Abstract
In the present study, the factors influencing density of granular sludge particles were evaluated. Granules consist of microbes, precipitates and of extracellular polymeric substance. The volume fractions of the bacterial layers were experimentally estimated by fluorescent in situ hybridisation staining. The volume fraction occupied by precipitates was determined by computed tomography scanning. PHREEQC was used to estimate potential formation of precipitates to determine a density of the inorganic fraction. Densities of bacteria were investigated by Percoll density centrifugation. The volume fractions were then coupled with the corresponding densities and the total density of a granule was calculated. The sensitivity of the density of the entire granule on the corresponding settling velocity was evaluated by changing the volume fractions of precipitates or bacteria in a settling model. Results from granules originating from a Nereda reactor for simultaneous phosphate COD and nitrogen removal revealed that phosphate-accumulating organisms (PAOs) had a higher density than glycogen-accumulating organisms leading to significantly higher settling velocities for PAO-dominated granules explaining earlier observations of the segregation of the granular sludge bed inside reactors. The model showed that a small increase in the volume fraction of precipitates (1–5 %) strongly increased the granular density and thereby the settling velocity. For nitritation–anammox granular sludge, mainly granular diameter and not density differences are causing a segregation of the biomass in the bed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aerobic granular sludge offers an interesting alternative for conventional activated sludge systems. This technology relies on compact and self-immobilised granulated biomass. By applying short settling times in a sequencing batch process, only big and rapidly settling biomass aggregates are selected, while flocculent sludge is washed out (Morgenroth et al. 1997; Beun et al. 1999; Etterer and Wilderer 2001; de Kreuk et al. 2005). The parameters determining the settling velocity of particles and in turn biomass washout are of crucial importance to granular sludge technology. The settling velocity is influenced by particle size and shape, and the difference between the density of the water and the particles (Winkler et al. 2012). Recently, we demonstrated that in aerobic granular sludge, vertical segregation of granules occurs, resulting from small differences in granular settling velocity. Fast- or slow-settling particles showed different microbial composition and activities. Selective sludge withdrawal from either the top or bottom of the sludge bed was used to control the microbial community structure and enhance desired removal processes (Winkler et al. 2011a, b; Bassin et al. 2012; Volcke et al. 2012). In a heterotrophic system, it was shown that glycogen-accumulating organisms (GAO) dominated the slower settling granules, and the preferred phosphate-accumulating bacteria dominated the fast-settling granules which were hence favoured for substrate since the reactor was fed in a plug flow regime from the bottom of the reactor. In an autotrophic reactor system, the fast-settling granules consisted of aerobic (ammonium-oxidising bacteria (AOB)) and anaerobic ammonium-oxidising (anammox) bacteria, whereas slow-settling particles harboured the undesired nitrite-oxidising bacteria (NOB) (Vlaeminck et al. 2010; Winkler et al. 2011b; Volcke et al. 2012). In both cases, sludge withdrawal from the top of the sludge bed ensured a stable and well-working process. Segregation was also observed in fluidized bed biofilm reactors where bigger granules were reported at the bottom of the reactor setup (Ro and Neethling 1994; DiFelice et al. 1997; Sliekers et al. 2003). The major factors for fast-settling granules are size and density (Nicolella et al. 2000; Nor Anuar et al. 2007; Winkler et al. 2011b, 2012). Factors influencing granular density are chemical precipitates and the microbial community structure itself. Calcium phosphate precipitations are formed easily in the enhanced biological phosphorous removal (EBPR) process (Arvin 1983; Carlsson et al. 1997; Clark et al. 1997; Maurer et al. 1999; Angela et al. 2011) and are also reported in granular systems (Yamaguchi et al. 2001; de Kreuk et al. 2005; Angela et al. 2011; Bassin et al. 2012). Precipitates in the inner core of the granule are known for anaerobic and aerobic granules and are in both systems discussed to be induced due to pH shifts within the granules caused by microbiological activities (Yamaguchi et al. 2001; Angela et al. 2011). Reported values of granular densities vary between 1,005 and 1,070 kg/m3 (Batstone and Keller 2001; Etterer and Wilderer 2001; Bassin et al. 2012). However, not only precipitates influence the overall granular density. Bacteria can occupy large volume fractions of the granules, and although less dense than precipitates, they will contribute to granular densities and hence settling characteristics. Storage polymers such as polyphosphate, glycogen and polyhydroxyalkanoates will contribute to the overall density (Oshiki et al. 2010). Given the importance of particle density for segregation of biomass in granular sludge beds, it is interesting to investigate the factors influencing the density of sludge particles (Bin et al. 2011; Winkler et al. 2011b; Volcke et al. 2012). The goal of this study was therefore to determine the influence of precipitates and the microbial community structure on the density of granular sludge and in turn evaluate their effect on settling velocity.
Materials and methods
Biomass characterisation
Fluorescent in situ hybridisation
Granules from completely autotrophic nitrogen removal over nitrite (CANON) and EBPR reactors as previously described (Winkler et al. 2011a, b) were inspected by microscopic analysis to assess morphology and microbiological composition. Slicing was performed after fixation in 4 % paraformaldehyde. Granules were embedded in a tissue-freezing medium (Leica Microsystems) hardened by freezing (−20 °C) and cut in the frozen state with a microtome–cryostat (Leica CM1900-Cryostat) into 25 μm thin slices. Dried slices were kept on a microscopic glass slide and fluorescent in situ hybridisation (FISH) was performed for determination of anammox (Cy3) AOB (Cy5) and NOB (Fluos) (for CANON reactor) as well as phosphate-accumulating organisms (PAOs) (Cy5), GAOs (Fluo) and nitrifyers (Cy3) (for the EBPR reactor) following the same procedure as previously described (Winkler et al. 2011b). Sequences are listed in Table 1.
Origin of bacteria and Percoll density distribution
Different bacteria were either grown in our laboratories or provided by other researchers. All strains provided by others were fixed in 4 % paraformaldehyde and incubated for 120 min at room temperature. After fixation, samples were centrifuged for 2 min at 16,000 rpm, washed twice in 1× phosphate-buffered saline (PBS) and resuspended in a volume of 1:1 ethanol/PBS buffer for storage at −20 °C. Nitrobacter and Nitrosomonas were harvested from chemostat reactors, and their purity and cell concentration was controlled via direct cell counts using a Bright-Line haemocytometer (Hausser Scientific, Horsham PA). For the bacteria from other laboratories, purity was given by the researchers, who determined their purity by FISH as well as qPCR and results are given in Table 2. Percoll density centrifugation is a useful tool to measure bacterial densities and has been mainly used for cell separation (Scherer 1983; Beaty et al. 1987; Strous et al. 1999) and by some researchers also to directly measure the density of cells (Woldringh et al. 1981; Putzer et al. 1991) or the density of extracted storage polymers (Oshiki et al. 2010). Percoll particles have a very dense (2.2 g/ml) inner core of silica with an average particle size of 29–30 nm (in 0.15 M NaCl). These particles partially settle during centrifugation, forming an uneven distribution of particles by which a density gradient is formed (Laurent et al. 1979). The bacteria loaded on this gradient will sediment to an equilibrium position, at which the gradient density is equal to the density of the bacteria. During this process, the bacteria are separated solely on the basis of differences in their densities, irrespective of their size (Amersham Biosciences 1995). An isotonic stock solution was made by mixing 45 ml of Percoll (GE, Healthcare) with 5 ml of 1.5 M NaCl solution. Next, this isotonic Percoll stock solution was used to create an 80 % (v/v) working solution by using NaCl (1.5 mM). The tubes were centrifuged in a fixed angle rotor (Beckmann Optima TL Ultracentrifuge) at 27,000×g for 45 min at room temperature to allow a self-forming gradient to develop by isopycnic centrifugation. All cell pellets and aggregates were crushed by the means of a glass mortar (Glas-Col), and 25 μl of the resulting cell suspension was loaded on the gradient tubes. Five density marker beads (Cosmopheric) in the range of 1.028–1.13 (in grams per millilitre) were used as internal standards, and their height was recorded to fit the measured bacterial densities by interpolation using a polynomial regression fit. All measurements were repeated five to ten times to determine a standard deviation.
Chemical precipitation
CT scan
Granules of bottom and top sludge were analysed by a nanofocus computed tomography Micro Scanner (phoenix nanotom s, GE). Granules were placed into a straw and fixed on the rotating plate and were exposed to a 180 kV/15 W ultra-high performance nanofocus X-ray tube enabling a non-destructive visualisation of the granules. Components of higher densities appeared white on the images (Fig. 2).
Microelectrode measurements
pH profiles were measured with a 25-μm microelectrode (Unisense , Aarhus, Denmark). Medium concentrations were as described previously (Winkler et al. 2011a, b). The data acquisition system consisted of a picoamperometer (Unisense, Denmark; model PA2000), an A/D converter (Pico Technology Ltd, UK; model ADC-101) and a computer to log the sensor data and the microsensor motion. Motion control consists of a motorised two-dimensional stage (Phytron Inc., USA, model MT-65) and a motorised micromanipulator (Unisense, Denmark, model MM3M), controlling the position of the microelectrode in three axis. Sensors were equipped with silver/silver chloride anode and a gold-plated platinum cathode. Bulk dissolved oxygen concentration was controlled using a mass flow controller for air and dinitrogen gas and the medium was then fed into to a chamber, which was used for microelectrode measurements.
PHREEQC
PHREEQC for Windows (version 2) was used to calculate the saturation index for potential precipitates. The programme uses a Newton–Raphson numerical method to solve a system of non-linear equations. The sensitivity to pH and phosphate concentration was evaluated. The saturation index was calculated based on the concentration as present in the media of our lab reactors (Winkler et al. 2011a) and concentrations are listed in Supplementary material (Table S1). The sensitivity analysis for acidity was performed in the range from 5.5 until 10, in step sizes of 0.5 pH units. The phosphate concentrations were varied as well to see the effect of biological phosphate release on precipitation equilibria. Saturation indices (SI) larger than 1 indicate supersaturation and hence the increased ability of precipitation of components.
Settling model
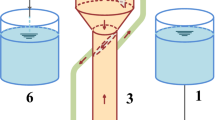
In our observations, granules can be divided in two to three layers consisting of either bacteria, extracellular polymeric substances (EPS) and/or chemical precipitates (Fig. 1). According to these observations, we evaluated our settling model. Heterotrophic granules from our reactors generally showed a densely populated outer rim of nitrification and heterotrophic bacteria (De Kreuk et al. 2007; Xavier et al. 2007). Underneath this outer layer, a low-density layer was found consisting mainly of EPS. The inner core of the granules generally contained large amounts of precipitates (Fig. 1, A). Autotrophic, nitrogen-removing granules generally consisted of a dense outer rim with AOB and a dense inner core with anammox bacteria. When nitrite oxidisers were present, they usually resided between the AOB and anammox bacteria (Ni et al. 2009; Volcke et al. 2010a, 2010b; Winkler et al. 2011b) (Fig. 1, B). For all simulations performed, the settling velocity was calculated based on granular diameter and granular densities. The thickness of each layer was estimated by CT scan and FISH pictures (Fig. 2) to calculate the volume fractions occupied by each compound. The structure as schematically drawn in Fig. 1 was used to predict the settling velocity of granules within an EBPR system and CANON system. The average density of the whole granule (ρ p ) was calculated by multiplying the different volume fraction by the density of the components consisting of either bacteria, EPS or precipitates \( {\rho_p}=\left( {V_{\mathrm{A}}^{\prime}\cdot {\rho_{\mathrm{A}}}} \right)+\left( {V_{\mathrm{B}}^{\prime}\cdot {\rho_{\mathrm{B}}}} \right)+\left( {V_{\mathrm{C}}^{\prime}\cdot {\rho_{\mathrm{C}}}} \right) \) (Appendix). A density of EPS could not be found in literature (), but since EPS largely consists of water, it was assumed to be equal to the density of water (Flemming et al. 2007; Flemming and Wingender 2010). The density of calcium phosphate (2.2 g/ml) was used for the precipitates (International Union of Pure and Applied Chemistry 1979). The overall density (ρ p ) of the granule was then used to calculate the theoretical settling behaviour of a granule according to the procedure by Winkler et al. (2012).
Schematic view of a granule originating from an enhanced biological phosphorous removal (EBPR) system (1) consisting of three layers of bacteria (A), EPS (B) and chemical precipitates (C) as well as a granule originating from an completely autotrophic nitrogen removal over nitrite (CANON) process (2) consisting of three layers of bacteria: AOB (A), NOB (B) and anammox (C)
Results
Characterisation of the sludge granules
The results from the CT scan, pH microelectrode measurements as well FISH for heterotrophic and autotrophic sludge granules are presented in Fig. 2. The CT scan originating from the heterotrophic EBPR system (left) and autotrophic CANON system (right) indicated that the granular makeup was very different for these two cases. In the EBPR system, higher densities were measured in the middle of the granule, whereas this was not observed for the nitritation–anammox granules originated form a CANON reactor. FISH on sliced granules confirmed these differences. The autotrophic granules showed AOB in the outer layers and anammox bacteria growing equally distributed in the inner core, whereas the EBPR granules contained few bacteria in the core of the granule. The EBPR granules showed the typical spatial distribution of nitrifiers in the oxygen-penetrated layers (Cy3) as well as PAOs (Cy5) and GAOs (Fluos) in the outer layers (Fig. 2c, d). The pH profiles from granules obtained from a measurement mimicking anaerobic conditions within a EBPR system showed a more significant pH increase towards the core of the granule, as compared to an anammox granule (0.6 instead of 0.1) (Fig. 2e, f).
Density of bacteria
The effect of fixation with 4 % paraformaldehyde was tested on four different strains and the density difference (in grams per millilitre) was negligibly small (less than 0.1 %). The densities of different bacteria are summarised in Table 3. The highest density was measured for the nitrifying bacteria whereas the lowest for the GAOs. There was no significant density differences between GAO bacteria harvested from the aerobic or anaerobic phase. However, a clear difference of 12 g/ml was measured for PAO bacteria harvested from the aerobic and anaerobic phase of the system. Anammox bacteria (Candidatus Brocardia fulgida) had a density of 1.048 ± 0.002 g/ml and were hence denser than GAO bacteria but had a lower density than the rest of all bacteria.
Chemical precipitation
We calculated the saturation index values with PHREEQC based on the media composition as given in the online material (Supplementary Table S1). PHREEQC calculated circa 40 minerals but we only showed those which had the highest (positive) SI values. If the SI is equal to 1, the equilibrium between the mineral and the solution is reached. In the case that a SI of a mineral increases above 1, it is thermodynamically possible that the mineral can precipitate. A higher pH and phosphate concentration increase the SI for all minerals and led to supersaturation especially for fluoroapatite, hydroxydicalciumphosphate and hydroxyapatite (Fig. 3).
Evaluations of the settling model
We evaluated three different cases with our settling model (Fig. 4a–c). All cases are based on the observed volume fractions from CT scan and FISH pictures (Fig. 2) as well as based on evaluated densities (Table 3; Fig. 3). Cases A and B are based on the results gained from the EBPR system (Fig. 1, picture 1). In case A, we assumed that there is no precipitation and the outer layer (A) is either occupied by PAOs or by GAOs. The thickness was chosen to be not deeper than 200 μm (corresponds to a volume fraction of 70 % at a fixed diameter of 1.2 mm; Fig. 4a) to be consistent with the observed bacterial layer from sliced granules stained by FISH (Fig. 2c). We chose the densities of the organisms in the end of the aerobic phase (Table 3) because in granular reactors the settling occurs immediately after the aeration stops. During this phase, PAOs are expected to be enriched with polyphosphate granules. Our results show that the settling velocity of PAOs granule was significantly increased when compared to GAO-dominated granules by up to a factor 2. In case B, the thickness of the bacterial layer (aerobic PAOs and GAOs) was fixed at 50 μm (corresponding to 23 % volume fraction) and only the volume fraction occupied by precipitates was varied showing that small changes in the inorganic volume fraction (5 %) are severely affecting settling velocities by a factor of up to 6.
Calculated settling velocities and granular densities with respect to varying volume fractions of: a bacteria and b precipitates for a PAO- (squares) and GAO-dominated (diamonds) granules as well as c calculated settling velocities (solid line) and corresponding granular densities (dashed line) for a CANON granule with respect to varying diameter of a granules dominated by either AOB and anammox (squares) or AOB and NOB (diamonds)
Case C refers to the CANON reactor (Fig. 1, picture 2). Since nitrifiers only occupy the oxygen-penetrated outer layer, which is typically not deeper than 80 μm we assumed for AOBs and NOBs a maximal thickness of 80 μm (corresponding to a volume fraction of 35 %) (Winkler et al. 2011b). Here, we once assumed a granule consisting of an outer layer of AOB (80 μm) and an inner anoxic layer consisting of anammox and once a granule consisting of an outer layer of AOB (50 μm) followed by NOB (30 μm) and an inner layer consisting of EPS. We then tested the effect on settling velocity by changing the diameter of the granule. Results showed that at smaller granular diameters, the settling velocities and granular densities were very similar for both types of granules and increased for the anammox-dominated granules when granular diameter was increased (Fig. 4c).
Discussion
The results presented here show that differences in density of different types of bacteria and differences in the inorganic volume fraction can significantly alter settling velocity of granules and hence influence the earlier described segregation effect within granular sludge systems (Winkler et al. 2011a, b; Bassin et al. 2012; Volcke et al. 2012). The higher density of PAOs in combination with intracellular stored poly-P resulted in higher settling velocities of PAO-dominated granules when compared to a GAO-dominated granule (Fig. 4a). Another factor influencing the settling velocity is the inorganic fraction. The contribution of precipitates significantly increased granular settling velocity as shown by our settling model (Fig. 4b). A change in volume fraction from 1 to 5 % accounts for a change in settling velocity from 20 to 90 m/h. The small volume fraction occupied by inorganic components will account for 10–30 % of the total dry weight as indicated by general ash contents measured in other granular studies (Batstone and Keller 2001; Etterer and Wilderer 2001; Bassin et al. 2012). The precipitation of components is provoked at higher pH and phosphate concentration (Fig. 3). The observation that precipitation was detected mainly in the inner core is in line with earlier research (Maurer et al. 1999; Yamaguchi et al. 2001; Angela et al. 2011) and can be explained by the higher pH in the middle of the granules and higher phosphate concentrations caused by phosphate release of PAOs during the anaerobic feeding period (Fig. 2e, f). The strong increase of the pH inside an EBPR granule can be explained by the uptake (H+ removal from bulk) of the acidic component acetic acid by PAOs and GAOs. However magnesium- and potassium phosphates (Mg2+, K+ are counter ions from the poly-P) released by PAOs will have a buffering effect, reducing the pH. Although this buffering effect disfavours precipitation, the higher phosphate release by PAOs will favour precipitation of phosphate minerals (Fig. 3) (Smolders et al. 1994; Lopez-Vazquez et al. 2008; Lopez-Vazquez et al. 2009a, b). The complexity of precipitation equilibria within a granule merely dominated by PAOs or GAOs would need to be modelled mathematically to further investigate in which granule one can expect more precipitates. Experimental data showed a considerably higher PAO fraction and higher ash contents at the bottom of the settled sludge bed (Winkler et al. 2011a; Bassin et al. 2012). A directed change in operational conditions to favour precipitation, e.g. by adding higher phosphate concentrations for several cycles, might be used to favour chemical precipitation and by this a segregation in granular reactors. However, care must be taken since too dense granules can cause mixing problems or clogging (Nicolella et al. 2000; Safferman and Bishop 1996). Segregation in an EBPR system is not only influenced by a higher inorganic fraction within the granules but also by PAO densities and internally stored polyphosphate, which will be accumulated in the biomass after the aerobic period (Fig. 4a). Internal storage compounds can influence the density of bacteria. This was also found previously by others who measured the effect of sulphur inclusions on the density of bacteria Chromatium warmingii (this resulted in a density range of 1.071–1.108 g/ml) (Guerrero et al. 1984). Our results show that in granular sludge systems segregation of biomass can easily occur due to slight variations in density of bacteria and precipitates.
For the CANON system, our results from FISH and CT scan show that precipitation is playing a less important role than in an EBPR system and also the detected pH shift was lower than in EBPR granules, indicating that the internal pH plays an important role in the formation of precipitates in granular systems (Figs. 2 and 3). The settling model shows that in the competition for settling speed between a granule occupied by either anammox and AOB or by NOB and AOB, granular diameter is playing an important role (Fig. 4c). Experimental and mathematical models have shown that in bigger granules NOB bacteria are outcompeted by anammox bacteria. NOB are expected to grow in smaller granules due to a higher aerobic volume fraction (Vlaeminck et al. 2010; Winkler et al. 2011b; Volcke et al. 2012). Since the settling velocity of a granule is influenced by size and density and NOB are expected to grow in smaller granules, this fact will lead to slower settling velocities. While anammox bacteria can grow in deeper layers of the granules, AOB and NOB are restricted to grow in oxygen-penetrated layers of the granules. This will hence lead to a decrease in density with increasing diameter (Fig. 4c). Oxygen penetration depth can be calculated by standard formulas and can be expected to not be higher than circa 80 μm in CANON systems since it is run under lower oxygen concentrations to keep the accumulation of NOB bacteria low (Harremoës 1977; Hao et al. 2005; Winkler et al. 2011b). Therefore, the contribution of the density from nitrifying bacteria will be limited in systems consisting of mainly bigger granules. However in systems consisting of mostly granules with a small diameter, a segregation will be difficult to achieve because the density of NOB is much higher than the density of AOB or anammox (Table 3). For this reason, it is important to select for bigger granules in CANON system (Fig. 4c).
We observed significant density differences among the autotrophic bacteria. Nitrobacter winogradskyi (NOB) had the highest density and Candidatus B. fulgida (anammox) had the lowest density. It is likely that these differences can be explained by the cellular makeup of these organisms, but it is presently impossible to say which factor may determine these differences and whether the observed densities are intrinsic properties of these organisms or caused by the cultivation conditions. For Escherichia coli, different studies have reported densities between 1.05 and 1.11 g/ml which is most likely due to different cultivation methods, strains and different Percoll procedures used (Koch and Blumberg 1976; Martinez-Salas et al. 1981; Woldringh et al. 1981).
Selective sludge removal has successfully been implemented to control microbial populations in aerobic granular sludge systems (Winkler et al. 2011a, b; Bassin et al. 2012). Since anaerobic granules also have a multistructural layer of microorganisms, a segregation based on different physical–chemical differences of granules can be equally expected in these systems underlining the importance of this research also for other reactor systems (Macleod et al. 1990). Our results show that microorganisms which adhere to the granule strongly influence subsequent physical (densities) and chemical (precipitates) properties of the granule and hence their settling velocity.
References
Amersham Biosciences (1995) Handbook from Amersham Biosciences, applications for Percoll. ISBN 18-1115-69
Angela M, Béatrice B, Mathieu S (2011) Biologically induced phosphorus precipitation in aerobic granular sludge process. Water Res 45(12):3776–3786
Arvin E (1983) Observations supporting phosphate removal by biologically mediated chemical precipitation. A review. Water Sci Technol 15(3–4):43–63
Bassin JP, Winkler M-KH, Kleerebezem R, Dezotti M, van Loosdrecht MCM (2012) Relevance of selective sludge removal in segregated aerobic granular sludge reactors to control PAO-GAO competition at different temperatures. Biotechnol Bioeng 109(8):1919–1928
Batstone DJ, Keller J (2001) Variation of bulk properties of anaerobic granules with wastewater type. Water Res 35(7):1723–1729
Beaty PS, Wofford NQ, McInerney MJ (1987) Separation of Syntrophomonas wolfei from Methanospirillum hungatii in syntrophic cocultures by using Percoll gradients. Appl Environ Microb 53(5):1183–1185
Beun JJ, Hendriks A, Van Loosdrecht MCM, Morgenroth E, Wilderer PA, Heijnen JJ (1999) Aerobic granulation in a sequencing batch reactor. Water Res 33(10):2283–2290
Bin Z, Zhe C, Zhigang Q, Min J, Zhiqiang C, Zhaoli C, Junwen L, Xuan W, Jingfeng W (2011) Dynamic and distribution of ammonia-oxidizing bacteria communities during sludge granulation in an anaerobic–aerobic sequencing batch reactor. Water Res 45(18):6207–6216
Carlsson H, Aspegren H, Lee N, Hilmer A (1997) Calcium phosphate precipitation in biological phosphorus removal systems. Water Res 31(5):1047–1055
Chandran K, Love NG (2008) Physiological state, growth mode, and oxidative stress play a role in Cd(II)-mediated inhibition of Nitrosomonas europaea 19718. Appl Environ Microb 74(8):2447–2453
Clark T, Stephenson T, Pearce PA (1997) Phosphorus removal by chemical precipitation in a biological aerated filter. Water Res 31(10):2557–2563
Crocetti GR, Hugenholtz P, Bond PL, Schuler A, Keller J, Jenkins D, Blackall LL (2000) Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. Appl Environ Microb 66(3):1175–1182
Crocetti GR, Banfield JF, Keller J, Bond PL, Blackall LL (2002) Glycogen-accumulating organisms in laboratory-scale and full-scale wastewater treatment processes. Microbiol 148(11):3353–3364
Daims H, Nielsen JL, Nielsen PH, Schleifer KH, Wagner M (2001) In situ characterization of Nitrospira-like nitrite oxidizing bacteria active in wastewater treatment plants. Appl Environ Microb 67(11):5273–5284
de Kreuk MK, Pronk M, van Loosdrecht MCM (2005) Formation of aerobic granules and conversion processes in an aerobic granular sludge reactor at moderate and low temperatures. Water Res 39(18):4476–4484
De Kreuk MK, Picioreanu C, Hosseini M, Xavier JB, Van Loosdrecht MCM (2007) Kinetic model of a granular sludge SBR: influences on nutrient removal. Biotechnol Bioeng 97(4):801–815
DiFelice R, Nicolella C, Rovatti M (1997) Mixing and segregation in water fluidised-bed bioreactors. Water Res 31(9):2392–2396
Etterer T, Wilderer PA (2001) Generation and properties of aerobic granular sludge. Water Sci Technol 43(3):19–26
Flemming HC, Wingender J (2010) The biofilm matrix. Nature Rev Microbiol 8(9):623–633
Flemming HC, Neu TR, Wozniak DJ (2007) The EPS matrix: the “House of Biofilm Cells”. J Appl Bacteriol 189(22):7945–7947
Guerrero R, Mas J, Pedros-Alio C (1984) Buoyant density changes due to intracellular content of sulfur in Chromatium warmingii and Chromatium vinosum. Arch Microbiol 137(4):350–356
Hao XD, Cao XQ, Picioreanu C, van Loosdrecht MCM (2005) Model-based evaluation of oxygen consumption in a partial nitrification–anammox biofilm process. Water Sci Technol 52(7):155–160
Harremoës P (1977) Half-order reactions in biofilm and filter kinetics. Vatten 33:122–143
International Union of Pure and Applied Chemistry (1979) Nomenclature of organic chemistry. Pergamon, Oxford. ISBN 0-08022-3699
Koch AL, Blumberg G (1976) Distribution of bacteria in the velocity gradient centrifuge. Biophys J 16(5):389–405
Laurent TC, Alexander G, Ogston HP, Carlsson B (1979) Physical chemical characterization of Percoll. II. Size and interaction of colloidal particles. J Colloid Interf Sci 76(1):133–141
Lopez-Vazquez CM, Song YI, Hooijmans CM, Brdjanovic D, Moussa MS, Gijzen HJ, Van Loosdrecht MCM (2008) Temperature effects on the aerobic metabolism of glycogen-accumulating organisms. Biotechnol Bioeng 101(2):295–306
Lopez-Vazquez CM, Hooijmans CM, Brdjanovic D, Gijzen HJ, van Loosdrecht MCM (2009a) Temperature effects on glycogen accumulating organisms. Water Res 43(11):2852–2864
Lopez-Vazquez CM, Oehmen A, Hooijmans CM, Brdjanovic D, Gijzen HJ, Yuan Z, van Loosdrecht MCM (2009b) Modeling the PAO-GAO competition: effects of carbon source, pH and temperature. Water Res 43(2):450–462
Macleod FA, Guiot SR, Costerton JW (1990) Layered structure of bacterial aggregates produced in an upflow anaerobic sludge bed and filter reactor. Appl Environ Microb 56(6):1598–1607
Martinez-Salas E, Martin JA, Vicente M (1981) Relationship of Escherichia coli density to growth rate and cell age. J Appl Bacteriol 147(1):97–100
Maurer M, Abramovich D, Siegrist H, Gujer W (1999) Kinetics of biologically induced phosphorus precipitation in waste-water treatment. Water Res 33(2):484–493
Mobarry BK, Wagner M, Urbain V, Rittmann BE, Stahl DA (1996) Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl Environ Microb 62(6):2156–2162
Morgenroth E, Sherden T, Van Loosdrecht MCM, Heijnen JJ, Wilderer PA (1997) Aerobic granular sludge in a sequencing batch reactor. Water Res 31(12):3191–3194
Ni BJ, Chen YP, Liu SY, Fang F, Xie WM, Yu HQ (2009) Modeling a granule-based anaerobic ammonium oxidizing (ANAMMOX) process. Biotechnol Bioeng 103(3):490–499
Nicolella C, van Loosdrecht MCM, Heijnen SJ (2000) Particle-based biofilm reactor technology. Trends Biotechnol 18(7):312–320
Nor Anuar A, Ujang Z, van Loosdrecht MCM, de Kreuk MK (2007) Settling behaviour of aerobic granular sludge. Water Sci Technol 56:55–63
Oshiki M, Onuki M, Satoh H, Mino T (2010) Separation of PHA-accumulating cells in activated sludge based on differences in buoyant density. J Gen Appl Microbiol 56(2):163–167
Putzer KP, Buchholz LA, Lidstrom ME, Remsen CC (1991) Separation of methanotrophic bacteria by using Percoll and its application to isolation of mixed and pure cultures. Appl Environ Microb 57(12):3656–3659
Ro KS, Neethling JB (1994) Biological fluidized-beds containing widely different bioparticles. J Environ Eng 120(6):1416–1426
Safferman SI, Bishop PL (1996) Aerobic fluidized bed reactor with internal media cleaning. J Environ Eng 122:284–291
Scherer P (1983) Separation of bacteria from a methanogenic wastewater population by utilizing a self-generating Percoll gradient. J Appl Bacteriol 55(3):481–486
Schmid M, Walsh K, Webb R, Rijpstra WIC, van de Pas-Schoonen K, Verbruggen MJ, Hill T, Moffett B, Fuerst J, Schouten S, Damste JSS, Harris J, Shaw P, Jetten M, Strous M (2003) Candidatus “Scalindua brodae”, sp nov., Candidatus “Scalindua wagneri”, sp nov., two new species of anaerobic ammonium oxidizing bacteria. Syst Appl Microbiol 26(4):529–538
Sliekers AO, Third KA, Abma W, Kuenen JG, Jetten MSM (2003) CANON and Anammox in a gas-lift reactor. FEMS Microbiol Let 218(2):339–344
Smolders GJF, Van Der Meij J, Van Loosdrecht MCM, Heijen JJ (1994) Stoichiometric model of the aerobic metabolism of the biological phosphorus removal process. Biotechnol Bioeng 44(7):837–848
Strous M, Fuerst JA, Kramer EHM, Logemann S, Muyzer G, Van De Pas-Schoonen KT, Webb R, Kuenen JG, Jetten MSM (1999) Missing lithotroph identified as new planctomycete. Nature 400(6743):446–449
Van Der Star WRL, Miclea AI, Van Dongen UGJM, Muyzer G, Picioreanu C, Van Loosdrecht MCM (2008) The membrane bioreactor: a novel tool to grow anammox bacteria as free cells. Biotechnol Bioeng 101(2):286–294
Vlaeminck SE, Terada A, Smets BF, De Clippeleir H, Schaubroeck T, Bolca S, Demeestere L, Mast J, Boon N, Carballa M, Verstraete W (2010) Aggregate size and architecture determine microbial activity balance for one-stage partial nitritation and anammox. Appl Environ Microb 76(3):900–909
Volcke EIP, Picioreanu C, De Baets B, van Loosdrecht MCM (2010a) Effect of granule size on autotrophic nitrogen removal in a granular sludge reactor. Environ Technol 31(11):1271–1280
Volcke EIP, Picioreanu C, De Baets B, van Loosdrecht MCM (2010b) How to deal with particle size distribution in granular sludge reactor models? In: Proceedings IWA/WEF Wastewater Treatment Modelling Seminar—WWTmod 2010 (28–30). International Water Association, Québec. pp. 79–86
Volcke EIP, Picioreanu C, De Baets B, van Loosdrecht MCM (2012) The granule size distribution in an anammox-based granular sludge reactor affects the conversion - implications for modelling. Biotechnol Bioeng 109(7):1629–1636
Wagner M, Rath G, Koops HP, Flood J, Amann R (1996) In situ analysis of nitrifying bacteria in sewage treatment plants. Water Sci Technol 34(1–2):237–244
Winkler M-KH, Bassin JP, Kleerebezem R, de Bruin LMM, van den Brand TPH, Van Loosdrecht MCM (2011a) Selective sludge removal in a segregated aerobic granular biomass system as a strategy to control PAO-GAO competition at high temperatures. Water Res 45(11):3291–3299
Winkler M-KH, Kleerebezem R, Kuenen JG, Yang J, Van Loosdrecht MCM (2011b) Segregation of biomass in cyclic anaerobic/aerobic granular sludge allows the enrichment of anaerobic ammonium oxidizing bacteria at low temperatures. Environ Sci Technol 45(17):7330–7337
Winkler M-KH, Bassin JP, Kleerebezem R, van der Lans RGJM, van Loosdrecht MCM (2012) Temperature and salt effects on settling velocity in granular sludge technology. Water Res 46(12):3897–3902
Woldringh CL, Binnerts JS, Mans A (1981) Variation in Escherichia coli buoyant density measured in Percoll gradients. J Appl Bacteriol 148(1):58–63
Xavier JB, De Kreuk MK, Picioreanu C, Van Loosdrecht MCM (2007) Multi-scale individual-based model of microbial and bioconversion dynamics in aerobic granular sludge. Environ Sci Technol 41(18):6410–6417
Yamaguchi T, Yamazaki S, Uemura S, Tseng IC, Ohashi A, Harada H (2001) Microbial-ecological significance of sulfide precipitation within anaerobic granular sludge revealed by micro-electrodes study. Water Res 35(14):3411–3417
Acknowledgments
This study was partly funded by STOWA and DHV in the framework of the Dutch national research program. Percoll density centrifugation was conducted at Columbia University and was supported by the National Fish and Wildlife Foundation and the National Science Foundation CAREER award to Kartik Chandran. The authors would like to acknowledge Jingjing Yang, Nienke Bruinsmar, Tessa van den Brandt, Gavin McStay and Birk Hahne for their help in the laboratory and Cristian Picioreanu for useful discussions. Further, we greatly appreciate the supply of the biomass. Special thanks go to Laurens Wellens, Yang Jiang, Tommasso Lotti and Wendell Khunjar.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 23 kb)
Appendix
Thickness of different layers
Volume fractions of different layers
Volume percentages of different layers
Volume fractions coupled with densities
Rights and permissions
About this article
Cite this article
Winkler, MK.H., Kleerebezem, R., Strous, M. et al. Factors influencing the density of aerobic granular sludge. Appl Microbiol Biotechnol 97, 7459–7468 (2013). https://doi.org/10.1007/s00253-012-4459-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4459-4