Abstract
Production characteristics of the microalgal Dunaliella salina were studied. The maximum rate of carotenoid synthesis was observed at high light intensity and nitrogen deficiency. The applicability of cytometric and fluorescent indicators to assess the degree of β-carotene accumulation and to estimate the D. salina functional state was studied. The shift in growth conditions, which stimulated carotenoid accumulation, affected the algae photosynthetic apparatus and weakly affected cell enzyme activity and cell viability. Fluorescein diacetate (FDA) fluorescence and the ratio of live cells can be used to assess the degree of lethal effect of an external factor on the physiological state of microalgal. The measurement of cell autofluorescence at a wavelength of 575 nm was a highly effective approach to the rapid assessment of β-carotene content in D. salina and to the prediction of β-carotene formation. The correlations were obtained between cell autofluorescence ratios in the orange (575 nm) and red (680 nm) spectrum areas and the ratio content of carotenoid and chlorophyll in algae cells. A linear relationship was established between the Fm/D750 ratio (the ratio of the maximum chlorophyll fluorescence at closed PS II reaction centers to the suspension optical density at 750 nm) and intracellular chlorophyll content in microalgal. These relationships allow assessing pigment content in the cells as well as predicting β-carotene formation in D. salina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The green microalga Dunaliella salina is capable of synthesizing about 15–20 mg of carotenoids per liter of culture suspension (García-González et al. 2003). It is one of the most promising sources for β-carotene industrial production (Zhu et al. 2018). β-carotene content in its cells is up to 95% of the total carotenoid content (Jimenez and Pick 1994; Prieto et al. 2011). Excessive carotenoid accumulation in D. salina cells should be considered a physiological mechanism of algae adaptation to unfavorable growth conditions, specifically high light intensity, high salinity, and nitrogen deficiency in the medium (Ben-Amotz and Avron 1983; Solovchenko 2013). Coping with abiotic stress, D. salina accumulates a large amount of β-carotene as an antioxidant, preventing the excessive accumulation of reactive oxygen species in chloroplasts. The generation of singlet molecular oxygen increases with the damage of components or disruption of the functioning of PS II (photosystem II) complexes (Borowitzka 2018; D'Alessandro and Havaux 2019; Polle et al. 2020).
So, D. salina is an ideal organism for large-scale biotechnological production of β-carotene. Various approaches are applied in D. salina cultivation (Borowitzka 1990; Richmond 2000; Borovkov et al. 2020). One of them is the one-stage intensive culture cultivation, characterized by a lower intracellular β-carotene content in algae but higher cell density and culture productivity (García-González et al. 2003). The second widely used approach is based on two-stage cultivation. At the first stage, the conditions for active culture growth are established. β-carotene synthesis is induced by the stress effect (high light intensity, high salinity, and nitrogen deficiency in the medium) at the second stage (Ben-Amotz 1995; Prieto et al. 2011; Sui et al. 2019; Borovkov et al. 2020).
There is a difficulty: the stress effect of stimulating carotenogenesis can result in the cessation of microalgal growth and culture death. Moreover, β-carotene accumulation in the cells is a non-linear process. It is intense for the first 2 days after the shift in conditions, and then, there is a decrease followed by an increase (Lers et al. 1990; Pisal and Lele 2005). The complex dependence of β-carotene content on medium conditions requires operational monitoring and control of the technological process of pigment acquisition. To date, the functional state of a cultivated object, both during cumulative growth and under conditions of intense carotenogenesis, is usually assessed considering production indicators and intracellular pigment concentration; their determination requires lengthy and time-consuming measurements. Thus, the development of new methodological approaches for automating the registration of detailed data on microalgal physiological state is a task of key importance. In this direction, the application of flow cytometry coupled with the use of various vital dyes and PAM fluorometry is in perspective (Falkowski and Kolber 1995; Antal et al. 2001; Peperzak and Brussaard 2011; Hyka et al. 2013).
The use of informative express indicators will provide continuous monitoring of microalgal physiological state and pigment content in the cells. Moreover, it will allow predicting the rate of β-carotene formation in the cells and the optimal timing of product harvesting.
The aim of this study was to assess the applicability of cytometry and fluorescent indicators (a) to control Dunaliella salina’s physiological state and (b) to rapidly determine the carotenoid content of Dunaliella salina cells under various conditions of algae growth.
Materials and methods
The object of research was the pure culture of green microalgal Dunaliella salina (Teod.)—the strain IBSS-1—from the collection of living cultures of marine planktonic microalgal at the “Hydrobionts of the World Ocean” core facility (WDCM No. 1201) at the A.O. Kovalevsky Institute of Biology of the Southern Seas (IBSS) of the RAS.
Experimental conditions
At the first stage, we studied the capability of the D. salina strain to intensively synthesize carotene under the effect of well-known stimulating factors: salinity, high light intensity, and low nitrogen concentration in the medium. For 8 days before the experiment, the growth of the collection culture and its stabilization were carried out at a light intensity of 120 μE·m−2·s−1, a temperature of + 28 °C, and a salinity of 105 ‰ (seawater 18 ‰ + 1.5 M NaCl). The division rate was maintained at 0.2–0.4 div.·day−1 by quantitative dilution of the culture with a nutrient medium. One day before the experiment, the algae were resuspended in a complete F/2 nutrient medium and in a medium with a low nitrogen content, equalizing initial cell concentrations in the two variants.
During the experiment, algae were cultivated in a cumulative mode at two levels of light intensity and two nitrogen concentrations (the values are given in Table 1). The content of other elements in the nutrient medium corresponded to the F/2 medium protocol (Andersen 2005).
Algal cultivation was carried out in 300-mL Erlenmeyer flasks (150 mL of suspension in each) placed in plexiglass flow-through cells. The flasks were illuminated from below with cold-light LEDs. Initial concentration of D. salina cells was about (0.9·106 ± 0.1·106) cells·mL−1, with β-carotene content of (1.2 ± 0.3) pg·cell−1. In each variant of the experiment, three flasks were used.
At the second stage, we studied the applicability of fluorescent and cytometric indicators to assess the degree of β-carotene accumulation in the cells and to control algae’s general functional state during cumulative growth under optimal conditions and under conditions of intense carotenogenesis. During the experiment, algae were cultivated in a 1200-mL vertical flat panel vessel with a suspension thickness of 2 cm under one-sided round-the-clock artificial illumination with high-brightness white light LEDs (its different levels were reached by varying the distance from the light source and applying shading filters). The surface level of irradiance increased with the culture compaction: inside the vessels, light intensity varied within the range of 100–180 μE·m−2·s−1 during the accumulation phase and 1000–1800 μE·m−2·s−1 in carotene synthesis mode. The irradiance level was determined by a probing 4π sensor of the QSL-2101 quantometer (Biospherical Instruments Inc., USA). To form the necessary temperature conditions, a thermostat box (refrigerated display case) with adjustable temperature was used. Lighting equipment was placed outside this box, and insolation was carried out through a glass wall. The cultures were aerated with aquarium compressors; pH of the culture medium was between 8.2 and 8.5.
From the 1st to the 10th day of the experiment, the algae were cultivated under optimal conditions: an initial light intensity of 180 μE·m−2·s−1, a temperature of + 28 °C, and a F/2 nutrient medium with a two-fold reduced nitrogen content. On the eleventh day of the experiment, cultivation of one part of the culture was continued under the same conditions, and cultivation of another part was continued under shifted conditions: light intensity of 1800 μE·m−2·s−1, temperature of + 18 °C, and residual mineral nitrogen content of about 10 μM. To determine the indicators studied, sampling was carried out every other day throughout the experiment.
Measurement methods
For cytometric analysis, 3-mL samples were taken from the cultivation flasks. The Cytomics FC500 Flow Cytometer (Beckman Coulter, USA) was used, equipped with a 488-nm single-phase argon laser, and the CXP software was applied. The total microalgal abundance in the cultures was determined in a cluster on 2 parametric cytograms by forward light scattering (FS) and fluorescence of individual cells in the red spectrum area (FL4, 675 nm) on dimensionless logarithmic scales. Cell concentration was calculated considering the sample flow rate (60 μL·min−1), the time of counting (33–100 s), and the abundance of cells recorded during this time interval. The intensity of cell autofluorescence in the orange and red spectrum areas was measured in the FL2 channel (575 nm) and FL4 channel, respectively. Algae were stained with the vital dye fluorescein diacetate (FDA), an indicator of metabolic activity in living cells, according to the protocol published in (Solomonova and Mykhanov 2011). Metabolic activity and pigment concentration in the cells were evaluated on two parametric cytograms by FDA fluorescence (FL1 channel in the green spectrum area, 525 nm) and autofluorescence (FL4 in the red spectrum area, 675 nm) on dimensionless logarithmic scales. The figures can be found in the publication (Solomonova and Mykhanov 2011); that work was performed with the authors involved. The mean values of accumulated FDA fluorescence per cell were calculated, and the indicator was denoted as FDAfl.
Taking into account chlorophyll an autofluorescence (FL4 in the red spectrum area, 675 nm), the quantity of living and dead cells was determined (Solomonova and Akimov 2013).
Pigment concentration in the samples was determined by standard spectrophotometry on the SF-2000 spectrophotometer (“OKB Spectr”, Saint Petersburg, Russia). Chlorophyll a concentration was calculated by the generally accepted formula (Jeffrey and Humphrey 1975).
where OD is the optical density of the extracts at the indicated wavelength, taking into account the correction for nonspecific absorption at 750 nm; Vex is the volume of acetone extract, mL; Vf is the volume of the filtered culture, L; Lk is the cuvette length, sm.
The total carotenoid content was determined using the formulas given in (Lichtenthaler and Buschmann 2001), using optical density values at the wavelengths corresponding to the absorbance maximums of the pigments:
where A is absorbancy.
As mentioned above, β-carotene content in D. salina cells is about 93–95% of the total carotenoid concentration (Jimenez and Pick 1994); therefore, the term “β-carotene” is used to denote carotenoid content in this work.
Variable chlorophyll a fluorescence Fv/Fm (PS II maximum quantum efficiency) was measured on the Mega-25 m fluorometer with pulse modulation of excitation light at 455 nm (Pogosyan and Matorin 2005), developed at the Biophysics Department of Biology Faculty of Lomonosov Moscow State University. The calculation was performed according to the formula:
where Fm is the maximum chlorophyll fluorescence after saturating the photosynthesis reaction centers; F0 is the chlorophyll fluorescence value at open photosynthesis reaction centers.
The optical density of the cell suspension was quantified at 750 nm (OD750): at this wavelength, light is absorbed by the cell structural elements and there is no selective light absorption by pigments of the algae’s photosynthetic apparatus. The value of the optical density of the algae suspension is related to the algae concentration in the medium, which allows estimating the cell abundance in the culture through optical measurements. Earlier, we studied the applicability of the optical density as a biomass indicator for different algae species (Shoman and Akimov 2013). Specifically, in a certain range of values, there is a linear relationship between this value and the organic carbon content measured by gas adsorption chromatography on the CHN analyzer in an algae suspension. During the experiment, the optical density of algae was quantified in a 10-cm cylindrical cuvette installed in the cuvette compartment of the SF-26 spectrophotometer, and the absorbance relative to the cuvette was measured with a non-absorbing solution—seawater.
The data was statistically processed using standard software packages, Microsoft Excel 7.0 and Grapher 16. The dots on the graphs represent the means of 3–4 measurements. The bars on the charts denote the standard deviation of the values obtained. The significance of the differences between the samples was assessed using the Student’s t-test at p < 0.05.
Results
Figure 1 shows D. salina cell concentration and β-carotene content on the 3rd, 5th, and 7th days of cumulative algae growth under various cultivation conditions described in Table 1. The initial algae concentration in all variants of the experiment was the same and amounted to about 0.9·106 cells·mL−1, with a β-carotene content of (1.2 ± 0.3) pg·cell−1. The maximum increase in D. salina abundance was observed when algae were fully supplied with mineral nutrition (F/2 medium) and light intensity was 120 μE·m−2·s−1 (variant 1, Fig. 1a). In a nitrogen-depleted medium, the mean growth rate at this light intensity remained high in the first 3 days. However, on the 3rd–5th day of exposure, a significant decrease was recorded, which was probably associated with an increasing deficiency of this biogenic element. High light intensity caused growth rate inhibition, approximately the same for cases of high and low nitrogen content in the medium. The intracellular carotenoid content was stable under favorable growth conditions and rose under nitrogen limitation (Fig. 1b). High light intensity stimulated intense β-carotene accumulation, which had the highest value with nitrogen deficiency in the medium (variants 3 and 4).
a Cell abundance in the suspension. b Specific β-carotene content per cell; and c its total concentration in the suspension on the 3rd, 5th, and 7th days of Dunaliella salina growth; d the mean daily increase in carotenoid concentration in the cells (the Figs. 1–4 on the graphs indicate the variants of the experiment; see Table 1)
As seen from Fig. 1c, the total β-carotene content per unit of culture suspension increased by 4 times from the variant 1 of the experiment to the variant 4, whilst the intracellular pigment content increased by 13 times. This is mainly due to a growth rate slowdown during the shift to stressful cultivation conditions, which is especially evident on the 3rd–5th day of exposure.
In Fig. 1d, the values which are the mean daily increase in carotenoid content are shown as a percentage of the initial level for the first 3 days and the following two. In all variants, a decrease was recorded in the relative rate of their accumulation on the 3rd–5th day compared to the initial period. Specifically, in variant 4, where the maximum absolute increase in pigment content was observed, the relative rate decreased by more than 4 times. This is due to a decline in algae growth rate during this period that was not compensated by an increase in intracellular β-carotene content.
At the second stage of the work, as already mentioned, D. salina growth was studied in cumulative mode and under conditions of intense carotenoid synthesis. Moreover, the applicability of fluorescent and cytometric indicators to estimation of algae functional state was assessed. Figure 2 shows the dynamics of cell abundance and intracellular pigment content during D. salina cumulative growth. The maximum cell density was of 14·106 cells·mL−1 on the 13th–15th day of the experiment. The mean growth rate for the first four days of exposure was 0.65 div.·day−1. The growth rate at the beginning of the stationary phase (8th–10th day) was 0.2 div.·day−1. From this moment on, intracellular chlorophyll a concentration decreases and carotenoid content in the cells rises. The range of their values is relatively small. Thus, the Car/Chl ratio increased from 0.68 during the exponential growth phase to 1.4 in the stationary state; under conditions of intense carotene accumulation, this ratio is significantly higher than 10 (Ben-Amotz et al. 1989; Fachet et al. 2016).
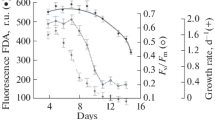
Figure 3 shows the dynamics of the values of the Fm/D750 ratio, the Fv/Fm parameter, the FDAfl, and the ratio of viable cells in the population during the experiment. As can be seen from the data presented, a progressive decrease in the values of these indicators was recorded with the development of the stationary state of the culture. The Fm/D750 ratio characterizing specific chlorophyll content in algae cells varied insignificantly during the exponential growth and at the beginning of the stationary phase. At later stages of the stationary state, the decrease in Fm/D750 ratio was observed, which correlated with a decrease in chlorophyll a content in D. salina during this period. Similar dynamics of values during the experiment were noted for Fv/Fm parameter. Up to the 10th day of the experiment, Fv/Fm was in the range of 0.6–0.7. On the 10th–23rd day of exposure, the values decreased linearly to 0.3. During the shift to the stationary state, FDA fluorescence indicated the level of cell metabolic activity decreased as well, which was especially noticeable on the 16th day of the experiment. In this case, the total decrease in the value during the experiment was about 40%. On the 16th day of the experiment, the decrease in the number of viable cells in the culture was also observed, reaching 30% at the end of the stationary growth phase.
On the 11th day of the cumulative growth, the part of the culture was shifted to new growth conditions corresponding to maximum β-carotene accumulation in the cells: light intensity of 1800 μE·m−2·s−1, temperature of + 18 °C, and residual NaNO3 content in the medium of about 10 μM. The culture density remained approximately at the same level for 5 days under the shifted conditions, with a subsequent decrease in algae cell concentration of about 15% by the end of the experiment. The change in the intracellular content of chlorophyll a and β-carotene had the opposite direction and was non-linear. The first three, days chlorophyll content in the cells rapidly decreased, and β-carotene content increased. In the following 9 days, the rate of β-carotene accumulation gradually decreased. At the same time, the intracellular Car/Chl ratio varied from 0.7 at the moment of the shift in growth conditions (the 11th day) to 21 on the 24th day of the experiment (Fig. 4).
After the change in growth conditions, the decrease in the Fm/D750 ratio, the Fv/Fm parameter, the FDAfl, and the percent of living cells in D. salina were recorded (Fig. 5). Thus, the Fm/D750 ratio decreased by about 5 times during exposure under new growth conditions. The value of the Fv/Fm parameter characterizing the efficiency of the algae photosynthetic apparatus decreased threefold in the first three days after the shift in growth conditions. Then, the parameter stabilized at 0.2, which indicates a significant suppression of cell photosynthetic activity due to nitrogen depletion in the medium and the inhibitory effect of high light intensity. The FDAfl value and the percent of viable cells in the culture remained high; the total decreases for the entire algae exposure under the new conditions were 50 and 30%, respectively.
The dynamics of the Fm/D750 ratio, the FDA fluorescence, the Fv/Fm parameter, and the percent of living cells in the culture of Dunaliella salina under conditions of intense carotenoid synthesis. The vertical dashed blue line indicates the moment of the shift in algae growth conditions (the 11th day of the experiment)
The data obtained from the results of measuring cell autofluorescence in the orange (FL2, 575 nm) and red (FL4, 675 nm) spectrum areas under various growth conditions allowed establishing several correlations between the pigment content in Dunaliella salina and the fluorescent and optical indicators of their cells. Specifically, a linear relationship was obtained between the Fm/D750 ratio and intracellular chlorophyll content in microalgae (Fig. 6a).
As shown, cell autofluorescence in D. salina is observed at 575 nm (FL2 channel), resulting from high carotenoids in the cells. The measurements of the indicator during the experiment made it possible to establish a linear relationship between the β-carotene content in the cells and their autofluorescence at 575 nm, with a high coefficient of determination (R2 = 0.91) (Fig. 6b). In turn, a linear relationship between the ratio of β-carotene to chlorophyll a and FL2/FL4 (ratio of autofluorescence in the orange and red spectrum areas) (see Fig. 6c) allows applying the FL2/FL4 ratio for monitoring carotenoid synthesis in D. salina and assessing the shifts in the relationship between β-carotene and chlorophyll in the cells during intense carotenoid synthesis.
Figure 7 shows the dynamics of the mean daily increase in β-carotene in the suspension during the experiment and the corresponding shift in the mean daily increase in FL2/FL4. Thus, the value of the mean daily increase in FL2/FL4 (2.5) obtained on the 20th day of the experiment characterizes the final stage of exposure; after that, D. salina cultivation is economically irrational.
Discussion
In this work, we studied β-carotene accumulation in halotolerant green microalga D. salina in two cultivation modes. In the first one, algae were cultivated in a cumulative mode with four combinations of light intensity and nitrogen content in the medium. It was found as follows: the maximum value of carotene accumulation is observed when high light intensity (1200 μE·m−2·s−1) is coupled with mineral nitrogen deficiency. To date, it is a well-known fact (Ben-Amotz and Avron 1983; Lamers et al. 2010; Dhanam and Dhandayuthapani 2013). Under such cultivation conditions, a high relative rate of carotenoid synthesis (more than 400%) was recorded in the first 3 days. A rapid slowdown in algae growth rate and the relative rate of pigment accumulation was registered, although its intracellular concentration continued to increase. In the second mode, the two-stage cultivation method was applied. At the first stage, growth conditions were created, contributing to the maximum accumulation of microalgal biomass. At the second stage, those initiating β-carotene accumulation in the cells were created. Currently, this method is used in biotechnology for D. salina mass cultivation (Prieto et al. 2011; Sui et al. 2019; Borovkov et al. 2020). Table 2 compares the values of mean daily increase in β-carotene for two cultivation modes on the third, fifth, and seventh days of the experiment. As can be seen from the presented data, the current mean daily increase in β-carotene with the two-stage cultivation method applied was higher throughout the experiment, and the final β-carotene content in the suspension was twice as high as that during cumulative cultivation.
In the presented study, the main goal was to be able to use cytometric and fluorescent indicators to assess the physiological state of D. salina and determine the content of pigments in cells. The cultivation of D. salina under conditions of high light intensity and nitrogen deficiency led to a sharp decrease in the efficiency of the algae photosynthetic apparatus (Fv/Fm) and a five-fold decrease in the value of the Fm/D750 ratio. Importantly, similar dynamics of Fv/Fm values were obtained in the publication (Fachet et al. 2016) when D. salina was cultivated under similar conditions. However, it differs from the result of the experiment on D. salina cumulative growth with no shift in light intensity, with its decrease in Fm/D750 about 30% and a decrease in the Fv/Fm value down to 0.3 only at the late stages of the stationary state of the culture. Apparently, the sensitivity of photosynthetic apparatus to light intensity rises with nitrogen deficiency (Han et al. 2000; Shoman 2015). As mentioned above, the FDAfl value and the ratio of viable cells remained high. Earlier, on several algae species representing different taxonomic groups, we showed that shifts in light intensity, temperature, and mineral nutrition (in the range of values not leading to cell death) practically do not affect the value of FDAfl and the number of viable cells in the population (Solomonova et al. 2018; Solomonova and Akimov 2021; Shoman et al. 2021). The applicability of FDA fluorescence to assess algae viability was shown in the publications (Brookes et al. 2000; Onji et al. 2000; Peperzak and Brussaard 2011). The obtained result can help in biotechnology when the accumulation of biologically valuable substances is stimulated in algae cells in the zone of extreme effect of the factor on the cultivation object.
To date, extensive experimental material has been accumulated estimating the relationship between intracellular chlorophyll content and its autofluorescence at 680 nm (Sosik et al. 1989; Hyka et al. 2013; Alvarez et al. 2017; Olson et al. 2018). There are few publications considering the calculation of intracellular carotenoid content by fluorescence. Different carotenoid forms emit fluorescence at certain wavelengths within 530–580 nm. As shown (Ukibe et al. 2008; Rioboo et al. 2011), fluorescence at 570–575 nm is caused by astaxanthin emission. The literature data on the wavelength at which β-carotene extinction is recorded are contradictory. According to the study (Kleinegris et al. 2010), the peak of β-carotene emission in D. salina was registered at 560 nm; the presence of green fluorescence was also noted, which can be used in fluorescence microscopy to obtain images of the cells and the carotenoid content in them (Lee et al. 2012); the FL1 channel (529 nm) alone was used to analyze pigment content in Symbiodinium. In Chen et al. (2017), for real-time monitoring of carotenogenesis in the cells of the green microalga Chromochloris zofingiensis, it was proposed to use both FL2 (585 nm) and FL1 (533 nm) fluorescence since the indicators linearly correlated with carotenoid content in microalgae with a determination coefficient above 0.8. However, to evaluate astaxanthin synthesis in C. zofingiensis, a FL2 fluorescence indicator was used in the work (Mendoza et al. 2008); a slightly different approach was proposed for assessing carotenoid content in D. salina cells by applying cytometric indicators. As shown by the authors, the shifts in D. salina lipid composition can be detected using the Nile Red (NR) lipophilic stain. Considering the fact that the intracellular content of lipids and carotenoids is interdependent, lipid staining with NR can be used as an indirect method for determining carotenoid content in the cells. The authors were unable to establish a relationship between intracellular carotenoid content and carotenoid fluorescence in the orange spectrum area directly due to low values of the latter. The results of our work showed the presence of pronounced carotenoid autofluorescence at 575 nm (FL2 channel). It allowed us to obtain correlations between the values of FL2 fluorescence and β-carotene content, as well as between the FL2/FL4 and intracellular ratio of β-carotene to chlorophyll a in the D. salina strain studied.
Conclusion
The Dunaliella salina strain IBSS-1’s maximal carotenoid synthesis rate was seen in conditions of high light intensity, high salinity, low temperature, and nitrogen deficit in the medium. With the initial culture of algae under conditions that promote maximum biomass accumulation and a subsequent switch to settings that promote carotenogenesis, the mean daily increase in β-carotene and its ultimate amount in the suspension is larger than with cumulative cultivation. In the latter instance, a rapid decline in algal growth rate at relatively low culture densities is the primary factor limiting pigment accumulation. Nitrogen deficit in the medium had a less impact on D. salina’s ability to accumulate β-carotene in any of the culture modes than did light intensity. The study’s findings revealed the following: Algal photosynthetic apparatus is impacted by changes in growth circumstances that promote carotenoid accumulation, and weaker effects are seen on cell viability and enzyme function. During the stationary growth phase, FDA fluorescence and the percentage of viable cells in the culture gradually decline, but the dynamics of these indicators do not fundamentally change as a result of the change in abiotic growth circumstances not causing algal death. In light of this, FDA fluorescence and the quantity of enzyme-active cells in the culture can be used to gauge the severity of an external factor’s lethal impact on the physiological state of microalgae; however, they are inapplicable as precise indicators in the course of intense β-carotene synthesis. The correlations between the Fm/D750 ratio and chlorophyll content, the FL2/FL4 ratio and the intracellular ratio of β-carotene to chlorophyll a, and the correlations between the FL2/FL4 ratio and β-carotene content allow for real-time access to pigment content and the prediction of β-carotene formation in D. salina.
Data availability
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Alvarez E, Nogueira E, Lopez-Urrutia A (2017) In vivo single-cell fluorescence and size scaling of phytoplankton chlorophyll content. Appl Environ Microbiol 83:e03317-e3416. https://doi.org/10.1128/AEM.03317-16
Andersen RA (2005) Algal culturing techniques. Elsevier Academic Press, New York
Antal TK, Venediktov PS, Matorin DN, Ostrowska M, Wozniak B, Rubin AB (2001) Measurement of phytoplankton photosynthesis rate using a pump-and-probe fluorometer. Oceanologia 43(3):291–313
Ben-Amotz A (1995) New mode of Dunaliella biotechnology: two-phase growth for β-carotene production. J Appl Phycol 7:65–68. https://doi.org/10.1007/BF00003552
Ben-Amotz A, Avron M (1983) On the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol 72:593–597. https://doi.org/10.1104/pp.72.3.593
Ben-Amotz A, Shaish A, Avron M (1989) Mode of action of the massively accumulated β-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol 91(3):1040–1043. https://doi.org/10.1104/pp.91.3.1040
Borovkov AB, Gudvilovich IN, Avsiyan AL (2020) Scale-up of Dunaliella salina cultivation: from strain selection to open ponds. J Appl Phycol 32(3):1545–1558. https://doi.org/10.1007/s10811-020-02104-5
Borowitzka MA (1990) The mass culture of Dunaliella salina. In: Technical resource papers regional workshop on the culture and utilization of Seaweads, vol. 2. http://www.fao.org/3/ab728e/. Accessed 16 Feb 2020
Borowitzka MA (2018) The ‘stress’ concept in microalgal biology—homeostasis, acclimation and adaptation. J Appl Phycol 30:2815–2825. https://doi.org/10.1007/s10811-018-1399-0
Brookes JD, Geary SM, Ganf GG, Burch MD (2000) Use of FDA and flow cytometry to assess metabolic activity as an indicator of nutrient status in phytoplankton. Mar Freshw Res 51:817–823. https://doi.org/10.1071/MF00048
Chen J, Wei D, Pohnert G (2017) Rapid estimation of astaxanthin and the carotenoid-to-chlorophyll ratio in the green microalga Chromochloris zofingiensis using flow cytometry. Mar Drugs 15(7):231–254. https://doi.org/10.3390/md15070231
d’Alessandro S, Havaux M (2019) Sensing β-carotene oxidation in photosystem II to master plant stress tolerance. New Phytol 223(4):1776–1783. https://doi.org/10.1111/nph.15924
Dhanam DS, Dhandayuthapani K (2013) Optimization of β-carotene production by marine microalga - Dunaliella salina. Int J Curr Microbiol Appl Sci 2:37–43
Fachet M, Hermsdorf D, Rihko-Struckmann L, Sundmacher K (2016) Flow cytometry enables dynamic tracking of algal stress response: a case study using carotenogenesis in Dunaliella salina. Algal Res 13:227–234. https://doi.org/10.1016/j.algal.2015.11.014
Falkowski PG, Kolber Z (1995) Variations in chlorophyll fluorescence yields in phytoplankton in the world oceans. Funct Plant Biol 22(2):341–355. https://doi.org/10.1071/PP9950341
García-González M, Moreno J, Cañavate JP, Anguis V, Prieto A, Manzano C, Florencio FJ, Guerrero MG (2003) Conditions for open-air outdoor culture of Dunaliella salina in southern Spain. J Appl Phycol 15:177–184. https://doi.org/10.1023/A:1023892520443
Han P, Virtanen M, Koponen J, Straskraba M (2000) Effect of photoinhibition on algal photosynthesis: a dynamic model. J Plankton Res 22(5):865–885. https://doi.org/10.1093/PLANKT/22.5.865
Hyka P, Lickova S, Přibyl P, Melzoch K, Kovar K (2013) Flow cytometry for the development of biotechnological processes with microalgal. Biotechnol Adv 31(1):2–16. https://doi.org/10.1016/j.biotechadv.2012.04.007
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194. https://doi.org/10.1016/S0015-3796(17)30778-3
Jimenez C, Pick U (1994) Differential stereoisomer compositions of β, β-carotene in thylakoids and in pigment globules in Dunaliella. J Plant Physiol 143(3):257–263. https://doi.org/10.1016/S0176-1617(11)81628-7
Kleinegris DM, van Es MA, Janssen M, Brandenburg WA, Wijffels RH (2010) Carotenoid fluorescence in Dunaliella salina. J Appl Phycol 22(5):645–649. https://doi.org/10.1007/s10811-010-9505-y
Lamers PP, Van de Laak CCW, Kaasenbrood PS, Lorier J, Janssen M, De Vos RC, Bino RJ, Wijffels RH (2010) Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol Bioeng 106:638–648. https://doi.org/10.1002/bit.22725
Lee CS, Yeo YSW, Sin TM (2012) Bleaching response of Symbiodinium (zooxanthellae): determination by flow cytometry. Cytom Part A 81(10):888–895. https://doi.org/10.1002/cyto.a.22111
Lers A, Biener Y, Zamir A (1990) Photoinduction of massive β-carotene accumulation by the alga Dunaliella bardawil: kinetics and dependence on gene activation. Plant Physiol 93(2):389–395. https://doi.org/10.1104/pp.93.2.389
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1(1):F4.3.1-F4.3.8. https://doi.org/10.1002/0471142913.faf0403s01
Mendoza H, De la Jara A, Freijanes K, Carmona L, Ramos AA, de Sousa DV, Serafim Varela JC (2008) Characterization of Dunaliella salina strains by flow cytometry: a new approach to select carotenoid hyperproducing strains. Electron J Biotechnol 11:1–6. https://doi.org/10.4067/S0717-34582008000400005
Olson RJ, Zettler ER, DuRand MD (2018) Phytoplankton analysis using flow cytometry. Handbook of methods in aquatic microbial ecology, 1st edn. CRC Press, pp 175–186
Onji M, Sawabe T, Ezura Y (2000) An evaluation of viable staining dyes suitable for marine phytoplankton. Bull Fac Fish Mie Univ 51:151–158
Peperzak L, Brussaard CPD (2011) Flow cytometric applicability of fluorescent vitality probes on phytoplankton. J Phycol 47:692–702. https://doi.org/10.1111/j.1529-8817.2011.00991.x
Pisal DS, Lele SS (2005) Carotenoid production from microalga, Dunaliella salina. Indian J Biotechnol 4:476–483
Pogosyan SI, Matorin DN (2005) Variability in the condition of the photosynthetic system of the Black Sea phytoplankton. Oceanology 45:139–148
Polle JE, Jin E, Ben-Amotz A (2020) The alga Dunaliella revisited: looking back and moving forward with model and production organisms. Algal Res 49:1–5. https://doi.org/10.1016/j.algal.2020.101948
Prieto A, Cañavatea JP, García-González M (2011) Assessment of carotenoid production by Dunaliella salina in different culture systems and operation regimes. J Biotechnol 151:180–185
Richmond A (2000) Microalgal biotechnology at the turn of the millennium: a personal view. J Appl Phycol 12:441–451. https://doi.org/10.1023/A:1008123131307
Rioboo C, González-Barreiro Ó, Abalde J, Cid Á (2011) Flow cytometric analysis of the encystment process induced by paraquat exposure in Haematococcus pluvialis (Chlorophyceae). Eur J Phycol 46:89–97. https://doi.org/10.1080/09670262.2011.561775
Shoman NY (2015) The dynamics of the intracellular contents of carbon, nitrogen, and chlorophyll a under conditions of batch growth of the diatom Phaeodactylum tricornutum (Bohlin, 1897) at different light intensities. Russ J Mar Biol 41:356–362. https://doi.org/10.1134/S1063074015050132
Shoman NYU, Akimov AI (2013) Effect of irradiance and temperature on specific growth rate of diatoms Phaeodactulum tricornutum and Nitzschia sp. No 3. Morskoj Ehkologicheskij Zhurnal 12:85–91 (In Russian)
Shoman N, Solomonova E, Akimov A (2021) Application of structural, functional, fluorescent, and cytometric indicators for assessing physiological state of marine diatoms under different light growth conditions. Turk J Bot 45:511–521. https://doi.org/10.3906/bot-2102-39
Solovchenko AE (2013) Physiology and adaptive significance of secondary carotenogenesis in green microalgae. Russ J Plant Physiol 60:1–13. https://doi.org/10.1134/S1021443713010081
Solomonova ES, Akimov AI (2013) The assessment of functional status of Chlorella vulgaris suboblonga by flow cytometry and variable fluorescence. Morskoj Ehkologicheskij Zhurnal 1:73–81 (In Russian)
Solomonova ES, Akimov AI (2021) Assessing the physiological state of microalgal using cytometric and fluorescent indicators. Russ J Plant Physiol 68:981–987. https://doi.org/10.1134/S1021443721050204
Solomonova ES, Mykhanov VS (2011) Flow cytometry for the assessment of physiological active cells in batch cultures of Phaeodactylum tricornutum and Nitzschia specia. Morskoj Ehkologicheskij Zhurnal 10:67–72 (In Russian)
Solomonova ES, Akimov AI, Shoman NYu (2018) Investigation of applicability of relative variable chlorophyll fluorescence and diacetate fluorescein staining for estimation and control of the state of algae culture on the example Phaeodactylum tricornutum (Phaeodactylaceae). Botanicheskij Zhurnal 103:1177–1191. https://doi.org/10.7868/S0006813618090089. (In Russian)
Sosik HM, Chisholm SW, Olson RJ (1989) Chlorophyll fluorescence from single cells: interpretation of flow cytometric signals. Limnol Oceanogr 8:1749–1761. https://doi.org/10.4319/lo.1989.34.8.1749
Sui Y, Muys M, Van de Waal DB, D’Adamo S, Vermeir P, Fernandes TV, Vlaeminck SE (2019) Enhancement of co-production of nutritional protein and carotenoids in Dunaliella salina using a two-phase cultivation assisted by nitrogen level and light intensity. Bioresour Technol 287:121398. https://doi.org/10.1016/j.biortech.2019.121398
Ukibe K, Katsuragi T, Tani Y, Takagi H (2008) Efficient screening for astaxanthin-overproducing mutants of the yeast Xanthophyllomyces dendrorhous by flow cytometry. FEMS Microbiol Lett 286:241–248. https://doi.org/10.1111/j.1574-6968.2008.01278.x
Zhu C, Zhai X, Jia J, Wang J, Han D, Li Y, Yajie T, Chi Z (2018) Seawater desalination concentrate for cultivation of Dunaliella salina with floating photobioreactor to produce β-carotene. Algal Res 35:319–324. https://doi.org/10.1016/j.algal.2018.08.035
Funding
This work was supported by the Russian Foundation for Basic Research (grant number 16–34-00388) and A.O. Kovalevsky Institute of Biology of the Southern Seas of RAS (state project № 121041400077–1 “Functional, metabolic and toxicological aspects of aquatic organisms existence and their populations in habitats with different physical and chemical regime”).
Author information
Authors and Affiliations
Contributions
AA, formal analysis, investigation, and writing—review and editing. ES, methodology, investigation, formal analysis, and writing—original draft. NS, investigation, formal analysis, and writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
The present study does not need an ethical approval as the studied organism is microalgae.
Consent for publication
All authors mutually agreed to publish the work in this journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Ronan Sulpice
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akimov, A.I., Solomonova, E.S. & Shoman, N.Y. Estimation physiological state and carotenoid content of Dunaliella salina (Teod.) using flow cytometry and variable fluorescence methods. Aquacult Int 32, 161–174 (2024). https://doi.org/10.1007/s10499-023-01153-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01153-0