Abstract
We investigated the feasibility of rapid, nondestructive assay of carotenoid-to-chlorophyll (Car/Chl) ratio and total carotenoids (Car) in cell suspensions of the carotenogenic chlorophyte Haematococcus pluvialis Flotow under stressful conditions. Whole-cell spectra are characterized by variable nonlinear contributions of Car and chlorophylls (Chl), with a strong influence of Car packaging and sieve effect inherent to stressed H. pluvialis cells. Nevertheless, nondestructive assay of Car/Chl in the range of 0.55–31.2 (Car content up to 188 mg L−1; 5.4 % of the cell dry weight) turned to be achievable with a simple spectrophotometer lacking an integrating sphere upon deposition of the cells on glass fiber filters. The scattering-corrected optical density (OD) in the blue–green region of the whole-cell spectrum, normalized to that in the red maximum of Chl absorption (OD500/OD678), was tightly related (r 2 = 0.96) with the Car/Chl ratio found in extracts. Some features such as the amplitude and position of the minimum of the normalized first-derivative OD whole-cell spectra also exhibited a strong (r 2 > 0.90) nonlinear correlation with Car/Chl. These spectral indices were also tightly related with Car, but the slope of the relationship varied with the stressor intensity. The importance of calibration over the widest possible range of pigment contents and a correct choice of biomass load per filter are emphasized. The advantages and limitations of nondestructive monitoring of carotenogenesis in H. pluvialis are discussed in view of its possible application in optical sensors for laboratory cultivation and mass production systems of the algae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chlorophyte Haematococcus pluvialis Flotow is one of the most biotechnologically important microalgal species (Del Campo et al. 2007), the richest natural source of the ketocarotenoid astaxanthin (Ast) (Sussela and Toppo 2006). Ast exerts a plethora of beneficial effects on human health; it is also a valuable feed additive in aquaculture (Guerin et al. 2003; Boussiba 2000). In H. pluvialis, accumulation of Ast as a major (over 95 % of total carotenoids; Lemoine and Schoefs 2010) secondary carotenoid (Car) takes place under stressful conditions (high irradiance, nutrient limitation, high salinity, etc.), in parallel with a reduction in chlorophyll (Chl) content, and other cellular responses (Lemoine and Schoefs 2010). It is believed that Ast, mainly in the form of fatty acid esters (Zhekisheva et al. 2002; Boussiba 2000), localized in cytoplasmic lipid globules—or oil bodies (Peled et al. 2011)—plays a role in protection of microalgal cells against photooxidative damage via optical screening and/or elimination of reactive oxygen species (Solovchenko 2011; Wang et al. 2003; Li et al. 2008), although the extent of its involvement is sometimes difficult to assess (Fan et al. 1998). While the pattern of the physiological responses to stress described previously is conserved for various stress conditions, their extent depend strongly on the stress intensity, as it is sensed by the algal cells. As a consequence, quantitative relations between stress and response are often elusive and poorly reproducible (Hu et al. 2008; Sarada et al. 2002; Zhekisheva et al. 2005).
The investigation of Car accumulation occurring in H. pluvialis on the background of Chl degradation is of considerable importance for basic as well as applied research. Studies of the relation between accumulation of secondary Car, increase in carotenoid-to-chlorophyll (Car/Chl) ratio, and change in light absorption by the microalgal culture provide valuable information about acclimation of the photoautotrophic microorganisms to stressors (Hu et al. 2008; Sarada et al. 2002). In microalgal biotechnology, information on the dynamics of Car content in biomass is essential for timely and informed decisions on the adjustment of illumination conditions, on medium composition, and on the time for biomass harvesting. Traditionally, the Car and Chl contents are determined by “wet” chemical methods with the use of spectrophotometry or chromatography (Zhekisheva et al. 2005), which are time-consuming, expensive, and not readily available in the field or at mass cultivation facilities. These considerations emphasize the need for a reliable, rapid, and preferably nondestructive technique for fast appraisal of the relative Car content in microalgal cultures.
Remarkably, the engagement of protective mechanisms based on the buildup of Ast-containing oil bodies in the cell is accompanied by specific changes in the optical properties of the algal cells (Solovchenko 2011). Recent reports show that Ast presence and subcellular distribution in vivo could be characterized (Kaczor et al. 2011) and even distinguished from β-carotene (Collins et al. 2011) using advanced spectral techniques such as Raman spectroscopy. However, current understanding on the effects of Ast accumulation on the optical properties of H. pluvialis cell suspension is clearly insufficient to develop nondestructive spectroscopic tools suitable for on-line monitoring of Car in laboratory or industrial mass cultures of H. pluvialis.
A solution to this problem would require a better understanding of the spectroscopy of H. pluvialis cell suspensions and the relationships between whole-cell spectra and contributions of different pigments to overall light absorption in the process of carotenogenesis. Recently, we successfully employed cell suspension optical density (OD) measurements to develop an algorithm for gauging high-light tolerance of H. pluvialis cultures (Solovchenko 2011). In the present work, we show the possibility of obtaining a quantitative description of carotenogenesis in stressed H. pluvialis cultures with a simple spectrophotometer lacking integrating sphere. We also, for the first time, report the relationships between Car/Chl and spectral features of H. pluvialis whole-cell OD which form the foundation for the development of techniques for noninvasive assay of Car accumulation in H. pluvialis cells. It is important to realize that this approach is not intended to replace traditional pigment assay, but to complement it, providing a meaningful index for the extent of stress in Haematococcus cultures. We also believe that it would facilitate the development of algorithms for nondestructive real-time monitoring of other microalgae cultivated under stressful conditions.
Materials and methods
Cultivation conditions
The unicellular chlorophyte H. pluvialis Flotow 1844 em. Wille K-0084 was obtained from the Scandinavia Culture Center for Algae and Protozoa at the University of Copenhagen, Copenhagen, Denmark. The algae were cultivated on modified BG11 medium (Boussiba and Vonshak 1991) in 0.4-L glass columns (5 cm ID) under constant illumination provided by daylight fluorescent lamps. The exponentially growing cells (referred to as “green” cells) initially grown at the irradiance of 35 μE m−2 s−1 were transferred to nitrate-free medium at the irradiance of 350 μE m−2 s−1 as measured by a LiCor 850 quantometer (LiCor, USA) in the center of an empty column. The cultures were constantly bubbled with CO2/air mixture (2:98, v/v) at 25 °C and the pH of the culture under these conditions was in the range 7.0–7.8.
Pigments extraction and analysis
Total Chl and Car were extracted from microalgal cells pelleted by centrifugation with dimethyl sulfoxide (DMSO) for 5 min at 70 °C with 5 ml per approximately 3.5 mg dry weight (DW). The pigment concentrations were routinely determined spectrophotometrically in DMSO extracts with a Cary 50 Bio Spectrophotometer (Varian, Palo Alto, CA, USA) (Solovchenko et al. 2010). The extracts were diluted such that the absorbance at pigment maxima was kept in the range 0.2–0.8. In certain experiments, the pigment content in H. pluvialis cells was determined in acetone extracts after separation using an high-performance liquid chromatography system equipped with a photodiode array detector (Varian Analytical Instruments, Walnut Creek, CA, USA) as previously described (Zhekisheva et al. 2005; Peled et al. 2011).
Filter spectra measurement and processing
To reduce the influence of scattering and rapid sedimentation of cells on the cuvette floor, a technique for measuring turbid microalgal samples similar to that described in Solovchenko et al. (2009) was used. Cells were deposited on 25-mm GF/F glass wool filters (Schleicher & Schuell, Dassel, Germany). The wet filters were mounted with cells facing the detector on the output window of the cuvette compartment. The OD of the filters was expressed as −log10 T, where T is transmittance (Fig. 1a). The spectra were taken in the range 400–800 nm with subtraction of the values of a baseline provided by an empty filter soaked in the culture medium. The corrected spectra, OD(λ), were obtained by smoothing the measured spectra with three-point moving average algorithm and baseline shift to yield zero OD at 800 nm. The OD(λ) spectra were normalized to the red Chl OD maximum at 678 nm yielding OD(λ)/OD678 spectra (Fig. 1b). Finally, the first derivative of the OD(λ)/OD678 spectra (Fig. 1c) was calculated using routine spreadsheet equation to estimate the steepness of the slope in the range 520–600 nm and the position of the associated inflexion point. This part of the original spectra is further referred to as the “green edge.” The coefficient of variation on spectral measurements was routinely under 5 %.
OD spectra of red H. pluvialis cells (Car content = 188 mg L−1; 5.4 % DW) measured upon deposition on glass fiber filters at different biomass loads: a measured spectra, the amount of biomass on filter (in milligrams DW) is indicated near the respective curves; b measured spectra from a corrected to OD800 = 0 and normalized to OD678; and c the first derivative of the normalized OD spectra from b
Statistical treatment
The results of three independent experiments are presented in the figures. Where appropriate, averages and standard errors of the mean were calculated and displayed. All correlations are significant at P < 0.001 level.
Results
The dynamics of biomass accumulation and pigment content
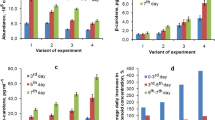
In routine experiments, the cells of H. pluvialis cultures were incubated under conditions favoring carotenogenesis (nitrogen-free medium, 350 μE m−2 s−1 irradiance) for 6 days. At the beginning of cultivation (day 0), the H. pluvialis cells were typically characterized by a high Chl content (1.8–2.0 % DW) and a correspondingly low Car content (0.5–1.1 % DW). The Car profile at this stage was dominated by lutein and β-carotene, containing also neoxanthin, violaxanthin, and zeaxanthin as the minor Car, but only traces of Ast were detectable (not shown). After 6 days under the stress conditions, the algae steadily accumulated biomass (increasing by fourfold), while the cell density remained relatively unchanged (Fig. 2a). The Chl content steadily decreased by more than 3-fold, while total carotenoid content increased by 17-fold (Fig. 2b); the same applies to specific Car content (Fig. 2c). Consequently, the Car/Chl weight ratio increased spectacularly (by more than 31-fold; Fig. 2b, curve 3), indicating that this is the most sensitive and unequivocal indicator of stress. The proportion of total Ast (in free and fatty-acid esterified forms), negligible before the onset of stress, increased sharply, reaching >95 % of the total Car by the end of the experiment (not shown).
Representative kinetics of a biomass accumulation, 1 and cell number, 2; b changes in volumetric pigment (Car, 1 and Chl, 2) contents and their ratio, 3; and c accumulation of Car in biomass (as DW percentage, 1 or per cell, 2) in a H. pluvialis culture under nitrogen starvation conditions employed in this work
OD spectra of whole H. pluvialis cells
Our preliminary attempts to record conventional OD spectra of H. pluvialis cell suspensions in a standard 1-cm cuvette gave unsatisfactory results due to rapid sedimentation of the algal cells on the cuvette floor and to the strong influence of light scattering (data not shown), stressing the need for means to correct the contribution of the latter.
One may circumvent the use of an integrating sphere for the measurement of OD in cell suspension by acquiring spectra from cells deposited on a wet glass fiber filter using a conventional spectrophotometer. The wet filter is positioned over the photomultiplier window, with cells away from the incident beam. In this case too, it is critical to correct for light scattering. Using the spectrum of a wet empty filter for baseline correction allowed obtaining reliable spectral data in a wide range of cell density and Car content of the culture and over the whole spectral range studied (see the “Materials and methods” section). A further correction proved to be necessary to eliminate the contribution of scattering by the cells, with the extent of the correction depending on the amount of biomass loaded on the filter (see, e.g., the 750- to 800-nm range in Fig. 1a). The OD at 800 nm (where no pigment absorbs) was used as a blank correction for this purpose. As a result, the corrected OD(λ) spectra were very close to baseline in the 750- to 800-nm range, indicative of a great decrease in the contribution of scattering.
The scattering-corrected OD spectra of H. pluvialis cells deposited on a wet filter, acquired without the use of integrating sphere, at different physiological states (from vegetative green cells to stressed red cysts with high Car/Chl) are presented in Fig. 3, in comparison with the conventional spectra of the pigments extracted from the same samples. The spectra of the whole cells (Fig. 3a) present profound differences with those for the extracts (Fig. 3b), in which pigments are in dilute solution. In particular, the amplitudes of the pigment absorption bands in the red and in the blue–green parts of the spectrum were lower, the bands were broader, and the peaks were less resolved in the whole-cell spectra than in the spectra of the pigment extracts, apparently due to a sieving effect and/or aggregation of the molecules of the pigments (see the “Discussion” section). Remarkably, the magnitude of the difference increased along with the accumulation of Car and loss of Chl; the highest difference between whole-cell and extract spectra was recorded for the cells with the highest Car content (cf. spectral curves in Fig. 3a, b).
Representative changes of OD of whole H. pluvialis cells (a, c) and the corresponding absorbance of DMSO extracts (b, d) in the course of nitrogen starvation (see Fig. 2). The spectra before (a, b) and after (c, d) normalization to Chl red absorption maximum are shown. The Car/Chl ratio is indicated. Biomass filter load was 1.33 mg DW (a), while the average for variable loads (0.4–4.5 mg DW) for each Car/Chl are presented in c, together with the standard errors of the mean (n=7) for the three upper curves. Finally, the specific values for OD (a) and absorbance (b) were calculated for whole cells and extracted pigments, respectively, equivalent to 1.33 mg DW
The data presented in Fig. 1 are representative of suspensions subjected to intense and prolonged stress, achieving very high Car content and Car/Chl to test the feasibility of the suggested approach. Different amounts of biomass were loaded upon filters. The raw spectra (Fig. 1a) indicate that, similar to spectra recorded with an integrating sphere (see spectra in Solovchenko 2011), only the Chl absorption is well-resolved (peak centered at 678 nm), while the combined contribution of Car and Chl in the blue–green region of the spectrum appears as a broad flat band in the range 400–550 nm. After subtraction of OD800 and normalization to OD678, the spectra essentially coincided (see, e.g., Fig. 1b), revealing the quasilinear dependence between OD and the amount of biomass loaded on the filter in the range 0.4–4.5 mg DW/filter, corresponding to OD500 of 0.04–0.78.
The analysis of the relationship between OD(λ) and the amount of biomass applied to the filter with different Car/Chl in the diluted suspensions showed that the relation “OD(λ) vs. DW on filter” was nearly linear in the range 0.4–4.5 mg DW in all cases studied (see, e.g., the data in Fig. 4). This relationship departed from linearity at higher OD500 (>0.9) in the case of “green” cell suspension (low Car/Chl), but less in the case of “red” cells with high Car/Chl, apparently due to stronger aggregation of Car and sieve effect in the latter case. Notably, the higher the Car/Chl ratio of the samples taken at different cultivation stages, the lower the slope of the linear part of the relationships “OD500 vs. DW on filter” (Fig. 4). This effect was also apparent with OD678 (not shown).
Relationship between scattering-corrected OD of the filter at 500 nm and the amount of biomass loaded on the filter for H. pluvialis whole cells with different Car/Chl ratios taken at different stages of nitrogen starvation (see Fig. 2)
Changes of the OD spectra of H. pluvialis cells in the course of carotenogenesis
Cultivation of H. pluvialis under stressful conditions brought about profound changes in the OD spectra of the microalga (Fig. 3). The stress-induced carotenogenesis was accompanied by the disappearance of spectral details in the blue–green region, resulting in the formation of a broad (approximately 150 nm wide), almost featureless band with an abrupt longer-wavelength slope. A considerable decrease of the amplitude in the band of the specific OD contributed by Chl in the red region (Fig. 3a) took place along with an overall reduction of that in the blue–green region, where absorption by both Car and Chl occurs, despite the increase in Car content. On the other hand, an increase in Car contribution to the absorbance was readily apparent in the extract spectra (Fig. 3b) at intermediate Car/Chl ratios; however, at higher Car/Chl, a saturation effect becomes obvious, equivalent to that expected from the Lambert–Beer law at high chromophore concentrations.
In order to rule out a possible interference from variable Chl background disturbing the relationship “OD(λ) vs. pigment content or ratio,” we tried to relate the OD(λ) spectra normalized to Chl red absorption maximum, OD500/OD678, with Car or Car/Chl. Normalization revealed, apart from a decline of Chl contribution to light absorption by the microalgal cells, a dramatic increase in the absorption in the 400- to 550-nm range with flattening of the spectrum shape and an increase in the longer-wavelength slope of this band (Fig. 3c, which may be called “green edge” by analogy to the “red edge” (Gitelson et al. 1996) in plants). This effect was even more pronounced in the extract spectra (Fig. 3d).
The amplitude of the OD500/OD678 ratio was correlated to the Car/Chl mass ratio and, to a lesser extent, to the Car content (not shown), in the broad band 400–550 nm. Thus, the ratio OD500/OD678 was linearly related with the Car/Chl ratio at Car/Chl < 20 (r 2 = 0.95); at higher values of the ratio, this relationship departed from linearity but remained uniform and tight under all conditions investigated (cf. solid and broken lines in Fig. 5). The relationships “OD500/OD678 vs. Car” were also linear in the whole range studied, but the slope of the relationship was variable and dependent on the stress intensity experienced by the cultures (data not shown), presenting, therefore, a lesser diagnostic value.
Relationship between the changes in OD at 500 nm normalized to the red chlorophyll maximum and the Car/Chl ratio in the H. pluvialis cells in the course of nitrogen starvation (see Figs. 2 and 3). The data of three independent experiments are shown by different symbols. Dashed line is the best fit function for the entire dataset; solid line is that for Car/Chl < 20 (n = 23, r 2 = 0.96)
Notably, due to stress-induced carotenogenesis, which brought about the broadening and flattening of the OD500/OD678 spectra, the longer-wavelength slope of the broad absorption band in the blue–green (green edge) became more abrupt and moved towards longer wavelengths (Fig. 6c). This effect was readily apparent as a bathochromic shift and an increase in the amplitude of the characteristic minimum in the range 520–600 nm on the first-derivative spectra (Fig. 6a). The position of the inflection point and the amplitude of the derivative minimum (i.e., the inclination of the slope of the unresolved absorption band governed by Car; Fig. 6) exhibited a uniform positive correlation with Car/Chl ratio under our experimental conditions (Fig. 6b, c, respectively). As in the case of OD500/OD678, the green edge parameters were tightly related with Car content, but the parameters of the relationships were different in different experiments, depending of the stress intensity (data not shown).
The changes in first derivative of the normalized OD spectra of whole cells on filter (a) and relationships between the position (b) or the amplitude (c) of the “green edge” feature (see hollow dots connected with a broken line in a) with Car/Chl ratio in the course of H. pluvialis nitrogen starvation (see Figs. 2 and 3c). Dashed line is the best fit function (n = 23, r 2 = 0.90)
Discussion
Current literature on H. pluvialis includes only a few reports on the systematic investigation of spectral properties of whole-cell suspension (see, e.g., Solovchenko 2011), especially cysts with high Car content (or Car/Chl). These circumstances obviously stem from the optical complexity of this system. In particular, H. pluvialis cells contain high amounts of pigments which are localized in specific structures (thylakoid membranes of chloroplast or cytoplasmic oil bodies in case of Chl and primary Car or secondary Car, respectively) nonuniformly distributed within the cell volume (Boussiba 2000; Peled et al. 2011). Under stressful conditions, H. pluvialis cells rapidly accumulate secondary Car concomitantly with a decline in Chl and primary (photosynthetic) Car. The increase in concentration of chromophore (such as Ast) molecules confined to the small volume of oil bodies could lead to broadening and bathochromic shifts of their maxima (Zsila et al. 2001). As a consequence, a number of serious obstacles for measurement of OD spectra in H. pluvialis cell suspension arise, including rapid cell sedimentation, significant influence of light scattering, strong pigment aggregation, and sieving effect.
Traditionally, integrating spheres are used to cope with incomplete light collection due to light scattering (Merzlyak et al. 2008). The application of more sophisticated approaches for light-scattering compensation requires more advanced and expensive spectrophotometers and additional spectrum scans for each sample (Merzlyak and Naqvi 2000) being, therefore, less suitable for rapid estimation of Car or Car/Chl. In this work, we used an alternative approach similar to the opal glass method developed by Shibata (1973). This method is based on the deposition of microalgal cells on glass fiber filters prior to measurement and was previously developed to record the OD spectra of the chlorophyte Parietochloris incisa (Solovchenko et al. 2009). Remarkably, the approach does not require any special sampling accessories such as solid sample holder since the wet filters are adhesive enough to be mounted on the vertical wall of the cuvette compartment in front of the light-exit window. There is no need for special software and/or processing modules as well since all the steps of spectral data processing are carried out with little effort using standard spreadsheet functions. Application of this technique made it possible to obtain reliable spectral data on H. pluvialis cell suspensions, compatible with those recorded with a more advanced spectrophotometer fitted with an integrating sphere (see, e.g., spectra in Solovchenko 2011).
The analysis of cell suspension spectra recorded using this approach revealed certain features characteristic of stress-induced pigment changes, primarily due to carotenogenesis in H. pluvialis cells. Noteworthy, the amplitude of the raw OD(λ) spectra was not directly correlated to their pigment content (Fig. 3a, b). Thus, the magnitude of the broad maximum in the blue–green region of the spectrum was often lower in the red cells with high Car/Chl (>3) than in samples with lower Car/Chl. However, this apparent paradox observed in raw whole-cell spectra (decrease in blue–green band upon increasing Car content) was fully resolved after normalization to the red Chl maximum (Fig. 3c), suggesting that the increase in Car content contributes more than the decrease in Chl to the course of stress-induced carotenogenesis.
We believe that the discrepancy observed with raw whole-cell spectra stems from a sieving effect which could be further exacerbated by fusion of Ast-containing oil bodies (Pilát et al. 2012) at advanced stages of carotenogenesis. Although there are some indications for the buildup of Ast in small and medium oil bodies at intermediate stages of carotenogenesis (Collins et al. 2011), it is difficult to infer the relation between oil body size and the extent of Ast packaging therein. Furthermore, the manifestations of packaging could also reflect the extension of the effective optical path due to light scattering (inversely related to the size of scattering particles). All these circumstances complicate the construction of a robust algorithm for direct estimation of Car content via OD(λ). It is unlikely that the previously mentioned discrepancy resulted from distortion of spectra due to deposition of the cells on glass fiber filters since the normalized spectra are largely independent of the biomass (Fig. 1b) routinely applied to the filters in our experiments; indeed, a quasilinear relation exists in “OD(λ) vs. DW on filter” (Fig. 4). On the other hand, one should carefully check the linearity of this relationship in each particular experimental system since it is influenced, apart from the optical properties of microalgal cells per se, by the scattering properties of the filters and the spectrophotometer geometry. Remarkably, the slope of “OD500 vs. DW on filter” relationship decreased with the onset of stress-induced carotenogenesis, reflecting the increase in sieve effect due to buildup of local Ast concentration and, possibly, fusion of oil bodies containing the pigment.
It is well-known that, in H. pluvialis cultures subjected to stressful conditions (Torzillo et al. 2003; Boussiba 2000), carotenogenesis occurs in parallel with degradation of Chl manifesting the reduction of photosynthetic apparatus in order to avoid photooxidative damage (Wang et al. 2003; Solovchenko 2011). It was found recently that the Car/Chl ratio, but not the absolute amount of Chl or Car, correlates directly with high light-stress tolerance in H. pluvialis (Solovchenko 2011), making Car/Chl an informative index of the cell physiological condition. At the same time, extra care should be exercised at very high Car/Chl since small errors in Chl assay in this case could lead to a considerable inaccuracy in the determination of the ratio. The normalization of OD(λ) to the red Chl maximum essentially equalized the contribution of Chl to light absorption, making apparent the relative contribution of Car which drastically increased in the course of carotenogenesis (Fig. 3c). Indeed, OD(λ)/OD678 exhibited a tight relationship with Car/Chl in the studied range (Fig. 5).
The relationships “OD(λ)/OD678 vs. Car content” and “OD(λ)/OD678 vs. Car/Chl” were linear in a wide range of Car changes and began to depart from linearity only at high Car (see Fig. 5). It is difficult to say whether it is an effect of saturation or the change of effective absorption coefficient of Ast taking place along with its accumulation. Plausible reasons include changes in Car composition (e.g., increase in proportion of Ast from <1 to >95 %; Zhekisheva et al. 2005) and in the degree of aggregation of Car molecules.
Analysis of the first-derivative spectra revealed that the characteristic spectral changes accompanying carotenogenesis in H. pluvialis include a remarkable increase in the green edge effect (the amplitude of the derivative minimum in the 520- to 600-nm range and its profound bathochromic shift; Fig. 6a). It was found that the amplitude of green edge slope and the magnitude of its shift towards longer wavelengths are exponentially related with Car/Chl, and these relationships are uniform under the experimental conditions used in the present work (Fig. 6b, c). On the contrary, the relationships of Car content with the green edge features (as well as with OD(λ)/OD678) were tight (r 2 > 0.95) but possessed different slopes under different stress intensities. One may speculate that this phenomenon is caused by different trends of Chl degradation significantly affecting OD(λ)/OD678, but not Car content. This further supports the suggestion of Car/Chl ratio as a preferable marker of stress in H. pluvialis.
We would like to note in conclusion that, despite the strong influence of pigment packaging and sieve effect inherent to the whole-cell spectra of stressed H. pluvialis cultures, the nondestructive rapid assay of Car/Chl ratio and Car content turned to be feasible in this system upon deposition of the cells on a glass fiber filter with a simple spectrophotometer without the use of an integrating sphere. It was found that the dramatic changes in scattering-corrected OD occurring in the blue–green region of the spectrum, upon normalization to variable Chl absorption, are tightly related with changes in pigment content and composition. Simple OD ratios as well as the first-derivative minimum amplitude and position in the range 520–600 nm exhibited a strong positive correlation with Car/Chl in the range 0.55–31.2 and Car up to 188 mg L−1. It should be noted, however, that, due to the extremely high local Car concentrations achieved in stressed H. pluvialis cells, the relationship with absolute Car content varied depending on the intensity of the stress. Nevertheless, in view of a similar quasilinear evolution of the Car/Chl mass ratio observed with other microalgae under stress (C. Aflalo, unpublished; see also Merzlyak et al. 2007; Solovchenko et al. 2009), this methodology could be extended to study cellular response in other systems.
The findings described in this work allow one to obtain a quantitative record of the development of stress-induced carotenogenesis in H. pluvialis nondestructively via OD measurements. In particular, the normalized OD in the broad band around 500 nm, as well as the green edge features, could be employed in the development of models for rapid assay of Car/Chl in the algal cells suspensions. It should be noted, however, that obtaining a calibration in the widest possible range of Car/Chl changes in any particular culture system and careful control of the biomass load per filter is crucial for the robustness of Car/Chl estimation.
The advantages of OD-based nondestructive monitoring of carotenogenesis in microalgae such as rapidity, simplicity, and affordability form a ground to believe that this approach could find use in the development of algorithms for real-time monitoring of carotenogenesis and physiological condition of the microalga in situ. Such algorithms could be used in solid-state optical sensor design for automation of large-scale laboratory cultivation and mass production systems of H. pluvialis and, possibly, other microalgae, provided that its limitations are taken into account.
Abbreviations
- A(λ):
-
Absorbance value at wavelength λ; we used this notation for dissolved pigments in extracts
- OD(λ):
-
Scattering-corrected optical density spectra of a microalgal cell sample deposited on a glass fiber filter
- OD λ :
-
OD(λ) value at wavelength λ
- OD(λ)/OD678 :
-
Spectrum normalized to red chlorophyll absorption maximum
- First derivative:
-
Partial derivative of OD(λ)/OD678 spectra: ∂[OD(λ) / OD678] / ∂λ
References
Boussiba S (2000) Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiol Plant 108(2):111–117. doi:10.1034/j.1399-3054.2000.108002111.x
Boussiba S, Vonshak A (1991) Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant Cell Physiol 32(7):1077–1082
Collins AM, Jones HDT, Han D, Hu Q, Beechem TE, Timlin JA (2011) Carotenoid distribution in living cells of Haematococcus pluvialis (Chlorophyceae). PLoS One 6(9):e24302. doi:10.1371/journal.pone.0024302
Del Campo J, García-González M, Guerrero M (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74(6):1163–1174. doi:10.1007/s00253-007-0844-9
Fan L, Vonshak A, Zarka A, Boussiba S (1998) Does astaxanthin protect Haematococcus against light damage? Z Naturforsch C 53(1–2):93
Gitelson A, Merzlyak M, Lichtenthaler H (1996) Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J Plant Physiol 148(3–4):501–508. doi:10.1016/S0176-1617(96)80285-9
Guerin M, Huntley M, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21(5):210–216. doi:10.1080/09670260802227736
Hu Z, Li Y, Sommerfeld M, Chen F, Hu Q (2008) Enhanced protection against oxidative stress in an astaxanthin-overproduction Haematococcus mutant (Chlorophyceae). Eur J Phycol 43(4):365–376. doi:10.1080/09670260802227736
Kaczor A, Turnau K, Baranska M (2011) In situ Raman imaging of astaxanthin in a single microalgal cell. Analyst 136(6):1109–1112. doi:10.1039/C0AN00553C
Lemoine Y, Schoefs B (2010) Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynthesis Res 106(1):155–177. doi:10.1007/s11120-010-9583-3
Li Y, Sommerfeld M, Chen F, Hu Q (2008) Consumption of oxygen by astaxanthin biosynthesis: a protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae). J Plant Physiol 165(17):1783–1797. doi:10.1016/j.jplph.2007.12.007
Merzlyak MN, Naqvi KR (2000) On recording the true absorption spectrum and the scattering spectrum of a turbid sample: application to cell suspensions of the cyanobacterium Anabaena variabilis. Journal of Photochemistry & Photobiology, B: Biology 58(2–3):123–129. doi:10.1016/S1011-1344(00)00114-7
Merzlyak M, Chivkunova O, Gorelova O, Reshetnikova I, Solovchenko A, Khozin-Goldberg I, Cohen Z (2007) Effect of nitrogen starvation on optical properties, pigments, and arachidonic acid content of the unicellular green alga Parietochloris incisa (Trebouxiophyceae, Chlorophyta). J Phycol 43(4):833–843
Merzlyak M, Chivkunova O, Maslova I, Naqvi K, Solovchenko A, Klyachko-Gurvich G (2008) Light absorption and scattering by cell suspensions of some cyanobacteria and microalgae. Russ J Plant Physiol 55(3):420–425. doi:10.1134/S1021443708030199
Peled E, Leu S, Zarka A, Weiss M, Pick U, Khozin-Goldberg I, Boussiba S (2011) Isolation of a novel oil globule protein from the green alga Haematococcus pluvialis (Chlorophyceae). Lipids 46(9):851–861. doi:10.1007/s11745-011-3579-4
Pilát Z, Bernatová S, Ježek J, Šerý M, Samek O, Zemánek P, Nedbal L, Trtílek M (2012) Raman microspectroscopy of algal lipid bodies: β-carotene quantification. J Appl Phycol 24(3):541–546. doi:10.1007/s10811-011-9754-4
Sarada R, Tripathi U, Ravishankar G (2002) Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem 37(6):623–627. doi:10.1016/S0032-9592(01)00246-1
Shibata K (1973) Dual wavelength scanning of leaves and tissues with opal glass. Biochim Biophys Acta 304(2):249
Solovchenko A (2011) Pigment composition, optical properties, and resistance to photodamage of the microalga Haematococcus pluvialis cultivated under high light. Russ J Plant Physiol 58(1):9–17. doi:10.1134/S1021443710061056
Solovchenko A, Khozin-Goldberg I, Cohen Z, Merzlyak M (2009) Carotenoid-to-chlorophyll ratio as a proxy for assay of total fatty acids and arachidonic acid content in the green microalga Parietochloris incisa. J Appl Phycol 21(3):361–366. doi:10.1007/s10811-008-9377-6
Solovchenko A, Merzlyak M, Khozin-Goldberg I, Cohen Z, Boussiba S (2010) Coordinated carotenoid and lipid syntheses induced in Parietochloris incisa (Chlorophyta, Trebouxiophyceae) mutant deficient in Δ5 desaturase by nitrogen starvation and high light. J Phycol 46(4):763–772. doi:10.1111/j.1529-8817.2010.00849.x
Sussela M, Toppo K (2006) Haematococcus pluvialis—a green alga, richest natural source of astaxanthin. Curr Sci 90(12):1602–1603
Torzillo G, Goksan T, Faraloni C, Kopecky J, Masojídek J (2003) Interplay between photochemical activities and pigment composition in an outdoor culture of Haematococcus pluvialis during the shift from the green to red stage. J Appl Phycol 15(2):127–136. doi:10.1023/A:1023854904163
Wang B, Zarka A, Trebst A, Boussiba S (2003) Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process under high irradiance. J Phycol 39(6):1116–1124. doi:10.1111/j.0022-3646.2003.03-043.x
Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z (2002) Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J Phycol 38(2):325–331. doi:10.1046/j.1529-8817.2002.01107.x
Zhekisheva M, Zarka A, Khozin-Goldberg I, Cohen Z, Boussiba S (2005) Inhibition of astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga Haematococcus pluvialis (Chlorophyceae). J Phycol 41(4):819–826. doi:10.1111/j.0022-3646.2005.05015.x
Zsila F, Deli J, Simonyi M (2001) Color and chirality: carotenoid self-assemblies in flower petals. Planta 213(6):937–942. doi:10.1007/s004250100569
Acknowledgments
This work was in part supported by the Ministry of Science and Education of the Russian Federation (contract no. 16.513.12.3028) and “Skolkovo” Scientific Fund. Financial support by the European Commission’s Seventh Framework Programme for Research and Technology Development (FP7), project SENSBIOSYN, grant no. 232522 is gratefully acknowledged. Dedicated technical assistance of Mrs. Larisa Lozovskaya is much appreciated. The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solovchenko, A., Aflalo, C., Lukyanov, A. et al. Nondestructive monitoring of carotenogenesis in Haematococcus pluvialis via whole-cell optical density spectra. Appl Microbiol Biotechnol 97, 4533–4541 (2013). https://doi.org/10.1007/s00253-012-4677-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4677-9