Abstract
The rational modification of the actinomycetes genomes has a variety of applications in research, medicine, and biotechnology. The use of site-specific recombinases allows generation of multiple mutations, large DNA deletions, integrations, and inversions and may lead to significant progress in all of these fields. Despite their huge potential, site-specific recombinase-based technologies have primarily been used for simple marker removal from a chromosome. In this review, we summarise the site-specific recombination approaches for genome engineering in various actinomycetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Site-specific recombinases (SSRs) catalyse recombination between two specific DNA sequences, resulting in DNA excision, inversion, integration, and translocation (Fig. 1). Because of their efficiency and simplicity, SSRs have become widespread tools for the genomic engineering of many bacteria and higher organisms (Baltz 2012; Branda and Dymecki 2004; Kuhn and Torres 2002; Leibig et al. 2008; Marx and Lidstrom 2002). The classification of SSRs as either tyrosine or serine enzymes depends on the catalytic amino acid that cleaves the DNA molecule. Serine recombinases (e.g. Int-phiC31) are usually unidirectional, mediating the integration of a circular DNA molecule into the genome. Serine recombinases recognise non-identical attP and attB sites (Thorpe and Smith 1998; Gregory et al. 2003; Baltz 2012). The tyrosine family enzymes (e.g. Cre) are usually bidirectional, acting reversibly on a DNA molecule and recognising target sites sharing very limited DNA sequence identity (Rajeev et al. 2009). The positions of the recombination target sites determine the recombination outcome. If two target sites are identically oriented, SSRs perform excision of the DNA between them. SSRs change the directionality of a DNA fragment between two inversely oriented target sites. The integration of a circular DNA fragment into the genome will occur if both molecules contain the corresponding target sites (Fig. 1).
Reactions catalysed by recombinases. a Excision of DNA fragment flanked by two recombination sites, located in the same orientation. b Inversion of DNA fragment located between two recombination sites, located in the opposite orientation. c Integration of circular DNA molecule into linear. d Integration of phage DNA into chromosome by recombination between attP and attB sites, with subsequent formation of attL and attR sites
SSR technology has been used in actinomycete genome manipulation for more than 20 years (Bierman et al. 1992; Baltz 2012). However, the utilisation of SSRs in actinomycetes has been primarily limited to the use of the phiC31 integration system. Although using phiC31-based vectors has allowed tremendous progress in actinomycete genetics, the potential of other SSRs has been underexplored. Recently, several additional SSRs (Cre, Dre, Flp, and Int-VWB) have been used to expand the suite of genome manipulation strategies. Currently, SSR-based genome engineering approaches include the construction of unmarked mutants (Suzuki et al. 2005), targeting of heterologous DNA to the chromosome (Kuhstoss et al. 1991), marker-free expression of foreign genes (Schweizer 2003), deletion of large genomic fragments (Suzuki et al. 2005), and in vivo cloning of genomic DNA regions (Schweizer 2003).
Recombinant DNA integration into the actinomycete chromosome
SSR-mediated integration can be used to insert a foreign DNA molecule into a predetermined location within an actinomycete genome. This approach has been intensively used to heterologously express antibiotic biosynthetic gene clusters to produce novel molecules. Several integration systems used in actinomycete genetics are reviewed here.
Phage phiC31
The temperate phage phiC31 was isolated from Streptomyces coelicolor in 1971 (Lomovskaya et al. 1971). The genome of phiC31 is approximately 41.5 kb. After infection of the cells, the phage integrates into a conserved gene that encodes a pirin homologue and is located approximately 90 kb upstream of the S. coelicolor oriC (Gregory et al. 2003). Less efficient integration in additional pseudo-attB sites has also been reported (Combes et al. 2002). The analysis of attB- and attP-containing regions revealed imperfect inverted repeats around the site of the crossover (Kuhstoss and Rao 1991). The minimal att sites required for efficient recombination were found (Table 2). Both sites contain a TT dinucleotide in the centre, where the crossover occurs (Thorpe and Smith 1998). Any nucleotide substitutions in this dinucleotide region completely abolish the recombination efficiency. Recombination between att sites is catalysed by the integrase protein, which belongs to a family of serine recombinases (Table 1). Interestingly, attP is located close to the 5′-end of the int-phiC31 gene, downstream of the int-phiC31 promoter. This configuration should lead to the spatial separation of the int-phiC31 gene and its promoter during the process of phage integration (Kuhstoss and Rao 1991). After recombination between the attB and attP sites and integration of the phage, the attL and attR sites are formed (Fig. 1). It is postulated that the integrase alone cannot recombine the attR and attL sites or non-permissive pairs of sites, such as attP/attP, attB, and attL (Thomason et al. 2001; Thorpe and Smith 1998). Furthermore, the TT dinucleotides in the centre of the att sites were shown to be responsible for determining the polarity of the attachment sites during recombination. The substitution of the TT dinucleotide in one of the att sites by AA results in the formation of an antiparallel synapse and the formation of L B –L P and R P –R B sites, where the left side of attB is joined with the left side of attP, and subsequently, the right side of attP is joined with the right side of attB (Smith et al. 2004). Several phiC31-based integrative vectors were constructed, which turned out to be the most popular in streptomycetes genetics. The pSET152 integrative plasmid vector contains int-phiC31 and attP, an apramycin-resistance gene aac(3)IV, the RK2 origin of conjugal transfer oriT, lacZα with a multiple cloning site and the pUC origin of replication in Escherichia coli. The cosmid vector pOJ436 contains all of the aforementioned elements as well as three cos sites for in vitro packaging (Bierman et al. 1992). The construction of a pTO1 plasmid, which is less popular than the two former vectors, was also reported. This plasmid contains a pBR322 origin for replication in E. coli, int-phiC31 and attP fragments from phiC31, the RK2 origin of conjugal transfer oriT, the ampicillin-resistance gene bla for selection in E. coli and the thiostrepton-resistance gene tsr for selection in Streptomyces. Recently, the construction of another phiC31-based integrative vector, pTES, for marker-free expression in actinomycetes was reported. This vector contains the apramycin-resistance gene aac(3)IV, the ColE1 origin for replication in E. coli, the RK2 origin of conjugal transfer oriT and the integrase gene int-phiC31. The phiC31 attP with a multiple cloning site was placed between two loxP sites. After the gene of interest was cloned in the vector and the resulting plasmid was integrated into the phiC31 attB site, the backbone of the plasmid, containing aac(3)IV, ColE1 ori, int-phiC31, and oriT, could be removed. This removal is accomplished by the expression of Cre recombinase from an additional vector and subsequent recombination between two loxP sites (Herrmann et al. 2012).
phiC31-based integrative plasmids have a broad host range, including not only the Streptomyces genus but also Actinomadura, Arthrobacter, Micromonospora, Nocardia, Rhodococcus, Nonomuraea, and Actinoplanes (Voeykova et al. 1998; Stinchi et al. 2003; Li et al. 2003; Ha et al. 2008; Baltz 2012). Among Streptomyces, the successful integration of phiC31-based plasmids has been reported for the following strains: Streptomyces albus, Streptomyces antibioticus, Streptomyces aureofaciens, Streptomyces bambergiensis, Streptomyces fradiae, Streptomyces griseus, Streptomyces hygroscopicus, Streptomyces lavendulae, Streptomyces lividans, Streptomyces purpureus, Streptomyces rimosus, Streptomyces venezuelae, Streptomyces viridochromogenes, Streptomyces virginiae, S. coelicolor, Streptomyces peucetius, Streptomyces diastatochromogenes, Streptomyces ansochromogenes, and Streptomyces ambofaciens (Voeykova et al. 1998; Eustaquio et al. 2005; Paranthaman and Dharmalingam 2003; Luzhetskyy et al. 2007; Liao et al. 2010; Kim et al. 2008; Baltz 2012). In most cases, the inheritance of the phiC31-based integrative plasmids had no effect on morphology or antibiotic production, but some negative effects on antibiotic production were observed (Baltz 1998; Luzhetskiĭ et al. 2001).
phiC31-based integrative constructs were used for the heterologous production of the following antibiotics: nikkomycin, moenomycin A, coumermycin A1, chlorobiocin, caprazamycins, novobiocin, aranciamycin, A54145, and daptomycin (Liao et al. 2010; Ostash et al. 2007; Flinspach et al. 2010; Eustaquio et al. 2005; Luzhetskyy et al. 2007; Alexander et al. 2010; Penn et al. 2006). Notably, in the case of novobiocin and chlorobiocin, the heterologous expression of wild-type biosynthetic clusters in S. coelicolor led to production levels comparable with those of native producers. After the modification of the novobiocin gene cluster by the introduction of the strong inducible promoter tcp830 upstream of the cluster, the level of antibiotic production was 3.4-fold higher than that with an unmodified cluster. S. lividans derivatives with the same expression constructs were at least five times less productive (Eustaquio et al. 2005; Dangel et al. 2010). In the case of nikkomycin, the introduction of an additional copy of its biosynthetic cluster in its native producer S. ansochromogenes led to a fourfold enhancement in the production of nikkomycin X and a 1.8-fold enhancement in the production of nikkomycin Z (Liao et al. 2010). Heterologous expression of the lpt gene cluster in S. ambofaciens BES2074 resulted in average yields of 385 mg/l of the fully modified A54145 B-core lipopeptides. This yield amounts to 92 % of the S. fradiae high-producer control (Alexander et al. 2010).
Phage phiBT1
The phiBT1 phage is a homoimmune relative of phiC31, and despite the high level of similarity between their genomes, integrase genes int-phiBT1 and int-phiC31 share only 26 % identity. Similar to Int-phiC31, Int-phiBT1 integrase belongs to the family of large serine recombinases (Table 1). phiBT1 attP and attP from phiC31 share no significant homology. Similar to phiC31, the attB and attP of phiBT1 are quite different and contain imperfect inverted repeats (Gregory et al. 2003). The minimal sizes of phiBT1 attB and attP have also been determined. The minimal size of attB for in vitro integration is 36 bp, while the minimal size of attP is 48 bp (Table 2). Both att sites share a GT dinucleotide in the centre (Zhang et al. 2008). In S. coelicolor, phiBT1 attB maps within sco4848, which encodes a putative integral membrane protein. This gene lies approximately 1 Mb to the right of oriC (Gregory et al. 2003). In the mouse and human genomes, multiple pseudo attB and attP sites were found, indicating that phiBT1-based integrative plasmids can integrate into pseudo attB sites in Streptomyces chromosomes in the same manner as phiC31-based integrative plasmids (Chen and Woo 2005, 2008). In 2010, an article devoted to the identification of 16 pairs of non-compatible phiBT1 attB and attP recombination sites was published (Zhang et al. 2010). As a result of this discovery, a new approach for DNA assembly, called site-specific recombination-based tandem assembly was developed (Zhang et al. 2011). The plasticity of phiBT1 integrase can be used for the introduction of additional incompatible phiBT1 attB sites in various chromosome regions with the subsequent integration of phiBT1-based vectors containing corresponding attP sites in the desired region of the chromosome or for the creation of multiple insertions.
Interestingly, there is evidence that phiBT1 integrase, with its low efficiency, catalyses the in vitro recombination between attL and attR (Zhang et al. 2008). This result represents the first example of serine recombinase catalysing a reverse reaction because serine recombinases were strongly believed to mediate attB–attP recombination only in a unidirectional manner (Thorpe and Smith 1998). This property of phiBT1 integrase might result in the instability of integrated plasmids in vivo, though no experimental evidence of such instability was reported (Baltz 2012).
Because the utilisation of phiC31-based integrative vectors is restricted in a number of strains owing to a lack of attB sites in non-streptomycetes actinomycetes or to detrimental effects on the level of antibiotic production, the phiBT1 phage was used for the construction of integrative vectors that should serve as good alternatives to phiC31-based vectors (Baltz 1998, 2012; Gregory et al. 2003).
Vector pRT801 was constructed by the substitution of a phiC31 attP/int-phiC31-containing BamHI/SphI fragment of pSET152 with a phiBT1 attP/int-phiBT1 fragment. The constructed vector contains the apramycin-resistance gene aac(3)IV, the origin of conjugal transfer oriT, a multiple cloning region, a phiBT1 attP and the int-phiBT1 gene. Additionally, derivatives of pRT801 with a resistance gene for hygromycin instead of apramycin were created by the ligation of a hygromycin-resistance-encoding HpaI fragment from Tn5099-10 with a 4.415 kb Ecl136II fragment of pRT801. The resulting plasmids, which differed only in the orientation of the hygromycin-resistance gene, were named pMS81 and pMS82 (Gregory et al. 2003).
The broad host range of phiBT1-based vectors was demonstrated by the introduction of pRT801 into Streptomyces avermitilis, Streptomyces cinnamonensis, S. fradiae, Streptomyces lincolnensis, Streptomyces nogalater, Streptomyces roseosporus, S. venezuelae, Streptomyces ghanaensis, and other Streptomyces strains. The frequencies of exconjugant formations with pRT801 and pSET152 were also comparable. The stability of phiBT1-integrative vectors was shown in S. coelicolor with pMS81/82 plasmids during two rounds of sporulation under non-selective conditions (Gregory et al. 2003; Ostash et al. 2009).
All of the above information indicates that phiBT1-based vectors are as convenient as phiC31-based vectors. Nevertheless, phiBT1-based integrative vectors have been much less popular in Streptomyces genetics than phiC31-based vectors. The most interesting examples of the application of the phiBT1 integrative system include the expression of biosynthetic clusters of the daptomycin-related compound A54145 in S. fradiae and of meridamycin in S. lividans. In the case of A54145, a BAC harbouring the entire cluster was modified by the insertion of a cassette containing oriT, aac(3)IV, and phiBT1 attP/int-phiBT1. The integration of the biosynthetic cluster at the phiBT1 attB resulted in a production level greater than or equal to that observed in the case of integration at the phiC31 attB site. Notably, in both cases of the ectopic expression of A54145, involving integration at the phiC31 and phiBT1 attB sites, the production level was higher than in the parental strain (Alexander et al. 2010). Expression of the meridamycin gene cluster in S. lividans was not as successful as in the previous example. After the integration of the BAC containing the meridamycin gene cluster at the phiBT1 attB site, no production of the compound was detected. The transcription of the meridamycin structural genes occurred at a much lower level than in the natural host. Detectable amounts of meridamycin were produced only after the introduction of a strong erm E promoter in front of the structural genes (Liu et al. 2009).
Phage VWB
The temperate bacteriophage VWB was isolated in 1984 using S. venezuelae as a test strain (Anné et al. 1984). Unlike phiC31 and phiBT1, integrase of phage VWB belongs to the integrase family of tyrosine recombinases (Table 1) showing homology to the phage lambda integrase. The integration site of the VWB phage is located in a putative tRNAArg gene (Van Mellaert et al. 1998). Intriguingly, the native tRNAArg gene contains a CCA terminus, which is removed after the integration of the phage. Because this CCA terminus is often missing in Streptomyces tRNA genes, the question of whether the tRNAArg gene retains its functionality after the integration of the phage needs to be answered. DNA fragments containing the attP site from VWB and the attB site from S. venezuelae were analysed (Table 2). Alignment of the DNA sequences containing the attB, attP, attL, and attR sequences revealed a 45-bp fragment common to all of the sites (Van Mellaert et al. 1998). A VWB attB site from S. ghanaensis was also revealed. This site was located in the tRNAArg gene, which differs from that of S. venezuelae only in the lack of a CCA terminus. Genome sequence analysis of S. ghanaensis did not reveal any other tRNAArg gene, indicating its essentiality (Ostash et al. 2009).
Using the integrase gene int-VWB and attP site of the VWB phage, the integrative plasmid pKT02 was constructed. This plasmid also contains the backbone of the E. coli vector pIC20R and the thiostrepton-resistance gene tsr. The main drawback of this plasmid is the absence of the oriT origin of conjugal transfer; thus, this plasmid can only be introduced into Streptomyces cells by protoplast transformation. This vector was successfully transformed into S. lividans and S. venezuelae cells. Notably, the frequency of transformant formation with pKT02 was much lower than with phiC31- and pSAM2-based plasmids (Van Mellaert et al. 1998).
Another VWB-based integrative vector, known as pSOK804, was created. This vector was constructed by the ligation of a 2.3 kb SphI/HindIII fragment from pKT02 to a 3.0 kb SphI/HindIII fragment of pSET152; therefore, this vector contains the origin of conjugal transfer oriT and an apramycin-resistance gene aac(3)IV that is functional in both E. coli and Streptomyces (Sekurova et al. 2004). The possibility of transferring pSOK804 by conjugation makes this vector more convenient than pKT02.
VWB-based integrative plasmids have been reported to be successfully, but occasionally with low efficiency, introduced into the following strains: S. natalensis, S. fradiae, S. sioyaensis, S. lividans, S. albus, S. coelicolor, and Actinoplanes teichomyceticus (Herrmann et al. 2012; Enríquez et al. 2006; Butler et al. 2002; Myronovskyy et al. 2009; Horbal et al. 2012). Challenges have also been encountered in working with pSOK804. Together with different replicative plasmids, the introduction of pSOK804 into S. verticillus has not been possible so far, whereas the introduction of phiC31- and phiBT1-based plasmids has been successful. The failure to introduce the pSOK804 vector or replicative vectors into S. verticillus is not surprising because this strain has long been known to be refractory to all means of introducing foreign DNA (Galm et al. 2008).
Recently, another VWB-based integrative vector was constructed, namely, pTOS. This vector was designed for the so-called marker-free expression of genes in actinomycetes. Vector pTOS contains two rox sites flanking attP and the multiple cloning site. After the gene of interest is cloned in a marker-free expression vector and integrated into the chromosome of the desired strain, the plasmid backbone with the antibiotic-resistance marker can be deleted. This deletion is accomplished by the expression of Dre recombinase from a second replicative vector, which will recombine two rox sites and delete the redundant sequence located between them. The utility of this vector was shown in S. lividans (Herrmann et al. 2012).
pSAM2 plasmid
pSAM2 is an 11 kb integrated element found in S. ambofaciens ATCC15154 that can also persist in a form of cccDNA. This element can replicate, is self-transmissible, and mobilises chromosomal markers (Kuhstoss et al. 1989). pSAM2 resembles temperate bacteriophages with its site-specific recombination system involved in integration. The DNA sequence of pSAM2 contains a gene int showing homology to the site-specific recombinases of the integrase family (Table 1). The introduction of point or frameshift mutations in this gene completely abolished the integrative function of pSAM2 (Boccard et al. 1989b). The putative excisionase gene xis is located upstream of the integrase gene. The stop codon of xis overlaps with the start codon of integrase, and together they constitute an operon (Sezonov et al. 1998).
pSAM2 integrates site specifically in a unique site of the S. lividans genome, whereas several integration sites have been reported for S. ambofaciens (Boccard et al. 1989a). Integration of the plasmid occurs through recombination between the attB and attP sites. In S. ambofaciens, the attB site of pSAM2 overlaps with the proline tRNA gene. A comparison of the attB and attP regions revealed a 58-bp similarity between the two sequences. Southern hybridisation showed that the genomes of S. lividans, Streptomyces bikiniensis, Streptomyces glaucescens, Streptomyces actuosus, S. coelicolor, S. antibioticus, and Streptomyces parvulus contain an att sequence in a conserved chromosomal region (Boccard et al. 1989a). The minimal fragment of pSAM2 attB sufficient for the successful recombination corresponds to the 26-bp long anticodon stem and loop of tRNAPro (Table 2; Raynal et al. 2002). Analysis of the pSAM2 attP organisation revealed two arm-type sites consisting of 17-bp direct repeats that lie between positions −100/−116 and +102/+118 according to the centre of the core sequence at position 0 (Raynal et al. 2002).
The study of the function of the int and xis gene products revealed that the Int of pSAM2 is the only protein required for the site-specific integration of the plasmid, whereas Int and Xis are both required for its excision (Raynal et al. 1998).
Based on pSAM2, the pPM927 integrative vector was constructed. This vector contains the pBR329 replicon-, thiostrepton-, and spectinomycin-resistance genes, the origin of conjugal transfer, pSAM2 int and xis genes and attP. In addition, the fd transcriptional terminator is followed by the tipA-inducible promoter for the regulated expression of cloned genes (Smokvina et al. 1990). The presence of the xis gene in pPM927 is redundant because Int alone is sufficient for integration in E. coli (Raynal et al. 1998). This requirement is supported by the successful integration of pSAM2-based plasmids containing only attP and the int gene in Mycobacterium smegmatis (Seoane et al. 1997). Utilisation of the xis-int operon for the construction of pPM927 raises the question of plasmid stability in Streptomyces. Nevertheless, no excision event could be detected (Smokvina et al. 1990).
The comparison of the transformation efficiencies of replicative plasmids and the pSAM2-based vector in S. ambofaciens shows at least a 100-fold decrease in the latter (Smokvina et al. 1990). phiC31-based integrative vectors also transform with higher efficiencies than pSAM2-based vectors: 60-fold higher with cosmid vectors and up to 600-fold higher for plasmid vectors (Kuhstoss et al. 1991). The successful integration of pSAM2-based plasmids has been reported in the following Streptomyces strains: S. ambofaciens, Streptomyces griseofuscus, Streptomyces lipmanii, S. lividans, Streptomyces toyocaensis, S. hygroscopicus, S. avermitilis, S. aureofaciens, S. coelicolor, and S. fradiae (Kuhstoss et al. 1991; Ichinose et al. 2001; Rodriguez et al. 2004; Wang et al. 2009; Novakova et al. 2010; Zhao et al. 2010, 2011; Liu et al. 2010). All of the data indicate that pSAM2-based integrative plasmids have broad host specificity and can be used when the utilisation of superior phiC31-based vectors is not wanted.
Phage R4
R4 is a broad host Streptomyces bacteriophage isolated from soil samples in 1979 (Chater and Carter 1979). The integration and excision functions are performed by the product of the single gene ORF469 (sre). R4 integrase belongs to a serine recombinase family (Table 1), showing homology to Tn2501 resolvase and SpoIVCA of Bacillus subtilis (Matsuura et al. 1996; Miura et al. 2011). The attB and attP sites share a 12 bp common core sequence, and the attP site is located 27 bp upstream of the integrase translational start codon (Miura et al. 2011). The integrative functions of R4 integrase and its cognate att sites were demonstrated using E. coli, S. parvulus, human cells, and insect cells (Miura et al. 2011; Matsuura et al. 1996; Olivares et al. 2001; Chompoosri et al. 2009). Although the minimal attB and attP sites determined in E. coli assays were 50 and 49 bp (Table 2), respectively, the longer 64 bp attP and 295 bp attB resulted in enhanced recombination efficiency in human cells (Miura et al. 2011; Olivares et al. 2001). R4 integrase was also shown to successfully perform not only intermolecular but also intramolecular recombination. Unfortunately, despite its well-characterised functionality, the R4 integrative system has not yet found application in Streptomyces genetics.
Phage μ1/6
Recently, a new site-specific integrase system based on phage μ1/6 was established. Originally, this phage was discovered in the S. aureofaciens genome as a DNA fragment of 38,194 bp (Farkasovská et al. 2007). A bioinformatics analysis of the μ1/6 genome sequence revealed a gene encoding a putative integrase. This basic protein of 416 amino acids belongs to the tyrosine recombinase/integrase family, which also includes lambda integrase, bacteriophage P1 Cre recombinase and bacterial XerD/C recombinases (Table 1). Downstream of the integrase gene, an attP site was detected (Table 2). Within its sequence, multiple direct and inverted repeats were observed, which most likely act like binding sites for the integrase and other factors that participate in recombination. The attB site (Table 2) for phage μ1/6 is located in the 3′-end of the putative tRNAThr gene. After phage integration in its cognate attB site, the sequence of the tRNA gene was completely restored. Although the sequence alignment of all attachment sites revealed a common 46-bp region, minimal attachment sites were not determined (Farkašovská and Godány 2012).
Based on μ1/6, the integrative vector pCTint was constructed. This vector contains a chloramphenicol marker for selection in E. coli, a thiostrepton marker for selection in Streptomyces, the E. coli origin of replication and the integrase- and attP-containing DNA fragment of μ1/6. This vector contains no origin for conjugal transfer; thus, it can be introduced in Streptomyces cells only by transformation. Using this vector, thiostrepton-resistant transformants of S. aureofaciens, S. lividans, and S. coelicolor were successfully obtained. The functionality of μ1/6 integrase was also demonstrated in an E. coli model, indicating the potential to use this integrational system with a broad host range. However, experiments in vitro revealed that in addition to integrase, some host factors are most likely also required for efficient recombination between the attB/P sites (Farkašovská and Godány 2012).
Phage pMLP1
Temperate bacteriophage pMLP1 was found in Micromonospora carbonacea var. africana. This phage can persist in both as a replicative element and an integrated into a chromosome. Detailed analysis of the pMLP1 sequence revealed the DNA region responsible for an integrative function. This region contains an integrase gene intM, an excisionase gene xisM and attP. 322 aa integrase shows similarity to the integrase family of proteins (Table 1). It contains three highly conserved regions (boxes A, B, and C) typical for tyrosine recombinases. xisM is located upstream of intM and encodes a putative protein similar to excisionases. attP is located downstream of intM. Integration of the bacteriophage occurs into the tRNAHis gene, which is regenerated at attL. Comparison of the attB sites of M. carbonacea var. africana and M. halophytica var. nigra with attP revealed 24 and 25 bp identical segments, respectively (Table 2). Based on pMLP1, two integrative vectors pSPRH840 and pSPRH910 were constructed. Vector pSPRH840 contains backbone of pUC19 with xisM, intM, and attP of pMLP1 as well as oriT, apamycin-, and hygromycin-resistance genes. pSPRH910 differs from pSPRH840 only in absence of hygromycin-resistance gene. Successful integration of pSPRH840 was shown for both M. carbonacea var. africana and M. halophytica var. nigra. The frequency of exconjugant formation of M. carbonacea var. africana with the vector pSPRH910 was higher than 1 × 10−3. Also it was shown that pSPRH840 and pSET152 do not interfere one with another when integrated in the same strain (Alexander et al. 2003).
Phage TG1
Temperate bacteriophage TG1 was isolated from soil samples on Streptomyces cattleya. It was reported that TG1 lysogenizes several other Streptomyces strains including S. avermitilis and S. coelicolor. Sequencing of the lysogenized and nonlysogenized strains of S. avermitilis led to the identification of the TG1 attachment sites. The attB site of TG1 is located within the dapC gene encoding N-succinyldiaminopimelate aminotransferase. Nevertheless, disruption of dapC by integration of the phage does not result in lysine or diaminopimelate auxotrophy. TG1 attB site in S. coelicolor was found in the gene sco3658, also encoding aminotransferase. The attP site was deduced from comparative studies of TG1 lysogenized and nonlysogenized strains of S. avermitilis. The attP site is located upstream of the int-TG1 gene encoding integrase. The integrase of TG1 shows high similarity to the integrase of phiC31, which belongs to the serine recombinase family (Table 1). Both, attB and attP sites contain incomplete inverted-repeat sequences with central TT dinucleotide where crossing-over occurs (Morita et al. 2009a). In vitro studies demonstrated that the minimal sequences of attP and attB required for recombination by the TG1 integrase are 43 and 39 bp, respectively. However, longer attP and attB sequences of 44 and 46 bp, respectively (Table 2) are required for recombination without loss of efficiency compared to the recombination efficiency of the full-length sites. Experiments in vitro also demonstrated that TG1 integrase does not mediate recombination between attL and attR (Morita et al. 2009b). Genome database survey with TG1 attB from S. avermitilis as a query revealed presence of respective homologous sequences in Streptomyces scabies 87.22, Salinispora tropica CNB-440, Salinispora arenicola CNS-205, S. griseus subsp and others. Indeed, successful TG1-based integration in S. scabies and S. griseus was reported (Morita et al. 2012). Construction of the TG1-based integrative vector pKU462 was reported. This vector contains int and attP of TG1, kanamycin-resistance gene (aphII), oriT, and pMB1 origin of replication in E. coli (Morita et al. 2009a). This vector was successfully used for DNA integration in S. avermitilis (Jiang et al. 2009).
Generation of the marker-free mutants
Gene inactivation methods most often retain a selectable marker within the genome. Because the number of available resistance markers for actinomycete genome manipulation is limited, their reutilisation within the same genome is highly desirable. In addition, resistance markers integrated into a chromosome may result in polar effects on the expression of genes located upstream and downstream. The expression of selectable markers may also influence bacterial fitness. Therefore, the elimination of the resistance marker after genome manipulation is very important. SSR was among the first methods to allow elimination of the resistance marker after mutant selection in a range of organisms (Branda and Dymecki 2004; Schweizer 2003; Fedoryshyn et al. 2008a, b; Zelyas et al. 2009). The SSR-mediated excision of the antibiotic-resistance cassette leaves a ‘scar’ in the chromosome and generates a single target site (Fig. 1). The length of this scar is usually less than 50 bp and does not exhibit polar transcriptional effects. Marker rescue and reuse offers a simple and efficient way to introduce multiple gene deletions. However, consecutive deletions of additional genes in the same genetic background may be limited because of undesired DNA rearrangements generated by the multiple target sites left after each excision. Therefore, different recombinases should be used for the generation of multiple gene deletions.
Cre recombinase
The 38 kDa Cre (cyclisation recombination) originates from bacteriophage P1 and belongs to a tyrosine recombinase family (Table 1). Cre catalyses a DNA recombination between two 34-bp recognition sites named loxP (locus of crossing over [X] of the P1 phage). The loxP sequence consists of two 13-bp inverted repeats linked together by an 8-bp internal region (Table 2). The reaction performed by Cre on the loxP sites is very efficient and does not require any accessory proteins or high-energy cofactors, thus making it suitable for use in many different organisms. In 2006, the successful expression of Cre recombinase from the phage vector in S. coelicolor A(3)2 was reported (Khodakaramian et al. 2006). The frequency of resistance marker excision was from 60 to 90 %. We have reported the successful expression of the synthetic gene encoding the Cre recombinase expressed from different plasmid vectors in several members of the Streptomyces, Micromonospora, Kitasatospora, and Saccharothrix genera (Herrmann et al. 2012; Fedoryshyn et al. 2008b). The frequency of resistance marker excision by Cre expressed from pALCRE, pNLCRE, pUWLHCRE, and pUWLCRE has been nearly 100 % in all strains we have tested. To minimise genetic instability, different heterotypic lox sites containing mutations within the inverted repeats (loxLE and loxRE) have been used for plants, chicken cell lines, and bacteria (Branda and Dymecki 2004; Leibig et al. 2008). The recombination of loxLE and loxRE results in a double-mutant loxLERE site, which is a poor substrate for Cre. We could show, however, that Cre recognises the double mutated site at very high frequency in actinomycetes. Thus, the risk of undesired chromosomal rearrangements is still very high if Cre is iteratively used in combination with loxLE and loxRE (Herrmann et al. 2012).
Flp recombinase
Flp recombinase is another tyrosine recombinases (Table 1) isolated from Saccharomyces cerevisiae. The Flp recognition site (frt) consists of two 13-bp palindromic sequences separated by an 8-bp spacer region (Table 2). Flp has been used to perform gene deletions, insertions, integrations, and inversions in various organisms (Schweizer 2003). The functional expression of the synthetic gene flp(a), encoding Flp recombinase, has been reported in several actinomycetes, including S. coelicolor M145, S. lividans TK24, and Saccharothrix espanaensis, but not in Micromonospora Tü6368 (Fedoryshyn et al. 2008a; Herrmann et al. 2012). However, the frequency of frt-flanked apramycin-resistance gene excision from the chromosome of actinomycetes is lower than those of Cre, ranging from 10 to 40 %. The native flp gene has also been expressed, showing an even lower marker excision frequency of approximately 2 % (Zelyas et al. 2009). However, even at this frequency, marker-free mutants can be obtained relatively easily.
Dre recombinase
Recently, we reported the very efficient expression of Dre recombinase in actinomycetes. Similarly to Cre, Dre is a tyrosine-type recombinase (Table 1) recognising a target site called rox (Table 2). All three recombinases (Flp, Cre, and Dre) are heterospecific: Cre does not recognise rox or frt, Flp does not recognise loxP or rox, and Dre does not recognise loxP or frt. This characteristic makes Dre an ideal partner for Flp and Cre in performing multiple mutations in a single genetic background. The frequency of the marker removal from chromosomes of different actinomycetes was nearly 100 %. Thus, Dre recombinase is comparable with Cre in its high efficiency. Similar to Cre, Dre recombinase was successfully expressed in the Saccharothrix, Streptomyces, and Micromonospora genera (Herrmann et al. 2012).
int and xis system of pSAM2
In Prof. Pernodet’s group, the xis and int genes from the pSAM2 plasmid have been used to delete antibiotic-resistance markers from the chromosomes of streptomycetes. Several cassettes that contain antibiotic-resistance genes inserted between the attL and attR sites (attL antibiotic-resistance attR) and several vectors expressing the Int and Xis proteins from pSAM2 (Raynal et al. 2006) have been generated. The successful functional expression of four different recombinases (Cre, Flp. Dre, and Xis/Int) in actinomycetes enables the construction of marker-free multi-mutant strains. At least four consecutive resistance marker deletions can be generated in actinomycetes in a single genomic background.
Generation of large-scale deletions
The deletion of large genomic DNA fragments is becoming an increasingly important way to generate bacteria with minimized genomes and improved characteristics (e.g. small molecule overproduction; Jewett and Forster 2010). In addition, the ability to delete and reintegrate large DNA fragments within a genome facilitates gene functional studies. Large genome rearrangements in many organisms have been accomplished using the Cre/loxP system (Branda and Dymecki 2004; Komatsu et al. 2010). Recently, Cre-mediated large fragment excisions were utilised to construct improved S. avermitilis host strains for the heterologous production of natural products. Cre recombinase was able to excise a fragment of greater than 1.5 Mb in one step. The obtained mutant was used for the improved production of the natural products streptomycin, cephamycin C, and pladienolide after the heterologous expression of three corresponding biosynthetic gene clusters (Komatsu et al. 2010).
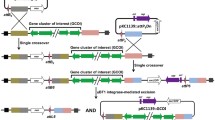
Another strategy for the generation of biosynthetic gene cluster deletions based on the Cre/loxP system was recently reported (Herrmann et al. 2012). Two loxP sites should be introduced at both ends of a gene cluster as direct repeats for the subsequent recombination. The usefulness of this method has been demonstrated recently by generating three deletions spanning the α-lipomycin, phenalinolactone, and monensin biosynthetic gene clusters in S. aureofaciens Tü117, S. sp. Tü6071, and S. cinnamonensis A519, respectively. The successful complementation of phenalinolactone production by the introduction of the corresponding gene cluster allowed the modification of a cosmid directly in E. coli via recombineering techniques and subsequent fast-gene deletion in situ using mutated cosmids (Herrmann et al. 2012).
In summary, a variety of different genome-engineering strategies, including the construction of unmarked multiple mutations, marker-free expression of target genes, large-fragment deletions, and chromosomal integration of biosynthetic gene clusters, have been performed in actinomycetes using the site-specific recombinases Int-phiC31, Int-phiBT1, Int-VWB, Cre, Dre, Flp, and Int/Xis of pSAM2. These techniques have a broad application in actinomycete genetics and will further facilitate functional studies of a large number of genes from the increasing set of sequenced genomes.
References
Alexander DC, Devlin DJ, Hewitt DD, Horan AC, Hosted TJ (2003) Development of the Micromonospora carbonacea var. africana ATCC 39149 bacteriophage pMLP1 integrase for site-specific integration in Micromonospora spp. Microbiology 149:2443–2453
Alexander DC, Rock J, He X, Brian P, Miao V, Baltz RH (2010) Development of a genetic system for combinatorial biosynthesis of lipopeptides in Streptomyces fradiae and heterologous expression of the A54145 biosynthesis gene cluster. Appl Environ Microbiol 76:6877–6887
Anné J, Wohlleben W, Burkardt HJ, Springer R, Pühler A (1984) Morphological and molecular characterisation of several actinophages isolated from soil which lyse Streptomyces cattleya or Streptomyces venezuelae. J Gen Microbiol 130:2639–2649
Baltz RH (1998) Genetic manipulation of antibiotic-producing Streptomyces. Trends Microbiol 6:76–83
Baltz RH (2012) Streptomyces temperate bacteriophage integration systems for stable genetic engineering of actinomycetes (and other organisms). J Ind Microbiol Biotechnol 39:661–672
Bierman M, Logan R, O’Brien K, Seno ET, Rao RN, Schoner BE (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49
Boccard F, Smokvina T, Pernodet JL, Friedmann A, Guérineau M (1989a) Structural analysis of loci involved in pSAM2 site-specific integration in Streptomyces. Plasmid 21:59–70
Boccard F, Smokvina T, Pernodet JL, Friedmann A, Guérineau M (1989b) The integrated conjugative plasmid pSAM2 of Streptomyces ambofaciens is related to temperate bacteriophages. EMBO J 8:973–980
Branda CS, Dymecki SM (2004) Talking about a revolution: the impact of site-specific recombinases on genetic analyses in mice. Dev Cell 6:7–28
Butler AR, Bate N, Kiehl DE, Kirst HA, Cundliffe E (2002) Genetic engineering of aminodeoxyhexose biosynthesis in Streptomyces fradiae. Nat Biotechnol 20:713–716
Chater KF, Carter AT (1979) A new, broad host-range, temperate bacteriophage (R4) of Streptomyces and its interaction with some restriction-modification systems. J Gen Microbiol 115:431–442
Chen L, Woo SL (2005) Complete and persistent phenotypic correction of phenylketonuria in mice by site-specific genome integration of murine phenylalanine hydroxylase cDNA. Proc Natl Acad Sci USA 102:15581–15586
Chen L, Woo SL (2008) Site-specific transgene integration in the human genome catalyzed by phiBT1 phage integrase. Hum Gene Ther 19:143–151
Chompoosri J, Fraser T, Rongsriyam Y, Komalamisra N, Siriyasatien P, Thavara U, Tawatsin A, Fraser MJ Jr (2009) Intramolecular integration assay validates integrase phiC31 and R4 potential in a variety of insect cells. Southeast Asian J Trop Med Public Health 40:1235–1253
Combes P, Till R, Bee S, Smith MC (2002) The streptomyces genome contains multiple pseudo-attB sites for the (phi)C31-encoded site-specific recombination system. J Bacteriol 184:5746–5752
Dangel V, Westrich L, Smith MC, Heide L, Gust B (2010) Use of an inducible promoter for antibiotic production in a heterologous host. Appl Microbiol Biotechnol 87:261–269
Enríquez LL, Mendes MV, Antón N, Tunca S, Guerra SM, Martín JF, Aparicio JF (2006) An efficient gene transfer system for the pimaricin producer Streptomyces natalensis. FEMS Microbiol Lett 257:312–318
Eustaquio AS, Gust B, Galm U, Li SM, Chater KF, Heide L (2005) Heterologous expression of novobiocin and clorobiocin biosynthetic gene clusters. Appl Environ Microbiol 71:2452–2459
Farkašovská J, Godány A (2012) Analysis of the site-specific integration system of the Streptomyces aureofaciens phage μ1/6. Curr Microbiol 64:226–233
Farkasovská J, Klucar L, Vlcek C, Kokavec J, Godány A (2007) Complete genome sequence and analysis of the Streptomyces aureofaciens phage mu1/6. Folia Microbiol 52:347–358
Fedoryshyn M, Petzke L, Welle E, Bechthold A, Luzhetskyy A (2008a) Marker removal from actinomycetes genome using FLP recombinase. Gene 419:43–47
Fedoryshyn M, Welle E, Bechthold A, Luzhetskyy A (2008b) Functional expression of the Cre recombinase in actinomycetes. Appl Microbiol Biotechnol 78:1065–1070
Flinspach K, Westrich L, Kaysser L, Siebenberg S, Gomez-Escribano JP, Bibb M, Gust B, Heide L (2010) Heterologous expression of the biosynthetic gene clusters of coumermycin A(1), clorobiocin and caprazamycins in genetically modified Streptomyces coelicolor strains. Biopolymers 93:823–832
Galm U, Wang L, Wendt-Pienkowski E, Yang R, Liu W, Tao M, Coughlin JM, Shen B (2008) In vivo manipulation of the bleomycin biosynthetic gene cluster in Streptomyces verticillus ATCC15003 revealing new insights into its biosynthetic pathway. J Biol Chem 283:28236–28245
Gregory MA, Till R, Smith MC (2003) Integration site for Streptomyces phage phiBT1 and development of site-specific integrating vectors. J Bacteriol 185:5320–5323
Groth AC, Olivares EC, Thyagarajan B, Calos MP (2000) A phage integrase directs efficient site-specific integration in human cells. Proc Natl Acad Sci USA 97:5995–6000
Ha HS, Hwang YI, Choi SU (2008) Application of conjugation using phiC31 att/int system for Actinoplanes teichomyceticus, a producer of teicoplanin. Biotechnol Lett 30:1233–1238
Herrmann S, Siegl T, Luzhetska M, Petzke L, Jilg C, Welle E, Erb A, Leadlay PF, Bechthold A, Luzhetskyy A (2012) Site-specific recombinase strategies for engineering actinomycetes genome. Appl Environ Microbiol 78:1804–1812
Horbal L, Zaburannyy N, Ostash B, Shulga S, Fedorenko V (2012) Manipulating the regulatory genes for teicoplanin production in Actinoplanes teichomyceticus. World J Microbiol Biotechnol 28:2095–2100
Ichinose K, Taguchi T, Bedford DJ, Ebizuka Y, Hopwood DA (2001) Functional complementation of pyran ring formation in actinorhodin biosynthesis in Streptomyces coelicolor A3(2) by ketoreductase genes for granaticin biosynthesis. J Bacteriol 183:3247–3250
Jewett MC, Forster AC (2010) Update on disigning and building minimal cells. Curr Opin Biotechnol 21:697–703
Jiang J, Tetzlaff CN, Takamatsu S, Iwatsuki M, Komatsu M, Ikeda H, Cane DE (2009) Genome mining in Streptomyces avermitilis: a biochemical Baeyer–Villiger reaction and discovery of a new branch of the pentalenolactone family tree. Biochemistry 48:6431–6440
Khodakaramian G, Lissenden S, Gust B, Moir L, Hoskisson PA, Chater KF, Smith MC (2006) Expression of Cre recombinase during transient phage infection permits efficient marker removal in Streptomyces. Nucleic Acids Res 34:e20
Kim MK, Ha HS, Choi SU (2008) Conjugal transfer using the bacteriophage phiC31 att/int system and properties of the attB site in Streptomyces ambofaciens. Biotechnol Lett 30:695–699
Komatsu M, Uchiyama T, Omura S, Cane DE, Ikeda H (2010) Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc Natl Acad Sci USA 107:2646–2651
Kuhn R, Torres RM (2002) Cre/loxP recombination system and gene targeting. Methods Mol Biol 180:175–204
Kuhstoss S, Rao RN (1991) Analysis of the integration function of the streptomycete bacteriophage phiC31. J Mol Biol 222:897–908
Kuhstoss S, Richardson MA, Rao RN (1989) Site-specific integration in Streptomyces ambofaciens: localization of integration functions in S. ambofaciens plasmid pSAM2. J Bacteriol 171:16–23
Kuhstoss S, Richardson MA, Rao RN (1991) Plasmid cloning vectors that integrate site-specifically in Streptomyces spp. Gene 97:143–146
Leibig M, Krismer B, Kolb M, Friede A, Götz F, Bertram R (2008) Marker removal in staphylococci via Cre recombinase and different lox sites. Appl Environ Microbiol 74:1316–1323
Li X, Zhou X, Deng Z (2003) Vector systems allowing efficient autonomous or integrative gene cloning in Micromonospora sp. strain 40027. Appl Environ Microbiol 69:3144–3151
Liao G, Li J, Li L, Yang H, Tian Y, Tan H (2010) Cloning, reassembling and integration of the entire nikkomycin biosynthetic gene cluster into Streptomyces ansochromogenes lead to an improved nikkomycin production. Microb Cell Fact 9:6
Liu H, Jiang H, Haltli B, Kulowski K, Muszynska E, Feng X, Summers M, Young M, Graziani E, Koehn F, Carter GT, He M (2009) Rapid cloning and heterologous expression of the meridamycin biosynthetic gene cluster using a versatile Escherichia coli-streptomyces artificial chromosome vector, pSBAC. J Nat Prod 72:389–395
Liu G, Ou HY, Wang T, Li L, Tan H, Zhou X, Rajakumar K, Deng Z, He X (2010) Cleavage of phosphorothioated DNA and methylated DNA by the type IV restriction endonuclease ScoMcrA. PLoS Genet 6:e1001253
Lomovskaya ND, Emeljanova LK, Alikhanian SI (1971) The genetic location of the prophage on Streptomyces coelicolor A3(2) chromosome. Genetics 68:341–347
Luzhetskiĭ AN, Ostash BE, Fedorenko VA (2001) Interspecies conjugation of Escherichia coli–Streptomyces globisporus 1912 using integrative plasmid pSET152 and its derivatives. Genetika 37:1340–1347
Luzhetskyy A, Mayer A, Hoffmann J, Pelzer S, Holzenkämper M, Schmitt B, Wohlert SE, Vente A, Bechthold A (2007) Cloning and heterologous expression of the aranciamycin biosynthetic gene cluster revealed a new flexible glycosyltransferase. ChemBioChem 8:599–602
Marx CJ, Lidstrom ME (2002) Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. Biotechniques 33:1062–1067
Matsuura M, Noguchi T, Yamaguchi D, Aida T, Asayama M, Takahashi H, Shirai M (1996) The sre gene (ORF469) encodes a site-specific recombinase responsible for integration of the R4 phage genome. J Bacteriol 178:3374–3376
Miura T, Hosaka Y, Yan-Zhuo Y, Nishizawa T, Asayama M, Takahashi H, Shirai M (2011) In vivo and in vitro characterization of site-specific recombination of actinophage R4 integrase. J Gen Appl Microbiol 57:45–57
Morita K, Yamamoto T, Fusada N, Komatsu M, Ikeda H, Hirano N, Takahashi H (2009a) The site-specific recombination system of actinophage TG1. FEMS Microbiol Lett 297:234–240
Morita K, Yamamoto T, Fusada N, Komatsu M, Ikeda H, Hirano N, Takahashi H (2009b) In vitro characterization of the site-specific recombination system based on actinophage TG1 integrase. Mol Genet Genomics 282:607–616
Morita K, Morimura K, Fusada N, Komatsu M, Ikeda H, Hirano N, Takahashi H (2012) Site-specific genome integration in alphaproteobacteria mediated by TG1 integrase. Appl Microbiol Biotechnol 93:295–304
Myronovskyy M, Ostash B, Ostash I, Fedorenko V (2009) A gene cloning system for the siomycin producer Streptomyces producer Streptomyces sioyaensis NRRL-B5408. Folia Microbiol 54:91–96
Novakova R, Kutas P, Feckova L, Kormanec J (2010) The role of the TetR-family transcriptional regulator Aur1R in negative regulation of the auricin gene cluster in Streptomyces aureofaciens CCM 3239. Microbiology 156:2374–2383
Olivares EC, Hollis RP, Calos MP (2001) Phage R4 integrase mediates site-specific integration in human cells. Gene 278:167–176
Ostash B, Saghatelian A, Walker S (2007) A streamlined metabolic pathway for the biosynthesis of moenomycin A. Chem Biol 14:257–267
Ostash B, Makitrinskyy R, Walker S, Fedorenko V (2009) Identification and characterization of Streptomyces ghanaensis ATCC14672 integration sites for three actinophage-based plasmids. Plasmid 61:171–175
Paranthaman S, Dharmalingam K (2003) Intergeneric conjugation in Streptomyces peucetius and Streptomyces sp. strain C5: chromosomal integration and expression of recombinant plasmids carrying the chiC gene. Appl Environ Microbiol 69:84–91
Penn J, Li X, Whiting A, Latif M, Gibson T, Silva CJ, Brian P, Davies J, Miao V, Wrigley SK, Baltz RH (2006) Heterologous production of daptomycin in Streptomyces lividans. J Ind Microbiol Biotechnol 33:121–128
Rajeev L, Malanowska K, Gardner JF (2009) Challenging a paradigm: the role of DNA homology in tyrosine recombinase reactions. Microbiol Mol Biol Rev 73:300–309
Raynal A, Tuphile K, Gerbaud C, Luther T, Guérineau M, Pernodet JL (1998) Structure of the chromosomal insertion site for pSAM2: functional analysis in Escherichia coli. Mol Microbiol 28:333–342
Raynal A, Friedmann A, Tuphile K, Guerineau M, Pernodet JL (2002) Characterisation of the attP site of the integrative element pSAM2 from Streptomyces ambofaciens. Microbiology 148:61–67
Raynal A, Karray F, Tuphile K, Darbon-Rongère E, Pernodet JL (2006) Excisable cassettes: new tools for functional analysis of Streptomyces genomes. Appl Environ Microbiol 72:4839–4844
Rodriguez E, Ward S, Fu H, Revill WP, McDaniel R, Katz L (2004) Engineered biosynthesis of 16-membered macrolides that require methoxymalonyl-ACP precursors in Streptomyces fradiae. Appl Microbiol Biotechnol 66:85–91
Sauer B, McDermott J (2004) DNA recombination with a heterospecific Cre homolog identified from comparison of the pac-c1 regions of P1-related phages. Nucleic Acids Res 32:6086–6095
Schweizer HP (2003) Applications of the Saccharomyces cerevisiae Flp-FRT system in bacterial genetics. J Mol Microbiol Biotechnol 5:67–77
Sekurova ON, Brautaset T, Sletta H, Borgos SE, Jakobsen MØM, Ellingsen TE, Strøm AR, Valla S, Zotchev SB (2004) In vivo analysis of the regulatory genes in the nystatin biosynthetic gene cluster of Streptomyces noursei ATCC 11455 reveals their differential control over antibiotic biosynthesis. J Bacteriol 186:1345–1354
Seoane A, Navas J, García Lobo JM (1997) Targets for pSAM2 integrase mediated site-specific integration in the Mycobacterium smegmatis chromosome. Microbiology 143:3375–3380
Sezonov G, Duchêne AM, Friedmann A, Guérineau M, Pernodet JL (1998) Replicase, excisionase, and integrase genes of the Streptomyces element pSAM2 constitute an operon positively regulated by the pra gene. J Bacteriol 180:3056–3061
Smith MC, Till R, Smith MC (2004) Switching the polarity of a bacteriophage integration system. Mol Microbiol 51:1719–1728
Smokvina T, Mazodier P, Boccard F, Thompson CJ, Guérineau M (1990) Construction of a series of pSAM2-based integrative vectors for use in actinomycetes. Gene 94:53–59
Stinchi S, Azimonti S, Donadio S, Sosio M (2003) A gene transfer system for the glycopeptide producer Nonomuraea sp. ATCC39727. FEMS Microbiol Lett 225:53–57
Suzuki N, Tsuge Y, Inui M, Yukawa H (2005) Cre/loxP-mediated deletion system for large genome rearrangements in Corynebacterium glutamicum. Appl Microbiol Biotechnol 67:225–233
Thomason LC, Calendar R, Ow DW (2001) Gene insertion replacement in Schizosaccharomyces pombe mediated by Streptomyces bacteriophage phiC31 site-specific recombination system. Mol Genet Genomics 265:1031–1038
Thorpe HM, Smith MC (1998) In vitro site-specific integration of bacteriophage DNA catalyzed by a recombinases of the resolvase/invertase family. Proc Natl Acad Sci USA 95:5505–5510
Van Mellaert L, Mei L, Lammertyn E, Schacht S, Anné J (1998) Site-specific integration of bacteriophage VWB genome into Streptomyces venezuelae and construction of a VWB-based integrative vector. Microbiology 144:3351–3358
Voeykova T, Emelyanova L, Tabakov V, Mkrtumyan N (1998) Transfer of plasmid pTO1 from Escherichia coli to various representatives of the order Actinomycetales by intergeneric conjugation. FEMS Microbiol Lett 162:47–52
Wang SB, Cantlay S, Nordberg N, Letek M, Gil JA, Flärdh K (2009) Domains involved in the in vivo function and oligomerization of apical growth determinant DivIVA in Streptomyces coelicolor. FEMS Microbiol Lett 297:101–109
Zelyas N, Tahlan K, Jensen SE (2009) Use of the native flp gene to generate in-frame unmarked mutations in Streptomyces spp. Gene 443:48–54
Zhang L, Ou X, Zhao G, Ding X (2008) Highly efficient in vitro site-specific recombination system based on streptomyces phage phiBT1 integrase. J Bacteriol 190:6392–6397
Zhang L, Wang L, Wang J, Ou X, Zhao G, Ding X (2010) DNA cleavage is independent of synapsis during Streptomyces phage phiBT1 integrase-mediated site-specific recombination. J Mol Cell Biol 2:264–275
Zhang L, Zhao G, Ding X (2011) Tandem assembly of the epothilone biosynthetic gene cluster by in vitro site-specific recombination. Sci Rep 1:141
Zhao C, Huang T, Chen W, Deng Z (2010) Enhancement of the diversity of polyoxins by a thymine-7-hydroxylase homolog outside the polyoxin biosynthesis gene cluster. Appl Environ Microbiol 76:7343–7347
Zhou X, Wu H, Li Z, Zhou X, Bai L, Deng Z (2011) Over-expression of UDP-glucose pyrophosphorlyase increases validamycin A but decreases validoxylamine A production in Streptomyces hagroscopicus var. jinggangensis 5008. Metab Eng 13:768–776
Acknowledgments
The work in the laboratory of AL was supported by the BMBF (GenBioCom), DFG (Lu1524/2-1) and ERC (EXPLOGEN) grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Myronovskyi, M., Luzhetskyy, A. Genome engineering in actinomycetes using site-specific recombinases. Appl Microbiol Biotechnol 97, 4701–4712 (2013). https://doi.org/10.1007/s00253-013-4866-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4866-1