Abstract
Polyhydroxybutyrate (PHB) is an attractive substitute for petrochemical plastic due to its similar properties, biocompatibility, and biodegradability. The cost of scaled-up PHB production inhibits its widespread usage. Intensive researches are growing to reduce costs and improve thermomechanical, physical, and processing properties of this green biopolymer. Among cheap substrates which are used for reducing total cost of PHB production, some C1 carbon sources, e.g., methane, methanol, and CO2 have received a great deal of attention due to their serious role in greenhouse problem. This article reviews the fundamentals of strategies for reducing PHA production and moves on to the applications of several cheap substrates with a special emphasis on methane, methanol, and CO2. Also, some explanation for involved microorganisms including the hydrogen-oxidizing bacteria and methanotrophs, their history, culture condition, and nutritional requirements are given. After description of some important strains among the hydrogen-oxidizing and methanotrophic producers of PHB, the article is focused on limitations, threats, and opportunities for application and their future trends.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyhydroxyalkanoates (PHAs) are the biopolymers which accumulate as a carbon/energy or reducing power storage material in various microorganisms usually when there is a growth-limiting component, such as O, N, P, S, or trace elements, e.g., Mg, Ca, and Fe in the presence of excess carbon source (Anderson and Dawes 1990; Doi 1990; Brandi et al. 1990; Braunegg et al. 1998; Lee et al. 1999; Sudesh and Doi 2000; Kessler and Witholt 2001, Zinn 2001; Chanprateep 2010; Kunasundari and Sudesh 2011; Ramadas et al. 2010; Morgan-Sagastume et al. 2010). In some Bacillus spp., it supplies energy for sporulation (Slepecky and Law 1961). The low molecular weight polyhydroxybutyrate (PHB) is a part of bacterial Ca2+ channels (Lara and Huisman 1999).
Among the various biodegradable polymers, PHAs are attractive substitutes for conventional petrochemical plastics due to their similar properties to common thermoplastics, biocompatibility, and biodegradability under various environments (Lee 1996; Steinbüchel and Füchtenbusch 1998). The use of PHA in a wild range of applications has been hampered mainly by their high production cost (Choi and Lee 1997; Ackermann and Babel 1998; Bormann et al. 1998; Choi and Lee 1999b; Du et al. 2001; Hori et al. 2002; Povolo and Casella 2003; Du et al. 2004; Wang and Yu 2007; Van-Thuoc et al. 2008; Papaneophytou et al. 2009; Chanprateep 2010; Ibrahim and Steinbüchel 2010; Kozhevnikov et al. 2010; Povolo 2010; Povolo et al. 2010; Budde et al. 2011).
More than 150 different monomers can be combined within PHA family to give materials with extremely different properties (Doi and Steinbüchel 2002). The mechanical and biocompatibility of PHA can also be changed by blending, modifying the surface, or combining PHA with other polymers, enzymes, and inorganic materials, making it possible for a wider range of applications. PHA synthases which are key enzymes of PHA production use the coenzyme A-thioester of (r)-hydroxy fatty acids as substrates. The two classes of PHA synthases differ in the specific use of hydroxyfattyacids of short- (SCL) or medium-chain length (MCL). So, three types of SCL, MCL, and long-chain length (LCL) PHA may result from hydroxy fatty acids including 3–5, 6–14, and more than 15 carbon atoms, respectively. SCL-PHA are synthesized by numerous bacteria, including Ralstonia eutropha and Alcaligenes latus (PHB), and MCL-PHA can be made for example, by fluorescent Pseudomonas like Pseudomonas putida. A few bacteria, including Aeromonas hydrophila and Thiococcus pfennigii, synthesize copolyester, from the SCL- and MCL-PHA.
Copolymer of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) is less stiff and tougher, and it may be used as a packaging material. Depending on the monomer composition, PHAs properties can range from thermoplastic to elastomeric materials. Increase in the ratio of 3-hydroxybutanoic acid to 3-hydroxypentanoic acid results in an increase in melting point, water permeability, glass transition temperature and tensile strength. However, impact resistance is reduced (Rudnik 2008; Pilla 2011). Recently, the physicochemical properties of PHAs produced from various carbon sources have been reviewed (Du et al. 2012).
Also, functionalized PHAs have been ductile and showed signs of side-chain crosslinking, resulting in reduced degrees of crystallinity. Incorporation of special monomer into the polymeric chains produced desirable thermal properties with enhanced thermal stability and reduced melting temperatures (Höfer et al. 2011). Hazer et al (2012) summarize the modification reactions, which include functionalization and grafting reactions, to improve the mechanical, thermal, and hydrophilic properties of PHAs.
PHB is the first discovered PHA and also the most widely studied and best characterized one. It is accumulated inside a membrane enclosed inclusion in many bacteria at up to 80 % of the dry cell weight. PHB has mechanical properties very similar to conventional plastics like polypropylene or polyethylene. Although PHB can be extruded, molded, spun into fibers, made into films, and used to make heteropolymer, but typically, SCL-PHAs like PHB are highly crystalline and brittle with poor elastic properties. Whereas, LCL-PHA are more ductile and easier to mold (Kabilan et al. 2012).
The fermentation performances, high cell density, carbon substrate, metabolic and genetic engineering, as well as process optimization, modeling, and recovery methods affect the total cost of PHB (Heinzle and Lafferty 1980; Mulchandani et al. 1989; Haywood et al. 1990; Yoo and Kim 1994; Yamane et al. 1996b; Ryu et al. 1997; Raje and Srivastava 1998; Grothe et al. 1999; Tohyama et al. 2002; Lu et al. 2003; Steinbüchel and Lutke-Eversloh 2003; Reddy et al. 2003; Patwardhan and Srivastava 2004; Khanna and Srivastava 2005a, b, c; Koller et al. 2008; Pantazaki et al. 2009; Papaneophytou et al. 2009).
Also, recombinant organisms (Choi and Lee 1999a; Park et al. 2001; Hofer et al. 2011) as well as co-culture systems have been developed for increased polymer production (Ganduri et al. 2005; Patnaik 2005; Lemos et al. 2006; Jing and Jiaying 2011). The utilization of cheap substrates and the development of different fermentation strategies for PHA production (Khosravi-Darani and Vasheghani-Farahani 2005a, 2005b; Akaraonye et al. 2010) as well as opportunities and threats for their competition in the global market have recently been addressed (Chanprateep 2010). We have also studied on reducing these costs by modeling (Shah-Hosseini et al. 2003), proper experimental design (Khosravi-Darani et al. 2003a, 2004b), and development of a new recovery method (Khosravi-Darani et al. 2003b, 2004a; Khosravi-Darani and Vasheghani-Farahani 2005b).
The cost of the carbon source is approximately 40 % of the total operating cost (Yamane 1993; Choi and Lee 1997; Halami 2008). The various cheap carbon sources have been used for PHB production include whey (Wong and Lee 1998; Kim 2000; Koller et al. 2005; Nikel et al. 2006; Koller et al. 2011; Di Donato et al. 2009; López-Cuellar et al. 2011a), wheat bran (Van-Thuoc et al. 2008; Ramadas et al. 2009) and rice bran (Huang et al. 2006), corn steep liquor (Gouda et al. 2001) as well as starch (Quillaguamán et al. 2005; Chen et al. 2006), molasses (Gouda et al. 2001; Omar et al. 2011; Solaiman et al. 2006; Albuquerque et al. 2007; Santimano et al. 2009), waste water from olive mills and starch (Choi and Lee 1999b; Yan et al. 2006; Bengtsson et al. 2007), waste glycerol (João et al. 2009; Dobroth et al. 2011), waste of vegetable (Fukui and Doi 1998; Ribera et al. 2001; Bhubalan et al. 2008; Simon-Colin et al. 2008), sweet sorghum (Kaewkannetra et al. 2008), enzyme hydrolyzate of potato starch, sesame oil cake, groundnut oil cake, cassava powder, jackfruit seed powder and corn flour (Ramadas et al. 2009), waste of potato starch (Haas et al. 2008), canola oil (López-Cuellar et al. 2011b), and waste oil (Wong et al. 2000). Cyanobacteria as the sole photoautotrophs accumulating PHB visualize the desired perspective for reduction of the cost incurred for expensive carbon source and oxygen supply in commercial production of PHB through bacterial fermentation.
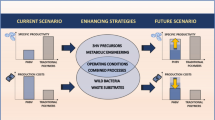
This review has been focused on PHB production from some C1 carbon sources like methane, methanol and CO2 as low cost substrates which all are serious pollutants in atmosphere of earth).
PHB production from CO2 by hydrogen-oxidizing bacteria
Main characteristic of hydrogen-oxidizing bacteria or Knallgas bacteria is ability of utilization of H2 as electron donor and O2 as electron acceptor to fix CO2 into cell materials via ribulose biphosphate or reverse tricarboxilic cycle (Aragno and Schlegel 1992). R. eutropha is one of the most famous PHB producers of hydrogen-oxidizing bacteria. This bacterium has gone through a series of name changes. At first it was named Hydrogenomonas eutrophus due to its ability to utilize hydrogen. Then it was renamed Alcaligenes eutropha because of its ability for degeneration peritrichous flagellation. Then its name was changed to R. eutropha due to lipid and fatty acid composition as well as 16S rRNA analysis and pheynotype of microbe. The new genus Wautersia was created from one of two phenotypically distinct clusters of R. eutropha. In turn, R. eutropha was renamed Wautersia eutropha. Looking at DNA–DNA hybridization and phyenotype comparison with Cupridavidus necator, W. eutropha was found to be the same species as the previously described C. necator. C. necator was named in 1987 far before all other name; it was assigned to R. eutropha according to Rule 23a of the International Code of Nomenclature of Bacteria (Vandamme and Coenye 2004).
Hydrogen-oxidizing organisms often inhabit oxic–anoxic interfaces in nature where O2 and H2 are supplied. Anaerobic organisms are well known as one of the supplier of O2 and H2. The culture of hydrogen-oxidizing bacteria is a matter worth considering as a possible tool for consumption of CO2. Initial research for growth characteristics and culture method in autotrophic condition of hydrogen-oxidizing bacteria was studied by many researchers (Foster and Litchfield 1964; Repask 1966; Ammann et al. 1968; Schink and Schlegel 1978; Malik and Schlegel 1980; Pinkwart et al. 1983). After their works, many types of the bacteria have been found in several genera and species. Many studies for the autotrophic bacteria have also been reported on taxonomy, physiology, ecology, metabolism, and genetics. However there have not been so many reports about culture technology for autotrophic growth and industrial usage of the bacteria because insoluble and explosive gaseous substrate, H2 is used as the substrate. Two types of closed culture systems are reported for increasing product yield for PHB production, dead-end (Bongers 1970) and recycled gas culture (Schlegel et al. 1961; Kodama et al. 1975). The dead-end culture system has disadvantages in gas mass transfer due to its lack of aeration. The recycled gas closed circuit culture (RGCCC) system allows many other benefits, such as reducing loss of substrate gas, operation safety for detonation, and continuous process (Ishizaki and Tanaka 1990, 1991; Ishizaki et al. 2001; Nishihara et al. 1991; Takeshita and Ishizaki 1996; Takeshita et al. 1993a, b; Tanaka and Ishizaki 1994; Tanaka et a. 1993; Hayashi et al. 1994; Sugimotoet al. 1999; Bae et al. 2001). In their works, theory, techniques, stoichiometry, and practical fermentation system for PHB production from CO2 by autotrophic culture of C. necator were also investigated. The fermentation technology for this microorganism with industrial aspects was also studied to solve many difficulties like detonation and low efficiency of gas usage due to flow out of exhaust gas from fermenter by using an explosion-proof-type fermentation system (Tanaka et al. 1993, 1995). They achieved also a high cell density cultivation of C. necator with production of 91.3 g/L cells and 61.9 g/L PHB in this autotrophic condition using an agitator with very high k L a in the system. Addition of carboxymethyl cellulose to medium in two stage chemolithoautotroph culture of C. necator was effective to increase mass transfer coefficient in RGCCC in an air lift type fermentor (Taga et al. 1997). Khosravi-Darani et al. (2006) also has also investigated the bioengineering aspects of RGCCC for industrial application to the culture of hydrogen-oxidizing bacteria as PHB producer from cheap, available, and frequent gas substrate. Their studies on the showed that high concentration of oxygen suppressed the specific growth rate of C. necator while limited concentration of gas can promote it. Recently, the cell growth and PHB accumulation in autotrophic batch culture of a newly isolated hydrogen-oxidizing bacterium Ideonella spp. strain O-1 in the presence of CO has been reported (Tanaka et al. 2011). This bacterium can grow in high O2 concentration of 30 % (v/v), while the growth of C. necator and A. latus was seriously inhibited. In culture medium containing 1 g/L (NH4)2SO4, PHB, and biomass concentration reached to 5.26 and 6.75 g/L, respectively, while PHB content of increased to 77.9 % (w/w) in dried cell. The strain O-1 was also grew in the present of 70 % (v/v) CO, while the growth C. necator and A. latus were seriously inhibited at 5 % (v/v) CO. Such a CO-resistant hydrogen-oxidizing bacterium is expected to useful for PHB production using directly the industrial exhaust gas containing CO2, H2, and also as the substrate.
C. necator B5786 as a CO-resistant strain of the hydrogen bacteria was also able to grow on gas mixtures containing CO at 5–25 % (v/v), and accumulate up to 70–75 % (w/w) PHA. No suppressed activity of the key enzymes of PHA synthesis, i.e., acetoacetyl-CoA-reductase, beta-ketothiolase, poly-3-hydroxybutyrate synthase, and butyrate dehydrogenase was reported. Also, no significant difference in crystallinity, molecular weight, and temperature characteristics have been shown compared with polymer produced on electrolytic hydrogen (Volova et al. 2002). In culture of this bacterium, kinetic indices of growth, PHA accumulation, and gas exchange have also been investigated on a gaseous substrate obtained by lignite gasification. Various strategies of gas supply to the culture were studied to increase the gas consumption. In controlled condition, high polymer yields of 75 % and substrate utilization of up to 90 % was achieved (Volova et al. 2002).
PHB production from methane
Methane is abundantly available in many oilfields and also can be produced in the biological degradation of organic matter by methanogenes. Approximately 50 % of the total organic carbon which can be degraded by anaerobic microorganisms is converted to methane (Asenjo and Suk 1986). Due to prohibitive cost of storage and transport, this valuable gas is simply flared or released to the atmosphere. In 2009, the USA released about 15 billion pounds as methane to the atmosphere (US Environmental Protection Agency 2011). Production of PHB from waste methane may sequester a potent greenhouse effect gas. Listewnik et al (2007) estimated a price of 6.35 UK pound/kg for produced PHB from natural gas in a two-stage plant producing 500 t PHB/year. Extrapolation for downstream procedures resulted in that the prices will be 11.50–14.00 UK pound/kg. Their estimations showed that a scaled up process of 5,000 t/year may cause a price reduction of approximately 30–35 %. It is interesting that PHB biodegradation in controlled anaerobic environments also leads to methane production (Morse et al. 2011). Some reported methylotrophs which are recognized as PHB producers from CO2, methane, and methanol as well as the content, yield, and productivity have been summarized in Table 1.
Microorganism and metabolic pathway
Methanotrophic bacteria are able to use oxidize methane as a sole carbon and energy source. (Whittenbury et al. 1970). Methanotrophs as a subset of the methylotrophs can grow on C1 compounds (Lidstrom 2006) and may be found in different natural ecosystems, bioreactors, gas pipelines, as well as soil near leaky gas pipes and water-treatment systems (Vecherskaya et al. 2001). Their great potential in environmental biotechnology are (1) methane escaping from atmosphere or soil, (2) co-metabolizing of contaminants, such as chlorinated hydrocarbons in the presence of methane by microorganisms, e.g., Methylocystis spp. GB 14, and (3) accumulation of PHB. Figures 1 (Van Dien and Lidstrom 2002) and 2 (Yaman 1993) show ribulose monophosphate (RuMP) and serine pathways for production of PHB from methane and methanol. Methanotrophs have been classified into two groups of types I and II; on the basis of their primary carbon assimilation pathway, the RuMP and serine pathway, respectively, different membrane arrangements, and the ability to fix molecular nitrogen (Bowman et al. 1993). Type X methanotrophs, a subset of type I methanotrophs use the RuMP pathway and also possess low levels of serine-pathway enzymes (Hanson and Hanson 1996). The recently discovered acidiphilic Verrucomicrobia methanotrophs of the genus Methylacidiphilum possess serine-pathway genes and apparently lack RuMP-pathway genes (Dunfield et al. 2007; Pol et al. 2007). Islam et al. (2008) has reported a methane oxidation at 55 °C and pH 2 by a thermo-acidophilic bacterium belonging to the Verrucomicrobia phylum. Also, Op den Camp et al. (2009) has studied environmental, genomic, and taxonomic perspectives of methanotrophic Verrucomicrobia.
Metabolism of methanol in M. extorquens AM1 (Van Dien and Lidstrom 2002)
Serine pathway for production of PHB from methane and methanol (Yamane 1993)
Both types have different survival strategies. Methanotrophs of group I are known by rapid growth under favorable condition and a rapid rate of die-off under stress conditions. While type II methanotrophs grow slower, survive better, and outcompete type I methanotrophs under oxygen- and nitrogen-limiting conditions. The literature contains conflicting evidence for PHB producers of methanotrophs. Both groups of methanotrophs can assimilate reduced C1 compounds and produce PHB via two pathways of RuMP and the serine pathway (Yamane et al 1996a). Type II bacteria which perform metabolization using the serine route, are more effective than type I bacteria (Wendlandt et al. 2001). To date, pure and mixed cultures of methanotrophs are used for PHB production. Most quantitative PHB production studies are reported from type II genera Methylocystis and Methylosinus, Methylococcus, and Methylomonas spp. (Asenjo and Suk 1986; Shah et al. 1996a, b; Wendlandt et al. 2001, 2005; Vecherskaya et al. 2001; Dedysh et al. 2004; Helm et al. 2006, 2008; Xin et al. 2007; Zhang et al. 2008; Pieja et al. 2011b). They and some other researchers (Bowman et al. 1993, 2001; Heyer et al. 2005) also reported also qualitative study for PHB production by type I methanotrophs. Anyway, the majority of PHB producers of methanotrophs type II are, e.g., Methylocystis paravus, Methylosinus trichosporium, Methylosinus sporium, Methylocystis spp. GB25, MTS, and Methylocella tundrae. Wendlandt et al. (1998, 2001) reported production of PHB with a very high molecular mass above 1 MDa.
At least three genes, phaCAB, are known crucial for PHB production (Madison and Huisman 1999) and have been used to screen for synthesis capacity (Sheu et al. 2000). As Fig. 3 shows that the route for the formation of PHB starts from acetyl-CoA molecules and proceeds via three distinct sequential, enzyme-mediated reactions as follows (Vincenzini and De Philippis 1999): (1) condensation of two molecules of acetyl-CoA to yield acetoacetyl-CoA by enzyme of the 3-ketothiolase; (2) reduction of acetoacetyl-CoA to d(-)-3-hydroxybutyryl-CoA catalyzed by enzyme of NADPH-dependent acetoacetyl-CoA reductase; and (3) polymerization of the 3-hydroxybutyrate to yield PHB by enzyme of PHA synthase. Of course the biosynthesis pathway for different genera and species of cyanobacteria may differ and further modifications on the pathway will be needed. For PHB production in Synechococcus sp. MA19, a hypothesized regulation has been reported (Asada et al. 1999). In case of hydrogen-oxidizing bacteria, CO2 fixation mechanism is mainly Calvin–Benson cycle or reductive TCA cycle (Ishii et al. 1997). In the former case, glyceraldehyde-3-phospahte in Calvin–Benson cycle and EMP is converted to pyruvate then it is changed to acetyl-CoA.
Metabolic pathway of PHB from acetyl-CoA-yielding substrate (Vincenzini et al. 1999)
PHB synthesis genes are not reported in type I methanotrophs or in Methylacidiphilum strains (Dunfield et al. 2007; Pol et al. 2007). PHB production has been investigated in obligate and facultative methanol-utilizing methylotrophs. Babel hypothesized that under unbalanced growth conditions, RuMP and serine pathways lead to production of PHB and exopolysaccharides, respectively (Babel 1992). In M. extorquens AM1 (Korotkova and Lidstrom 2001), linking of the PHB cycle to the serine cycle is reported. In another survey, in several RuMP pathway, methanol-utilizing methylotrophs (containing PHA-biosynthesis genes) (Follner et al. 1993), no PHB production was reported. While, transconjugants of these bacteria could synthesize PHB, suggesting that metabolic limitations do not prevent RuMP pathway. The level of copper in the growth media is one factor that may favor type II over type I methanotrophs due to their enzymatic requirements (Graham et al. 1993; Zahn and DiSpirito 1996; Nguyen et al. 1998; Dunfield et al. 2003; Lidstrom 2006). All type II and some type I genera are able to fix nitrogen; Oakley and Murrell 1988; Hanson and Hanson 1996; Auman et al. 2001; Bowman 2006), but type II methanotrophs may be more useful due to their faster growth on gaseous nitrogen (Murrell and Dalton 1983).
Graham et al. (1993) reported that the provision of N2 (gaseous) as the sole source of nitrogen favored the type II methanotroph, M. trichosporium OB3b, over Muscodor albus BG8. They showed that under copper- and nitrate-limited conditions, M. trichosporium OB3b was dominant. M. trichosporium synthesized soluble methane monooxygenase and nitrogenase under copper and nitrate limitation, respectively. M. albus predominated under methane limitation, especially during low-level feeding of methanol. The results imply that nitrogen limitation can be used to select for type II strains, such as M. trichosporium OB3b.
Nutrient concentration (Wise et al. 1999; Cebron et al. 2007) and medium pH affect type I methanotrophs, but type II are acidophilic and acid tolerant (Dedysh 2002). Low pH and high concentration of organic carbon source lead to high concentrations of dissolved CO2, which is a requirement for serine pathway and so favor for type II methanotrophs.
Pieja et al. (2011a) have investigated encoding for PHB synthase (phaC) as well as PHB production under nitrogen-limited conditions in types I and II proteobacterial methanotrophs. All type I and II strains were found as disable and able for both responses. Preferentially, medium conditions for PHB production in type II methanotrophs has been defined as low pH (4–5) medium or culture containing N2 (gaseous) or dilutes mineral salts in the absence of Cu.
Pieja et al (2011b) have also shown that methanotrophic PHB metabolism is linked to the supply of reducing power as opposed to the supply of C2 units for synthesis. They showed that type II methanotroph Methylocystis parvus OBBP did not replicate using stored PHB in the absence of methane, even in excess amount of other medium components. PHB was a source of reducing equivalents and not a sole growth substrate.
In another study, Pieja et al. (2012) also investigated on favor strategies for PHB accumulation in type II methanotrophic proteobacteria. So, three sequencing batch reactors inoculated by type II methanotrophs were subjected to (1) repeated nitrogen limitation, (2) repeated nitrogen and oxygen limitation, and (3) repeated nitrogen and methane limitation. PHB levels were measured over 11 cycles of 24 h. The results showed that repeated nitrogen and methane limitations favored PHB accumulation in strain OBBP and provided it with a competitive advantage under the conditions imposed.
Pfluger et al. (2011) have recently reported the feasibility of growing methanotrophic biomass in a fed batch reactor at ambient temperature and non-sterile conditions. Their results show that by increased concentration of nitrate and dissolved O2led to a type I methanotroph-dominated biofilm. While decreased O2 concentrations of 2 mg/L lead to increased growth of type II methanotrophic biofilms of PHB producer.
Stoichiometry
Biochemical pathways in type II methanotrophs have been analyzed firstly by Asenjo and Suk (1986) in order to establish the preliminary kinetic analysis, stoichiometry of PHB synthesis and determination of condition necessary for PHB accumulation from methane. The overall equation of methane to cell mass (C4H8O2N) for methanotrophs that use the serine pathway is found:
where FPH2 is reduced form of flavoproteins (FP) succinate dehydrogenase. Also, the overall equation obtained for cell mass biosynthesis from nitrate is:
The net equation for PHB synthesis is:
The overall equation for PHB accumulation for methanotrophs that use the serine cycle is found:
When the cells are synthesized using ammonia as a nitrogen source, 0.61 mol ATP/mol CH4 would be stored. Using nitrate, the value is 1.01 mol ATP/mol CH4 and for the synthesis of PHB from methane, 1.05 mol ATP/mol CH4 would be stored. In all the cases, the ratio of O2/CH4 metabolized is constant and equal to 1.5, whereas CO2 evaluation varies. Ueda et al (1992) suggested Eq. 5 for conversion of CH3OH to PHB in methylotrophic bacteria that accumulate PHB belong to the group having the serine pathway as methanol assimilatory pathway:
which q is moles of ATP and is assumed to be produced through the conversion of CH3OH to HCHO. However, when methane is used, the Eq. 6 is added to Eq. 5, and the result was different from that reported by Asenjo and Suk (1986). In fact production of methanol from methane will be primarily added to biosynthesis pathway of conversion of methanol to PHB.
Yield
One of the most important factors of PHB production is yield of substrate. Theoretically, yield can be predicted by a stoichiometric reaction scheme (Ueda et al. 1992; Anthony 1982). By using Eq. 4, theoretical yield for bioconversion of methane to PHB can be estimated to be 67 % (86/128 g).
In type II methanotrophic bacteria, however, a large fraction of the methane and oxygen consumed has to be converted to CO2 in order to generate the NADP+ of acetoacetyl-CoA reductase in the PHB biosynthetic pathway. By using Eqs. 5 and 6, it can be obtained that the theoretical yield for bioconversion of methane to PHB is 54 % without neglect of the regeneration of NADP+ of acetoacetyl-CoA reductase in the PHB biosynthetic pathway.
Empirical PHB yield was calculated on the basis of the PHB content of biomass and methane consumption by Wendlandt et al. (2001). Yield and productivity of PHB produced from methane by Methylocystis spp. GB250 SM (type II methanotrophs) were amounted to 0.54 g PHB g−1 CH4 and 2.85 g PHB L−1 h−1, respectively. This result is very close to theoretical yields reported by Asenjo and Suk (1986) and Ueda et al (1992).
Culture conditions
Many researchers performed cultivation of methanotrophs in a nonsterile manner. A mineral salt medium have been used in a series of researches. Also, controlling of gas composition of head space in culture vessel is also important. In fact, in controlled temperature, pH, and methane concentration, the process of PHB production can be conducted non-sterile due to kind of medium and establishment of a stable microbial community where the dominant species is Methylocystis spp. GB 25. Two-stage pilot-scale of PHB production from methane may lead to a maximum polymer content of 52 % of dried cell weight (Wendlant et al. 2010).
Asenjo and Suk (1986) conducted growth of cells in closed shake flask hermitically sealed with a rubber stopper. Two glass tubes were used to flow gas mixture of CH4/O2 50 % (v/v). Cultures were incubated in a shaker with 150–200 rpm at 30 °C. The culture maintained on plates using the same medium including 15 g/L agar and a sealed methane/oxygen chamber 50 % (v/v).
Wendlandt et al. (2001) investigated for increasing productivity and yield of PHB by a methanotrophic strain Methylocystis spp. GB 25 DSMZ 7674 in a 7- and 70-L pressure bioreactor. Cultivation was performed in two stages: a continuous growth phase at dilution rate 0.17 h−1 and a PHB accumulation phase under deficiency conditions of an essential nutrient, e.g., ammonium, P, or Mg in batch culture. In their research, the PHB content of biomass reached to 51 %; the maximum PHB yield (relative to consumption of methane) was 0.55 g g−1 with high molecular mass of 2.5 × 106 Da.
Zuniga et al. (2011) applied a two-phase partitioning bioreactor in limited nitrogen concentration to promote PHB production. Under this condition, the accumulated PHB in the reactor was 38 % (w/w) for isolated bacterium Methylobacterium organophilum and the highest productivity of 1.61 mg PHB g−1 h−1 was obtained with PHB content of 57 % (w/w).
Methanol
Methanol as one of the building blocks in the chemical industry can be used in bioprocess technology with methylotrophic bacteria for production of value added products. Schrader et al. (2009) have recently reviewed the potential of methylotrophs for the development of economically competitive biotechnological processes based on methanol as an alternative carbon source. Stoichiometric equation for biomass formation on methanol through the RuMP pathway is as Eq. 7, in which, carbon balance shows that 62 % of methanol is used for biomass formation:
Methanol also can be used as an alternative substrate for PHB production due to several advantages of low price, moderate requirement of oxygen, complete water miscibility, etc. (Byrom 1987). The maximum theoretical yield of PHB production from methanol is equal to 0.18 g/g in comparison to 0.33 g/g for glucose. In production of “Biopol” (PHA commercially produced by Monsanto Company), methanol was applied as carbon source. But low molecular weight polymer showed difficult extraction. More research showed that methylotrophs can produce high molecular weight PHB (Bourque et al. 1992, 1995) and even its copolymer, poly (3-hydroxybutyrate-3hydroxyvalerate), by addition of n-amyl alcohol or valeric acid while propionic acid was less effective (Ueda et al. 1992). However, 136 g/L PHB with 66 % of dry weight of the cells was achieved by Suzuki et al. (1986a) using computer-controlled fed-batch fermentation, which is the highest reported yield for any methylotroph. It can be suggested that genetic manipulation of flux control mechanisms may be necessary to match the yields currently achieved with C. necator. Methylobacterium spp. was the most famous strain for PHB production via the serine pathway (Fidler and Dennis 1992). This bacterium has a complete TCA cycle, unlike restricted facultative methylotrophs which apply the RuMP pathway. Growth-associated production of PHB in Methylobacterium rhodesianum (Ackermann and Babel 1997) and possess of two acetoacetyl-CoA reductases in M. rhodesianum MB 126 (Babel and Mothes 1994) has been reported. Suzuki et al. (1986a) examined eleven components and found NH4 +, SO4 2−, Mg2+, Fe2+, and Mn2+ ions are crucial deficient ions for PHB accumulation by Pseudomonas spp. K. For Methylobacterium organophilium, NH4 +, Mg2+, PO4 3−, and K+ were found to be crucial deficient ions for the stimulation of PHB accumulation (Choi et al. 1989). Daniel et al. (1992) has examined nutrient-deficient media on PHB accumulation by Pseudomonas 135. Among the several nutrient tested, it was found that PHB accumulation was most significantly stimulated by NH4 +, Mg2+, or PO4 3− deficient medium.
Several studies have been also carried out with various microorganisms and fermentation processes for production of PHB from methanol (Suzuki et al. 1986b, c, 1988, 2000; Byrom 1987; Choi et al. 1989; Haywood et al. 1989; Govorukhina and Trotsenko 1991; Hilger et al. 1991; Anderson et al. 1992; Daniel et al. 1992; Mothes et al. 1997, 1998; Zhao et al. 1993; Taidi et al. 1994; Yezza et al. 2006). Although in some of these studies, media optimization for PHB production from methanol by different methylotrophs were considered but in none of them the statistical design of experiment was applied for screening of all of media components. There are scattered and different concentrations for media components which have been reported in literature and the importance of minerals for maximal biomass and/or PHB production from methanol has been recognized by several investigators (Suzuki et al. 1986a; Choi et al. 1989; Hilger et al. 1991; Daniel et al. 1992; Bourque et al. 1992, 1995). The presence of trace elements is very necessary to achieve high concentration of cell mass (Suzuki et al. 2000). In addition, some of them may have accelerative effect on PHB accumulation. It is desirable to maintain the concentration of every elements at a suitable level at which the cell growth is not limited.
Suzuki et al. (1986a, b, c) studied the effect of type and concentration of nitrogen and phosphorus sources as well as seven trace elements on growth and PHB production of Protomonas extroquence spp. K. High concentration of PHB was attained in fed batch culture. Produced polymer showed a high and broad distribution of molecular weight. In another study, Suzuki et al. (1988) tested influence of process variables including temperature, pH, molar ratio of methanol to ammonia, and concentration of methanol on PHB production. They showed impact of methanol concentration on molecular weight of PHB. Daniel et al. (1992) obtained an optimal medium for growth of Pseudomonas 135 and reported similar results for nitrogen sources to Suzuki’s report (1986a). In their optimal medium, phosphate salts are more influential than phosphoric acid on cell growth. The optimum concentration of MgSO4 was found to be 0.45 g/L. However, increasing of trace elements concentration two fold did not show any significant improvement in cell growth. Bourque et al. (1995) also reported an optimum medium for growth of Methylobacterium extorquens ATCC 55366. They made several modification to medium 784 (ATCC 1985) reported by Choi et al. (1989). This medium is richer than medium 784 considering several composing salts but contains no NH4Cl, K2HPO4, MnCl2, CuCl2, or NiCl2. This medium contains less CaCl2 than medium784 in order to prevent significant precipitation problems. They also reported that concentration of trace elements has effect on cell growth in spite of Suzuki et al. (1986a) and Daniel’s (1992) opinion.
Mokhtari et al. (2009a) applied Plackett–Burmann design as a screening experimental plan for the first time to assess the relative importance of medium components including carbon and nitrogen sources, phosphate and minerals for maximal biomass and biopolymer production of M. extorquens DSMZ 1340 by methanol. Optimization of growth media by CCD resulted in the increased growth with an OD = 2.15 compared with an OD = 1.35 for Choi’s media as control. PHB production media was also optimized by RSM. It was found that a deficiency of MgSO4 and (NH4)2SO4 is crucial for PHB accumulation. Fed-batch cultivation at optimum condition resulted in PHB production as high as 62.3 % of DCW, which is higher than those reported in the literature for PHB production from methanol by Methylobacterium species. Also, fermentation was conducted with feed composition (639 g/L methanol, 4 g/L MgSO4·7H2O, 41 mL/L trace elements, 5.6 g/L NaH2PO4·H2O,and 24.3 g/L K2HPO4) in limited dissolved oxygen (Mokhtari et al. 2009b). After 35 h, PHB production phase was start and biomass and PHB productivities of 2.8 and 0.98 g L−1 h−1 were obtained, respectively.
PHB accumulation and growth of M. trichosporium IMV3011 on methanol has been investigated (Song et al. 2011; Xin et al. 2011). The carbon source concentration in fermenter is maintained at the lower level by a reasonable feeding strategy in order to reduce the inhibiting effect of substrate in the production of PHB as well as other types of products. The initial concentration of methanol was controlled at 1 g/L. Also, it was added at 0.1 % (v/v) of the total volume of medium in 5 times separately in the whole fermentation process, which would shorten the lag phase of growth. The concentration of cells dry weight reached at 2.91 g/L and the PHB concentration was 47.6 % in the fed-batch fermentation of IMV3011. By addition of 0.2 g/L malic acid as optimal organic acid for stimulation of intracellular PHB synthesis, the 3.32 g/L cells dry weight and 58.5 % PHB was obtained.
It had been shown that acetyl-CoA, citric acid, and malic acid caused an increase of PHB production as a key intermediate in the metabolism. The inhibition of TCA cycle increased PHB production of methanotrophic bacteria; 0.2 g/L malic acid was an optimal organic acid addition for intracellular PHB synthesis. The 3.32-g/L cells dry weight and 58.5 % PHB could be obtained under the condition. The corresponding change of M w would appear in various incubation stages of IMV3011 (Xin et al. 2011)
Cosubstrate of methane and methanol
Methanol can maintain methanotrophic activity in some conditions. Zhang et al. (2008) reported PHB production by methanotrophic strain M. trichosporium IMV3011 from methane and methanol in a brief non-sterile process. In this research, methanol at 0.1 % (v/v) was added to improve the oxidization of methane. The experiments were conducted in two stages cultivation for continuous growth phase and PHB accumulation (with limited concentration of NH4 +, NO3 −, P, Cu+2, Fe3+, Mg2+, or ethylenediamine tetraacetate in batch culture. In the most suitable condition, PHB concentration reached to 0.6 g/L. Addition of citric acid as an inhibitor of tricarboxylic acid cycle and simulator of PHB production was also investigated. This strategy caused an increased PHB yield from 12 to 40 % (w/w). The produced PHB had molecular weight up to 1.5 × 106 Da.
Conclusions
Due to high consumption of plastics and landscape problem in the world, government and industries of developed countries have intensified their efforts in the production and application of PHAs as biodegradable polymers roughly estimated at 50,000 tons/year. The intensive research are going to reduce costs and improve thermo-mechanical, physical and processing properties of these green biopolymers to allow them to become a leading biodegradable polymer in the near future.
Of course, at present, PHB has not been commercially produced from gas and the price will differ due to the resource from which is derived. Choi and Lee (1999a) studied on economical consideration of PHB production by various bacteria on some substrates. Table 2 shows the results of their economical evaluation for production of 100,000 ton PHB/year from methane and methanol in compare to glucose. Among the parameters influencing cost of PHB production, constant and current investments, upstream, and labor costs significantly increase with decreased productivity. Comparison of two processes with different productivities for PHB production by recombinant Escherichia coli shows the significant impact of productivity on final cost (Table 2). By increasing of productivity from 1.98 to 3.20 g L−1 h−1, production cost decreases from 5.37 to 4.91 US$/kg PHB. Low cost of methanol and energy in Iran (0.045 US$/kg), as well as high solubility of this gas and possibility of reaching high cell density may lead to decreased product cost. Table 2 shows cost estimation for PHB production from methanol by M. extroquens DSMZ 1340 which have been calculated base on global and national (Iran) cost of methanol and energy according to Choi and Lee (1999a). Applying a low-cost efficient process of separation will lead to a more decreased total cost of methylotrophic production of PHB.
Methane could be utilized as the carbon source of mixed culture to grow and produce PHB in a brief non-sterile process. Concentration of nutrient in the medium has great effect on biomass growth and synthesis of PHB. Mixed culture usage for the biotechnological production of PHB is an important step towards the development of a viable large-scale process for the production of PHB using cheap substrates like methane. Some inherent physicochemical properties such as brittleness and hydrophobicity can be improved by various methods, especially blending with another kind of polymer. An important prerequisite for bulk chemical production with methylotrophs will be a robust over expression of anaplerotic key enzymes together with a stable deactivation of oxidative pathways.
Also pulsing of nitrogen into a sequencing batch reactor with a sustained supply of methane has been known as a successful plausible strategy for stimulate the production and utilization of PHB. Further study is needed to examine the effects of repeated nitrogen limitation on methanotrophic cultures and the potential of such strategies to enhance PHB production.
Research on the genetics of biosynthesis of MCL-PHA has led to polymer synthesis in recombinant organisms. Several medium and high cost applications are emerging in new decade. A combination of more efficient metabolically engineered strains capable of utilizing cheap carbon sources and efficient fermentation strategies is certainly a future trend for increase deficiency of PHA production.
Cultivation of hydrogen-oxidizing bacteria from H2 (with sources of wind, natural gas, nuclear, and biomass) with average price of $2.6/gallons of gasoline equivalent, CO2 (liquid), and CH4 (22.0 and $2.169/m3) have also potential for economic production of PHB. Anyway if mentioned gas can be applied in situ as industrial output of a plant, the prices are very low beside great advance of solving greenhouse problem. Anyway, there are no bacteria that can produce copolymers from C1 compounds and only a few strains can produce them even from sugars as sole carbon source. We are studying for construction of recombinant of hydrogen-oxygen bacteria to produce MCL-PHAs from CO2.
Screening research also will aid to improve and select better candidates for increased biopolymer production. New isolated bacteria should be O2 and CO tolerant to be useful in the manufacture of MCL-PHA from industrial exhaust gas containing CO2, H2, and CO. Technology of autotrophic culture of hydrogen-oxidizing bacteria for production of PHB from CO2 has been progressed in the recent 20 years as described above however research and innovation is still necessary for practical application of autotrophic culture of the bacteria for the production of MCL-PHA in industrial scale. Structural studies are needed to improve understanding about the mechanism of action of involved enzymes in methanotrophs and hydrogen-oxidizing bacteria. Metabolic engineering thereafter promises to bring a feasible solution for the production of green plastic. Although some C1 substrates are used for PHB production with several kinds of microorganisms but comparison of yield and structure of produced PHB is yet unknown.
References
Ackermann JU, Babel W (1997) Growth-associated synthesis of poly (hydroxybutyric acid) in Methylobacterium rhodesianum as an expression of internal bottleneck. Appl Microbiol Biotechnol 47:144–149
Ackermann JU, Babel W (1998) Approaches to increase the economy of the PHB production. Polymer Degrad Stabil 59:183–186
Akaraonye E, Keshavarz T, Roy I (2010) Production of polyhydroxyalkanoates: the future green materials of choice (review). J Chem Technol Biotechnol 85:732–743
Albuquerque MGE, Eiro M, Torres C, Nunes BR, Reis MAM (2007) Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J Biotechnol 130:411–421
Ammann ECB, Reed L, Durichek JE (1968) Gas consumption and growth rate of Hydrogenomonas eutropha in continuous culture. Appl Microbiol 16:822–826
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Anderson AJ, Williams DR, Taidi B, Dawes EA, Ewing DF (1992) Studies on copolyester synthesis by Rhodoccocus ruber and factors influencing the molecular mass of polyhydroxybutyrate accumulated by Methylobacterium extorquens and Alcaligenes etrophus. FEMS Microbiol Rev 103:93–102
Anthony C (1982) The biochemistry of methylotrophs. Academic, New York
Aragno M, Schlegel HG (1992) The mesophilic hydrogen oxidizing (Knallgas) bacteria. In: Balows A, Triiper HG, Dworkin M, Harder W, Schleifer KH (eds) The prokaryotes, 2nd edn. Springer, New York, pp 344–384
Asada Y, Miyake M, Miyake J, Kurane R, Tokiwa Y (1999) Photosynthetic accumulation of poly(hydroxybutyrate) by cyanobacteria the metabolism and potential for CO2 recycling. Int J Biol Macromol 25:37–42
Asenjo JA, Suk J (1986) Microbial conversion of methaneintopoly-beta-hydroxybutrate (PHB)-growth and intracellular product accumulation in a type-II methanotroph. J Ferment Technol 64:271–278
Auman AJ, Speake CC, Lidstrom ME (2001) nifH sequences and nitrogen fixation in type I and type II methanotrophs. Appl Environ Microbiol 67:4009–4016
Babel W (1992) Pecularities of methylotrophs concerning over flow metabolism, especially the synthesis of polyhydroxyalkanoates. FEMS Microbiol Rev 103:141–148
Babel W, Mothes G (1994) Methylobacterium rhodesianum MB 126 possesses two acetoacetyl-CoA reductases. Arch Microbiol 161:277–280
Bae S, Kwak K, Kim S, Chung S, Igarashi Y (2001) Isolation and characterization of CO2-fixing hydrogen-oxidizing marine bacteria. J Biosci Bioeng 91:442–448
Bengtsson S, Hallquist J, Werker A, Welander T (2007) Acidogenic fermentation of industrial wastewaters: effects of chemostat retention time and pH on volatile fatty acids production. J Biochem Eng 40:492–499
Bhubalan K, Loo CY, Lee WH, Yamamoto T, Doi Y, Sudesh K (2008) Controlled biosynthesis and characterization of poly(3-hydroxybutyrate-3-hydroxyvalerate-co-3-hydroxyhexanoate) from mixtures of palm kernel oil and 3HV-precursors. Polymer Degrad Stabil 93:17–23
Bongers L (1970) Energy generation and utilization in hydrogen bacteria. J Bacteriol 104:145–151
Bormann EJ, Leißner M, Roth M, Beer B, Metzner K (1998) Production of polyhydroxybutyrate by Ralstonia eutropha from protein hydrolysates. Appl Microbiol Biotechnol 50:604–607
Bourque D, Ouellette B, Andre G, Groleau D (1992) Production of poly-β-hydroxybutyrate from methanol: characterization of a new isolate of Methylobacterium extorquens. Appl Microbiol Biotechnol 37:7–12
Bourque D, Pomerleau Y, Groleau D (1995) High-cell-density production of poly-β-hydroxybutyrate (PHB) from Methylobacterium extorquens: production of high-molecular-mass PHB. Appl Microbiol Biotechnol 44:367–376
Bowman JP (2001) Family I. Methylococcaceae and Family V. Methylocystaceae. In: Bergey’s manual of systematic bacteriology. Williams & Wilkins, Baltimore, pp 256–270, pp. 411–420
Bowman JP (2006) The methanotrophs-the families Methylococcacceae and Methylocystaceae. In: The prokaryotes a: handbook on the biology of bacteria. Springer, NewYork, pp 266–289
Bowman JP, Sly LI, Nichols PD, Hayward A (1993) Revised taxonomy of the methanotrophs: description of Methylobacter gen. nov., emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and a proposal that the family Methylococcaceae includes only the group I methanotrophs. Int J Syst Bacteriol 43:735–753
Brandi H, Gross RA, Lenz RW, Fuller RC (1990) Plastic from bacteria and for bacteria: poly (β-hydroxyalkanoates) as natural, biocompatible, and biodegradable polyesters. Adv Biochem Eng Biotechnol 41:77–93
Braunegg G, Lefebvre G, Genser KF (1998) Poly hydroxyalkanoate, biopolyesters from renewable resources: physiological and engineering aspects. J Biotechnol 65:127–161
Budde CF, Riedel SL, Hübner F, Risch S, Popović MK, ChoKyun R, Sinskey AJ (2011) Growth and polyhydroxybutyrate production by Ralstonia eutropha in emulsified plant oil medium. Appl Microbiol Biotechnol 89:1611–1619
Byrom D (1987) Polymer synthesis by microorganisms: technology and economics. Trends Biotechnol 5:246–250
Cebron A, Bodrossy L, Stralis-Pavese N, Singer AC, Thompson IP, Prosser JI, Murrell JC (2007) Nutrient amendments in soil DNA stable isotope probing experiments reduce the observed methanotroph diversity. Appl Environ Microbiol 73:798–807
Chanprateep S (2010) Current trends in biodegradable polyhydroxyalkanoates (review). J Biosci Bioeng 110:621–632
Chen CW, Don TM, Yen HF (2006) Enzymatic extruded starch as a carbon source for the production of poly(3-hydroxybutyrateco-3-hydroxyvalerate by Haloferax mediterranei. Process Biochem 41:2289–2296
Choi J, Lee SY (1997) Process analysis and economic evaluation for PHB production by fermentation. Bioprocess Eng 17:335–342
Choi J, Lee SY (1999a) Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl Microbiol Biotechnol 51:13–21
Choi J, Lee SY (1999b) High-level production of poly(3-hydroxybutyrateco-3-hydroxyvalerate) by fed-batch culture of recombinant Escherichia coli. Appl Environ Microbiol 65:4363–4368
Choi J, Kim JH, Daneial M, Lebeault JM (1989) Optimization of growth medium and poly-β-hydroxybutyric acid production from methanol in Methylobacterium organophilium. Korean J Appl Microbiol Bioeng 17:392–396
Dalton H (1981) Methane mono-oxygenase from a variety of microbes. In: Microbial growth on C, compounds.Heyden & Son, London, pp 1–10
Daniel M, Choi JH, Kim JH, Lebeault JM (1992) Effect of nutrient deficiency on accumulation and relative molecular weight of poly-β-hydroxybutyric acid by methylotrophic bacterium, Pseudomonas 135. Appl Microbiol Biotechnol 37:702–706
Dedysh SN (2002) Methanotrophic bacteria of acids phagnum bogs. Mikrobiologiia 71:741–754
Dedysh SN, Berestovskaya YY, Vasylieva LV, Belova SE, Khmelenina VN, Suzina NE, Trotsenko YA, Liesack W, Zavarzin GA (2004) Methylocella tundrae sp. nov., a novel methanotrophic bacterium from acidic tundra peatlands. Int J Syst Evol Microbiol 54:151–156
Di Donato P, Anzelmo G, Tommonaro G, Fiorentino G, Nicolaus B, Poli A (2009) Vegetable wastes as suitable biomass feedstock for biorefineries. New Biotechnol 25(Suppl):S257
Dobroth ZT, Hu S, Coats ER, McDonald RG (2011) Polyhydroxybutyrate synthesis on biodiesel wastewater using mixed microbial consortia. Bioresour Technol 102:3352–3359
Doi Y (1990) Microbial polyesters. VHC Publishers, New York
Doi Y, Steinbüchel A (2002) Biopolymers. Wiley, Weinheim
Doronina NV, Ezhov VA, Trotsenko YA (2008) Growth of Methylobacteriumtrichosporium OB3b on methanol and poly-β-hydroxybutyrate biosynthesis. Appl Biochem Microbiol 44:182–184
Du G, Chen J, Yu J, Lun S (2001) Continuous production of poly-3-hydroxybutyrate by Ralstonia eutropha in a two-stage culture system. J Biotechnol 88:59–65
Du G, Chen LXL, Yu J (2004) High-efficiency production of bioplastics from biodegradable organic solids. J Polym Environ 12:89–94
Du C, Sabirova J, Soetaert W, Lin SKC (2012) Polyhydroxyalkanoates production from low-cost sustainable raw. Materials Curr Chem Biol 6(1):14–25
Dunfield PF, Khmelenina VN, Suzina NE, Trotsenko YA, Dedysh SN (2003) Methylocella silvestris sp nov., a novel methanotroph isolated from an acidic forest cambisol. Int J Syst Evol Microbiol 53:1231–1239
Dunfield PF, Yuryev A, Senin P, Smirnova AV, Stott MB, Hou S, Ly B, Saw JH, Zhou Z, Ren Y, Wang J, Mountain BW, Crowe MA, Weatherby TM, Bodelier PL, Liesack W, Feng L, Wang L, Alam M (2007) Methane oxidation by an extremely acidophilic bacterium of the phylum Verrucomicrobia. Nature 450:879–882
Fidler S, Dennis D (1992) Polyhydroxyalkanoate production in recombinant Escherichia coli. FEMS Microbiol Rev 103:231–236
Follner CG, Babel W, Valentin HE, Steinbüchel A (1993) Expression of polyhydroxy alkanoic-acid-biosynthesis genes in methylotrophic bacteria relying on the ribulose monophosphate pathway. Appl Microbiol Biotechnol 40:284–291
Foster JF, Litchfield JH (1964) A continuous culture apparatus for the microbial utilization of hydrogen produced by electrolysis of water in closed-cycle space systems. Biotechnol Bioeng 6:44l–456l
Fukui T, Doi Y (1998) Efficient production of polyhydroxyalkanoates from plant oils by Alcaligenes eutrophus and its recombinant strain. Appl Microbiol Biotechnol 49:333–336
Ganduri VSRK, Ghosh S, Patnaik PR (2005) Mixing control as a device to increase PHB production in batch fermentation with co-cultures of lactobacillus delbrueckii and Ralstonia eutropha. Process Biochem 40:257–264
Ghatnekar MS, Pai JS, Ganesh M (2002) Production and recovery of poly-3-hydroxybutyrate from Methylobacterium sp V49. J Chem Technol Biotechnol 77:444–448
Gouda MK, Swellam AE, Omar SH (2001) Production of PHB by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol Res 15:201–207
Govorukhina NI, Trotsenko YA (1991) Poly-β-hydroxybutyrate contents of methylotrophic bacteria with different routes methanol assimilation. Appl Biochem Microbiol 27:80–83
Graham DW, Chaudhary JA, Hanson RS, Arnold RG (1993) Factors affecting competition between type I and type II methanotrophs in two-organism, continuous-flow reactors. Microb Ecol 25:1–17
Grothe E, Moo-Young M, Chisti Y (1999) Fermentation optimization for the production of poly (β-hydroxybutyric acid) microbial thermoplastic. Enz Microbial Technol 25:132–141
Haas R, Jin B, Zepf FT (2008) Production of poly(3-hydroxybutyrate) from waste potato starch. Biosci Biotechnol Biochem 72:253–256
Halami PM (2008) Production of polyhydroxyalkanoate from starch by the native isolate Bacillus cereus CFR06. World J Microbiol Biotechnol 24:805–812
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471
Hayashi NR, Peerapornpisal Y, Nishihara H, Ishii M, Igarashi Y, Kodama T (1994) Isolation and cultivation of thermophilic cyanobacteria from hot springs of northern Thailand. J Ferment Bioeng 78:179–181
Haywood GW, Anderson AJ, Dawes EA (1989) A survey of the accumulation of novel polyhydroxyalkanoates by bacteria. Biotechnol Lett 11:471–476
Haywood GW, Anderson AJ, Ewing DF, Dawes EA (1990) Accumulation of a polyhydroxyalkanoates containing primarily 3-hydroxydecanoate from simple carbohydrate substrates by Pseudomonas sp. Strain NCIMB 40135. Appl Environ Microbiol 56:3354–3359
Hazer DB, Kılıçay E, Hazer B (2012) Poly(3-hydroxyalkanoate)s: diversification and biomedical applications: a state of the art review. Mater Sci Eng 32:637–647
Heinzle E, Lafferty RM (1980) A kinetic model for growth and syntheseis of poly-β-hydroxybutyric acid (PHB) in Alkaligenes etruphus H16. Eur J Appl Microbiol Biotechnol 11:8–16
Helm J, Wendlandt KD, Rogge G, Kappelmeyer U (2006) Characterizing a stable methane-utilizing mixed culture used in the synthesis of a high-quality biopolymer in an open system. J Appl Microbiol 101:387–395
Helm J, Wendlandt KD, Jechorek M, Stottmeister U (2008) Potassium deficiency results in accumulation of ultra-high molecular weight poly-beta-hydroxybutyrate in a methane utilizing mixed culture. J Appl Microbiol 105:1054–1061
Heyer J, Berger U, Hardt M, Dunfield PF (2005) Methylohalobius crimeensis gen. nov., sp. nov., a moderately halophilic, methanotrophic bacterium isolated from hyper-saline lakes of Crimea. Int J Syst Evol Microbiol 55:1817–1826
Hilger U, Sattler K, Littkowsky U (1991) Studies on the growth associated accumulation of poly-hydroxybutyric acid with Methylobacterium rhodesianum Z. Zentralbl Mikrobiol 146:83–88
Hofer P, Vermette P, Groleau D (2011) Production and characterization of polyhydroxyalkanoates by recombinant Methylobacterium extorquens: combining desirable thermal properties with functionality. Biochem Eng J 54:26–33
Hori K, Kaneko M, Tanji Y, Xing XH, Unno H (2002) Construction of self-disruptive Bacillus megaterium in response to substrate exhaustion for polyhydroxybutyrate production. Appl Microbiol Biotechnol 59:211–216
Huang TY, Duan KJ, Huang SY, Chen CW (2006) Production of polyhydroxyalkanoates from inexpensive extruded rice bran and starch by Haloferax mediterranei. J Ind Microbiol Biotechnol 33:701–706
Ibrahim MHA, Steinbüchel A (2010) High-cell-density cyclic fed-batch fermentation of a poly(3-hydroxybutyrate)-accumulating thermophile, Chelatococcus sp. strain MW10. Appl Environ Microbiol 76:7890–7895
Ishii M, Miyake T, Satoh T, Sugiyama H, Oshima Y, Kodama T, Igarashi Y (1997) Autotrophic carbon dioxide fixation in Acidianus brierleyi. Arch Microbiol 166:368–371
Ishizaki A, Tanaka K (1990) Batch culture of Alcaligenes eutrophus ATCC 17697T using recycled gas closed circuit culture system. J Ferment Bioeng 69:170–174
Ishizaki A, Tanaka K (1991) Production of poly-β-hydroxybutyric acid from carbon dioxide by Alcaligenes eutrophus ATCC 17697T. J Ferment Bioeng 70:254–25
Ishizaki A, Tanaka K, Taga N (2001) Microbial production of poly(hydroxybutyrate) from CO2. Appl Microbiol Biotechnol 57:6–12
Islam T, Jensen S, Reigstad LJ, Larsen O, Birkeland NK (2008) Methane oxidation at 55 degrees C and pH 2 by a thermo acidophilic bacterium belonging to the Verrucomicrobia phylum. Proc Natl Acad Sci USA 105:300–304
Jing D, Jiaying X (2011) Biosynthesis of PHB, a new packaging material by methane-utilizing mixed culture HD6T. Adv Mater Res 380:244–247
João MBT, Cavalheiro M, Catarina MD, Grandfils C, Fonseca MMR (2009) Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Proc Biochem 44:509–515
Kabilan S, Ayyasamy M, Jayavel S, Paramasamy G (2012) Pseudomonas sp. as a source of medium chain length polyhydroxyalkanoates for controlled drug delivery: perspective. Int J Microbiol 2012:317828
Kaewkannetra P, Tanonkeo P, Tanamool V, Imai I (2008) Biorefinery of sweet sorghum juice into value added product of biopolymer. J Biotechnol 136:S412
Kallio RE, Harrington AA (1960) Sudanophilic granules and lipid of Pseudomonas methanica. J Bacteriol 80:321–324
Kessler B, Witholt B (2001) Factors involved in the regulatory network of polyhydroxyalkanoate metabolism. J Biotechnol 86:97–104
Khanna S, Srivastava AK (2005a) Statistical media optimization studies for growth and PHB production by Ralstosnia eutropha. Process Biochem 40:2173–2182
Khanna S, Srivastava AK (2005b) Recent advances in microbial polyhydroxyalkanoates. Process Biochem 40:607–619
Khanna S, Srivastava AK (2005c) A simple structured mathematical model for biopolymer (PHB) production. Biotech Prog 21:830–838
Khosravi-Darani K, Vasheghani-Farahani E (2005a) Microorganisms and systems for production of poly(hydroxybutyrate) as a biodegradable polymer. Iran J Chem Chemical Eng 24:1–19
Khosravi-Darani K, Vasheghani-Farahani E (2005b) Application of supercritical fluid extraction in biotechnology. Crit Rev Biotechnol 25:1–12
Khosravi-Darani K, Vasheghani-Farahani E, Shojaosadati SA (2003a) Application of the Plackett–Burman design for the optimization of poly(hydroxybutyrate) production by Ralstonia eutropha. Iran J Biotechnol 1:155–161
Khosravi-Darani K, Vasheghani-Farahani E, Yamini Y (2003b) Solubility of poly hydroxybutyrate in supercritical carbon dioxide. J Chem Eng Data 48:860–863
Khosravi-Darani K, Vasheghani-Farahani E, Shojaosadati SA (2004a) Application of the Taguchi design for production of poly(hydroxybutyrate) by Ralstonia eutropha. Iran J Chem Chemical Eng 23:131–136
Khosravi-Darani K, Vasheghani-Farahani E, Shojaosadati SA, Yamini Y (2004b) The effect of process variable on poly(hydroxybutyrate) recovery by supercritical fluid cell disruption. Biotechnol Prog 20:1757–1765
Khosravi-Darani K, Vasheghani-Farahani E, Tanaka K (2006) Hydrogen-oxidizing bacteria as poly(hydroxybutyrate) producers. Iran J Biotechnol 4:193–196
Kim SB (2000) Production of poly(hydroxybutyrate) from inexpensive substrates. Enz Microb Technol 27:774–777
Kim SW, Kim P, Lee HS, Kim JH (1996) High production of poly-β-hydroxybutyrate (PHB) from Methylobacterium organophilum under potassium limitation. Biotechnol Lett 18:25–30
Kodama T, Igarashi Y, Minoda Y (1975) Isolation and culture conditions of a bacterium grown on hydrogen and carbon dioxide. Agr Biol Chem 36:77–82
Koller M, Bona R, Braunegg G, Hermann C, Horvat P, Kroutil M, Martinz J, Neto J, Pereira L, Varila P (2005) Production of polyhydroxyalkanoates from agricultural waste and surplus materials. Biomacromol 6:561–565
Koller M, Bona R, Chiellini E, Fernandes EG, Horvat P, Kutschera C, Hesse P, Braunegg G (2008) Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora. Bioresour Technol 99:4854–4863
Koller M, Hesse P, Salerno A, Reiterer A, Braunegg G (2011) A viable antibiotic strategy against microbial contamination in biotechnological production of polyhydroxyalkanoates from surplus whey. Biomass Bioenerg 35:748–753
Korotkova N, Lidstrom ME (2001) Connection between poly-betahydroxybutyrate biosynthesis and growth on C1 and C2 compounds in the methylotroph Methylobacterium extorquens AM1. J Bacteriol 183:1038–1046
Kozhevnikov IV, Volova TG, Hai T, Steinbüchel A (2010) Cloning and molecular organization of the polyhydroxyalkanoic acid synthase gene (phaC) of Ralstonia eutropha strain B5786. Appl Biochem Microbiol 46:140–147
Kunasundari B, Sudesh K (2011) Isolation and recovery of microbial polyhydroxyalkanoates. Exp Poly Lett 5:620–634
Lafferty RM (1979) Microbiological method. US Patent 4138291
Lara LM, Huisman GW (1999) Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63:21–53
Lee SY (1996) Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49:1–14
Lee SY, Choi J, Wong HH (1999) Recent advances in poly(hydroxylalkanoate) production by bacterial fermentation: mini-review. Int J Biol Macromol 25:31–36
Lemos PC, Serafim LS, Reis MAM (2006) Synthesis of polyhydroxyalkanoates from different short-chain fatty acids by mixed cultures submitted to aerobic dynamic feeding. J Biotechnol 122:226–238
Lidstrom ME (2006) Aerobic methylotrophic prokaryotes. In: The prokaryotes, volume 2: ecophysiology and biochemistry. Springer, New York, pp 618–634
Listewnik HF, Wendlandt KD, Jechorek M, Mirschel G (2007) Process design for the microbial synthesis of poly-β-hydroxybutyrate (PHB) from natural gas. Eng Life Sci 7:278–282
López-Cuellar MR, Alba-Flores J, Gracida Rodríguez JN, Pérez-Guevara F (2011a) A viable antibiotic strategy against microbial contamination in biotechnologica production of polyhydroxyalkanoates from surplus whey. Biomass Bioenerg 35:748–753
López-Cuellar MR, Alba-Flores J, Gracida Rodríguez JN, Pérez-Guevara F (2011b) Production of polyhydroxyalkanoates (PHAs) with canola oil as carbon source. Int J Biol Macromol 48:74–80
Lu X, Zhang J, Wu Q, Chen GQ (2003) Enhanced production of poly (hydroxybutyrate-co-hydroxyhexanoate) via manipulation the fatty acid β-oxidation pathway in E. coli. FEMS Microbiol Lett 221:97–101
Madison LL, Huisman GW (1999) Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63:21–53
Malik KA, Schlegel HG (1980) Enrichment and isolation of new nitrogen-fixing hydrogen oxidizing bacteria. FEMS Microbiol Lett 8:101–104
Miyake M, Erata M, Asada Y (1996) A thermophilic cyanobacterium, Synechococcus sp. MA19, capable of accumulating poly-β-hydroxybutyrate. J Ferment Bioeng 82(5):512–514
Miyake M, Takase K, Narato M, Khatipov E, Schnackenberg J, Shirai M, Kurane R, Asada Y (2000) Polyhydroxybutyrate production from carbon dioxide by cyanobacteria. Appl Biochem Biotechnol A Enzy Eng Biotechnol 84–86:991–1002
Mokhtari-Hosseini ZB, Vasheghani-Farahani E, Heidarzadeh-Vazifekhoran A, Shojaosadati SA, Karimzadeh R, Khosravi-Darani K (2009a) Statistical media optimization for growth and PHB production from methanol by a methylotrophic bacterium. Bioresour Technol 100:2436–2443
Mokhtari-Hosseini ZB, Vasheghani-Farahani E, Shojaosadati SA, Karimzadeh R, Heidarzadeh-Vazifekhoran A (2009b) Effect of feed composition on PHB production from methanol by HCDC Methylobacterium extorquens (DSMZ 1340). J Chem Technol Biotechnol 84:1136–1139
Morgan-Sagastume F, Karlsson A, Johansson P, Pratt S, Boon N, Lant P, Werker A (2010) Production of polyhydroxyalk in open, mixed cultures from a waste sludge stream containing high levels of soluble organics, nitrogen and phosphorus. Water Res 44:5196–5211
Morse M, Liao Q, Criddle CS, Frank CW (2011) An aerobic biodegradation of the microbial copolymer poly(3-hydroxybutyrate-co-3-hydroxyhexanoate): effects of comonomer content, processing history, and semi-crystalline morphology. Polym 52:547–555
Mothes G, Rivera HS, Babel B (1997) Competition between β-ketothiolase and citrate synthase during poly (hydroxybutyrate) synthesis in Methylobacterium rhodesianum. Arch Microbiol 166:405–410
Mothes G, Ackermann JU, Babel W (1998) Regulation of poly(β-hydroxybutyrate) synthesis in Methylobacterium rhodesianum MB 126 growing on methanol or fructose. Arch Microbiol 169:360–363
Mulchandani A, Luong JHT, Grom C (1989) Substrate inhibition kinetics for microbial growth and syntheseis of poly-β-hydroxybutyric acid in Alkaligenes etruphus ATCC17679. Appl Microbiol Biotechnol 30:11–17
Murrell J, Dalton H (1983) Nitrogen-fixation in obligate methanotrophs. J Gen Microbiol 129:3481–3486
Nguyen HH, Elliott SJ, Yip JH, Chan SI (1998) The particulate methane monooxygenase from M. capsulatus (Bath) is a novel copper-containing three-subunit enzyme. J Biol Chem 273:7957–7966
Nikel PI, Almeida AD, Melillo EC, Galvagno MA, Pettinari MJ (2006) New recombinant Escherichia coli strain tailored for the production of poly(3-hydroxybutyrate) from agro-industrial by-products. Appl Environ Microbiol 72:3949–3954
Nishihara H, Igarashi Y, Kodama T (1991) Growth characteristics and high cell-density cultivation of a marine obligately chemolithoautotrophic hydrogen-oxidizing bacterium Hydrogenovibrio marinus strain MH-110 under a continuous gas-flow system. J Ferment Bioeng 72:358–361
Oakley C, Murrell J (1988) Nifh genes in the obligate methane oxidizing bacteria. FEMS Microbiol Lett 49:53–57
Omar S, Rayes A, Eqaab A, Viss I, Steinbüchel A (2011) Optimization of cell growth and poly(3-hydroxbutyrate) accumulation on date syrup by a Bacillus megaterium strain. Biotechnol Lett 23:1119–1123
Op den Camp HJM, Islam T, Stott MB, Harhangi HR, Hynes A, Schouten S, Jetten MSM, Birkeland NK, Pol A, Dunfield PF (2009) Environmental, genomic, and taxonomic perspectives on methanotrophic Verrucomicrobia. Env Microbiol Rep 1:293–306
Pantazaki AA, Papaneophytou CP, Pritsa AG, Liakopoulou-Kyriakides M, Kyriakidis DA (2009) Production of polyhydroxyalkanoates from whey by Thermus thermophilus HB8. Process Biochem 44:847–853
Papaneophytou CP, Pantazaki AA, Kyriakidis DA (2009) An extracellular polyhydroxybutyrate depolymerase in Thermus thermophilus HB8. Appl Microbiol Biotechnol 83:659–668
Park SJ, Ahn WS, Green PR, Lee SY (2001) Biosynthesis of Poly(hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) by metabolically engineered Escherichia coli strains. Biotechnol Bioeng 74:81–86
Patnaik PR (2005) Perspectives in the modelling and optimization of PHB production by pure and mixed cultures. CritRev Biotechnol 25:153–171
Patwardhan PR, Srivastava AK (2004) Model-based fed-batch cultivation of R. eutropha for enhanced biopolymer production. Biochem Eng J 20:21–28
Pfluger AR, Wu WM, Pieja AJ, Wan J, Rostkowski KH, Criddle CS (2011) Selection of type I and type II methanotrophic proteobacteria in a fluidized bed reactor under non-sterile conditions. Bioresour Technol 102:9919–9926
Pieja AJ, Rostkowski KH, Criddle CS (2011a) Distribution and selection of poly-hydroxybutyrate production capacity in methanotrophic proteobacteria. Microb Ecol 62:564–573
Pieja AJ, Sundstrom ER, Criddle CS (2011b) Poly-hydroxybutyrate metabolism in the type II methanotroph Methylocystis parvus OBBP. Appl Environ Microbiol 77:6012–6019
Pieja AJ, Sundstrom ER, Criddle CS (2012) Cyclic, alternating methane and nitrogen limitation increases PHB production in a methanotrophic community. Bioresour Technol 107:385–392
Pilla S (2011). Handbook of bioplastics and biocomposites engineering applications. Wiley, New York, pp. 373–396
Pinkwart M, Schneider K, Schlegel HG (1983) Purification and properties of the membrane-bound hydrogenase from N2-fixing Alcaligenes latus. Biochim Biophys Acta Protein Struct Mol Enzymol 745:267–278
Pol A, Heijmans K, Harhangi HR, Tedesco D, Jetten MS, OpdenCamp HJ (2007) Methanotrophy below pH1 by a new Verrucomicrobia species. Nature 450:874–878
Povolo S (2010) Production of polyhydroxyalkanoates (PHAs) with canola oil as carbon source. Int J Biol Macromol 48:74–80
Povolo S, Casella S (2003) Bacterial production of PHA from lactose and cheese whey permeate. Macromol Symp 197:1–9
Povolo S, Toffano P, Basaglia M, Casella S (2010) Polyhydroxyalkanoates production by engineered Cupriavidus necator from waste material containing lactose. Bioresour Technol 101:7902–7907
Powell KA, Collinson BA, Richardson KR (1980) Microbiological process for the production of poly(beta-hydroxybutyric acid) and microorganisms for use therein. Eur Patent Appl 80300432.4
Quillaguamán J, Hashim S, Bento F, Mattiasson B, Hatti-Kaul R (2005) Poly(β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1 using starch hydrolysate as substrate. J Appl Microbiol 99:151–157
Raje P, Srivastava AK (1998) Updated mathematical model fed-batch strategies for poly-β-hydroxybutyrate (PHB) ptoduction by Alkaligenes etruphus. Bioresour Technol 64:185–192
Ramadas NV, Singh SK, Soccol CR, Pandey A (2009) Polyhydroxybutyrate production using agro-industrial residue as substrate by Bacillus sphaericus NCIM 5149. Braz Arch Biol Technol 52:17–23
Ramadas NV, Soccol CR, Pandey A (2010) A statistical approach for optimization of polyhydroxybutyrate production by Bacillus sphaericus ncim 5149 under submerged fermentation using central composite design. Appl Biochem Biotechnol 162:996–1007
Reddy CSK, Ghai R, Rashmi R, Kalia VC (2003) Polyhydroxyalkanoates: an overview. Bioresour Technol 87:137–146
Repask R (1966) Characteristics of hydrogen bacteria. Biotechnol Bioeng 8:217–235
Ribera RG, Monteoliva-Sanchez M, Ramos-Cormenzana A (2001) Production of polyhydroxyalkanoates by Pseudomonas putida KT2442 harbouring pSK2665 in waste water from olive oil mills (alpechin). J Biotechnol 4:116–119
Rudnik E (2008). Compostable polymer materials. Elsevier, Amsterdam, p. 21
Ryu HW, Hahn SK, Chang YK, Chang HN (1997) Production of poly(3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligenes eutrophus with phosphate limitation. Biotechnol Bioeng 55:28–32
Santimano MC, Prabhu NN, Garg S (2009) PHA production using low-cost agro-industrial wastes by Bacillus sp. strain COL1/A6. J Microbiol 4:89–96
Schink B, Schlegel H (1978) Hydrogen, metabolism in aerobic hydrogen oxidizing bacteria. Biochimie 60(3):297–305
Schlegel HG, Gottschalk G, Von Bartha R (1961) Formation and utilization of poly-β-hydroxybutyic acid by Knallgas bacteria (Hydrogenomonas). Nature 191:463
Schrader J, Schilling M, Holtmann D, Sell D, Filho MV, Marx A, Vorholt JA (2009) Methanol-based industrial biotechnology: current status and future perspectives of methylotrophic bacteria (review). Trends Biotechnol 27:107–115
Scott D, Brannan J, Higgins IJ (1981) The effect of growth conditions on intracytoplasmic membranes and methane mono-oxygenase activities in Methylosinus trichosporium OB3b. J Gen Microbiol 125:63–72
Shah NN, Hanna ML, Jackson KJ, Taylor RT (1996a) Batch cultivation of Methylosinus trichosporium OB3B: IV production of hydrogen-driven soluble or particulate methane monooxygenase activity. Biotechnol Bioeng 45:229–238
Shah NN, Hanna ML, Taylor RT (1996b) Batch cultivation of Methylosinus trichosporiumOB3b. 5: characterization of poly(hydroxybutyrate) production under methane-dependent growth conditions. Biotechnol Bioeng 49:161–171
Shah-Hosseini S, Sadeghi MT, Khosravi-Darani K (2003) Simulation and model validation of batch poly(β-hydroxybutyrate) production process using Ralstonia eutropha. Iran J Chem Chemical Eng 22:35–41
Sharma L, Mallick N (2008) Exploitation of municipal and aquacultural discharges for poly-β-hydroxybutyrate production in cyanobacterium, Nostoc muscorum. Res J Biotechnol 3:282–287
Sheu DS, Wang YT, Lee CY (2000) Rapid detection of polyhydroxyalkanoate-accumulating bacteria isolated from the environment by colony PCR. Microbiol 146:2019–2025
Simon-Colin C, Raguenes G, Crassous P, Moppert X, Guezennec J (2008) A novel MCL-PHA produced on coprah oil by Pseudomonas guezennei biovar.tikehau, isolated from a ‘kopara’ mat of French Polynesia. Int J BiolMacromol 43:176–181
Slepecky RA, Law JH (1961) Synthesis and degradation of poly-b-hydroxybutyric acid in connection with sporulation of Bacillus megaterium. J Bacteriol 82:37–42
Solaiman D, Ashby R, Hotchkiss A, Foglia T (2006) Biosynthesis of medium-chain-length poly(hydroxyalkanoates) from soy molasses. Biotechnol Lett 28:57–162
Song H, Xin J, Zhang Y, Kong W, Xia C (2011) Poly-3-hydroxybutyrate production from methanol by Methylosinus trichosporium IMV3011 in the non-sterilized fed-batch fermentation. Afr J Microbiol Res 5:5022–5029
Sonnleitner B, Heinzle E, Braunegg G, Lafferty RM (1979) Formal kinetics of poly-β-hydroxybutyric acid (PHB) production in Alkaligenes etruphus H16 and Mycoplana rubera R14 with respect to the dissolved oxygen tension in ammonium limited batch-cultures. Eur J Appl Microbiol Biotechnol 7:1–10
Steinbüchel A, Füchtenbusch B (1998) Bacterial and other biological systems for polyester production. Trends Biotechnol 16:419–427
Steinbüchel A, Lutke-Eversloh T (2003) Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem Eng J 16:81–96
Sudesh K, Doi AY (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1503–1555
Sugimoto T, Tsuge T, Tanaka K, Ishizaki A (1999) Control of acetic acid concentration by pH-stat continuous substrate feeding in heterotrophic culture phase of two-stage cultivation of Alcaligenes eutrophus for production of PHB from CO2, H2 and O2 under non-explosive condition. Biotechnol Bioeng 62:625–631
Suzuki T, Yamane T, Shimizu S (1986a) Mass production of poly-β-hydroxybutyric acid by fully automatic fed-batch culture of methylotroph. Appl Microbiol Biotechnol 23:322–329
Suzuki T, Yamane T, Shimizu S (1986b) Kinetics and effect of nitrogen source feeding on production of poly(hydroxybutyric acid) by fed-batch culture. Appl Microbiol Biotechnol 23:366–369
Suzuki T, Yamane T, Shimizu S (1986c) Mass production of (poly-hydroxybutyric acid) by fed-batch culture with controlled carbon/nitrogen feeding. Appl Microbiol Biotechnol 24:370–374
Suzuki T, Deguchi H, Yamane T, Shimizu S, Gekko K (1988) Control of molecular weight of (poly-hydroxybutyric acid) produced in fed-batch culture of Protomonas extorquence. Appl Microbiol Biotechnol 27:487–491
Suzuki H, Kishimoto M, Kamoshita Y, Omasa T, Katakura Y, Suga KI (2000) On-line control of feeding of medium components to attain high cell density. Bioprocess Eng 22:433–440
Taga N, Tanaka K, Ishizaki A (1997) Effects of rheological change by addition of carboxymethylcellulose in culture media of an air-lift fermentor on poly-d-3-hydroxybutyric acid productivity in autotrophic culture of hydrogen-oxidizing bacterium. Alcaligenes eutrophus. Biotechnol Bioeng 53:529–533
Taidi B, Anderson AJ, Dawes EA, Byrom D (1994) Effect of carbon source and concentration on the molecular mass of poly(3-hydroxybutyrate) production by Methylobacterium extorquens and Alcaligenes etrophus. Appl Microbiol Biotechnol 40:786–790
Takeshita T, Ishizaki A (1996) Influence of hydrogen limitation on gaseous substrate utilization in autotrophic culture of Alcaligenes eutrophus ATCC 17697T. J Ferment Bioeng 81:83–86
Takeshita T, Tanaka K, Ishizaki A, Stanbury PF (1993a) Development of a dissolved hydrogen sensor and its application to evaluation of hydrogen mass transfer. J Ferment Bioeng 76:148–150
Takeshita T, Tanaka K, Ishizaki A, Stanbury PF (1993b) Studies on dissolved hydrogen behavior in autotrophic culture of A. eutrophus 17697T. J Fac Agr Kyushu Univ 38:55–64
Tanaka K, Ishizaki A (1994) Production of poly-d-3-hydroxybutyric acid from carbon dioxide by a two-stage culture method employing Alcaligenes eutrophus ATCC 17697T. J Ferment Bioeng 77:425–427
Tanaka K, Ishizaki A, Takeshita T, Kanemaru T, Shimoji T, Kawano T (1993) Equipment and operation for fermentative PHB production using gaseous substrate to guarantee safety from explosion. J Chem Eng Japan 26:225–227
Tanaka K, Ishizaki A, Kanamaru T, Kawano T (1995) Production of poly(d-3-hydroxybutyrate) from CO2, H2, and CO2 by high cell density autotrophic cultivation of Alcaligenes eutrophus. Biotechnol Bioeng 45:268–275
Tanaka K, Miyawaki K, Yamaguchi A, Khosravi-Darani K, Matsusaki H (2011) Cell growth and P(3HB) accumulation from CO2 of a carbon monoxide-tolerant hydrogen-oxidizing bacterium, Ideonella sp. O-1. Appl Microbiol Biotechnol 92:1161–1169
Tohyama M, Patarinska T, Qiang Z, Shimizu K (2002) Modeling of the mixed culture and periodic control for PHB production. Biochem Eng J 10:157–173
US Environmental Protection Agency. Methane: sources and emissions. http://www.epa.gov/outreach/sources.html. Accessed April21, 2011
Ueda S, Matsumoto S, Takagi A, Yamane T (1992) Synthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from methanol and n-amyl alkohol by methylotrophic bacteria Paraccocus denitrificans and Methylobacterium extorquens. Appl Environ Microbiol 58:3574–3579
Van Dien SJ, Lidstrom ME (2002) Stiochimetric model for evaluating the methabolic capabilities of the facultative methylotroph Methylobacterium extorquens AM1, with application to reconstruction of C3 and C4 methabolism. Biotechnol Bioeng 78:296–312
Vandamme P, Coenye T (2004) Taxonomy of the genus Cupriavidus: a tale of lost and found. Int J Syst Evol Microbiol 54:2285–2289
Van-Thuoc D, Quillaguamán J, Mamo G, Mattiasson B (2008) Utilization of agricultural residues for poly(3-hydroxybutyrate) production by Halomonas boliviensis LC1. J Appl Microbiol 104:420–428
Vecherskaya M, Dijkema C, Stams AJ (2001) Intracellular PHB conversion in a type II methanotroph studied by13CNMR. J Ind Microbiol Biotechnol 26:15–21
Vincenzini M, De Philippis R (1999) Polyhydroxyalkanoates. In: Chemicals from Microalgae, Cohen Z, London, Taylor and Francis, pp 292-352
Volova TG, Voĭnov NA (2004) Study of Ralstonia eutropha culture producing polyhydroxyalkanoates on products of coal processing. Prikl Biokhim Mikrobiol 40:296–300
Volova TG, Kalacheva GS, Altukhova OV (2002) Autotrophic synthesis of polyhydroxyalkanoates by the bacteria Ralstonia eutropha in the presence of carbon monoxide. App Microbiol Biotechnol 58:675–678
Wang J, Yu HQ (2007) Biosynthesis of polyhydroxybutyrate and extracellular polymeric substances by Ralstonia eutropha ATCC 17699 in batch cultures. Appl Microbiol Biotechnol 75:871–878
Wendlandt KD, Jechorek M, Helm J, Stottmeister U (1998) Production of PHB with a high molecular mass from methane. Poly Degrad Stabil 59:191–194
Wendlandt KD, Jechorek M, Helm J, Stottmeister U (2001) Producing poly-3-hydroxybutyrate with a high molecular mass from methane. J Biotechnol 86:127–133
Wendlandt KD, Geyer W, Mirschel G, Al-HajHemidi F (2005) Possibilities for controlling a PHB accumulation process using various analytical methods. J Biotechnol 117:119–129
Wendlandt KD, Stottmeister U, Helm J, Soltmann B, Jechorek M, Beck M (2010) The potential of methane-oxidizing bacteria for applications in environmental biotechnology (review). Eng Life Sci 10:87–102
Whittenbury R, Phillips KC, Wilkinson JF (1970) Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol 61:205–218
Wise MG, McArthur JV, Shimkets LJ (1999) Methanotroph diversity in land fill soil: isolation of novel type I and type II methanotrophs whose presence was suggested by culture independent 16S ribosomal DNA analysis. Appl Environ Microbiol 65:4887–4897
Wong HH, Lee SY (1998) Poly(3-hydroxybutyrate) production from whey by high density cultivation of recombinant Escherichia coli. Appl Microbiol Biotechnol 50:30–33
Wong AL, Chua H, Yu PH (2000) Microbial production of polyhydroxyalkanoates by bacteria isolated from oil wastes. App Biochem Biotechnol 84–86:843–857
Xin JY, Zhang YX, Zhang S, Xia CG, Li SB (2007) Methanol production from CO2 by resting cells of the methanotrophic bacterium Methylosinus trichosporium IMV3011. J Basic Microbiol 47:426–435
Xin J, Zhang Y, Dong J, Song H, Xia C (2011) An experimental study on molecular weight of polyhydroxybutyrate (PHB) accumulated in Methylosinus trichosporium IMV 3011. Afr J Biotechnol 10:7078–7087
Yamane T (1993) Yield of poly-D-3-hydroxybutyrate from various, carbon sources: a theoretical study. Biotechnol Bioeng 41:165–170
Yamane T, Chen XF, Ueda S (1996a) Growth associated production of poly(3-hydroxyvalerate) from n-pentanol by a methylotrophic bacterium. Paracoccus denitrificans. Appl Environ Microbiol 62:380–384
Yamane, Fukunaga M, Dee YW (1996b) Increase PHB production by high-cell-density fed-batch culture of Alcaligunes latus, a growth associated PHB producer. Biotechnol Bioeng 50:197–202
Yan S, Tyagi RD, Surampalli RY (2006) Polyhydroxyalkanoates (PHA) production using wastewater as carbon source and activated sludge as microorganisms. Water Sci Technol 53:175–180
Yezza A, Fournier D, Halasz A, Hawari J (2006) Production of polyhydroxyalkanoates from methanol by a new methylotrophic bacterium Methylobacterium sp. GW2. Appl Microbiol Biot 73:211–218
Yoo S, Kim WS (1994) Cybernetic model for synthesis of poly-β-hydroxybutyric acid in Alcaligenes etrophus. Biotechnol Bioeng 43:1043–1051
Zahn JA, DiSpirito AA (1996) Membrane-associated methane monooxygenase from M. capsulatus (Bath). J Bacteriol 178:1018–1029
Zhang Y, Xin J, Chen L, Song H, Xia C (2008) Biosynthesis of poly-3-hydroxybutyrate with a high molecular weight by methanotroph from methane and methanol. J Natural Gas Chem 17:103–109
Zhao S, Fan C, Hu X, Chen J, Feng H (1993) The microbial production of polyhydroxybutyrate from methanol. Appl Biochem Biotechnol 39(40):191–199
Zinn M, Witholt B, Egli T (2001) Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv Drug Deliv Rev 53:5–21
Zuniga C, Morales M, Le Borgne S, Revah S (2011) Production of poly-β-hydroxybutyrate (PHB) by Methylobacterium organophilum isolated from a methanotrophic consortium in a two-phase partition bioreactor. J Hazard Mater 190:876–882
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khosravi-Darani, K., Mokhtari, ZB., Amai, T. et al. Microbial production of poly(hydroxybutyrate) from C1 carbon sources. Appl Microbiol Biotechnol 97, 1407–1424 (2013). https://doi.org/10.1007/s00253-012-4649-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4649-0