Abstract
We constructed a biosynthetic pathway of isoprene production in Escherichia coli by introducing isoprene synthase (ispS) from Populus alba. 1-deoxy-d-xylulose 5-phosphate synthase (dxs), 1-deoxy-d-xylulose 5-phosphate reductoisomerase (dxr) and isopentenyl diphosphate (IPP) isomerase (idi) were overexpressed to enhance the isoprene production. The isoprene production was improved 0.65, 0.16, and 1.22 fold over the recombinant BL21 (pET-30a-ispS), respectively, and idi was found to be a key regulating point for isoprene production. In order to optimize the production of isoprene in E. coli, we attempted to construct polycistronic operons based on pET-30a with genes dxs, dxr, and idi in various orders. The highest isoprene production yield of 2.727 mg g−1 h−1 (per dry weight) was achieved by E. coli transformed with pET-30a-dxs/dxr/idi. Interestingly, the gene order was found to be consistent with that of the metabolic pathway. This indicates that order of genes is a significant concern in metabolic engineering and a sequential expression pattern can be optimized according to the biosynthetic pathway for efficient product synthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isoprene is the monomeric building block of the most diverse and abundant groups of biological organic compounds referred to as isoprenoids. As a feedstock in the synthetic chemistry industry, it has significant potential and is widely used in the synthesis of rubber, spices, medicines, and pesticides. Currently, the industrial supply of isoprene is limited to petrochemical sources. Considering the growing energy crisis and environmental pressures, there is an urgent need to seek feasible substitute approaches.
The biosynthesis of isoprene was first described in plants in 1957 (Sanadze 1957). A series of plants were reported to emit 1–2 % of their fixed carbon as isoprene (Kesselmeier and Staudt 1999; Monson and Fall 1989). Nevertheless, as isoprene is gaseous above 34 °C, it is impractical to harvest this hydrocarbon from plants. With the development of biosynthesis, isoprene produced by microorganisms provides a feasible approach. Isoprene is poorly soluble in water, so it can be condensed from the gas phase in fully enclosed bioreactors, with no need for additional purification of the liquid product. Although no ispS (isoprene synthase) gene has been elucidated in any microorganism, over-expression of ispS gene from plants can effectively endow microorganisms with the property of isoprene production from their own metabolism. At present, ispS has been cloned and characterized from Poplar (Populus alba, Populus tremuloides) (Miller et al. 2001; Sasaki et al. 2005) and kudzu vine (Pueraria montana) (Sharkey et al. 2005).
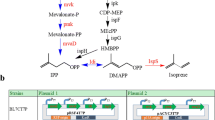
There are two distinct pathways leading to the biosynthesis of key isoprenoid intermediates, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP): the mevalonic acid pathway, which operates in eukaryotes, archaea and the cytosol of higher plants; and the methyl-erythritol-4-phosphate (MEP) pathway, which is present in prokaryotic bacteria, green algae and the chloroplasts of higher plants (Kuzuyama 2002; Lichtenthaler 2000). As the common engineering strain, Escherichia coli uses the MEP pathway to produce isoprenoids. The MEP pathway, which initiates with the glycolytic intermediates pyruvate and glyceraldehyde-3-phosphate and ends with the DMAPP and IPP, consists of seven subsequent enzymatic steps. As numerous reports have shown, the main rate-limiting enzymes within the MEP pathway were found to be 1-deoxyxylulose-5-phosphate synthase, 1-deoxyxylulose-5-phosphate reductoisomerase, IPP–DMAPP isomerase (Albrecht et al. 1999; Kajiwara et al. 1997; Kim and Keasling 2001; Matthews and Wurtzel 2000). Success has been achieved in the enhancement of isoprenoid production through regulation methods such as eliminating the rate-limiting steps and engineering regulation networks. However, research on isoprene production by regulating the metabolic flux of the MEP pathway has been limited, and has focused only on the overexpression of dxs or dxr for isoprene production enhancement. Xue et al. reported that the yield of isoprene in Bacillus subtilis was increased by 40 % over the wild-type strain by overexpressing dxs, but no improvement was observed in the overexpression of the dxr (Xue and Ahring 2011). It was also found that heterologous expression of dxs and dxr from B. subtilis in the E. coli harboring ispS resulted in a 2.3-fold enhancement of isoprene production (Zhao et al. 2011). Until now, no report about the effect of idi on the production of isoprene has been released, nor has there been any coexpression of the genes dxs, dxr, and idi to study their synergetic effect.
Usually, two methods are applied to coexpress heterologous genes. One is using a vector with independent promoters or internal ribosome entry site (IRES). Promoter interference sometimes occurs with the use of heterologous promoters, i.e., transcription from one promoter suppresses transcription from another (Emerman and Temin 1984). Using the IRES method, more than two genes connected by IRES sequences can be efficiently expressed from a single promoter (Ghattas et al. 1991). Nevertheless, the target genes’ number is limited to its restriction sites. The other one is using two compatible systems. ColE1-derived plasmid is the most attractive candidate due to its wide application and high copy number. However, many ColE1-compatible plasmids (such as p15A-derived plasmids) are of low copy numbers, which makes it difficult and time-consuming to construct vectors and also reduces the level of mRNA transcription (Johnston et al. 2000). Using two incompatible plasmids was reported as a new method for protein coexpression in E. coli and compensates for the deficiency of using two compatible plasmids (Yang et al. 2001). Therefore, a combination of using incompatible plasmids and the IRES method might provide a feasible strategy to coexpress several genes.
In this work, an isoprene synthase from P. alba was introduced to construct a pathway of isoprene production in E. coli. To achieve the coexpression of multiple genes, an expression system was established by combining two incompatible plasmids (pET-32a and pET-30a) and the IRES method together. Based on this expression system, the genes dxs, dxr, and idi were overexpressed and coexpressed for enhancing the production of isoprene. In addition, we attempted to optimize the isoprene production in transformed E. coli by constructing polycistronic operons with rearranged gene orders to investigate whether the gene order affects the final product yield.
Materials and methods
Bacterial strains, plasmids, and culture conditions
E. coli BL21 (DE3) (Novagen, USA) was used for gene cloning and expression. E. coli K12 AB200068 (CCTCC) was used for amplification of dxs, dxr, and idi genes. Plasmids pET-32a and pET-30a (Novagen, USA) were used as vectors for ispS, dxs, dxr, and idi expression. All E. coli strains were cultivated in Luria–Bertani (LB) medium at 37 °C with shaking (200 rpm), supplemented with antibiotics (100 μg/ml of ampicillin or 50 μg/ml of kanamycin) as needed.
Construction of recombinant plasmids and transformation
The nucleotide sequence of the P. alba isoprene synthase (GenBank accession No. AB198180) without its predicted chloroplast transit peptide was optimized to the preferred codon usage of E. coli and synthesized by Sangon Biotech (Shanghai. China). The gene was amplified using ispS-BamHI (F) and ispS-HindIII(R) as primers and cloned into pET-32a between restriction sites of BamHI and HindIII. The resulting plasmid was designated as pET-32a-ispS (1, Fig. 1). The genes dxs, dxr, and idi were amplified by PCR using E. coli K12 genomic DNA as a template. The dxs gene was amplified using primers dxs-NcoI (F) and dxs-BamHI (R) and then ligated into the pET-30a digested with NcoI and BamHI to create pET-30a-dxs (2, Fig. 1). The dxr gene was amplified using primers dxr-BamHI (F) and dxr-HindIII (R) and inserted into pET-30a between the restriction sites of BamHI and HindIII to create pET-30a-dxr (3, Fig. 1). The idi gene was amplified using primers idi-HindIII (F) and idi-XhoI (R) and inserted into pET-30a between the restriction sites of HindIII and XhoI to create pET-30a-idi (4, Fig. 1).
In the construction of these polycistronic operons (5–10, Fig. 1), the RBS and its surrounding sequence originated from pET-30a (AATAATTTTGTTTAACTTTAAGA AGGAGATATACAT) (Liu and Yu 2012) were introduced upstream of the second and third gene for coexpressing all the genes as separate proteins. These genes dxs, dxr, and idi were inserted into pET-30a among the restriction sites of KpnI, BamHI, SacI, and XhoI randomly, generating the operons: pET-30a-dxs/dxr/idi, pET-30a-dxs/idi/dxr, pET-30a-dxr/dxs/idi, pET-30a-dxr/idi/dxs, pET-30a-idi/dxs/dxr and pET-30a-idi/dxr/dxs (5–10, Fig. 1). For the genes cloning, dxs was amplified by PCR from E. coli K12 genomic DNA using primers dxs-KpnI (F) and dxs-BamHI (R) (for pET-30a-dxs/dxr/idi, pET-30a-dxs/idi/dxr), rbs-dxs-BamHI (F) and rbs-dxs-SacI (R) (for pET-30a-dxr/dxs/idi, pET-30a-idi/dxs/dxr), rbs-dxs-SacI (F) and rbs-dxs-XhoI (R) (for pET-30a-dxr/idi/dxs, pET-30a-idi/dxr/dxs); dxr was amplified by PCR from E. coli K12 genomic DNA using primers dxr-KpnI (F) and dxr-BamHI (R) (for pET-30a-dxr/dxs/idi, pET-30a-dxr/idi/dxs), rbs-dxr-BamHI (F) and rbs-dxr-SacI (R) (for pET-30a-dxs/dxr/idi, pET-30a-idi/dxr/dx s), rbs-dxr-SacI (F) and rbs-dxr-XhoI (R) (for pET-30a-dxs/idi/dxr, pET-30a-idi/dxs/dxr); idi-KpnI (F) and idi-BamHI (R) (for pET-30a-idi/dxs/dxr, pET-30a-idi/dxr/dxs), idi was amplified by PCR from E. coli K12 genomic DNA using primers rbs-idi-BamHI (F) and rbs-idi-SacI (R) (for pET-30a-dxs/idi/dxr, pET-30a-dxr/idi/dxs), rbs-idi-SacI (R) and idi-XhoI (R) (for pET-30a-dxs/dxr/idi, pET-30a-dxr/dxs/idi), respectively. All the primers were shown in Table 1.
These recombinant plasmids with kanamycin resistance were separately transformed into E. coli BL21 (pET-32a-ispS) competent cells using a heat pulse at 42 °C and then spread on LB agar plate containing kanamycin (selection for pET-30a) and ampicillin (selection for pET-32a).
Method to confirm incompatible plasmids’ coexistence and coexpression under antibiotic pressure in E. coli
The method for confirming the coexistence of incompatible plasmids is based on the method reported by Yang et al. (2001). Colonies (harboring incompatible plasmids) were picked from plates containing fresh cotransformed cells and cultured in liquid LB medium with selective pressure (ampicillin and kanamycin). Samples were taken from liquid cultures every 4 h and diluted appropriately. Each sample was spread both on selective LB agar plate and non-selective control plate. The ratio of the number of colonies on the selective agar plate to that on the non-selective agar plate represents the proportion of E. coli cells carrying both plasmids to total E. coli cells when the samples were taken. Co-transformants were also inoculated into liquid LB medium without antibiotics as control. Plating was done on the two kinds of plates in the same way as described above, and the colonies were also counted. Coexpression of recombinant plasmids was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Expression and purification of recombinant proteins
E. coli BL21 (DE3) strains containing recombinant vectors were grown in 50 ml LB medium to an OD600 of 0.5 and induced by 0.5 mM isopropyl β-d-thiogalactoside (IPTG) for 4 h. After cultivation, cells were harvested by centrifugation (4,000 rpm for 10 min), washed with PBS buffer and resuspended in 50 mM Tris–HCl (pH 8.0). Cells were mechanically disrupted by ultrasonication. The supernatants were then analyzed by 15 % SDS-PAGE. Gel band intensity was determined using a Gel-Pro Analyzer 6.0 (Media Cybernetics, Bethesda, MD). For isoprene synthase purification, the supernatants were pretreated with filters, purified with ÄKTA Purifier FPLC purification system equipped with HisTrap™ HP column (GE Healthcare, Sweden) and desalted with gel column.
Isoprene synthase assay
Isoprene synthase activity was assayed using a modified procedure of Lehning et al. (1999): by mixing 0.1 ml of 1 M MgCl2, 2.2 ml of ISB, 0.5 ml of 0.1 M DMAPP (in ISB) and 2.2 ml of purified isoprene synthase. ISB consists of 50 mM Tris–HCl, 12 mM MgCl2, 5 % glycerol and 2 mM dithiothreitol. The mixture was incubated in 17 ml gas-tight vial at 37 °C for 2 h. Gas from the headspace of sealed cultures was sampled with gas-tight syringe and analyzed by gas chromatography (GC) equipped with a flame ionization detector and HP-FFAP column.
Quantification of isoprene production
The strains containing recombinant vectors were grown in 50 ml LB culture (100 μg/ml ampicillin and 50 μg/ml kanamycin) at 37 °C with shaking until OD600 reached 0.5. 2 ml of culture were transferred to sealed vials and added with 0.1 mM IPTG at 37 °C with shaking (200 rpm) for 2 h. Gas sample from the headspace of the sealed cultures was analyzed with GC.
Growth curve experiments of recombinant strains
The recombinant strains were grown in 5 ml LB medium (100 μg/ml of ampicillin or 50 μg/ml of kanamycin) overnight at 37 °C with shaking and then diluted into 50 ml LB medium (100 μg/ml of ampicillin or 50 μg/ml of kanamycin) to an OD of 0.015. The OD was tested at 600 nm every 30 min. Each sample was measured in triplicate. And when OD600 of the culture reached approximately 0.3, IPTG was added to a final concentration of 0.1 mM.
Results
Construction of the isoprene metabolic pathway in E. coli
IspS from P. alba was cloned into pET-32a and the expression was analyzed by SDS-PAGE. The band corresponding to the size of ispS (81KDa), which harboring the N-terminal Trxis•Tag™/His•Tag®/S•Tag™/enterokinase configuration of pET-32a, was visible in SDS-PAGE gel (Fig. 2a, lane 2). The enzyme assay was performed as described. After a 2-h incubation at 37 °C, an isoprene-specific peak was detected with the same retention time (4.1 min) as the authentic standard. To identify whether the isoprene metabolic pathway is constructed successfully in E. coli (pET-32a-ispS), gas samples from the headspace of sealed vial cultures were analyzed by gas chromatography. It was found that E. coli BL21 (pET-32a-ispS) can accumulate 1.0 mg/g isoprene in 2 h, while the control strain produced little isoprene about 0.05 mg/g (Fig. 2b). Then, the enhancement of isoprene production should be attributed to the codon optimized isoprene synthase introduced.
Coexistence of pET-32a and pET-30a
Due to having the same pBR322 origin, pET-32a and pET-30a cannot coexist without selected pressure. Therefore, we maintained them in the same cell by taking advantage of different selective antibiotic resistance. As shown in Fig. 3, after continuous 24 h incubation, the two types of vectors in 70 % of strains cultured with two antibiotics were found to coexist stably, while 80 % of the strains cultured in liquid LB medium without antibiotics lost the vectors. The gene expressions were confirmed by SDS-PAGE analysis. Figure 4 showed that the genes ispS, dxs, dxr, and idi were all well expressed in recombinant strains. All the above results demonstrated the availability of incompatible plasmids system for heterologous genes’ coexpression.
Effect of antibiotic pressure on coexistence of pET-32a-ispS and pET-30a-dxs in E. coli. The columns show the proportions of cells that are resistant to both kanamycin and ampicillin in total E. coli cells after culturing the cotransformants for certain time: the blue represents liquid culture medium containing 50 μg/ml kanamycin and 100 μg/ml ampicillin; the blank represents liquid culture medium without antibiotics. Error bars represent standard deviations from the means
Overexpression of E. coli dxs, dxr, and idi
To identify the effect of dxs, dxr, and idi on isoprene production, vectors pET-30a-dxs, pET-30a-dxr and pET-30a-idi were constructed and introduced into the strain BL21 (pET-32a-ispS) respectively. The expression bands of dxs, dxr, and idi were visible on SDS-PAGE at the predicted sizes of 72, 47, and 24 kDa. The sizes were all 5 kDa larger than that of the natural proteins due to the configuration of N-terminal His•Tag®/thrombin/S•Tag™/enterokinase in PET-30a. As shown in Fig. 4, the relative expression of the genes in these recombinant vectors were present as follows: dxs (32.7 %), ispS (8.6 %) in pET-32a-isps&pET-30a-dxs; dxr (20.5 %), ispS (17.2) in pET-32a-isps&pET-30a-dxr; idi (55.2 %) and ispS (11.3 %) in pET-32a-isps&pET-30a-idi. The production of isoprene was analyzed with GC. Overexpression of dxs, dxr, and idi can produce 0.77, 0.54, 1.04 mg g−1 h−1 isoprene respectively (Fig. 5), increasing isoprene production 0.65, 0.16, 1.22 fold over the BL21 (pET-32a-ispS). The order of these genes effect on the isoprene production was found to follow the corresponding trend: dxr < dxs < idi.
Ordered coexpression of dxs, dxr, and idi using polycistronic operons
In this study, polycistronic operons consisting of three isoprene biosynthetic genes (dxs, dxr, and idi), in which the genes can be assembled as one transcriptional unit, were constructed based on pET-30a. As shown in the Fig. 6, the genes dxs, dxr, and idi were coexpressed efficiently as separate proteins. Due to the configuration of N-terminal His•Tag®/thrombin/S•Tag™/enterokinase upstream of the MCS in PET-30a, the top gene of each polycistronic operon is 5 kDa larger than its natural size. For example, the size of dxs is 72 kDa in pET-30a-dxs/dxr/idi and pET-30a-dxs/dxr/idi, 5 kDa larger than that in the other operons. The natural sizes of dxs, dxr, and idi were 67, 42, and 18 kDa, respectively. The relative expressions of the genes in these recombinant operons were present in Table 2. For all transformants no matter of the genes order, the dxs relative expression is higher than that of dxr and idi. Therefore, the gene expression is related to the protein structure to some extent. The expression was high when the gene was ordered at the top rather than at the downstream. For example, the dxs relative expression in pET-30a-dxs/dxr/idi and pET-30a-dxs/dxr/idi, in which dxs was ordered as the top gene, was more than that of the other four operons; the dxr relative expression in pET-30a-dxr/dxs/idi and pET-30a-dxr/idi/dxs, in which dxr was ordered as the top gene, was more than that of the other 4 operons and so did idi. For isoprene production, it was found that tandem coexpression of these limiting genes can stimulate the isoprene production up to 2.727, 2.463, 2.461, 1.647, 1.046, and 1.142 mg g−1 h−1 in recombinant vectors ordered 1–6, respectively (Fig. 7), increasing isoprene production 4.8, 4.23, 4.23, 2.5, 1.2, 1.4 fold over the BL21 (pET-32a-ispS). Compared with single gene overexpression, the synergy of multiple limiting enzymes contributed more to the final product yield. Interestingly, the gene order of transform (pET-30a-dxs/dxr/idi) with the highest isoprene production corresponded to that of the metabolic pathway.
SDS-PAGE analysis of crude protein fractions coexpressed in recombinant E. coli BL21. M Protein molecular weight marker; 0 E. coli BL21; 1 pET-30a-dxs/dxr/idi&pET-32a-ispS; 2 pET-30a-dxs/idi/dxr&pET-32a-ispS; 3 pET-30a-dxr/dxs/idi &pET-32a-ispS; 4 pET-30a-dxr/idi/dxr&pET-32a-ispS; 5 pET-30a-idi/dxs/dxr &pET-32a-ispS; 6 pET-30a-idi/dxr/dxs &pET-32a-ispS. filled blue circle dxs, filled orange circle dxr, filled green circle idi
The effect of protein expression on cell growth
To test the effect caused by expression of isps, dxs, dxr, idi, and isps/dxs/dxr/idi, a growth curve experiment was performed. As shown in Fig. 8, the growth of strains with protein overexpression was significantly slower than the control, especially the BL21 (isps/dxs/dxr/idi). This is surmised to be caused by great amount of energy and resource consumption for protein synthesis.
Discussion
Applications in fuel and product biosynthesis require efficient gene expression and robust performance. Therefore, the method for the effective coexpression of multiple genes is particularly important. In this work, we established an expression system by combining incompatible plasmids system with IRES method, and achieved the efficient coexpression of the genes (ispS, dxs, dxr, idi). In IRES method, the genes connected by IRES sequences can be efficiently expressed from a single promoter (Ghattas et al. 1991). Nevertheless, the target genes’ number is generally limited to restriction sites. The combination of incompatible plasmid system (Yang et al. 2001) not only increased target genes’ number but also guaranteed the high copy number of both plasmids. This strategy can generally also be applied for the coexpression of multiple genes (more than three) in other product biosynthesis.
Based on this expression system, the genes dxs, dxr, and idi were overexpressed to eliminate the repression of these limiting genes on IPP and DMAPP supply, which is a limiting factor on isoprenoids production. Overexpression of dxs, the first bottleneck in MEP pathway (Lois et al. 2000; Miller et al. 2000), resulted in significant enhancement of isoprene production, which indicated that adequate precursor was the base of high metabolic flux. In the previous study, the effect of idi on isoprene production was generally neglected (Xue and Ahring 2011; Zhao et al. 2011). Interestingly, our results showed that idi exhibited more significant effect on isoprene production than dxs and dxr. In the MEP pathway, idi appeared function as a salvage enzyme, adjusting the intracellular concentrations of IPP and DMAPP (Kajiwara et al. 1997). It has been extensively reported that IPP and DMAPP were produced in a 5:1 ratio by the catalysis of IspH (MEP pathway branch enzyme) in E. coli (Gräwert et al. 2004; Rohdich et al. 2002; Xiao et al. 2008). It has been proposed that the idi favors the production of DMAPP, adjusting this ratio to physiologically balanced level (Ajikumar et al. 2010). Since DMAPP is the immediate precursor of isoprene, overexpression of idi might increase the DMAPP concentration so as to significantly enhance the isoprene production. It is suggested that idi could be regarded as a key regulating point for isoprene production.
To further enhance isoprene production, genes dxs, dxr, and idi were coexpressed. The results showed that the isoprene production was further improved. It was revealed that the synergy of several rate-limiting enzymes made more contribution to the overall yield of final product than overexpression of any single gene.
When the limiting genes were overexpressed, the balance of the MEP pathway was important for high isoprene yield. A series of polycistronic operons were constructed via rearranging the gene (dxs, dxr, and idi) order, which resulted in different isoprene production. Moreover, among the six combinatorial orders of the three genes, the gene order with the highest isoprene production yield was consistent with that of the metabolic pathway. This phenomenon also appeared in the research on crtE, crtB, crtI, crtY, crtZ order assembly for carotenoid biosynthesis reported by Nishizaki et al. (2007). Because the gene transcription and translation are generally coupled in prokaryotic expression system, the gene ranked in front will be translated primarily, followed by the translation of the subsequent genes along with the mRNA transcription for polycistronic operons in E. coli. When the order of the limiting genes in polycistronic operons corresponds to that of isoprene metabolic pathway (dxs–dxr–idi), the upstream bottleneck could be eliminated, followed by the downstream flux enhancement. Thus, the quantity of final product could be improved. It fits the natural fine regulation mechanism of microorganisms that the closer the enzyme to the beginning of the pathway, the shorter the response time of the activation of its promoter (Zaslaver et al. 2004). Additionally, as shown in Fig. 7 and Table 2, isoprene production was closely related to relative dxs expression. This suggested that dxs became a key regulating point when downstream bottlenecks (dxr and idi) were eliminated. In conclusion, the gene order in this biosynthetic pathway is a very important determinant for final product yield. In addition, a sequential expression pattern according to the biosynthetic pathway could be optimized for more efficient synthesis of the product in metabolic pathway.
Although isoprene production was improved significantly in this study, many efforts are also needed to achieve the industrial production with higher yield and lower cost. On one hand, the productivity can be further improved by metabolic flux regulation (such as blocking the competing pathway through gene knockout method) and improving isoprene enzyme activity. On the other hand, to decrease the process cost, the fermentation conditions can be optimized. For example, LB medium is not suitable for industrial processes due to its price, and should be replaced by a more industrial medium such as marine mineral culture (M9). Moreover, although incompatible plasmids system can be used for the coexpression of several genes, different antibiotics led to the growth inhibition for industrial long-term productivity. To overcome this problem, the recombinant genes may be integrated into the chromosome replicon of a host for industrial fermentation which will be conducted in our future work.
References
Ajikumar PK, Xiao WH, Tyo KEJ, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G (2010) Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 330:70
Albrecht M, Misawa N, Sandmann G (1999) Metabolic engineering of the terpenoid biosynthetic pathway of Escherichia coli for production of the carotenoids β-carotene and zeaxanthin. Biotechnol Lett 21:791–795
Emerman M, Temin HM (1984) Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell 39:459–467
Ghattas IR, Sanes J, Majors J (1991) The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol 11:5848–5859
Gräwert T, Kaiser J, Zepeck F, Laupitz R, Hecht S, Amslinger S, Schramek N, Schleicher E, Weber S, Haslbeck M (2004) IspH protein of Escherichia coli: studies on iron–sulfur cluster implementation and catalysis. J Am Chem Soc 126(40):12847–12855
Johnston K, Clements A, Venkataramani RN, Trievel RC, Marmorstein R (2000) Coexpression of proteins in bacteria using T7-based expression plasmids: expression of heteromeric cell-cycle and transcriptional regulatory complexes. Protein Expr Purif 20(3):435–443
Kajiwara S, Fraser PD, Kondo K, Misawa N (1997) Expression of an exogenous isopentenyl diphosphate isomerase gene enhances isoprenoid biosynthesis in Escherichia coli. Biochem J 324:421
Kesselmeier J, Staudt M (1999) Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem 33:23–88
Kim SW, Keasling J (2001) Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol Bioeng 72:408–415
Kuzuyama T (2002) Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Biosci Biotechnol Biochem 66:1619–1627
Lehning A, Zimmer I, Steinbrecher R, Bruggemann N, Schnitzler JP (1999) Isoprene synthase activity and its relation to isoprene emission in Quercus robur L. leaves. Plant Cell Environ 22:495–504
Lichtenthaler HK (2000) Non-mevalonate isoprenoid biosynthesis: enzymes, genes and inhibitors. Biochem Soc Trans 28:785–789
Liu M, Yu H (2012) Cocktail production of an endo-β-xylanase and a β-glucosidase from Trichoderma reesei QM 9414 in Escherichia coli. Biochem Eng J 68:1–6
Lois LM, Rodríguez-Concepción M, Gallego F, Campos N, Boronat A (2000) Carotenoid biosynthesis during tomato fruit development: regulatory role of 1-deoxy-d-xylulose 5-phosphate synthase. Plant J 22:503–513
Matthews P, Wurtzel ET (2000) Metabolic engineering of carotenoid accumulation in Escherichia coli by modulation of the isoprenoid precursor pool with expression of deoxyxylulose phosphate synthase. Appl Microbiol Biotechnol
Miller B, Heuser T, Zimmer W (2000) Functional involvement of a deoxy–xylulose 5-phosphate reductoisomerase gene harboring locus of Synechococcus leopoliensis in isoprenoid biosynthesis. FEBS Lett 481:221–226
Miller B, Oschinski C, Zimmer W (2001) First isolation of an isoprene synthase gene from poplar and successful expression of the gene in Escherichia coli. Planta 213:483–487
Monson RK, Fall R (1989) Isoprene emission from aspen leaves—influence of environment and relation to photosynthesis and photorespiration. Plant Physiol 90:267–274
Nishizaki T, Tsuge K, Itaya M, Doi N, Yanagawa H (2007) Metabolic engineering of carotenoid biosynthesis in Escherichia coli by ordered gene assembly in Bacillus subtilis. Appl Microbiol Biotechnol 73:1355–1361
Rohdich F, Hecht S, Gärtner K, Adam P, Krieger C, Amslinger S, Arigoni D, Bacher A, Eisenreich W (2002) Studies on the nonmevalonate terpene biosynthetic pathway: metabolic role of IspH (LytB) protein. PNAS 99:1158
Sanadze J (1957) Emission of organic matters by leaves of Robinia pseudoacacia L. Soobshch Akad Nauk Gruz SSR 19:83–86
Sasaki K, Ohara K, Yazaki K (2005) Gene expression and characterization of isoprene synthase from Populus alba. FEBS Lett 579(11):2514–2518
Sharkey TD, Yeh S, Wiberley AE, Falbel TG, Gong DM, Fernandez DE (2005) Evolution of the isoprene biosynthetic pathway in Kudzu. Plant Physiol 137:700–712
Xiao Y, Zhao ZK, Liu P (2008) Mechanistic studies of IspH in the deoxyxylulose phosphate pathway: heterolytic CO bond cleavage at C4 position. J Am Chem Soc 130:2164–2165
Xue JF, Ahring BK (2011) Enhancing isoprene production by genetic modification of the 1-deoxy-d-xylulose-5-phosphate pathway in Bacillus subtilis. Appl Environ Microbiol 77:2399–2405
Yang W, Zhang L, Lu Z, Tao W, Zhai Z (2001) A new method for protein coexpression in Escherichia coli using two incompatible plasmids. Protein Expr Purif 22:472–478
Zaslaver A, Mayo AE, Rosenberg R, Bashkin P, Sberro H, Tsalyuk M, Surette MG, Alon U (2004) Just-in-time transcription program in metabolic pathways. Nat Genet 36:486–491
Zhao YR, Yang JM, Qin B, Li YH, Sun YZ, Su SZ, Xian M (2011) Biosynthesis of isoprene in Escherichia coli via methylerythritol phosphate (MEP) pathway. Appl Microbiol Biotechnol 90:1915–1922
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (grant no. 21176215), Outstanding Young Scholar of Zhejiang Province (grant no. R4110092) and the Program for Zhejiang Leading Team of S&T Innovation (grant no.2011R50007). We are grateful for all editor and reviewers for their helpful advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, X., Xu, H. & Yu, H. Significantly enhanced production of isoprene by ordered coexpression of genes dxs, dxr, and idi in Escherichia coli . Appl Microbiol Biotechnol 97, 2357–2365 (2013). https://doi.org/10.1007/s00253-012-4485-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4485-2