P. furiosus were investigated with ginsenoside Rd for 6 h and ginseng root extract for 7 h (Fig. 7). The maximal conversion of Rd to compound K was noted at 1 h with a conversion yield of 82.5% (mol/mol) and a productivity of 2,010 mg l−1 h−1. After 5 h, Rd and compound K were converted completely to APPD with a productivity of 364 mg l−1 h−1.
Time courses for the production of compound K and APPD from ginsenoside Rd and ginseng root extract by β-glycosidase from P. furiosus. a Production of compound K and aglycon PPD from ginsenoside Rd: the reactions were performed in 50 mM citrate buffer (pH 5.5) containing 4 mM Rd and 48 U ml−1 of enzyme at 95 °C. Rd (filled square), compound K (empty circle), and APPD (filled circle). b Production of compound K and aglycon PPD from root extract: The reactions were performed in 50 mM citrate buffer (pH 5.5) containing 10% (w/v) root extract and 96 U ml−1 of enzyme at 95°C. Rb1 (empty triangle), Rb2 (empty square), Rc (empty diamond), Rd (filled square), compound K (empty circle), and APPD (filled circle). Data are expressed as the means of three experiments and the error bars represent standard deviation
The ginsenosides in ginseng root extract that were convertible to APPD via compound K by β-glycosidase from P. furiosus were Rb1, Rb2, Rc, and Rd, the concentrations of each ginsenoside in 10% (w/v) ginsenoside root extract were 1.8, 1.1, 0.6, and 0.4 mM Rd, respectively, and the total amount of these ginsenosides was 3.9 mM. Ginsenosides Rb1, Rb2, and Rc were converted to Rd, which was transformed to APPD via compound K. The maximal concentration of compound K was 3.1 mM at 1.2 h, corresponding to a conversion yield of 79.5% (mol/mol) and a productivity of 1,610 mg l−1 h−1. After 6 h, ginsenosides Rb1, Rb2, Rc, and Rd were converted completely to APPD via compound K, corresponding to a productivity of 300 mg l−1 h−1.
Discussion
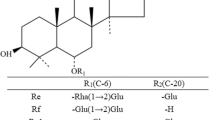
The PPD ginsenosides harbor different sugar moieties at C-3 and C-20 in the aglycon PPD (Fig. 3). The sugars linked to C-3 in the PPD ginsenosides are β-d-glucopyranose and β-d-glucopyranosyl-(1→2)-β-d-glucopyranose, whereas those linked to C-20 are β-d-glucopyranose, α-l-arabinopyranosyl-(1→6)-β-d-glucopyranose, and α-l-arabinofuranosyl-(1→6)-β-d-glucopyranose. The specific activity of β-glycosidase from P. furiosus for the ginsenoside substrates followed the order Rd > Rb1 > Rb2 > Rc > compound K. These results demonstrated that the enzyme evidenced higher hydrolytic activity toward the sugar moiety at C-3 (Rd → compound K) than toward the sugar moiety at C-20 (Rb1, Rb2, or Rc → Rd, compound K → APPD). The specific activity order β-d-glucopyranose linkage of 20-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranose in panaxadiol (Rb1 → Rd) > α-l-arabinopyranose linkage in 20-O-α-l-arabinopyranosyl-(1→6)-β-d-glucopyranose (Rb2 → Rd) > α-l-arabinofuranose linkage in 20-O-α-l-arabinofuranosyl-(1 → 6)-β-d-glucopyranose (Rc → Rd) is consistent with the order pNP-β-d-glucopyranoside > pNP-α-l-arabinopyranoside > pNP-α-l-arabinofuranoside.
One β-d-glycosidase can hydrolyze ginsenosides with different hydrolytic pathways via a multiple-step process. The hydrolytic pathway is determined by the stereo-specific preference of an enzyme for sugars linked to C-3 or C-20 in the PPD type. Several pathways from the glycosylated major ginsenosides to compound K have been previously reported. β-d-Glucosidases from Fusobacterium sp. (Park et al. 2001) and P. bainier (Yan et al. 2010) exploited the hydrolytic pathway of Rb1 → gypenoside XVII → F2 → compound K. β-d-Glucosidase from T. ginsenosidimutans showed two hydrolytic pathways of Rb1 → gypenoside XVII → gypenoside LXXV → compound K and Rd → F2 → compound K (An et al. 2010). β-d-Glycosidase from S. solfataricus exploited two hydrolytic pathways of Rb1 or Rb2 → Rd → F2 → compound K and Rc → compound Mc → compound K (Noh et al. 2009). β-Glycosidase from S. acidocaldarius exploited two hydrolytic pathways of Rb1 → Rd → compound K and Rb2 → compound Y → compound K (Noh and Oh 2009). β-d-Glucosidases from P. bainier (Yan et al. 2008b) and Cladosporium fulvum (Zhao et al. 2009) exploited the hydrolytic pathway of Rb1 → Rd → F2 → compound K and β-glycosidase from Aspergillus sp. exploited the hydrolytic pathways of Rb1, Rb2, or Rb3 → F2 → compound K (Yu et al. 2007).
Only one hydrolytic pathway to APPD has been reported in β-glucosidase from T. ginsenosidimutans as Rg3 → Rh2 → APPD (An et al. 2010). However, the enzyme did not convert compound K into APPD. β-Glycosidase from P. furiosus in the present study exploited different hydrolytic pathway to that from T. ginsenosidimutans as Rb1, Rb2, or Rc → Rd → compound K → APPD. This hydrolytic pathway of Rb1 and Rb2 to compound K was similar to that associated with β-glycosidase from Aspergillus sp. However, the hydrolytic pathway of Rc to compound K via Rd and the conversion of compound K to APPD have not been previously reported, thereby indicating that the hydrolytic pathway involving β-glycosidase from P. furiosus of Rb1, Rb2, or Rc → Rd → compound K → APPD is novel.
The production of compound K by several microorganisms and enzymes is summarized in Table 2. The previous maximal production of compound K from reagent-grade ginsenoside was reported in β-glycosidase from S. acidocaldarius (Noh and Oh 2009). The enzyme converted 0.90 mM reagent-grade Rb1 to 0.85 mM compound K for 3 h, with a productivity of 177 mg l−1 h−1. The concentration and productivity of compound K from 4.0 mM Rd in this study were 3.3 mM and 2,010 mg l−1 h−1 (Fig. 6a); these values were 3.9- and 11-fold higher than those obtained using β-glycosidase from S. acidocaldarius. The previous maximal production of compound K from ginseng root extract was 2.77 mM using β-glycosidase from S. solfataricus with a productivity of 136 mg l−1 h−1 (Noh et al. 2009). The production and productivity of compound K from ginseng root extract in the present study were 3.1 mM and 1,610 mg l−1 h−1, respectively (Fig. 6b); these values were 1.2- and 12-fold higher than those observed using β-glycosidase from S. solfataricus. The concentrations and productivities of compound K and APPD obtained in the present study by β-glycosidase from P. furiosus are the highest ever reported.
The major ginsenosides were decomposed to APPD under both acidic and high-temperature conditions. However, these chemical methods are generally poorly selective, elicit side reactions such as epimerization, hydration, and hydroxylation and generate environmental pollution (Tanaka et al. 1972; Chi and Ji 2005; Park et al. 2010). Significantly low yield of APPD from ginsenosides was exhibited in the hydrolysis of ginseng extract by treatment of acids such as acetic, citric, lactic, tartaric, and hydrochloric acids (Bae et al. 2004). In the experiments, 6 mg ml–1 of ginseng extract, which is corresponding to approximately 250 μg ml–1 ginsenosides (Son et al. 2008), was hydrolyzed to less than 1 μg ml–1 APPD, indicating that the yield of APPD from ginsenosides is less than 0.4%. The human intestinal bacterium Bacteriodes HJ15 converted 0.3 mM ginsenoside Rg3 to 0.07 mM APPD after 40 h of incubation time with a productivity of 0.81 mg l−1 h−1 (Bae et al. 2002b). However, in the icrobial transformation of ADDP from Rg3, the enzymes involved are unknown, and the produced concentration, productivity, and conversion yield of APPD are too low. Ginsenosides Rb1, Rb2, Rc, Rd, and compound K produced via enzymatic transformation using P. furiosus β-glycosidase are hydrolyzed completely to APPD and its concentration and productivity of APPD are 57- and 452-fold higher, respectively, than those obtained by microbial transformation.
The findings of this study demonstrated that APPD can be produced from ginsenoside Rd or ginseng root extract via compound K by a thermostable recombinant β-glycosidase from P. furiosus. The thermostable recombinant enzyme has several advantages for the production of compound K and APPD, including ease of purification by heat treatment, genetic improvement through directed evolution, an increased reaction velocity, increased solubility of compound K and APPD, which evidence low solubility in water at room temperature, and reduced contamination risk. The levels of compound K and APPD productivities achieved using β-glycosidase from P. furiosus are the highest ever reported. Moreover, the ginsenosides Rb1, Rb2, Rc, and Rd in ginseng root extract were hydrolyzed completely to APPD by β-glycosidase from P. furiosus.
References
An DS, Cui CH, Lee HG, Wang L, Kim SC, Lee ST, Jin F, Yu H, Chin YW, Lee HK, Im WT, Kim SG (2010) Identification and characterization of a novel Terrabacter ginsenosidimutans sp. nov. beta-glucosidase that transforms ginsenoside Rb1 into the rare gypenosides XVII and LXXV. Appl Environ Microbiol 76:5827–5836
Bae EA, Choo MK, Park EK, Park SY, Shin HY, Kim DH (2002a) Metabolism of ginsenoside Rc by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull 25:743–747
Bae EA, Han MJ, Choo MK, Park SY, Kim DH (2002b) Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull 25:58–63
Bae EA, Han MJ, Kim EJ, Kim DH (2004) Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm Res 27:61–67
Chae S, Kang KA, Chang WY, Kim MJ, Lee SJ, Lee YS, Kim HS, Kim DH, Hyun JW (2009) Effect of compound K, a metabolite of ginseng saponin, combined with gamma-ray radiation in human lung cancer cells in vitro and in vivo. J Agric Food Chem 57:5777–5782
Chen GT, Yang M, Song Y, Lu ZQ, Zhang JQ, Huang HL, Wu LJ, Guo DA (2008) Microbial transformation of ginsenoside Rb1 by Acremonium strictum. Appl Microbiol Biotechnol 77:1345–1350
Chi H, Ji GE (2005) Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol Lett 27:765–771
Han Y, Sun B, Hu X, Zhang H, Jiang B, Spranger MI, Zhao Y (2007) Transformation of bioactive compounds by Fusarium sacchari fungus isolated from the soil-cultivated ginseng. J Agric Food Chem 55:9373–9379
Hasegawa H, Sung JH, Huh JH (1997) Ginseng intestinal bacterial metabolite IH901 as a new anti-metastatic agent. Arch Pharm Res 20:539–544
Huang C, Wang G, Li H, Xie H, Sun J, Lv H, Lv T (2006) Sensitive and selective liquid chromatography-electrospray ionisation-mass spectrometry analysis of astragaloside-IV in rat plasma. J Pharm Biomed Anal 40:788–793
Kim DH, Jung JS, Moon YS, Sung JH, Suh HW, Kim YH, Song DK (2003) Inhibition of intracerebroventricular injection stress-induced plasma corticosterone levels by intracerebroventricularly administered compound K, a ginseng saponin metabolite, in mice. Biol Pharm Bull 26:1035–1038
Kim MK, Lee JW, Lee KY, Yang DC (2005) Microbial conversion of major ginsenoside Rb1 to pharmaceutically active minor ginsenoside Rd. J Microbiol 43:456–462
Kim BH, Lee SY, Cho HJ, You SN, Kim YJ, Park YM, Lee JK, Baik MY, Park CS, Ahn SC (2006) Biotransformation of Korean Panax ginseng by pectinex. Biol Pharm Bull 29:2472–2478
Ko SR, Suzuki Y, Suzuki K, Choi KJ, Cho BG (2007) Marked production of ginsenosides Rd, F2, Rg3, and compound K by enzymatic method. Chem Pharm Bull (Tokyo) 55:1522–1527
Noh KH, Oh DK (2009) Production of the rare ginsenosides compound K, compound Y, and compound Mc by a thermostable beta-glycosidase from Sulfolobus acidocaldarius. Biol Pharm Bull 32:1830–1835
Noh KH, Son JW, Kim HJ, Oh DK (2009) Ginsenoside compound K production from ginseng root extract by a thermostable beta-glycosidase from Sulfolobus solfataricus. Biosci Biotechnol Biochem 73:316–321
Park SY, Bae EA, Sung JH, Lee SK, Kim DH (2001) Purification and characterization of ginsenoside Rb1-metabolizing beta-glucosidase from Fusobacterium K-60, a human intestinal anaerobic bacterium. Biosci Biotechnol Biochem 65:1163–1169
Park CS, Yoo MH, Noh KH, Oh DK (2010) Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol 87:9–19
Son JW, Kim HJ, Oh DK (2008) Ginsenoside Rd production from the major ginsenoside Rb1 by beta-glucosidase from Thermus caldophilus. Biotechnol Lett 30:713–716
Suda K, Murakami K, Hasegawa H, Saiki I (2000) Induction of apoptosis in Lewis lung carcinoma cells by an intestinal bacterial metabolite produced from orally administered ginseng protopanaxadiol saponins. J Trad Med 17:236–244
Tanaka N, Nagai M, Ohsawa T, Tanaka O, Kawai K, Shibata S (1972) Chemical studies on the oriental drugs. XXVII. The acid catalyzed reactions and the absolute configuration at C20 of dammarane type triterpenes. Chem Pharm Bull (Tokyo) 20:1204–1211
Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M (2003) Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos 31:1065–1071
Wakabayashi C, Hasegawa H, Murata J, Saiki I (1997) In vivo antimetastatic action of ginseng protopanaxadiol saponins is based on their intestinal bacterial metabolites after oral administration. Oncol Res 9:411–417
Wu JY, Gardner BH, Murphy CI, Seals JR, Kensil CR, Recchia J, Beltz GA, Newman GW, Newman MJ (1992) Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J Immunol 148:1519–1525
Yan Q, Zhou W, Li X, Feng M, Zhou P (2008a) Purification method improvement and characterization of a novel ginsenoside-hydrolyzing beta-glucosidase from Paecilomyces bainier sp. 229. Biosci Biotechnol Biochem 72:352–359
Yan Q, Zhou XW, Zhou W, Li XW, Feng MQ, Zhou P (2008b) Purification and properties of a novel beta-glucosidase, hydrolyzing ginsenoside Rb1 to compound K, from Paecilomyces bainier. J Microbiol Biotechnol 18:1081–1089
Yan Q, Zhou W, Shi XL, Zhou P, Ju DW, Feng MQ (2010) Biotransformation pathways of ginsenoside Rb1 to compoundK by beta-glucosidases in fungus Paecilomyces bainier sp. 229. Process Biochem 45:1550–1556
Yoshikawa M, Morikawa T, Kashima Y, Ninomiya K, Matsuda H (2003) Structures of new dammarane-type triterpene saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal ginseng saponins. J Nat Prod 66:922–927
Yu H, Zhang C, Lu M, Sun F, Fu Y, Jin F (2007) Purification and characterization of new special ginsenosidase hydrolyzing multi-glycisides of protopanaxadiol ginsenosides, ginsenosidase type I. Chem Pharm Bull (Tokyo) 55:231–235
Zhao X, Gao L, Wang J, Bi H, Gao J, Du X, Zhou Y, Tai G (2009) A novel ginsenoside Rb1-hydrolyzing beta-D-glucosidase from Cladosprium fulvum. Process Biochem 44:612–618
Zhou W, Yan Q, Li JY, Zhang XC, Zhou P (2008) Biotransformation of Panax notoginseng saponins into ginsenoside compound K production by Paecilomyces bainier sp. 229. J Appl Microbiol 104:699–706
Acknowledgments
This work was supported by a grant (PA090939) from the Seoul R&BD Program and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (No. 2010-0019306).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoo, MH., Yeom, SJ., Park, CS. et al. Production of aglycon protopanaxadiol via compound K by a thermostable β-glycosidase from Pyrococcus furiosus. Appl Microbiol Biotechnol 89, 1019–1028 (2011). https://doi.org/10.1007/s00253-010-2960-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2960-1