Abstract
Several alcohol dehydrogenase (ADH)-related genes have been identified as enzymes for reducing levels of toxic compounds, such as, furfural and/or 5-hydroxymethylfurfural (5-HMF), in hydrolysates of pretreated lignocelluloses. To date, overexpression of these ADH genes in yeast cells have aided ethanol production from glucose or glucose/xylose mixture in the presence of furfural or 5-HMF. However, the effects of these ADH isozymes on ethanol production from xylose as a sole carbon source remain uncertain. We showed that overexpression of mutant NADH-dependent ADH1 derived from TMB3000 strain in the recombinant Saccharomyces cerevisiae, into which xylose reductase (XR) and xylitol dehydrogenase (XDH) pathway of Pichia stipitis has been introduced, improved ethanol production from xylose as a sole carbon source in the presence of 5-HMF. Enhanced furan-reducing activity is able to regenerate NAD+ to relieve redox imbalance, resulting in increased ethanol yield arising from decreased xylitol accumulation. In addition, we found that overexpression of wild-type ADH1 prevented the more severe inhibitory effects of furfural in xylose fermentation as well as overexpression of TMB3000-derived mutant. After 120 h of fermentation, the recombinant strains overexpressing wild-type and mutant ADH1 completely consumed 50 g/L xylose in the presence of 40 mM furfural and most efficiently produced ethanol (15.70 g/L and 15.24 g/L) when compared with any other test conditions. This is the first report describing the improvement of ethanol production from xylose as the sole carbon source in the presence of furan derivatives with xylose-utilizing recombinant yeast strains via the overexpression of ADH-related genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Second-generation biofuels sustainably produced from lignocellulosic biomass are indispensable for avoiding competition with international food supplies. In the conversion of lignocellulosic materials primarily composed of cellulose, hemicellulose, and lignin to ethanol using microorganisms, such as, Saccharomyces cerevisiae, pretreatment is an essential process to generate potentially fermentable sugars from the renewable biomass (Alvira et al. 2010).
Hydrothermal treatment is a pretreatment technology that allows selective production of two fractions: soluble hydrolysates essentially comprising hemicellulose derivatives and solid materials comprising cellulose and lignin residue (Boussarsar et al. 2009). The hemicellulosic fraction extracted by solubilization of lignocelluloses often contains monomeric xylose sugar. Although the compositions of the fractions differ depending on experimental conditions, hydrothermal treatment can generate certain amounts of xylose monomer and, in combination with enzymatic saccharification, can produce abundant monomeric sugars (Boussarsar et al. 2009; Sakamoto et al. 2012). As the dominant sugar component of these hydrolysates is xylose, hydrothermal solubilized fractions can subsequently be utilized for various applications, including xylose fermentation.
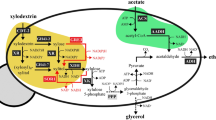
Although S. cerevisiae is a useful organism for ethanol production from glucose, it cannot utilize xylose as a sugar component (Hasunuma et al. 2011a; Kötter and Ciriacy 1993; Tantirungkij et al. 1993). Therefore, S. cerevisiae strains genetically engineered to express xylose reductase (XR) and xylitol dehydrogenase (XDH) genes derived from Pichia stipitis are typically utilized for ethanol production from xylose, which is converted into xylulose via xylitol by XR and XDH through reductive and oxidative reactions, respectively (Hasunuma et al. 2011a; Kötter and Ciriacy 1993; Tantirungkij et al. 1993). However, this recombinant strain develops a redox imbalance that can lead to decreased ethanol yield accompanied by unreacted xylitol due to different coenzyme specificities of XR (NADPH) and XDH (NAD+) from P. stipitis (Matsushika et al. 2008; Petschacher and Nidetzky 2008; Watanabe et al. 2005; Watanabe et al. 2007) (Fig. 1).
Strategy for improving ethanol production from xylose as a sole carbon source using recombinant S. cerevisiae strain with XR–XDH pathway from P. stipitis. The enhanced furan-reducing activity by overexpression of mutant NADH-dependent ADH1 (m6ADH1) is able to regenerate NAD+ via reduction of furan derivatives and relieve the redox imbalance within the XDH reaction. The triple line indicates omitted steps. XR xylose reductase, XDH xylitol dehydrogenase, ADH alcohol dehydrogenase, 5-HMF 5-hydroxymethylfurfural
Another usual problem with utilizing xylose derived from pretreated hydrolysates is formation of toxic compounds caused by monosaccharide degradation. The harsh conditions used for effective recovery of sugar components in the pretreatment of raw materials can release toxic compounds, such as, furfural and 5-hydroxymethylfurfural (5-HMF), which inhibit ethanol production and microbial cell growth, although their amounts depend on the composition of raw materials and the treatment conditions (Alvira et al. 2010). These furan derivatives are known to reduce the activities of alcohol dehydrogenase (ADH), aldehyde dehydrogenase (ALDH), and pyruvate dehydrogenase (PDH) (Modig et al. 2002). Furfural exhibits particularly strong inhibitory effects on fermentation when compared with 5-HMF (Sanchez and Bautista 1988), and hydrothermal pretreatment tends to produce larger amounts of furfural than 5-HMF (Sakamoto et al. 2012; Thomsen et al. 2009).
Recently, ADH isozymes that reduce furan derivatives have been identified. Microarray analysis and gene overexpression experiments have revealed that Adh6 from S. cerevisiae is able to reduce 5-HMF in a NADPH-dependent manner (Petersson et al. 2006), and later it was also shown that Adh6 has the activity for NADPH-dependent reduction of furfural (Almeida et al. 2008; Almeida et al. 2009). Moreover, an Adh1 variant (m6Adh1) of S. cerevisiae has been identified in industrial yeast strain TMB3000 as an NADH-dependent enzyme capable of reducing both furfural and 5-HMF (Laadan et al. 2008). Helpful capacities to reduce these toxic compounds have been also demonstrated with recombinant yeast strains overexpressing ADH6 or m6ADH1 on ethanol fermentation from glucose as a sole carbon source (Almeida et al. 2008; Laadan et al. 2008).
On the other hand, Bruinenberg et al. demonstrated that the addition of a hydrogen acceptor (i.e., acetoin) enabled xylose utilization in the XR–XDH-based Candida utilis (Bruinenberg et al. 1983). Later, Wahlbom et al. showed that the addition of furfural or acetoin decreased significantly xylitol yield in XR–XDH recombinant S. cerevisiae (Wahlbom and Hahn-Hägerdal 2002). Thus, the addition of electron acceptors seems to be surely beneficial for decrease in the accumulation of by-products. More recently, Almeida et al. displayed that NADH-dependent reduction of 5-HMF with m6ADH1-overexpressed xylose-fermenting strain resulted in decreased xylitol production from a glucose/xylose mixture, despite that no differences in ethanol productivity were seen between the yeast strains overexpressing NADPH-dependent ADH6 and NADH-dependent m6ADH1 (Almeida et al. 2009). However, even though xylose consumption is more severely suppressed by toxic compounds than glucose consumption (Almeida et al. 2009), the effects of overexpression of ADH isozymes on ethanol production from xylose as a sole carbon source remain uncertain.
In this study, we showed that overexpression of NADH-dependent m6ADH1 in the XR–XDH-based xylose-utilizing S. cerevisiae strain improved ethanol production from xylose in the presence of 5-HMF via enhanced furan-reducing activity. As expected, the reduction in 5-HMF by m6Adh1, which is able to regenerate NAD+ to relieve intracellular redox imbalance, led to increased ethanol yield and decreased xylitol accumulation during xylose fermentation (Fig. 1). In addition, we found that overexpression of wild-type ADH1 prevented inhibitory effects of furfural in xylose fermentation as well as overexpression of TMB3000-derived mutant, although overexpression of ADH6 was unable to rescue ethanol fermentation from xylose in the presence of furfural. Our results may help prevent the inhibitory effects of furan derivatives in hemicellulosic fractions generated from lignocellulosic hydrolysates, such as, hydrothermally treated biomass.
Materials and methods
Yeast strains and media
S. cerevisiae YPH499 was selected as a parental strain (Sikorski and Hieter 1989), and the derivatives used in this study are listed in Table 1. Synthetic dextrose (SD) medium contained 6.7 g/L yeast nitrogen base (YNB) without amino acids (BD Diagnostic Systems, Sparks, MD, USA) and 20 g/L glucose. SDC medium contained 6.7 g/L YNB, 20 g/L glucose, and 20 g/L casamino acids (BD Diagnostic Systems). SC fermentation medium contained 6.7 g/L YNB and 20 g/L casamino acids without carbon sources. Each medium was supplemented with 40 mg/L adenine, 20 mg/L histidine, 60 mg/L leucine, and 30 mg/L lysine for cultivation and fermentation.
Plasmid construction
Plasmids used in this study are listed in Table 1. Primers used in this study are listed in Table 2. ADH6 and wild-type ADH1 were attached to sequences encoding SGGGGS linkers and c-myc tags and were amplified by PCR from yeast genomic DNA with P1/P2 and P3/P4 oligonucleotide pairs. Blunt fragments were inserted into a pBlueScript II KS(+) (Agilent Technologies, Santa Clara, CA, USA) vector at the EcoRV site, producing the plasmids pBlue-ADH6-myc and pBlue-ADH1-myc.
QuikChange site-directed mutagenesis (Agilent Technologies) was carried out to introduce six-point mutations into ADH1 (V58T, S109P, L116S, Q147E, I151V, and Y294C) by referring to the original report (Laadan et al. 2008). For the S109P and L116S mutations, pBlue-ADH1-myc was used as a template for PCR with the P5/P6 oligonucleotides. After DpnI digestion, the obtained fragment was used as a template for the Y294C mutation with the P7/P8 oligonucleotides. After DpnI digestion and transformation, recovered plasmid was designated pBlue-m3ADH1-myc. For the V58T mutation, pBlue-m3ADH1-myc was used as a template for PCR with the P9/P10 oligonucleotides. After DpnI digestion, the obtained fragment was used as a template for the Q147E and I151V mutations with the P11/P12 oligonucleotides. After DpnI digestion and transformation, the resultant plasmid was designated pBlue-m6ADH1-myc.
The fragment containing the ADH1 promoter (5′-ADH1), NheI/EcoRI cloning sites and the ADH1 terminator (3′-ADH1) was amplified by PCR from yeast genomic DNA with the P13/P14 and P15/P16 oligonucleotide pairs. Fragments were digested with XhoI/NheI and NheI/NotI, respectively, and were then ligated and inserted into a pRS404 yeast shuttle vector (American Type Culture Collection, Manassas, VA, USA) at the XhoI/NotI sites, resulting in pADH404. The multicopy yeast of 2 μ origin was prepared by digestion of pRS406 + 2 μm (Ishii et al. 2009) with AatII, followed by ligation at the same site in pADH404 to yield pADH424.
ADH6, m6ADH1, and wild-type ADH1 with c-myc tags were prepared by digestion of pBlue-ADH6-myc, pBlue-m6ADH1-myc, and pBlue-ADH1-myc with NheI and EcoRI, followed by ligation at the same sites in pADH424. The resulting plasmids were designated pEWADH6, pEWm6ADH1, and pEWADH1.
Yeast transformation
Transformation was carried out according to the lithium acetate method (Gietz et al. 1992). The XR–XDH-based xylose-utilizing YPH499XU strain (Hasunuma et al. 2011b) was transformed with pADH424 (mock vector), pEWADH6, pEWm6ADH1, or pEWADH1, yielding YPH499XU/mock, YPH499XU/ADH6, YPH499XU/m6ADH1, or YPH499XU/ADH1, respectively (Table 1).
Ethanol fermentation from xylose
All transformants were grown in SD media and were then inoculated into 500 mL of SDC media to give an initial cell density (optical density) of 0.03 at 600 nm. Yeast cells were aerobically cultivated at 30 °C for 48 h. After harvesting and washing, cultured cells were inoculated into 50 mL of SC medium containing 50 g/L xylose as a sole carbon source to give an initial cell density (optical density) of 20 at 600 nm. For fermentation without toxic compounds, two independent experiments were performed. Furfural and/or 5-HMF were added into the fermentation medium to a final concentration of 40 mM depending on experimental conditions. All fermentation were performed at 30 °C under oxygen-limited conditions with mild agitation in closed 100-mL bottles equipped with a filter-tipped CO2 outlet and closable sampling tube.
Analytical procedures
In order to determine the concentrations of glycerol, xylitol, and xylose in the fermentation medium, the supernatant was analyzed using a Prominence high-performance liquid chromatograph (HPLC) (Shimadzu, Kyoto, Japan) equipped with a Shim-pack SPR-Pb column (250 mm × 7.8 mm; Shimadzu) and an RID-10A refractive index detector (Shimadzu). Supernatants and standards were diluted twofold with ultrapure water and were passed through a polytetrafluoroethylene (PTFE) filter. Then, 10 μL of all samples and standards was injected. The HPLC system was operated at 80 °C, with a mobile phase of ultrapure water at a flow rate of 0.6 mL/min.
For determination of the concentrations of ethanol, furfural, furfuryl alcohol, 5-HMF, and 2,5-furandimethanol, supernatants were analyzed with a model 2010 gas chromatograph (GC) coupled to a model QP2010 Plus mass spectrometer (MS) (Shimadzu) equipped with DB-FFAP column (60 m × 0.25 mm i.d., and 0.5 μm film thickness; Agilent Technologies). After centrifugation, supernatant was diluted tenfold with acetone. The injector temperature was 250 °C and 1 μL of all samples and standards was injected in split mode (1:50). Helium was used as the carrier gas at a flow rate of 1 mL/min. The ion source and interface temperatures were 230 °C and 250 °C, respectively. Oven temperature was programmed as follows: isothermal at 80 °C for 7 min, 120 °C/min for 1 min, isothermal at 200 °C for 7 min, 100 °C/min for 1 min, and isothermal at 250 °C for 15 min. Compounds were detected using selected ion monitoring (SIM) mode at m/z 31, 45, and 46 (for ethanol); m/z 96, 95, and 39 (for furfural); m/z 98, 41, and 39 (for furfuryl alcohol), m/z 97, 41, and 126 (for 5-HMF); and m/z 97, 128, and 41 (for 2,5-furandimethanol).
Results
Ethanol fermentation from xylose with NADPH- or NADH-specific ADH-overexpressing yeast strains
A recombinant S. cerevisiae strain YPH499XU utilizing xylose was previously developed by introducing the heterogenous XR and XDH genes from P. stipitis and the endogenous xylulokinase (XK) gene from S. cerevisiae into the genomic locus of YPH499 (Hasunuma et al. 2011b), a typical lab strain (Sikorski and Hieter 1989). ADH6, m6ADH1, wild-type ADH1, or empty vector was introduced into the YPH499XU yeast strain (Table 1). Primarily, overexpression of ADH genes was confirmed by Western blot analysis with anti-myc antibody (data not shown).
Xylose fermentation in the absence of furan derivatives was performed in SC medium containing 50 g/L xylose as a sole carbon source at 30 °C under oxygen-limited conditions after aerobic growth of recombinant yeast cells in SDC medium. Initial cell concentration was adjusted to an optical density of 20 at 600 nm. As shown in Fig. 2, all four transformants completely consumed xylose within 36 h and showed similar production levels of ethanol, whereas yeast strains overexpressing NADH-dependent m6ADH1 exhibited a slight decrease in the accumulation of xylitol (Table 3). Glycerol production by strains overexpressing m6ADH1 was slightly higher when compared with control strains harboring mock vector (Table 3).
Ethanol fermentation from xylose by recombinant S. cerevisiae; a YPH499XU/mock, b YPH499XU/ADH6, c YPH499XU/m6ADH1, and d YPH499XU/ADH1. Filled square xylose, unfilled circle ethanol, unfilled square glycerol, unfilled triangle acetate, filled circle xylitol. Each data point represents the mean of two independent experiments
Effects of ADH overexpressions on xylose fermentation in the presence of 5-HMF
In order to assess the effects of 5-HMF reduction by overexpression of NADPH- or NADH-dependent ADH genes on xylose fermentation, 40 mM 5-HMF was added to SC medium containing 50 g/L xylose (Fig. 3). After 36 h, the control strain had completely converted 40 mM 5-HMF to 2,5-furandimethanol, with a slight increase in glycerol and unchanged xylose consumption, as compared to the absence of 5-HMF (Fig. 3a, e and Table 3). The 5-HMF reduction and glycerol production profiles of the strain overexpressing NADH-dependent wild-type ADH1 were similar to those of the control strain (Fig. 3d, h and Table 3).
Reduction of 5-HMF during fermentation from xylose in the presence of 5-HMF by recombinant S. cerevisiae; a YPH499XU/mock, b YPH499XU/ADH6, c YPH499XU/m6ADH1, and d YPH499XU/ADH1. Filled square 2,5-furandimethanol, unfilled circle 5-HMF. Ethanol fermentation; e YPH499XU/mock, f YPH499XU/ADH6, g YPH499XU/m6ADH1, and h YPH499XU/ADH1. Filled square xylose, unfilled circle ethanol, unfilled square glycerol, unfilled triangle acetate, filled circle xylitol. 5-HMF was added to the fermentation at a concentration of 40 mM. Each data point represents the mean of three independent experiments, ±SD
On the other hand, yeast strains overexpressing ADH6 or m6ADH1 completely converted 5-HMF to 2,5-furandimethanol after only 12 h, clearly showing that overexpression of ADH6 and m6ADH1 is involved in 5-HMF reduction (Fig. 3b and c). Nevertheless, there were differences in the xylose fermentation profiles between the strains overexpressing ADH6 and m6ADH1. When compared with the absence of 5-HMF, addition of 5-HMF led to increased ethanol production and yield by the m6ADH1-overexpressing strain (Table 3). In addition, m6ADH1 overexpression provided positive effects that apparently reduced xylitol accumulation and increased ethanol production, as compared to the control strain (Fig. 3g and Table 3). In contrast, the trend of xylose fermentation in the ADH6-overexpressing strain in the presence of 5-HMF was identical to that of the control strain (Fig. 3f and Table 3). These distinct features of the recombinant yeast strains were also observed with 20 mM and 80 mM 5-HMF (data not shown).
When compared with the yields based on the consumed sugars, the difference of m6ADH1-overexpressing strain in ethanol production and xylitol accumulation during xylose fermentation in the presence of 5-HMF was more clear (Table 4). Especially, the difference of xylitol accumulation was significant compared with the control strain in the absence of 5-HMF (Table 4).
Effects of ADH overexpressions on xylose fermentation in the presence of furfural
In order to evaluate the effects of furfural reduction by NADPH- or NADH-dependent ADH genes on xylose fermentation, 40 mM furfural was added to SC medium containing 50 g/L xylose (Fig. 4). Approximately 4.9 mM furfural remained with the control strain after 48 h of fermentation (Fig. 4a). As the furfural remained in the medium at later phases, xylose utilization scarcely progressed, even after 120 h of fermentation (Fig. 4e). The ADH6-overexpressing strain exhibited similar conversion of furfural and xylose fermentation as the control strain (Fig. 4b and f).
Reduction of furfural during fermentation from xylose in the presence of furfural by recombinant S. cerevisiae; a YPH499XU/mock, b YPH499XU/ADH6, c YPH499XU/m6ADH1, and d YPH499XU/ADH1. Filled square furfuryl alcohol, unfilled circle furfural. Ethanol fermentation; e YPH499XU/mock, f YPH499XU/ADH6, g YPH499XU/m6ADH1, and h YPH499XU/ADH1. Filled square xylose, unfilled circle ethanol, unfilled square glycerol, unfilled triangle acetate, filled circle xylitol. Furfural was added to the fermentation at a concentration of 40 mM. Each data point represents the mean of three independent experiments, ±SD
In contrast, yeast strains overexpressing not only m6ADH1 but also wild-type ADH1 completely converted furfural to furfuryl alcohol after only 12 h of fermentation (Fig. 4c and d). Thus, we found the catalytic potential of wild-type Adh1 for reducing furfural. Overexpression of m6ADH1 and wild-type ADH1 in xylose-utilizing yeast strains made it possible to completely consume xylose and to efficiently produce ethanol after 120 h of fermentation (15.70 g/L and 15.24 g/L, respectively), despite interference with the rates of xylose consumption and ethanol production, as compared to fermentation without furfural (Fig. 4g, h and Table 3). The addition of furfural led to an apparent decrease in xylitol accumulation in these two strains (Table 3). These results suggest that NADH-dependent conversion of furfural contributes to decreased xylitol accumulation and increased ethanol production. In the presence of 20 mM furfural, xylose was completely consumed and production of ethanol, xylitol, and glycerol was not changed among the four recombinant strains (data not shown). However, the inhibitory effects of furfural are more severe when compared with 5-HMF; thus, Adh1 is useful for mitigating redox imbalance in xylose-fermenting yeast.
To determine the no inhibitory effect level for furfuryl alcohol, xylose fermentation in the presence of 40 mM furfuryl alcohol was tested with the control strain. There were almost no differences in fermentation profiles between absence and presence of furfuryl alcohol (data not shown). Finally, we confirmed that m6ADH1-overexpressing strain also permitted xylose fermentation in the presence of both 5-HMF and furfural (Fig. 5).
Reduction of 5-HMF during fermentation from xylose in the presence of both 5-HMF and furfural by recombinant S. cerevisiae; a YPH499XU/mock and d YPH499XU/m6ADH1. Filled square 2,5-furandimethanol, unfilled circle 5-HMF. Reduction of furfural; b YPH499XU/mock and e YPH499XU/m6ADH1. Filled square furfuryl alcohol, unfilled circle furfural. Ethanol fermentation; c YPH499XU/mock and f YPH499XU/m6ADH1. Filled square xylose, unfilled circle ethanol, unfilled square glycerol, unfilled triangle acetate, filled circle xylitol. 5-HMF and furfural were added to the fermentation at a concentration of 40 mM each. Each data point represents the mean of three independent experiments, ±SD
Discussion
The aim of this study was to investigate the effects of overexpression of ADH-related genes, which have been identified as the enzymes responsible for the reduction of furfural and/or 5-HMF on ethanol fermentation from xylose as a sole carbon source by xylose-utilizing recombinant yeast. As it has been confirmed that coenzyme specificities vary between Adh6 and m6Adh1 (Almeida et al. 2008; Almeida et al. 2009; Laadan et al. 2008; Petersson et al. 2006), we predicted that they would be able to decrease xylitol accumulation caused by redox imbalance and improve ethanol production in xylose fermentation (Fig. 1).
Overexpression of m6ADH1 had a significant effect, resulting in increased ethanol yield and decreased xylitol accumulation owing to NADH-dependent 5-HMF reduction (Table 3 and Fig. 3g). 5-HMF reduction by a wild-type ADH1-overexpressing strain was similar to that by control strains (Fig. 4a and d), indicating that wild-type Adh1 showed no 5-HMF-reducing activity. Slight increases of glycerol in all strains in the presence of 5-HMF probably reflected the regeneration of NAD+ by 5-HMF reduction. The recombinant strain overexpressing NADH-dependent m6Adh1 exhibited slight decreases in xylitol accumulation, even in the absence of toxic compounds, indicating that regenerated NAD+ in glycerol production due to the dehydrogenase activity of ADH1-derived enzymes facilitated conversion of xylitol to xylulose by NAD+-dependent XDH (Table 3). The observation that glycerol increased in the same strains supports the previous report on fermentation of a glucose/xylose mixture in the presence of 5-HMF (Table 3) (Almeida et al. 2009).
The most remarkable finding in the present study was the difference in ethanol production from xylose in the presence of furfural among ADH-overexpressing yeast strains (Fig. 4). While the ADH6-overexpressing strain and the control strain showed little fermentation, overexpression of m6ADH1 or ADH1 improved ethanol production in the presence of 40 mM furfural. This finding was supported by the enhancement of the furfural-reducing activity of yeast strains by the overexpression of m6ADH1 and wild-type ADH1 (Fig. 4c and d). This is the first report in which wild-type ADH1 could reduce furfural as well as TMB3000-derived m6ADH1. Although we confirmed equivalent ADH expression levels in the ADH6-overexpressing strain and the ADH1-overexpressing strain by Western blotting (data not shown), our results contrast with those of other reports, in which Adh6 showed furfural-reducing activity (Almeida et al. 2008; Almeida et al. 2009). This was probably attributed to a specific event for xylose fermentation in the presence of furfural. The possible explanation for this conflict is an insufficient amount of intracellular NADPH, which would cause low Adh6 activity, leading to more severe inhibition of xylose fermentation by furfural when compared with 5-HMF. The yeast strains overexpressing m6ADH1 and wild-type ADH1 displayed the most efficient production ethanol after 120 h of fermentation (15.70 g/L and 15.24 g/L, respectively). Thus, overexpression of m6ADH1 or wild-type ADH1 effectively improves ethanol production from xylose in the presence of furfural.
In summary, we demonstrated that the mutated Adh1 (m6Adh1) improves ethanol fermentation from xylose in the presence of 5-HMF or furfural owing to the NADH-dependent capacity for reduction of these toxic compounds. In addition, we first uncovered the activity of wild-type Adh1 to reduce furfural. Ethanol yields were higher than those in the absence of toxic compounds. Thus, overexpression of NADH-dependent ADH1-related genes, particularly m6ADH1 identified from TMB3000, is beneficial for ethanol fermentation from xylose in the presence of furan derivatives. These insights may be valuable for the utilization of hemicellulosic materials, such as, biomass subjected to hydrothermal or acid treatment.
References
Almeida JR, Röder A, Modig T, Laadan B, Lidén G, Gorwa-Grauslund MF (2008) Gorwa-Grauslund. NADH- vs. NADPH-coupled reduction of 5-hydroxymethyl furfural (HMF) and its implications on product distribution in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 78:939–945
Almeida JR, Bertilsson M, Hahn-Hägerdal B, Lidén G, Gorwa-Grauslund MF (2009) Carbon fluxes of xylose-consuming Saccharomyces cerevisiae strains are affected differently by NADH and NADPH usage in HMF reduction. Appl Microbiol Biotechnol 84:751–761
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis. Bioresour Technol 101:4851–4861
Boussarsar H, Rogé B, Mathlouthi M (2009) Optimization of sugarcane bagasse conversion by hydrothermal treatment for the recovery of xylose. Bioresour Technol 100:6537–6542
Bruinenberg PM, Debot PHM, Vandijken JP, Scheffers WA (1983) The role of redox balances in the anaerobic fermentation of xylose by yeasts. Eur J appl Microbiol Biotechnol 18:287–292
Gietz D, St Jean A, Woods RA, Schiestl RH (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20:1425
Hasunuma T, Sanda T, Yamada R, Yoshimura K, Ishii J, Kondo A (2011a) Metabolic pathway engineering based on metabolomics confers acetic and formic acid tolerance to a recombinant xylose-fermenting strain of Saccharomyces cerevisiae. Microb Cell Fact 10:2
Hasunuma T, Sung KM, Sanda T, Yoshimura K, Matsuda F, Kondo A (2011b) Efficient fermentation of xylose to ethanol at high formic acid concentrations by metabolically engineered Saccharomyces cerevisiae. Appl Microbiol Biotechnol 90:997–1004
Ishii J, Izawa K, Matsumura S, Wakamura K, Tanino T, Tanaka T, Ogino C, Fukuda H, Kondo A (2009) A simple and immediate method for simultaneously evaluating expression level and plasmid maintenance in yeast. J Biochem 145:701–788
Katahira S, Mizuike A, Fukuda H, Kondo A (2006) Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose- and cellooligosaccharide-assimilating yeast strain. Appl Microbiol Biotechnol 72:1136–1143
Kötter P, Ciriacy M (1993) Xylose fermentation by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 38:776–783
Laadan B, Almeida JR, Rådström P, Hahn-Hägerdal B, Gorwa-Grauslund M (2008) Identification of an NADH-dependent 5-hydroxymethylfurfural-reducing alcohol dehydrogenase in Saccharomyces cerevisiae. Yeast 25:191–198
Matsushika A, Watanabe S, Kodaki T, Makino K, Sawayama S (2008) Bioethanol production from xylose by recombinant Saccharomyces cerevisiae expressing xylose reductase, NADP+-dependent xylitol dehydrogenase, and xylulokinase. J Biosci Bioeng 105:296–299
Modig T, Lidén G, Taherzadeh MJ (2002) Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem J 363:769–776
Petersson A, Almeida JR, Modig T, Karhumaa K, Hahn-Hägerdal B, Gorwa-Grauslund MF, Lidén G (2006) A 5-hydroxymethyl furfural reducing enzyme encoded by the Saccharomyces cerevisiae ADH6 gene conveys HMF tolerance. Yeast 23:455–464
Petschacher B, Nidetzky B (2008) Altering the coenzyme preference of xylose reductase to favor utilization of NADH enhances ethanol yield from xylose in a metabolically engineered strain of Saccharomyces cerevisiae. Microb Cell Fact 7:9
Sakamoto T, Hasunuma T, Hori Y, Yamada R, Kondo A (2012) Direct ethanol production from hemicellulosic materials of rice straw by use of an engineered yeast strain codisplaying three types of hemicellulolytic enzymes on the surface of xylose-utilizing Saccharomyces cerevisiae cells. J Biotechnol 158:203–210
Sanchez B, Bautista J (1988) Effects of furfural and 5-hydroxymethylfurfural on the fermentation of Saccharomyces cerevisiae and biomass production from Candida guilliermondii. Enzyme Microb Technol 10:315–318
Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19–27
Tantirungkij M, Nakashima N, Seki T, Yoshida T (1993) Construction of xylose-assimilating Saccharomyces cerevisiae. J Ferment Bioeng 75:83–88
Thomsen MH, Thygesen A, Thomsen AB (2009) Identification and characterization of fermentation inhibitors formed during hydrothermal treatment and following SSF of wheat straw. Appl Microbiol Biotechnol 83:447–455
Wahlbom CF, Hahn-Hägerdal B (2002) Furfural, 5-hydroxymethyl furfural, and acetoin act as external electron acceptors during anaerobic fermentation of xylose in recombinant Saccharomyces cerevisiae. Biotechnol Bioeng 78:172–178
Watanabe S, Kodaki T, Makino K (2005) Complete reversal of coenzyme specificity of xylitol dehydrogenase and increase of thermostability by the introduction of structural zinc. J Biol Chem 280:10340–10349
Watanabe S, Saleh AA, Pack SP, Annaluru N, Kodaki T, Makino K (2007) Ethanol production from xylose by recombinant Saccharomyces cerevisiae expressing protein engineered NADP+-dependent xylitol dehydrogenase. J Biotechnol 130:316–319
Acknowledgments
The authors would like to thank Ms. Yoshimi Hori, Ms. Shizuka Matsumura, and Ms. Hiromi Nishigaki for technical assistance. This study was supported through project P07015 of the New Energy and Industrial Technology Development Organization (NEDO), under the sponsorship of the Ministry of Economy, Trade, and Industry (METI) of Japan. This work was also supported by the Global Warming Countermeasure Technology Development Program from the Ministry of the Environment of Japan; Grants-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports and Technology (MEXT) of Japan; and a Special Coordination Fund for Promoting Science and Technology, Creation of Innovative Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe) from MEXT, Japan.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishii, J., Yoshimura, K., Hasunuma, T. et al. Reduction of furan derivatives by overexpressing NADH-dependent Adh1 improves ethanol fermentation using xylose as sole carbon source with Saccharomyces cerevisiae harboring XR–XDH pathway. Appl Microbiol Biotechnol 97, 2597–2607 (2013). https://doi.org/10.1007/s00253-012-4376-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4376-6