Abstract
Burkholderia cepacia complex (Bcc) is a group of bacteria with conflicting biological characteristics, which make them simultaneously beneficial and harmful to humans. They have been exploited for biocontrol, bioremediation, and plant growth promotion. However, their capacity as opportunistic bacteria that infect humans restricts their biotechnological applications. Therefore, the risks of using these bacteria should be assessed. In this study, Burkholderia multivorans WS-FJ9 originally isolated from pine rhizosphere, which was shown to be efficient in solubilizing phosphate, was evaluated with respect to its biosafety, colonization in poplar rhizosphere, and growth-promoting effects on poplar seedlings. Pathogenicity of B. multivorans WS-FJ9 on plants was determined experimentally using onion and tobacco as model plants. Onion bulb inoculated with B. multivorans WS-FJ9 showed slight hypersensitive responses around the inoculation points, but effects were not detectable based on the inner color and odor of the onion. Tobacco leaves inoculated with B. multivorans WS-FJ9 exhibited slightly water-soaked spots around the inoculation points, which did not expand or develop into lesions even with repeated incubation. Pathogenicity of the strain in alfalfa, which has been suggested as an alternative Bcc model for mice, was not detectable. Results from gene-specific polymerase chain reactions showed that the tested B. multivorans WS-FJ9 strain did not possess the BCESM and cblA virulence genes. Scanning electron microscopy revealed that the colonization of the WS-FJ9 strain reached 1.4 × 104 colony forming units (cfu) g−1 rhizosphere soil on day 77 post-inoculation. The B. multivorans WS-FJ9 strain could colonize the rhizosphere as well as the root tissues and cells of poplars. Greenhouse evaluations in both sterilized and non-sterilized soils indicated that B. multivorans WS-FJ9 significantly promoted growth in height, root collar diameter, and plant biomass of inoculated poplar seedlings compared with controls. Phosphorus contents of roots and stems of treated seedlings were 0.57 and 0.55 mg g−1 higher than those of the controls, respectively. Phosphorus content was lower in the rhizosphere soils by an average of 1.03 mg g−1 compared with controls. The results demonstrated that B. multivorans WS-FJ9 is a nonpathogenic strain that could colonize the roots and significantly promote the growth of poplar seedlings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth-promoting rhizobacteria (PGPR) refer to the bacteria that colonize in the plant rhizosphere, helping the plants resist biotic and abiotic stress as well as promote plant growth (Brown 1974). Previous studies have demonstrated that Burkholderia cepacia complex (Bcc) bacteria promote plant growth and are potential human pathogens (Vandamme and Dawyndt 2011). Some Bcc genomovars prevent plant disease and promote plant growth (Parke et al. 1991), whereas some species degrade chemical substances in pesticides and herbicides that are potentially carcinogenic to humans, and are thus used in bioremediation (Sangodkar et al. 1988). Several Bcc genomovars cause cystic fibrosis (CF) in humans (Agodi et al. 2001). Hence, Bcc have high biological and economic values accompanied by biological risks. Several countries have applied Bcc as biological pesticides (Luo and Xie 2005). However, certain Bcc genomovars are potentially pathogenic to humans, which limit their application. Genomovars are species within Bcc without morphological differences. Alongside in-depth research on the bacteria, distinguishing strains in the natural environment from pathogenic strains in hospitals is difficult. The use of Bcc derived from plant rhizospheres or soil poses potential risks. Thus, the biosafety of these strains should be assessed repeatedly (Zhang et al. 2006). Bcc currently has 17 genomovars with low DNA hybridization rates (Payne et al. 2005; Vanlaere et al. 2009; Agnoli et al. 2012. To date, only genomovar 1 holds the specific name B. cepacia in Bcc, whereas genomovars 2 to 17 have been renamed (Agnoli et al. 2012). Burkholderia multivorans belongs to Bcc genomovar 2.
Some B. multivorans strains isolated from natural or hospital environments are opportunistic human bacterial pathogens (Vandamme et al. 2003; Baldwin et al. 2008; Varga et al. 2012), whereas others are antagonistic to certain pathogenic bacteria or fungi in animals and plants (Sijam and Dikin 2005) or function as PGPR (Hou 2012). Previous studies have shown that human pathogenic Bcc have corresponding virulence genes for human pathogenesis (BCESM and cblA), which are absent in nonpathogenic strains (Clode et al. 2000). Recent studies on Bcc have focused on antagonistic bacterial substances and enzymes (Lin et al. 2011; Peng et al. 2012). Only a few have focused on the function of Bcc in biocontrol and promotion of plant growth.
An efficient strain of phosphate-solubilizing bacterium, B. multivorans WS-FJ9, was isolated from a pine rhizosphere in our preliminary studies (Hou 2012). The present study is designed to evaluate the biosafety of this bacterial strain in model plants, namely onion, tobacco, and alfalfa. The presence of virulence genes was detected using polymerase chain reaction (PCR)-based tests. Screening using a rifampicin antibiotic marker and scanning electron microscopy (SEM) were performed to investigate the colonization dynamics of the bacteria in the poplar rhizosphere. The influence of Bcc on poplar seedling growth and phosphorus metabolism was evaluated under greenhouse conditions.
Materials and methods
Bacterial strains and preparation of bacterial suspensions
B. multivorans WS-FJ9 was isolated and screened from the rhizosphere soil of slash pine (Pinus elliotii Engelm.) in our laboratory prior to storage in the China Center for Type Culture Collection (CCTCC) (accession no. CCTCC M2011435). Two strains, namely B. cepacia LMG1222 with the virulence genes BCESM and cblA, and B. cepacia YM-GX3 with the gene cblA, were used as positive controls for the virulence gene test. B. cepacia LMG1222 was provided by the Belgian Coordinated Collections of Microorganisms (Ren et al. 2011). B. cepacia YM-GX3 collected from the same rhizosphere as B. multivorans WS-FJ9 was deposited in the Forest Pathology Lab of Nanjing Forestry University (accession no. NFUFPL B 2011002; Hou 2012). Pseudomonas fluorescens JW-JS1 was used as a control for the growth-promoting experiment. P. fluorescens JW-JS1 was previously collected from the poplar rhizosphere soil by our laboratory and showed good plant growth-promoting effect (Liu 2010). It was then deposited in the CCTCC (accession no. CCTCC M209027).
Each bacterial strain was inoculated into 50 mL nutrient broth in a 100-mL Erlenmeyer flask. The cultures were incubated at 28 °C for 48 h with shaking at 200 r min−1. The fermentation broth was centrifuged at 4 °C and 4,629 × g for 5 min. The bacterial cells were collected and washed thrice in sterile saline solution. The concentration of each bacterial suspension was adjusted to 1 × 108 colony forming units (cfu) mL−1 using plate colony-counting methods.
Preparation of poplar seedlings
Seeds of poplar NL-895 (Populus euramericana cv.) were from the poplar variety base of Chenwei Forest Farm, Sihong County, Jiangsu Province, China. The seeds were sterilized in 0.1 % KMnO4 solution for 2 h and rinsed thrice with sterile water before being sown in sterilized sand. Forty days after sowing, the poplar seedlings with consistent vigor growth were transplanted into plastic pots (volume of each pot, 500 cm3) containing a mixture of field soil and peat moss (volume ratio, 2:1). These plastic pots comprised equal ratios of two parts. One part contained non-sterilized potting medium, and the other part contained potting medium sterilized with high-pressure steam. A total of 50 poplar seedlings were planted separately in each pot with 1.5 kg of the mixture. The mixture contained 30.0 mg kg−1 of available nitrogen, 6.0 mg kg−1 of available phosphorus, and 23.5 mg kg−1 of available potassium. The poplar seedlings were grown in a greenhouse and watered once every 2 days. After a year, B. multivorans WS-FJ9 colonization was detected by rifampicin marking, and phosphorus metabolism was determined using the poplar seedlings in sterilized soil to avoid introduction of other microbes. The growth-promoting effect of B. multivorans WS-FJ9 was experimentally examined using the poplar seedlings in both sterilized and non-sterilized soil to assess the effect of bacteria on growth promotion and test whether the bacterial strain is competitive in a natural setting.
Biosafety assessment of B. multivorans WS-FJ9
Pathogenicity test with model plants
Onion (Allium cepa) and tobacco (Nicotiana benthniciana) were used as model plants to assess B. multivorans WS-FJ9 pathogenicity in plants. Alfalfa (Medicago sativa) was used as an alternative model plant to assess B. multivorans WS-FJ9 pathogenicity in mammals. B. multivorans WS-FJ9 pathogenicity test in onion was assessed using a previously described method (Sijam and Dikin 2005; Bernier et al. 2003). The pathogenicity tests in tobacco and alfalfa were based on the methods used by Ren (2009) and Silo-Suh et al. (2002). All treated groups used the virulence gene-containing B. cepacia LMG1222 strain and sterile water as the positive and negative controls, respectively. Tests were performed in growth chambers. Three replicates were prepared for each treatment. Six onion bulbs, 6 tobacco seedlings, and 30 seeds of alfalfa were inoculated in each replicate. Onion bulbs, 2-month-old tobacco seedlings, and 3-day-old germinative alfalfa were prepared, and bacterial suspensions were injected into the onion bulbs (1 mL), tobacco leaf (0.5 μL), and alfalfa cotyledon (20 μL) using sterile syringes. The model plants were incubated as follows: onion bulbs at 26 °C for 5 days; tobacco at 25 °C, 85 % relative humidity (RH), and 16 h day−1 (sunlight) for 3 days; and alfalfa at 37 °C, 70 % RH, and 12 h days−1 (sunlight) for 7 days. Subsequently, the changes in the inoculation sites on onion and tobacco, as well as the disease symptoms of alfalfa, were observed. The reactions are classified as follows: negative reaction, inoculation spots of onions did not expand and the internal onion showed no obvious changes; positive reaction, inoculation sites of tobacco leaf developed necrosis spots or water-soaked ring spots within 24 to 48 h; and negative response, yellow macula appeared after 72 h (Ren 1994). The disease symptoms of alfalfa were assessed by observing for necrosis near the inoculation site, etiolation, or abnormal root. The incidence of diseased alfalfa is calculated as follows: Disease incidence of alfalfa (%) = (number of alfalfa with disease symptoms / number of alfalfa inoculated with bacteria) × 100.
PCR detection of BCESM and cblA virulence genes in B. multivorans WS-FJ9
To detect BCESM and cblA genes in B. multivorans WS-FJ9, the PCR-based methods of Mahenthiralingam et al. (1997) and of Richardson et al. (2001) were used, respectively. The gene-specific primers for BCESM were BCESM1 (5′-CCACGGACGTGACTAACA-3′) and BCESM2 (5′-CGTCCATCCGAACACGAT-3′). For a 20-μL PCR reaction, the following amplification procedure was used: 94 °C for 1 min, followed by 30 cycles of 94 °C for 1 min, 63 °C for 1 min, and 72 °C for 2 min, with a final extension for 10 min at 72 °C. The gene-specific primers for cblA were cblA1 (5′-CCAAAGGACTAACCCA-3′) and cblA2 (5′-ACGCGATGTCCATCACA-3′). For a 20-μL reaction, the following amplification procedure was used: 94 °C for 3 min, followed by 35 cycles of 94 °C for 1 min, 60 °C for 1 min, and 72 °C for 1 min, with a final extension for 10 min at 72 °C. Amplification products were detected by gel electrophoresis on a 2 % agarose gel. B. cepacia LMG1222 and B. cepacia YM-GX3 strains were used as positive controls.
Detection of B. multivorans WS-FJ9 colonization in the poplar rhizosphere
The detection of the natural resistance of B. multivorans WS-FJ9 and the screening of the resistance marker were based on the method used by Liu (2010). The concentrations of rifampicin used were 100, 150, 200, 250, and 300 μg mL−1. The detection of the marker strain stability was designed using the method described by Xiao et al. (2009). The 18-h zymotic fluid of the marked strain was used to irrigate the roots of 1-year-old poplar NL-895 seedlings in plastic pots with sterilized potting medium and sterile water, which were used as controls. Bacterial suspension (50 mL, 5 × 108 cfu mL−1) were inoculated onto each seedling. The rhizosphere soil (1 g) was sampled on days 1, 3, 5, 7, 9, 11, 15, 19, 23, 27, 37, 47, 57, 67, and 77 post-irrigation, and the sample was added into a test tube with 9 mL sterile water. Rhizosphere soil samples were serially diluted to final concentrations of 10−2, 10−3, and 10−4 g mL−1. These soil diluents (100 μL) were spread onto nutrient agar (NA) plates with 300 μg mL−1 rifampin and incubated at 28 °C for 3 days, during which the number of cfu was counted. The numbers of bacterial cells recovered were expressed as colony forming units per gram rhizosphere soil weight.
For scanning electron microscope (SEM) studies, the samples were treated according to Ren et al. (2011) with minor modifications. Seeds of poplar NL-895 were soaked in 0.1 % KMnO4 for 30 min and rinsed thoroughly in six changes of sterile water. Then, the floating seeds were discarded, and the remaining seeds were surface-sterilized with 70 % ethanol for 3 min and then soaked in 0.1 % aqueous HgCl2 (w/v) for 1 min followed by six rinses with sterile water. Finally, the seeds were transferred to 750 mL glass bottles containing 100 mL 1/2 Murashige–Skoog medium (Meilan and Ma 2007) for germination. These seedling explants were transferred into fresh media once a month. After a series of transfer culture (about five times), bacteria were inoculated onto the plantlets. Aseptic techniques were used while breaking the culture medium, and 5 mL bacterial suspension of B. multivorans WS-FJ9 was injected onto the root surface of poplar plantlets. After 20 days, the plantlets were uprooted, washed with 70 % ethanol for 3 min, and rinsed thrice with sterile water. The roots were cut into 1.5-cm-long segments, fixed in 4 % glutaraldehyde in 0.1 M PBS buffer (pH 7.2) for 12 h, and rinsed with the same PBS buffer. Rinsed roots were dehydrated in an ethanol series and critical point-dried using EMITECH K850 (Quorum Technologies Ltd, East Grinstead, West Sussex, UK). Dried roots were then sliced in half longitudinally with a sterile razor, mounted on specimen holders, and coated with gold–palladium using HITACHI E-1010 (HITACHI, Tokyo, Japan). The samples were examined using an FEI QUANTA 200 scanning electron microscope (FEI Company, Hillsboro, USA).
Experiment on the effect of B. multivorans WS-FJ9 on poplar seedlings
One-year-old seedlings of poplar NL-895 that were planted in plastic pots containing sterilized or non-sterilized soil were used for this experiment, which involved three treatments. Each treatment used six seedlings (six replicates). B. multivorans WS-FJ9 bacterial suspension (20 mL, 1 × 108 cfu mL−1) was inoculated to each seedling. P. fluorescens JW-JS1 bacterial suspension (1 × 108 cfu mL−1) and sterile distilled water of the same volume were used as the positive and negative controls, respectively. All treatments were performed in a greenhouse, and the plants remained in the greenhouse to grow under the same conditions.
The seedling height, root collar diameter, and plant biomass were measured approximately 150 days post-poplar seedling inoculation. Plant biomass was measured as follows. Whole plants were harvested and washed initially. Excess water was removed by heating at 105 °C for 4 h, and the plant material was heated at 80 °C to reach a constant weight, which was measured as dry biomass. Biomass measurements for each treatment were performed in five replicates. To determine the amount of phosphorus, 0.3 g of the root, 0.3 g of the stem, and 0.5 g of the rhizosphere soil from each treatment were dried to a constant weight and placed into separate Kjeldahl bottles. Approximately 1 mL distilled water was added to moisten the samples before they were steeped overnight in 9 mL concentrated sulfuric acid (98.3 %) and 1 mL perchloric acid (72 %). The Kjeldahl bottles containing the samples were placed into an electric cooker, and the samples were digested until the solution became transparent. After cooling, the samples were moved into volumetric flasks with a metered volume of 50 mL. Inductively coupled plasma mass spectrometry (Optima 2100DV, Perkin Elmer, Waltham, USA) was used to measure the phosphorus content of each sample.
Data analysis and processing
Microsoft Excel 2003 (Microsoft Corporation, Redmond, USA) was used to collate and analyze the data and determine the colonization dynamics of the marked B. multivorans RWS-FJ9 strain, which was B. multivorans WS-FJ9 marked with rifampicin and inoculated in the poplar rhizosphere. SPSS (version 16.0) (IBM Inc., New York, USA) was used to analyze significant differences and perform multiple comparison analysis of the phosphorus content and alfalfa morbidity as well as the height, ground diameter, and biomass of the poplar seedlings (Zhang et al. 2012).
Results
Biosafety of B. multivorans WS-FJ9
WS-FJ9 pathogenicity in plants
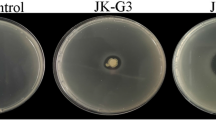
Results of the inoculation tests in the onions are shown in Fig. 1a, b. After inoculation and incubation at 26 °C for 5 days, none of the disease symptoms, including water soaking, maceration, and necrosis, were observed. These test results were not significantly different from those treated with sterile water. The onions inoculated with B. cepacia LMG1222, with virulence genes BCESM and cblA, generated hypersensitive spots in the inoculated points, which grew with time. The inner color of the LMG1222-treated onions turned yellow, and their tissues were soft and rotten with strong odor.
Plant pathogenicity tests of B. multivorans WS-FJ9. a Onion inoculated with B. multivorans WS-FJ9, B. cepacia LMG1222, and sterilized water, respectively. b Cross-section view of the onion inoculated with B. multivorans WS-FJ9, B. cepacia LMG1222, and sterilized water, respectively. c Tobacco leaf inoculated with B. multivorans WS-FJ9, B. cepacia LMG1222, and sterilized water, respectively
Results of the inoculation tests in tobacco are shown in Fig. 1c. The tobacco leaves inoculated with B. multivorans WS-FJ9 exhibited slight hypersensitive responses and developed small water-soaked spots. The water-soaked spots in the leaves did not expand or develop into lesions even with further incubation. No significant differences from the controls were observed. The tobacco leaves inoculated with B. cepacia LMG1222 exhibited large water-soaked spots and developed lesions after 24 h. The lesions expanded with further incubation. These conditions indicated that B. cepacia LMG1222 is a virulent tobacco pathogen. The results demonstrated that B. multivorans WS-FJ9 is nonpathogenic to onion bulb and tobacco.
B. multivorans WS-FJ9 pathogenicity in mammals as determined in alfalfa
The inoculation test results are listed in Table 1. Most alfalfa seedlings that were inoculated with B. cepacia LMG1220 exhibited necrosis, etiolation near the inoculation point, or root distortion. The incidence of infection was significantly more evident in B. cepacia LMG1220 than in B. multivorans WS-FJ9 or sterile water. The average disease symptoms of inoculating B. multivorans WS-FJ9, water, and B. cepacia LMG1222 were 8.9, 7.8, and 75.6 %, respectively. Among the treatments, seedlings inoculated with B. multivorans WS-FJ9, which only had two to three incidences of infection, were observed in one of three replicates. This finding was not significantly different from the control (water) having one infected seedling from one replicate (Table 1).
PCR detection of BCESM and cblA in B. multivorans WS-FJ9
BCESM and cblA were amplified to further detect the presence of virulence genes in the phosphate-solubilizing B. multivorans WS-FJ9 strain. The absence of PCR products in Fig. 2 indicated that these genes do not exist in B. multivorans WS-FJ9. BCESM is a Bcc strain-specific pathogenic gene. Similarly, cblA is specific to most of the prevalent pathogenic Bcc strains. The absence of both genes in B. multivorans WS-FJ9 suggested that the strain is safe to mammals.
Result of 2 % agarose gel electrophoresis of BCESM and cblA gene PCR products of B. cepacia YM-GX3, B. cepacia LMG1222, and B. multivorans WS-FJ9. M, Marker DL2000; lanes 1 and 4, B. cepacia YM-GX3; lanes 2 and 5, B. cepacia LMG1222; lanes 3 and 6, B. multivorans WS-FJ9; lanes 2 and 5 contained PCR products of BCESM and cblA gene, respectively, and lane 4 contained the cblA gene
Temporal colonization dynamics of B. multivorans WS-FJ9 in the poplar rhizosphere
B. multivorans WS-FJ9 did not show natural resistance to rifampicin. The growth and colony morphology of B. multivorans RWS-FJ9 in NA plates were consistent with those of the corresponding rifampicin-marked strain (Fig. 3a). B. multivorans WS-FJ9 and RWS-FJ9 colonies were light yellow, round, moist, viscous, with smooth surfaces and neat edges. Rifampicin did not influence the physiological traits of B. multivorans WS-FJ9 and RWS-FJ9, which verified the reliability of the observed colonization in later incubation periods. Figure 3b shows that B. multivorans RWS-FJ9 can colonize in the rhizosphere of 1-year-old poplar seedlings. The colonization dynamics of B. multivorans RWS-FJ9 in the poplar rhizosphere is characterized by a down–up–down fluctuation of the population. The recycled marked strain B. multivorans RWS-FJ9 reduced the biomass to the lowest level (1.8 × 104 cfu g−1 rhizosphere soil) on day 5, which then rapidly increased to its highest level (4.9 × 104 cfu g−1 rhizosphere soil) on day 11. This value showed a continuous decrease after 19 days, which slowed down only after 57 days. The degree of colonization eventually reached 1.4 × 104 cfu g−1 rhizosphere soil on day 77. Thus, B. multivorans WS-FJ9 showed a long survival time in the poplar rhizosphere, with potential applications in the field.
SEM observation of B. multivorans WS-FJ9 colonization
The adventitious root surfaces of poplar plantlets infected with bacteria were observed by SEM. B. multivorans WS-FJ9 were abundantly found on the surface of the plantlets (Fig. 4a, b). The strain was likewise present on the surface of cells within the root tissues as observed in the vertical sections of the roots (Fig. 4c, d). A large amount of bacterial cells was found within the root tissues. Furthermore, B. multivorans WS-FJ9 colonized in the interior of root tissue cells (Fig. 4e, f). These results demonstrated that the bacterium could colonize in the poplar rhizosphere, as well as the poplar root tissues and cells, by endophytism. The invasion of the tracheid by the bacteria is illustrated in Fig. 4e.
Growth-promoting effects of B. multivorans WS-FJ9 on poplar seedlings
B. multivorans WS-FJ9 was inoculated onto poplar seedlings in the greenhouse. The results after 150 days are shown in Table 2. The increment in seedling height, root collar diameter, and plant biomass of the seedlings inoculated with B. multivorans WS-FJ9 grown in sterilized soil were 23.8 cm, 1.43 mm, and 3.17 g, respectively, whereas those grown in non-sterilized soil were 22.5 cm, 1.53 mm, and 2.92 g, respectively. These values were all higher than those inoculated with P. fluorescens JW-JS1 and the uninoculated seedlings. The seedling height and plant biomass of the plants inoculated with B. multivorans WS-FJ9 and P. fluorescens JW-JS1 were significantly different (P < 0.05), but the root collar diameters were not significantly different at P < 0.05. Therefore, B. multivorans WS-FJ9 had more effects on the poplar seedlings compared with P. fluorescens JW-JS1 and the control.
The phosphorus contents of the root, stem, and rhizosphere soil of the seedlings were detected after 150 days. The results are summarized in Fig. 5. The phosphorus contents of the stems and roots treated with B. multivorans WS-FJ9 and P. fluorescens JW-JS1 were significantly higher than those of the control group, but that of the rhizosphere soil was lower than that of the control. Phosphorus contents of roots and stems treated with B. multivorans WS-FJ9 were 1.98 and 1.64 mg g−1, respectively, which were significantly higher than that of the control at P < 0.05 (The increments were 0.75 and 0.76 mg g−1, respectively). Phosphorus content of the rhizosphere soil was 1.03 mg g−1, which was less than that of the control. These results indicated that inoculation of B. multivorans WS-FJ9 and P. fluorescens JW-JS1 can promote plant absorption of phosphorus, thereby decreasing the phosphorus content of the rhizosphere soil treated with bacteria compared with the controls. Plant stems inoculated with B. multivorans WS-FJ9 had higher phosphorus concentrations than those inoculated with P. fluorescens JW-JS1 (P < 0.05). The phosphorus content of the roots was slightly higher, but was not significantly different at P < 0.05. By contrast, the phosphorus content of the rhizosphere soil was lower than that with P. fluorescens JW-JS1 (P < 0.05). These results showed that B. multivorans WS-FJ9 and P. fluorescens JW-JS1 have excellent phosphate solubilization capabilities. B. multivorans WS-FJ9 has a notably better effect on poplar seedlings than P. fluorescens JW-JS1 in promoting phosphorus absorption of poplars.
Discussion
Previous studies have shown that certain Bcc strains have excellent biocontrol and growth-promoting properties. Biocontrol-based products that have been registered in Europe and America include Intercepty, Blue Circle, Deny, and Type Wisconsin (Luo and Xie 2005). An example of such product in China is Yabao (Zhang et al. 2006). However, with the extensive application of PGPR strains and their products in agriculture, strains that are potential opportunistic human pathogens may have been overlooked and registered for commercial application. Although these microorganisms can aid in growth promotion, biocontrol, and biodegradation, their risk as human pathogens cannot be overlooked (Hao et al. 2006). Thus, several countries have limited the use of Bcc biocontrol bacteria. Japan and some European countries believe that measures should be undertaken to clarify the risks of using Bcc (Fang et al. 2006). These measures are very important in biocontrol bacteria detection and biosafety assessment (Luo and Xie 2005).
The advantages of plant models include short generation cycles, easy operation, and the need for no specific devices. Many researchers have been searching for suitable plant models to test the virulence of bacteria in mammals. In addition to the plant model, other established models use insects and nematodes for determining bacterial pathogenicity in mammals (Rahme et al. 1995; Fedhila et al. 2010; Mellies and Lawrence-Pine 2010). Compared with existing mouse models, these alternative models are rapidly generated, economic, reliable, stable, and easy to handle. Alfalfa is an alternative model that may be potentially used to assess the virulence of Bcc. Silo-Suh (2002) compared the results of different models and found that the wounded alfalfa seedling infection model is a useful tool in identifying factors that contribute to the persistence of Pseudomonas aeruginosa in CF. Bernier (2003) tested this result by comparing alfalfa and mouse models, and found that most of the strains that were virulent in the alfalfa infection model were also virulent in the lung infection model. Zhang (2008) and Ren (2011) used the alfalfa model to test the virulence of Bcc in mammals. Hence, alfalfa could be used as an alternative plant model to determine Bcc pathogenicity in animals. The test for pathogenicity of B. multivorans WS-FJ9 to alfalfa showed that the average morbidity of seedlings inoculated or treated with B. multivorans WS-FJ9, water, and B. cepacia LMG1222 were 8.9, 7.8, and 75.6 %, respectively. No significant difference was found between B. multivorans WS-FJ9 and the control. Thus, B. multivorans WS-FJ9 strain is not pathogenic to alfalfa, indicating that B. multivorans WS-FJ9 is relatively safe to mammals. Simultaneously, this study utilized onion and tobacco as plant models to test the virulence of bacteria to plants, and it was proven that B. multivorans WS-FJ9 is not virulent to plants. PCR detection of the virulence genes BCESM and cblA was utilized to further assess the biosafety of B. multivorans WS-FJ9. Results showed that this strain does not contain the two virulence genes. Thus, with broader biosafety assessments for B. multivorans WS-FJ9, the results provided a basis for the safe application of the strain in the future.
Exogenous microorganisms effectively promote plant growth, which depends on rhizosphere colonization and the relatively high survival of microorganisms in the whole plant growth cycle (Muralidhar and Panda 2000; Sheng 2003). Antibiotic marker method is typically used to trace the survival of microorganisms in soils (Glandorf et al. 1992). In this study, we adopted the anti-rifampicin marker method to study the colonization dynamics of B. multivorans WS-FJ9 in the poplar rhizosphere and utilized SEM to observe visually the bacterial colonization. The marked B. multivorans RWS-FJ9 strain was stable and able to long-term colonize in the poplar rhizosphere. The bacterial population even reached 1.4 × 104 cfu g−1 on day 77. This result was better than those of Ren (2009), Wang (2011), and Zhang (2012), whose observation periods of colonization were 66, 35, and 30 days, respectively, and bacterial populations were 102, 103, and 104 cfu g−1, respectively. Poplar root tissues and cells were observed by SEM. A very large amount of bacterial cells was found on the surface of roots and in the internal of root tissues and cells, eliminating the possibility that the bacterial cells were brought into the root tissues during operation.
The inoculation experiment showed that the B. multivorans WS-FJ9 strain had effective phosphate-solubilizing and plant-promoting effects on poplar seedlings. The results were consistent with the results of in vitro phosphate-solubilizing activity assays (Hou 2012; Liu 2010). B. multivorans WS-FJ9 and P. fluorescens JW-JS1 have excellent phosphate solubilization capabilities. B. multivorans WS-FJ9 had a notably better effect on poplar seedlings than P. fluorescens JW-JS1. Moreover, limiting the availability of phosphorus enhanced the phosphate-solubilizing ability of B. multivorans WS-FJ9 (Li 2013). In conclusion, B. multivorans WS-FJ9 is relatively safe to mammals and plants. It could stably colonize in poplar rhizosphere and significantly promote the growth of poplars at the seedling stage. Thus, it could potentially serve as a strain that can be used in the development of microbiological fertilizers. Moreover, preliminary study has demonstrated that this bacterial strain is highly antagonistic to several plant pathogens, particularly to poplar canker (Hou 2012), and has strong biodegradation ability for the autotoxic substance in poplar rhizosphere soil (Li et al. 2013). Existing reports have shown that PGPR can induce systemic resistance (Kloepper et al. 2004), which helps plants to resist the invasion of living organisms. Moreover, PGPR can improve systemic tolerance of plants against many forms of abiotic stress (Yang et al. 2009). The antagonistic effect of B. multivorans WS-FJ9 on plant pathogens should be further investigated.
References
Agnoli K, Schwager S, Uehlinger S, Vergunst A, Viteri DF, Nguyen DT, Sokol PA, Carlier A, Eberl L (2012) Exposing the third chromosome of Burkholderia cepacia complex strains as a virulence plasmid. Mol Microbiol 83:362–378. doi:10.1111/j.1365-2958.2011.07937.x
Agodi A, Mahenthiralingam E, Barchitta M, Giannino V, Sciacca A, Stefani S (2001) Burkholderia cepacia complex infection in Italian patients with cystic fibrosis: prevalence, epidemiology, and genomovar status. J Clin Microbiol 39:2891–2896. doi:10.1128/JCM.39.8.2891-2896.2001
Baldwin A, Mahenthiralingam E, Drevinek P, Pope C, Waine DJ, Henry DA, Speert DP, Carter P, Vandamme P, LiPuma JJ, Dowson CG (2008) Elucidating global epidemiology of Burkholderia multivorans in cases of cystic fibrosis by multilocus sequence typing. J Clin Microbiol 46:290–295. doi:10.1128/JCM.01818-07
Bernier SP, Silo-Suh L, Woods DE, Ohman DE, Sokol PA (2003) Comparative analysis of plant and animal models for characterization of Burkholderia cepacia virulence. Infect Immun 71:5306–5313
Brown ME (1974) Seed and root bacterization. Annu Rev Phytopathol 12:181–197
Clode FE, Kaufmann ME, Malnick H, Pitt TL (2000) Distribution of genes encoding putative transmissibility factors among epidemic and nonepidemic strains of Burkholderia cepacia from cystic fibrosis patients in the United Kingdom. J Clin Microbiol 38:1763–1766
Fang Y, Xie GL, Lu HX, Zhang LX, Luo YC (2006) Am opportunistic human bacterial pathogen: study status and risk analysis of Burkholderia cepacia in agriculture. Acta Agriculturae Zhejiangensis 18(4):284–288
Fedhila S, Buisson C, Dussurget O, Serror P, Glomski IJ, Liehl P, Lereclus D, Nielsen-LeRoux C (2010) Comparative analysis of the virulence of invertebrate and mammalian pathogenic bacteria in the oral insect infection model Galleria mellonella. J Invertebr Pathol 103:24–29
Glandorf DCM, Brand I, Bakker PAHM, Schippers B (1992) Stability of rifampicin resistance as a marker for root colonization studies of Pseudomonas putida in the field. Plant Soil 147:135–142
Hao XJ, Xie GL, Lu YL, Luo YC, Zhang LX, Luo JY, Zhao LH, Liu SY (2006) Identification and detection of two types of opportunistic human bacterial pathogens from rice. Chinese J Rice Sci 20(3):324–328
Hou L (2012) Studies on screening of efficient phosphate-solubilizing bacteria in the rhizosphere of pine trees and on their characteristics. Master thesis, Nanjing Forestry University, Nanjing, China
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266. doi:10.1094/PHYTO.2004.94.11.1259
Li GX (2013) Phosphate-solubilizing mechanism of Burkholderia multivorans WS-FJ9 and its growth-promoting effects on poplars. PhD thesis, Nanjing Forestry University, Nanjing, China
Li GX, Wu XQ, Ye JR (2013) Accumulation, toxic properties and bioremediation of autotoxic substance in poplar rhizosphere soil. J Nanjing F Univ (Nat Sci Ed) 37(3):71–76
Lin HH, Chen YS, Li YC, Tseng IL, Hsieh TH, Buu LM, Chen YL (2011) Burkholderia multivorans acts as an antagonist against the growth of Burkholderia pseudomallei in soil. Microbiol Immunol 55:616–624. doi:10.1111/j.1348-0421.2011.00365.x
Liu H (2010) Interactions between phosphobacteria and ectomycorrhizal fungi to improve the growth of poplar and it’s mechanisms. PhD thesis, Nanjing Forestry University, Nanjing, China
Luo YC, Xie GL (2005) Burkholderia cepacia: our enemy or friend? Acta Microbiol Sin 45(4):647–652
Mahenthiralingam E, Simpson DA, Speert DP (1997) Identification and characterization of a novel DNA marker associated with epidemic Burkholderia cepacia strains recovered from patients with cystic fibrosis. J Clin Microbiol 35:808–816
Meilan R, Ma C (2007) Poplar (Populus spp.). In: Wang K (ed) Agrobacterium Protocols Volume 2, 2nd edn. Totowa, New Jersey, pp 143–151
Mellies JL, Lawrence-Pine ER (2010) Interkingdom signaling between pathogenic bacteria and Caenorhabditis elegans. Trends Microbiol 18:448–454. doi:10.1016/j.tim.2010.07.002
Muralidhar RV, Panda T (2000) Fungal protoplast fusion—a revisit. Bioproc Eng 22:429–431
Parke JL, Rand RE, Joy AE, King EB (1991) Biological control of Pythium damping-off and Aphanomyces root rot of peas by application of Pseudomonas cepacia or P. fluorescens to seed. Plant Dis 75(10):987–992
Payne GW, Vandamme P, Morgan SH, Lipuma JJ, Coenye T, Weightman AJ, Jones TH, Mahenthiralingam E (2005) Development of a recA gene-based identification approach for the entire Burkholderia genus. Appl Environ Microbiol 71:3917–3927. doi:10.1128/AEM.71.7.3917-3927.2005
Peng R, Lin JP, Wei DZ (2012) A novel strain Pseudomonas aeruginosa CS-2 producing an organic solvent-tolerant lipase. Adv Mater Res 343:774–780
Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM (1995) Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899–1902
Ren JH (2009) Screening of poplar canker antagonistic bacterium and study on antibiotic substance. Dissertation, Nanjing Forestry University, Nanjing, China
Ren JH, Ye JR, Liu H, Xu XL, Wu XQ (2011) Isolation and characterization of a new Burkholderia pyrrocinia strain JK-SH007 as a potential biocontrol agent. World J Microb Biot 27:2203–2215
Ren XZ (1994) Classification and identification of plant pathogenic bacteria. Agriculture Press, Beijing, p 55
Richardson J, Stead DE, Coutts RH (2001) Incidence of the cblA major subunit pilin gene amongst Burkholderia species. FEMS Microbiol Lett 196:61–66
Sangodkar UM, Chapman PJ, Chakrabarty AM (1988) Cloning, physical mapping and expression of chromosomal genes specifying degradation of the herbicide 2,4,5-T by Pseudomonas cepacia AC1100. Gene 71:267–277
Sheng XF (2003) Colonization of silicate bacterium strain NBT in wheat roots. J Appl Ecol 14(11):1914–1916
Sijam K, Dikin A (2005) Biochemical and physiological characterization of Burkholderia cepacia as biological control agent. Int J Agric Biol 7:385–388
Silo-Suh L, Suh SJ, Sokol PA, Ohman DE (2002) A simple alfalfa seedling infection model for Pseudomonas aeruginosa strains associated with cystic fibrosis shows AlgT (sigma-22) and RhlR contribute to pathogenesis. Proc Natl Acad Sci USA 99:15699–15704. doi:10.1073/pnas.242343999
Vandamme P, Dawyndt P (2011) Classification and identification of the Burkholderia cepacia complex: past, present and future. Syst Appl Microbiol 34:87–95
Vandamme P, Holmes B, Coenye T, Goris J, Mahenthiralingam E, LiPuma JJ, Govan JR (2003) Burkholderia cenocepacia sp. nov.—a new twist to an old story. Res Microbiol 154:91–96. doi:10.1016/S0923-2508(03)00026-3
Vanlaere E, Baldwin A, Gevers D, Henry D, De Brandt E, LiPuma JJ, Mahenthiralingam E, Speert DP, Dowson C, Vandamme P (2009) Taxon K, a complex within the Burkholderia cepacia complex, comprises at least two novel species. Burkholderia contaminans sp. nov. and Burkholderia lata sp. nov. Int J Syst Evol Microbiol 59(1):102–111
Varga JJ, Losada L, Zelazny AM, Brinkac L, Harkins D, Radune D, Hostetler J, Sampaio EP, Ronning CM, Nierman WC, Greenberg DE, Holland SM, Goldberg JB (2012) Draft genome sequence determination for cystic fibrosis and chronic granulomatous disease Burkholderia multivorans isolates. J Bacteriol 194:6356–6357. doi:10.1128/JB.01306-12
Wang T, Duan YJ, Liu BT, Guo G, Zhou DB, Tan X, Zhang XY, Huang MJ (2011) The colonization of two antagonistic blight bacteria strains inside banana. Genet Appl Biol 30(3):342–350
Xiao XZ, Lu KX, Liao ZW (2009) Ampicillin-resistant Bacillus pumilus BX-4 and its colonization in the tomato rhizosphere. J Agro-Environ Sci 28(6):1172–1176
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4. doi:10.1016/j.tplants.2008.10.004
Zhang DT, Peng ZK, Cao QQ, Yang M, Zheng L, Zhou EX (2012) Colonization of three antagonistic strains on rice plant and their biocontrol effects on rice sheath blight. J Northwest A&F Univ (Nat Sci Ed) 40(2):97–102
Zhang LX, Song JH, Xie GL (2008) Identification of the Burkholderia cepacia complex genomovars and their virulence in an alfalfa infection model. Acta Microbiol Sin 48(11):1445–1450
Zhang LX, Xie GL, Lou MM (2006) Risk assessment to use Burkholderia cepacia as a biocontrol agent of plant disease. Chin J Biol Control 22(4):260–264
Acknowledgments
This study was funded by the Chinese Special Research Program for Forestry Sectors Beneficial to Public (201004061), Innovation Plan for Graduate Students of Jiangsu, China (CXLX11-0552), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, GX., Wu, XQ. & Ye, JR. Biosafety and colonization of Burkholderia multivorans WS-FJ9 and its growth-promoting effects on poplars. Appl Microbiol Biotechnol 97, 10489–10498 (2013). https://doi.org/10.1007/s00253-013-5276-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5276-0