Abstract
‘Streptomyces tsukubaensis’ was the first tacrolimus producer strain identified. Although it has been included in the Streptomyces genus, its taxonomic position has not been rigorously determined. By using a polyphasic approach, we have established that the tacrolimus producer strain ‘S. tsukubaensis’ NRRL 18488 represents a unique species in the Streptomyces genus, which is phylogenetically distant from other subsequently described producers. This fact means a horizontal transference of the tacrolimus-producing gene cluster. Physiology, nutrient requirement, and molecular genetics analyses of tacrolimus biosynthesis in ‘S. tsukubaensis’ necessitate chemically defined or semi-defined media, which work as a jigsaw puzzle and allow for pieces (nutrients) exchange. To date, studies related to ‘S. tsukubaensis’ have been mainly focused in the improvement of tacrolimus production using complex industrial fermentation media, which difficulty allows testing of tacrolimus overproduction enhancers or inhibitors because of the presence of non‐defined substances. In the present work, two semi-defined media were developed in order to study the main factors involved in tacrolimus production in ‘S. tsukubaensis’.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of organ transplants has increased exponentially during the last decades due to the development of surgical techniques and especially due to the use of new immunosuppressive therapies. Tacrolimus (FK506), a polyketide discovered in 1984 by scientists of Fujisawa Pharmaceutical Co. (Kino et al. 1987), was the first of a new family of immunosuppressants. After overcoming exhaustive clinical trials, tacrolimus was approved by FDA in 1994 to prevent graft rejection in patients who undergo organ transplantation (Meier-Kriesche et al. 2006). Besides, immunosuppressant drugs are also widely used to slow down the immune response of people suffering from certain autoimmune disorders (Fireman et al. 2004).
Among different immunosuppressant compounds (cyclosporin, rapamycin, mycophenolate) that are commercially available, tacrolimus has resulted to be one of the most effective drugs in graft rejection treatment. This explains the industrial interest around this compound. Thus, tacrolimus (commercial name “Progaf”) sales in 2011 yielded $1,886 million, which represented 27.6 % of the annual sales in the immunosuppressant market (EvaluatePharma®: http://www.evaluatepharma.com/default.aspx).
The immunosuppressant tacrolimus was initially discovered in the fermentation broth of ‘Streptomyces tsukubaensis’, which was isolated from Japan soil of the Tsukuba region. Most of the initial studies performed with ‘S. tsukubaensis’ had been focused on strain improvement and the development of fermentation procedures at industrial level (Kino et al. 1987; Okuhara et al. 1990; Turło et al. 2006). Recently, a few studies have been performed at the molecular level related to the tacrolimus biosynthesis cluster (Goranovic et al. 2010; Mo et al. 2011; Mo et al. 2012) and very little is known about the nutritional control and requirements of tacrolimus biosynthesis by ‘S. tsukubaensis’. On the other hand, no rigorous taxonomic study of this tacrolimus producer has been performed; hence, its name has not been validly published. It was, therefore, of great interest to clarify these points to establish the bases for molecular genetic studies of the regulation of gene expression in ‘S. tsukubaensis’.
The first part of our study is focused on the determination of the taxonomical status of ‘S. tsukubaensis’. Streptomyces is one of the largest genuses, actually composed of more than 600 species [http://www.bacterio.cict.fr/s/streptomycesa.html (Euzéby 1997)]. Relationships within this taxonomically complex genus have been clarified by the application of polyphasic approach, which integrates the analysis of genotypic and phenotypic features (Vandamme et al. 1996).
To the best of our knowledge, only complex fermentation media have been used for tacrolimus production by ‘S. tsukubaensis’ (Kino et al. 1987; Goranovic et al. 2010). These complex media do not allow a rigorous study of the effect of different precursors and nutritional or stress factors over tacrolimus biosynthesis. In the present study, two chemically semi-defined media were optimized for tacrolimus biosynthesis by ‘S. tsukubaensis’.
Materials and methods
Bacterial strains
‘S. tsukubaensis’ NRRL 18488 was obtained from the NRRL Culture Collection of the Agricultural Research Service (USA). Streptomyces clavuligerus ATCC 27064T was acquired from the American Type Culture Collection, while Streptomyces amakusaensis DSM 40219T and Streptomyces inusitatus DSM 41441T were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH Culture Collection.
Culture media and conditions
The taxonomical analysis required different media. ISP media (International Streptomyces project; Shirling and Gottlieb 1966) are briefly described: yeast extract/malt extract agar (ISP2), oatmeal agar (ISP3), inorganic salts/starch agar (ISP4), glycerol–asparagine agar (ISP5), peptone–yeast extract iron agar (ISP6), and tyrosine agar (ISP7). The medium used in the carbon source assimilation analysis comprises: 10 g/l d-glucose, 0.5 g/l MgSO4 ·7H2O, 0.5 g/l NaCl, 0.01 g/l FeSO4 ·7H2O, 1.0 g/l K2HPO4, and 12.0 g/l agar, adjusted to pH 7.4. The different carbon sources (see “Results”) were sterilized by filtration and added at a final concentration of 0.1 %. Modified Bennett's agar (Jones 1949) consists of 1 g/l yeast extract, 1 g/l meat extract, 2 g/l N-Z-amine A, and 10 g/l glycerol, adjusted to pH 7.3. The components per liter of the “172 medium” are 10 g glucose, 20 g glucidex-20, 5 g yeast extract, 5 g casein peptone, and 1.8 g agar, adjusted to pH 6.8, and then supplemented with 8 g CaCO3. The “172A medium” has the same components except that the CaCO3 is replaced with 2.5 g/l of 3-(N-morpholino) propanesulfonic acid (MOPS).
Tacrolimus production by ‘S. tsukubaensis’ was analyzed in two different liquid media: MG (Doull and Vining 1990; Rodríguez-García et al. 2007) and ISP4 (Shirling and Gottlieb 1966). The basal composition of these media and the fermentation conditions are as follows: MG medium contains: 50 g/l starch, 8.83 g/l glutamic acid, 2.5 mM KH2PO4/K2HPO4, 0.2 g/l MgSO4 ·7H2O, 1 mg/l CaCl2, 1 mg/l NaCl, 9 mg/l FeSO4 ·7H2O, 21 g/l MOPS, and 0.45 ml/l 10× trace elements, adjusted to pH 6.5. For 10 ml solution (100×) of trace elements, the components are 39.0 mg CuSO4 ·5H2O, 5.7 mg H3BO3, 3.7 mg (NH4)6Mo7O24 ·4H2O, 6.1 mg MnSO4 ·H2O, and 880.0 mg ZnSO4 ·7H2O. Fermentation was performed in 100 ml of MG medium using 500-ml unbaffled flasks inoculated with 107 spores/ml. Incubation was done at 28 °C and 220 rpm.
Fermentation in ISP4 was performed using a two-stage culture system. A modified BaSa medium (Dumont et al. 1992) was used as seed culture. It contains 2 g/l KNO3, 20 g/l yeast extract, 20 g/l mixed peptone (Biomedics), 0.5 g/l NaCl, 0.5 g/l MgSO4 ·7H2O, 0.005 g/l of MnSO4 ·H2O 1 %, 0.01 g/l of ZnSO4 ·7H2O, and 0.02 g/l of CaCl2 ·2H2O, adjusted to pH 7.0; after autoclaving 20.0 g/l glucose, 0.025 g/l FeSO4 ·7H2O was added. The original composition of the fermentation medium ISP4 was 10 g/l starch, 1 g/l K2HPO4, 1 g/l MgSO4, 1 g/l NaCl, 2 g/l (NH4)2SO4, 2 g/l CaCO3, 0.001 g/l FeSO4 ·7H2O, 0.001 g/l MnCl2 ·4H2O, and 0.001 g/l ZnSO4 ·7H2O, adjusted to pH 7.2. The seed medium (BaSa) was inoculated with a final concentration of 107 spores/ml and incubated at 28 °C and 220 rpm during 40 h in 500-ml unbaffled flasks. Seed culture was used for the inoculation of 100 ml of production medium ISP4 (final OD600nm 1) in 500-ml unbaffled flasks. Fermentation was performed at 28 °C and 220 rpm.
Determination of phenotypical characteristics
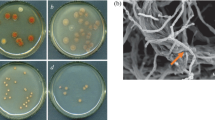
Morphological characterization of ‘S. tsukubaensis’ was performed following the standard protocol of ISP (Shirling and Gottlieb 1966). Colors were described according to the NBS-ISCC Colour System (www.anthus.com). Spore arrangement and spore surface ornamentation were observed by examining gold-coated, dehydrated preparations from 14-day-old cultures grown on ISP2 medium at 28 °C using a scanning electron microscope (JEOL 6100).
Physiological characteristics were determined following the methods described by Shirling and Gottlieb (1966) and Williams et al. (1983). NaCl tolerance was tested in the “172 medium” at the following concentrations: 1, 2, 3, 5, 7, and 10 % (w/v). In all the aforementioned tests, bacterial growth was recorded after 14 days of incubation at 28 °C (except where specified otherwise).
Determination of chemotaxonomic properties and G + C content of the DNA
Analysis of fatty acid composition was carried out using mycelium grown on solid medium. Therefore, ‘S. tsukubaensis’ was cultured on “172 medium” for 7 days at 28 °C. The analysis was performed with an Agilent 5898A gas chromatograph using an Ultra2 capillary column as described by Miller (1982) with the modifications of Miller and Berger (1985). Whole-cell sugar composition was determined by thin-layer chromatography (TLC) following the procedure described by Hasegawa et al. (1983). Analysis of the isomer of diaminopimelic acid was performed by TLC as described by Staneck and Roberts (1974) with ninhydrin staining. For this purpose, a cell suspension was hydrolyzed with sulphuric acid as described by Becker et al. (1964) and neutralized with a solution of saturated Ba(OH)2.
The G + C content of genomic DNA was determined by high-performance liquid chromatography (HPLC) (Agilent 1100 Series) following the procedure described by Tamaoka and Komagata (1984).
Phylogenetic analysis
The phylogenetic analysis was performed based on the sequence of the 16S rRNA. The corresponding sequence to ‘S. tsukubaensis’ was obtained from the Genome Sequencing Project carried out in INBIOTEC (Barreiro et al. 2012). This sequence was analyzed by using the BLAST network service provided by the European Bioinformatic Institute. Thus, these type strains of Streptomyces genus that showed ≥ 97.5 % of 16S rRNA sequence similarity with the corresponding sequence of ‘S. tsukubaensis’ were selected for the analysis. The phylogenetic tree was inferred by using the neighbor-joining method (Saitou and Nei 1987) from the MEGA 4 program (Tamura et al. 2007). Kimura's two-parameter correction model was used to generate evolutionary distance matrices for the neighbor-joining data (Kimura 1980). Topologies of the resultant trees were evaluated by boot strapping analysis (Felsenstein 1985) based on 1,000 replicates. The calculation of pairwise 16S rRNA gene sequence similarities was provided using EMBOSS Pairwise Alignment Algorithm (www.ebi.ac.uk/Tools/emboss/align/).
The level of DNA–DNA relatedness between ‘S. tsukubaensis’ and the phylogenetically closest type strain S. clavuligerus was determined following the procedure of De Ley et al. (1970) with the modifications described by Huss et al. (1983) using a model Cary 100 Bio UV/VIS spectrophotometer.
Fermentation analysis: growth, tacrolimus production, and phosphate determination
Growth of the fermentation cultures was measured by dry weight. Thus, 2 ml of bacterial culture was centrifuged, washed twice with MQ water, and dried at 80 °C during 48 h. The samples related to the fermentation in ISP4 medium were washed twice with MQ water at pH 1 (to remove insoluble CaCO3 from the broth).
For tacrolimus determination, the broth samples were mixed with equal volume of methanol (1:1). The mixture was stirred for 1 h and centrifuged. The supernatant was analyzed by HPLC using an Alliance equipped with an UV detector and XBridge-C18 column (2.1 × 150 mm, 3.5 μm, Waters). The mobile phase used for isocratic elution consisted of 65 % MQ water plus 0.01 % TFA, 28 % acetonitrile, and 7 % methyl tert-butyl ether. The flow rate was kept at 0.5 ml/min and the column temperature was set to 50 °C. The absorbance was monitored at 210 nm (UV detector). Standards of pure tacrolimus (Antibioticos SA) and ascomycin (Sigma-Aldrich) were used as controls.
The phosphate concentration of culture supernatants was measured using the malachite green assay (Lanzetta et al. 1979).
The GenBank[/EMBL/DDBJ] accession number for the 16S rRNA sequence of ‘S. tsukubaensis’ is HE656024.
Results
Taxonomical position of ‘S. tsukubaensis’
Morphological and chemotaxonomic characteristics of ‘S. tsukubaensis’
‘S. tsukubaensis’ showed branched substrate mycelium and sparse aerial hyphae which differentiated into straight to flexuous chains of short rod-shaped spores with smooth surface. At macroscopic level, the spores showed an intense gray pigment and in certain media, especially in ISP2, ‘S. tsukubaensis’ produced a pigment with red to pink tonality (Fig. 1a, b).
Analysis of the cell wall composition of ‘S. tsukubaensis’ revealed the presence of LL-diaminopimelic acid but with lack of diagnostic sugars. This is in accordance with the classification of the microorganism to the cell wall type I group (Lechevalier and Lechevalier 1970). The cellular fatty acid profile is composed of major amounts of iso-C14:0 (18.81 %), anteiso-C15:0 (17.05 %), iso-C16:0 (15.16 %), and iso-C15:0 (15.11 %), with lesser amounts (<10 %) of C16:0 (8.05 %), C16:1 (5.97 %), iso-C13:0 (3.81 %), iso-C17:0 (3.25 %), C12:0 (3.03 %), anteiso-C17:0 (2.91 %), iso-C17:1 (2.10 %), C15:0 (1.80 %), iso-C16:1 (1.46 %), and C14:0 (1.43 %). This composition is characteristic of the Streptomyces genus, where the cellular fatty acid profiles consisted mainly of saturated straight-chain and iso- and anteiso-branched-chain components (Locci 1989).
The DNA G + C content of ‘S. tsukubaensis’ is 70.2 mol %. All of these features confirm the inclusion of ‘S. tsukubaensis’ NRRL 18488 in the Streptomyces genus.
Phylogenetic analysis
In the phylogenetic tree based on the 16S rRNA sequence (Fig. 2), ‘S. tsukubaensis’ forms a distinct phyletic line loosely associated with the clade integrated by the type strains of S. clavuligerus, S. amakusaensis, and S. inusitatus. From this group, the strain S. clavuligerus was the most closely related to ‘S. tsukubaensis’ and the 16S rRNA sequence similarity between these two microorganisms was 98.4 %. Corresponding similarities with the other type strains of the clade were 97.8 % with S. amakusaensis and 97.7 % with S. inusitatus.
Neighbor-joining phylogenetic tree based on 16S rRNA gene sequences showing the relationships between ‘S. tsukubaensis’ and closely related type strains of species of the Streptomyces genus. Bootstrap values based on 1,000 replicates are shown at branch nodes. Bar 0.005 substitutions per nucleotide position
The taxonomic study was completed with a phenotypic analysis of ‘S. tsukubaensis’ and the most closely related type strains. The results of this study (Table 1) permitted one to readily distinguish the analyzed strains, notably by the color of the mycelium: ‘S. tsukubaensis’ showed a pinkish–reddish tonality in contrast with the yellowish–brownish color of the mycelium of the other analyzed strains (Fig. 1b).
The ‘S. tsukubaensis’ strain shares a DNA/DNA relatedness value of 10.1 % with the most closely related type strain S. clavuligerus, a value well below the 70 % cutoff point recommended for the assignment of strains to the same species (Wayne et al. 1987).
Moreover, at difference of ‘S. tsukubaensis’, the type strain S. clavuligerus ATCC 27064 does not contain the tacrolimus gene cluster on its genome sequence (Medema et al. 2010).
Optimization of a semi-defined medium that allows the study of different factors on the tacrolimus production
Initially, three media were chosen as the base for the development of a chemically defined medium for tacrolimus production by ‘S. tsukubaensis’: (1) the medium described by Yoon and Choi (1997) designed for tacrolimus production by S. tacrolimicus ATCC 55098, (2) the defined medium SA (Aidoo et al. 1994) optimized for the phylogenetically closed strain S. clavuligerus, and (3) the MG medium developed for Streptomyces coelicolor (Doull and Vining 1990). The SA medium did not allow the cell growth of ‘S. tsukubaensis’, and in the medium described by Yoon and Choi the tacrolimus production was around 5 μg/ml, but ‘S. tsukubaensis’ showed a clumped growth. Hence, these two defined media were ruled out.
Effect of starch on growth and tacrolimus production
The fermentation of ‘S. tsukubaensis’ in MG medium was started directly from a spore suspension. Initially, several starch preparations from different suppliers were analyzed since starch is produced from different raw materials and it could present diverse properties depending on the supplier. Surprisingly, the starch source had a strong effect on tacrolimus production by ‘S. tsukubaensis’ (data not shown). Thus, Difco's starch produces the highest tacrolimus titer, which reached values of around 20 μg/ml after 168 h of fermentation. Since the starch could contain different supplier-dependent impurities, we consider the medium as a semi-defined one.
Once the starch containing basal medium named modified MG (MGm) was established, tacrolimus production was analyzed in the presence of well-known factors that affect secondary metabolism, such as nutritional starvation [e.g., phosphate depletion (Martín 2004)] or other stresses [e.g., exposure to stressing compounds like dimetil sulfoxide (DMSO) (Chen et al. 2000) or thiosulfate (Nárdiz et al. 2011)].
Effect of phosphate concentration
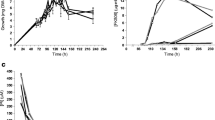
Three phosphate concentrations were analyzed: 2.5 mM, selected as phosphate-limiting condition based on the studies performed in S. coelicolor (Doull and Vining 1990; Santos-Beneit et al. 2008), and two higher concentrations of 10 and 20 mM. Figure 3a shows the negative effect that phosphate has on tacrolimus biosynthesis by ‘S. tsukubaensis’. Thus, tacrolimus production was triggered exactly at the same time that the phosphate was depleted in MGm-2.5 medium (Fig. 3b). Besides, around this time, a dark green pigment was produced in MGm-2.5 cultures, whereas it was not produced in the phosphate-replete conditions.
a Effect of phosphate concentration (inorganic phosphate, Pi—2.5, 10, and 20 mM) on volumetric production of tacrolimus by ‘S. tsukubaensis’ in MGm medium. b, c Growth and tacrolimus production in relation to the phosphate consumption in MGm medium with two different initial phosphate concentrations 2.5 mM (MGm-2.5) and 10 mM (MGm-10). The arrow indicates the time of phosphate depletion (below 0.5 mM). Data show the average of three replicates. d Tacrolimus production by ‘S. tsukubaensis’ in MGm medium supplemented with 2.5 g/l of lysine at different phosphate concentration (2.5, 10, and 20 mM). Vertical bars indicate standard deviation of the mean values
Cross-regulation of phosphate and lysine
In addition, a cross-talk between the phosphate concentration and the presence of lysine in the fermentation medium that affects tacrolimus production was observed. Lysine, after its conversion to pipecolic acid, is a precursor of the tacrolimus molecule; hence, it is expected that this amino acid increases tacrolimus production. Thus, the supplementation of MGm medium with 2.5 g/l of lysine (Goranovic et al. 2010) increased tacrolimus production from 20 μg/ml (titer reached in MGm; Fig. 3a) to 30 μg/ml at the same time of fermentation (Fig. 3d). Interestingly, in the presence of lysine, the highest amount of tacrolimus was produced at a phosphate concentration of 10 mM (Fig. 3d). This change of optimal concentration could be due to the formation of hydrogen bonds (N+H–O−P) between phosphate and lysine (Burget and Zundel 1986). Therefore, less amount of phosphate would be available for the growth of microorganisms.
Effect of stressing factors
Finally, the effect of stress agents [DMSO or sodium thiosulfate] on tacrolimus production was analyzed. The assayed concentrations of the stressing factors DMSO and thiosulfate were selected according to Chen et al. (2000) and Nárdiz et al. (2011), respectively. Thus, DMSO and sodium thiosulfate added in the middle of exponential phase caused an increase of tacrolimus production. Especially, supplementation with 1.5 % (v/v) of DMSO was particularly effective at enhancing tacrolimus production (twofold increase) and the synthesis of a pink pigment. Addition of these agents did not affect the total biomass generated during the fermentation (Fig. 4a).
a Effect of stress agents DMSO (1.5 and 3 %) and thiosulfate (3 and 5 mM) on growth. The arrow indicates the time of the addition of the stress agents to MGm medium. Effect of b DMSO and c thiosulfate on tacrolimus production by ‘S. tsukubaensis’ in MGm medium. Data are the average of three replicates. Vertical bars indicate the standard deviation of the mean values
The final optimized composition of the MGm for tacrolimus production is detailed in Table 2.
Optimization of an ISP4-based medium for improving tacrolimus production
Since ‘S. tsukubaensis’ grew well on ISP4 solid medium and presented good sporulation after 10 days, this medium was selected as basal medium in liquid cultures for the optimization of tacrolimus production by this microorganism. Fermentation was started from a 40-h preculture in BaSa medium. In the standard ISP4 medium, production of tacrolimus started after 48 h of inoculation and maximum production was at about 10 μg/ml (Fig. 5).
Analysis of a the growth rate and b tacrolimus production by ‘S. tsukubaensis’ in ISP4 medium. Effect of the addition of glucose (20 g/l) and MOPS (5 g/l). Data are the average of three replicates. Vertical bars indicate the standard deviation of the mean values. Note that the optimized conditions were accumulated in the next step of optimization
Effect of glucose supplementation and MOPS buffering
The first step of optimization was the modification of the carbon source. Thus, glucose at a final concentration of 20 g/l was added to the ISP4 medium (in addition to starch that is included in the original formulation of ISP4). Better growth and volumetric production of tacrolimus were observed in cultures grown in this modified medium (Fig. 5). Therefore, tacrolimus yield was improved by supplying this readily metabolized sugar (glucose) during the growth phase and a slowly metabolized carbon source (starch) during the production phase.
MOPS was added to the ISP4 medium at a final concentration of 5 g/l to prevent the decline of pH during fermentation. This buffer was able to maintain the pH at 6.8 throughout fermentation. Hence, growth was improved and the variability observed at the end of the production phase in unbuffered medium, probably due to changing pH values, was eliminated (Fig. 5).
Effect of starch source
As in the MGm medium described earlier, starches from different suppliers (Scharlau, Difco, and Panreac) were tested. In this case, starch from Scharlau supported the highest volumetric tacrolimus production (Fig. 6a). Sequentially, the next step was defining the optimal concentration of starch in the medium. As observed in Fig. 6b, the highest yield of tacrolimus was obtained by increasing the concentration of starch from 10 to 30 g/l. Higher concentrations of starch (40 and 60 g/l) decreased tacrolimus yield, although with increased cell growth (Fig. 6b).
Effect of the a type of starch (Prolabo, Panreac, Scharlau, and Difco) and b amount (10, 20, 30, 40, and 60 g/l) of starch on growth and tacrolimus production by ‘S. tsukubaensis’ in modified ISP4 medium. Data are the average of three replicates. Vertical bars indicate the standard deviation of the mean values
Effect of other slowly utilized carbon sources
Slow-processing carbon sources such as lactose (Singh and Behera 2009) or corn dextrin (Kumar et al. 2005) have been described as good nutrients for tacrolimus production in other Streptomyces species. These two carbon sources were tested for their abilities to support the growth of ‘S. tsukubaensis’ and tacrolimus production in this strain.
Since the best productivity of tacrolimus was observed at 30 g/l of starch, the same concentration was used for other carbon sources as well. The growth rate of ‘S. tsukubaensis’ was similar in corn dextrin and starch, but tacrolimus titer was significantly higher using the former (Sigma). On the contrary, growth rate and tacrolimus production were drastically decreased using lactose instead of dextrin as carbon source (data not shown).
To find the optimal concentration of corn dextrin for best growth condition and tacrolimus production, fermentation was done by growing ‘S. tsukubaensis’ in different concentrations of corn dextrin. As observed in Fig. 7a, the highest production was achieved with 30 to 40 g/l of corn dextrin. A concentration of 30 g/l of this carbon source allowed the highest production level. However, since tacrolimus production using 30 g/l of corn dextrin presents high variability (standard deviation), a concentration of 40 g/l was selected as the best one for both growth and tacrolimus production for further optimization steps.
a Analysis of growth and tacrolimus production by ‘S. tsukubaensis’ in modified ISP4 medium at different concentrations (20, 30, 40 50, and 60 g/l) of corn dextrin. b Effect of nitrogen source on growth and tacrolimus production in ‘S. tsukubaensis’ in modified ISP4 medium. The nitrogen sources analyzed were (NH4)2SO4 (1, 2, and 3 g/l), KNO3, yeast extract, and peptone. c Effect of pH on growth and tacrolimus production by ‘S. tsukubaensis’ in modified ISP4 medium. Data are the average of three replicates. Vertical bars indicate the standard deviation of the mean values
Effect of nitrogen source
In order to examine the effects of different nitrogen sources over tacrolimus production, three concentrations (1, 2, and 3 g/l) of ammonium sulfate [(NH4)2SO4], 2 g/l of potassium nitrate (KNO3), 10 g/l of yeast extract, and 10 g/l of peptone were analyzed. The concentration of corn dextrin was fixed at 40 g/l as carbon source in all tested media (Fig. 7b). Peptone and yeast extract significantly increased cell growth but completely suppressed tacrolimus production. Potassium nitrate reduced both cell growth and tacrolimus production. Ammonium sulfate was the best nitrogen source studied for tacrolimus biosynthesis and the optimal concentration of ammonium sulfate for tacrolimus production by ‘S. tsukubaensis’ was found to be 2 g/l.
Effect of pH
In initial experiments, it was observed that pH had a significant effect on tacrolimus production; therefore, a more detailed study was performed. Figure 7c shows the effect of pH on growth and tacrolimus production on the ISP4 modified medium. Growth was not affected in the pH range from 6.0 to 8.0, but the best volumetric and specific production of tacrolimus was obtained at pH 6.5. Tacrolimus production was significantly decreased when the pH turn to alkaline values.
The composition of the optimized ISP4 media, which is called ISPz, is detailed in Table 2.
Discussion
To the best of our knowledge, all tacrolimus producer strains have been described as members of Streptomyces genus, including ‘S. tsukubaensis’, Streptomyces tacrolimicus ATCC 55098 (Martínez-Castro et al. 2011; Dumont et al. 1992), Streptomyces sp. ATCC 53770 (MA 6548) (Garrity et al. 1993), Streptomyces sp. MA 6949 (Sigmund et al. 2003), Streptomyces kanamyceticus KCC-S0436 (Muramatsu et al. 2005), Streptomyces glaucescens (Kumar et al. 2005), and S. clavuligerus CKD 1119 (Kim and Park 2008). However, despite shearing the ability to synthesize the same secondary metabolite, most of these species are not closely phylogenetically related as it was shown by Garrity et al. (1993) and Muramatsu et al. (2005). On the other hand, the taxonomic position is quite far to be determined in almost all of the cases, which produces insecurity when referring to those strains as separate species.
On the basis of phenotypic, phylogenetic, and chemotaxonomic analyses, we propose that the strain ‘S. tsukubaensis’ represents a unique species of the Streptomyces genus. It was isolated from soil of the Tsukuba region (Japan), patented under the name ‘S. tsukubaensis’ No. 9993 (Okuhara et al. 1990) and deposited in the Fermentation Research Institute (Japan) with the deposit number FERM BP-927. Subsequently, it was redeposited in 1989 in the Agricultural Research Culture Collection International Depository (USA) with the deposit number NRRL 18488 (Baumann and Emmer 1999). According to our results, the most closely related species is the clavulanic acid producer S. clavuligerus ATCC 27064T, which presents 98.4 % of 16S rRNA similarity. Surprisingly, both strains presented a very low value (10.1 %) of DNA/DNA identity, as shown in a DNA/DNA hybridization study. Evolutionarily, the reported reorganization of the genome of S. clavuligerus in 6.8-Mb small chromosome and a 1.8-Mb plasmid may have occurred through the recombination of a smaller plasmid with the arms of the main chromosome (Medema et al. 2010). Hence, the lack of ends in the chromosome of S. clavuligerus could reduce the relatedness of DNA/DNA.
‘S. tsukubaensis’ is a Gram-positive aerobic actinomycete that forms extensively branched substrate and aerial hyphae which differentiate into straight to flexuous chains of short rod-shaped spores with smooth surface. It belongs to the gray spore color series (Tresner and Backus 1963) with pinkish–reddish mycelium, and in certain media, especially in ISP2, it produces a reddish pigment.
In order to study in detail the regulatory mechanisms of tacrolimus biosynthesis, the development of a reliable defined medium was necessary. The use of a simple and chemically defined media generally facilitates the investigation of the physiology and biochemistry of secondary metabolite production by a microorganism. In the present work, two chemically semi-defined media were optimized for tacrolimus production by ‘S. tsukubaensis’.
The basic original recipe of MG medium designed by Doull and Vinning (1990) is maintained and the effect of different starch suppliers has been analyzed in order to increase tacrolimus production by ‘S. tsukubaensis’. MGm is a minimal medium and fermentation is started with a spore suspension, which allows a precise study of the effect of different factors on secondary metabolism. In this medium, the organism reached the stationary phase of growth in 120 h after inoculation with a spore suspension. Onset of tacrolimus production under these conditions took place at 100 h.
Under stress conditions, like limitation in nutrients, morphological differentiation and secondary metabolism are triggered in Streptomyces species. Streptomycetes inhabit soil, where plant-derived material is the main nutrient input, i.e., phosphate-poor environment (Hodgson 2000). Thus, this nutritional factor exerts a strong control over secondary metabolism in order to survive to phosphate limitation (Martín 2004). Another factor that usually affects antibiotic production is the supplementation of fermentation medium with a precursor of the interesting molecule. l-Pipecolate, derived from lysine catabolism, serves as a terminator unit for the synthesis of the linear chain, after which lactonization occurs, yielding the macrolactone ring of FK506 (Motamedi and Shafiee 1998). Thus, MGm medium was modified in order to analyze the effect of different factors as phosphate concentration (a nutritional factor), DMSO or thiosulfate (toxic compounds), and lysine (precursor of tacrolimus molecule). Phosphate exerted a negative control over secondary metabolism as it has been shown in other Streptomyces species, like S. clavuligerus (Jhang et al. 1989), Streptomyces natalensis (Mendes et al. 2007), or Streptomyces hygroscopicus (Cheng et al. 1995). The MGm medium permitted one to determine that the onset of tacrolimus production in phosphate limitation conditions was produced exactly at the same time that the phosphate concentration was depleted. Moreover, an interaction between phosphate and lysine has been observed. Thus, the addition of lysine to MGm medium increased tacrolimus production and changed the phosphate limitation condition. On the other hand, the supplementation of the MGm medium with toxic compound, especially DMSO, altered the secondary metabolism, and tacrolimus production was enhanced, also producing a pink pigment. Apart from tacrolimus, ‘S. tsukubaensis’ has the genetic capacity to produce a vast number of secondary metabolites (Barreiro et al. 2012). The mechanism of action of DMSO is not known, but it has been reported that this compound stimulates polyketide biosynthesis (Chen et al. 2000; Butler and Cundliffe 2001). In summary, the simple composition of MGm permits one to analyze the effect of different factors, as well as interactions between the components of the fermentation medium, on tacrolimus biosynthesis by ‘S. tsukubaensis’.
On the other hand, ISPz, a more complex semi-defined medium, has been optimized to improve tacrolimus production. In this case, the stationary phase was reached at 72 h after inoculation from BaSa seed culture and the production was started at 20 h. The volumetric titer of tacrolimus in this medium was up to 90.9±13.5 μg/ml equivalent to a maximum specific productivity of 14.9±1.9 μg/mg. This value is well above those previously reported yields of 30–40 μg/ml for ‘S. tsukubaensis’ NRRL 18488 (Kino et al. 1987; Kim and Park 2008).
The carbon sources can be divided into two groups: quickly metabolized carbon sources and sustainable (slowly utilized) carbon sources—of which glucose is regarded as quickly metabolized carbon source, whereas dextrin and soluble starch are sustainable carbon sources. The results presented in this article suggest that glucose did not exert catabolite repression of tacrolimus production by ‘S. tsukubaensis’. On the other hand, soluble starch allowed tacrolimus production, but the use of corn dextrin substantially enhanced the immunosuppressant biosynthesis. The maximum volumetric titer of tacrolimus was obtained using 30 g/l of corn dextrin, which had very high variability between batches. The best growth and tacrolimus production with less variability were observed in cultures grown in a mixture of 40 g/l of corn dextrin plus 20 g/l of glucose.
Regarding the nitrogen source, addition of yeast extract or peptone increased the growth rate in ‘S. tsukubaensis’ but totally suppressed tacrolimus production, whereas these nitrogen sources have been reported to be used for tacrolimus (or tacrolimus-like compound) production in S. tacrolimicus B3178 (Singh and Behera 2009). Ammonium salts are the best of the tested nitrogen sources; 2 g/l of ammonium sulfate was the best option for tacrolimus production in ‘S. tsukubaensis’. Increase of ammonium sulfate concentration inhibited growth and tacrolimus production. Similar to the effect of phosphate, high ammonium concentrations exerted a negative control over tacrolimus biosynthesis; some kind of ammonium repression has been previously reported in S. clavuligerus (Aharonowitz 1980) and S. hygroscopicus (Lee et al. 1997).
In addition to carbon and nitrogen sources, pH also had an important role in tacrolimus production in ‘S. tsukubaensis’. As it was observed in this study, change in the pH of medium (even in a range of ±0.5 pH units) decreased the production of tacrolimus but did not have any effect on growth rate. Finally, pH 6.5 was the best condition to obtain high volumetric titers of tacrolimus in cultures of ‘S. tsukubaensis’.
In conclusion, this work has rigorously validated the taxonomical position of ‘S. tsukubaensis’ as a tacrolimus producer closely related to S. clavuligerus and also established the bases for further metabolic studies related to the regulatory mechanisms of tacrolimus biosynthesis. Besides, it offers a variety of semi-defined media for deep analyses of nutrient regulation influence over tacrolimus production.
References
Aharonowitz Y (1980) Nitrogen metabolite regulation of antibiotic biosynthesis. Annu Rev Microbiol 34:209–233
Aidoo KA, Wong A, Alexander DC, Rittammer RA, Jensen SE (1994) Cloning, sequencing and disruption of a gene from Streptomyces clavuligerus involved in clavulanic acid biosynthesis. Gene 147(1):41–46
Barreiro C, Prieto C, Sola-Landa A, Solera E, Martínez-Castro M, Perez-Redondo R, Garcia-Estrada C, Aparicio JF, Fernandez-Martínez LT, Santos-Aberturas J, Salehi-Najafabadi Z, Rodríguez-García A, Tauch A, Martín JF (2012) Draft genome of ‘Streptomyces tsukubaensis’ NRRL 18488, the producer of the clinical immnunosuppressant tacrolimus (FK506). J Bacteriol 194(14):3756–3757
Baumann K, Emmer G (1999) Heteroatoms-containing tricyclic compounds. US Patent 5,912,238
Becker B, Lechevalier MP, Gordon RE, Lechevalier HA (1964) Rapid differentiation between Nocardia and Streptomyces by paper chromatography of whole-cell hydrolysates. Appl Microbiol 12:421–423
Burget U, Zundel G (1986) Lysine–phosphate hydrogen bonds and hydrogen-bonded chains with large proton polarizability in polylysine–phosphate systems: IR investigations. J Mol Struct 145(1–2):93–109
Butler AR, Cundliffe E (2001) Influence of dimethylsulfoxide on tylosin production in Streptomyces fradiae. J Ind Microbiol Biotechnol 27(1):46–51
Chen G, Wang GY, Li X, Waters B, Davies J (2000) Enhanced production of microbial metabolites in the presence of dimethyl sulfoxide. J Antibiot (Tokyo) 53(10):1145–1153
Cheng YR, Hauck L, Demain AL (1995) Phosphate, ammonium, magnesium and iron nutrition of Streptomyces hygroscopicus with respect to rapamycin biosynthesis. J Ind Microbiol 14(5):424–427
De Ley J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem 12:133–142
Doull JL, Vining LC (1990) Nutritional control of actinorhodin production by Streptomyces coelicolor A3(2): suppressive effects of nitrogen and phosphate. Appl Microbiol Biotechnol 32(4):449–454
Dumont F, Garrity GM, Fernandez IM, Matas TD (1992) Process for producing FK-506. US Patent 5,116,756
Euzéby JP (1997) List of bacterial names with standing in nomenclature: a folder 243 available on the Internet. Int J Syst Bacteriol 47:590–592
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4):783–791
Fireman M, Di Martini AF, Armstrong SC, Cozza KL (2004) Immunosuppressants. Psychosomatics 45:354–360
Garrity GM, Heimbuch BK, Motamedi H, Shafiee A (1993) Genetic relationships among actinomycetes that produce the immunosuppressant macrolides FK506, FK520/FK523 and rapamycin. J Ind Microbiol 12(1):42–47
Goranovic D, Kosec G, Mrak P, Fujs S, Horvat J, Kuscer E, Kopitar G, Petkovic H (2010) Origin of the allyl group in FK506 biosynthesis. J Biol Chem 285(19):14292–14300
Hasegawa T, Takizawa M, Tanida S (1983) A rapid analysis for chemical grouping of aerobic actinomycetes. J Gen Appl Microbiol 29:319–322
Hodgson DA (2000) Primary metabolism and its control in streptomycetes: a most unusual group of bacteria. Adv Microb Physiol 42:47–238
Huss VAR, Festl H, Schleifer KH (1983) Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol 4:184–192
Jhang J, Wolfe S, Demain AL (1989) Phosphate regulation of ACV synthetase and cephalosporin biosynthesis in Streptomyces clavuligerus. FEMS Microbiol Lett 48(2):145–150
Jones KL (1949) Fresh isolates of actinomycetes in which the presence of sporogenous aerial mycelia is a fluctuating characteristic. J Bacteriol 57:141–145
Kim HS, Park YI (2008) Isolation and identification of a novel microorganism producing the immunosuppressant tacrolimus. J Biosci Bioeng 105(4):418–421
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H (1987) FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot 40:1249–1255
Kumar P, Sharma S, Shukla A, Kumar S, Maurya RK, Katial V, Mitra A, Gigras P (2005) Production of tacrolimus (FK-506) using new Streptomyces species. WO 2005/038009 A2
Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA (1979) Improved assay for nanomole amounts of inorganic phosphate. Anal Biochem 100:95–97
Lechevalier MP, Lechevalier H (1970) Chemical composition as a criterion in the classification of aerobic actinomycetes. Int J Syst Bacteriol 20:435–443
Lee MS, Kojima I, Demain AL (1997) Effect of nitrogen source on biosynthesis of rapamycin by Streptomyces hygroscopicus. J Ind Microbiol Biotechnol 19(2):83–86
Locci R (1989) Streptomyces and related genera. In: Williams ST, Sharpe ME, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 4. Williams & Wilkins, Baltimore, pp 2451–2508
Martín JF (2004) Phosphate control of the biosynthesis of antibiotics and other secondary metabolites is mediated by the PhoR–PhoP system: an unfinished story. J Bacteriol 186(16):5197–5201
Martínez-Castro M, Barreiro C, Romero F, Fernández-Chimeno RI, Martín JF (2011) Streptomyces tacrolimicus sp. nov., a low producer of the immunosuppressant tacrolimus (FK506). Int J Syst Evol Microbiol 61(Pt 5):1084–1088
Medema MH, Trefzer A, Kovalchuk A, van den Berg M, Müller U, Heijne W, Wu L, Alam MT, Ronning CM, Nierman WC, Bovenberg RA, Breitling R, Takano E (2010) The sequence of a 1.8-mb bacterial linear plasmid reveals a rich evolutionary reservoir of secondary metabolic pathways. Genome Biol Evol 2:212–224
Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, Barr ML, Leichtman AB (2006) Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant 6(5 Pt 2):1111–1131
Mendes MV, Tunca S, Antón N, Recio E, Sola-Landa A, Aparicio JF, Martín JF (2007) The two-component phoR-phoP system of Streptomyces natalensis: inactivation or deletion of phoP reduces the negative phosphate regulation of pimaricin biosynthesis. Metab Eng 9(2):217–227
Miller LT (1982) Single derivatization method for routine analysis of bacterial whole-cell fatty acid methyl esters, including hydroxyl acids. J Clin Microbio 16:584–586
Miller L, Berger T (1985) Bacteria identification by gas chromatography of whole cell fatty acids, Hewlett-Packard application note 228–241. Hewlett-Packard, Avondale
Mo S, Kim DH, Lee JH, Park JW, Basnet DB, Ban YH, Yoo YJ, Chen SW, Park SR, Choi EA, Kim E, Jin YY, Lee SK, Park JY, Liu Y, Lee MO, Lee KS, Kim SJ, Kim D, Park BC, Lee SG, Kwon HJ, Suh JW, Moore BS, Lim SK, Yoon YJ (2011) Biosynthesis of the allylmalonyl–CoA extender unit for the FK506 polyketide synthase proceeds through a dedicated polyketide synthase and facilitates the mutasynthesis of analogues. J Am Chem Soc 133(4):976–985
Mo S, Yoo YJ, Ban YH, Lee SK, Kim E, Suh JW, Yoon YJ (2012) Roles of fkbN in positive regulation and tcs7 in negative regulation of FK506 biosynthesis in Streptomyces sp. KCTC 11604BP. Appl Environ Microbiol 78(7):2249–2255
Motamedi H, Shafiee A (1998) The biosynthetic gene cluster for the macrolactone ring of the immunosuppressant FK506. Eur J Biochem 256(3):528–534
Muramatsu H, Mokhtar SI, Katsuoka M, Ezaki M (2005) Phylogenetic analysis of immunosuppressant FK506-producing streptomycete strains. Actinomycetologica 19(2):33–39
Nárdiz N, Santamarta I, Lorenzana LM, Martín JF, Liras P (2011) A rhodanese‐like protein is highly overrepresented in the mutant S. clavuligerus oppA2::aph: effect on holomycin and other secondary metabolites production. Microb Biotechnol 4(2):216–225
Okuhara M, Tanaka H, Goto T, Kino T, Hatanaka H (1990) Tricyclo compounds, a process for their production and a pharmaceutical composition containing the same. US Patent 4,894,366
Rodríguez-García A, Barreiro C, Santos-Beneit F, Sola-Landa A, Martín JF (2007) Genome-wide transcriptomic and proteomic analysis of the primary response to phosphate limitation in Streptomyces coelicolor M145 and in a DeltaphoP mutant. Proteomics 7(14):2410–2429
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Santos-Beneit F, Rodríguez-García A, Franco-Domínguez E, Martín JF (2008) Phosphate-dependent regulation of the low- and high-affinity transport systems in the model actinomycete Streptomyces coelicolor. Microbiology 154(Pt8):2356–2370.
Shirling SB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340
Sigmund JM, Clark DC, Rainey FA, Anderson AS (2003) Detection of eubacterial 3-hydroxy-3-methylglutaryl coenzyme a reductases from natural populations of actinomycetes. Microb Ecol 46(1):106–112
Singh BP, Behera BK (2009) Regulation of tacrolimus production by altering primary source of carbons and amino acids. Lett Appl Microbiol 49(2):254–259
Staneck JL, Roberts GD (1974) Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl Microbiol 28:226–231
Tamaoka J, Komagata K (1984) Determination of DNA base composition by reversed-phase high-performance liquid chromatography. FEMS Microbiol Lett 25:125–128
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tresner HD, Backus EJ (1963) System of color wheels for streptomycete taxonomy. Appl Microbiol 11:335–338
Turło J, Gutkowska B, Gajzlerska W (2006) Submerged cultivation of ‘Streptomyces tsukubaensis’ in media composed of waste products of food industry. Acta Pol Pharm 63(5):463–465
Vandamme P, Pot B, Gillis M, de Vos P, Kersters K, Swings J (1996) Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev 60(2):407–438
Wayne LG, Brenner DJ, Colwell RR, Grimont PAD, Kandler O, Krichevsky MI, Moore LH, Moore WEC, Murray RGE, Stackebrandt E, Starr MP, Trüper HG (1987) International Committee on Bacterial Systematics. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol 37:463–464
Williams ST, Goodfellow M, Alderson G, Wellington EMH, Sneath PHA, Sackin MJ (1983) Numerical classification of Streptomyces and related genera. J Gen Microbiol 129:1743–1813
Yoon YJ, Choi CY (1997) Nutrient effects on FK-506, a new immunosuppressant, production by Streptomyces sp. in a defined medium. J Ferment Bioeng 83(6):599–603
Acknowledgments
This work was supported by a grant of the CICYT Ministry of Innovation and Science (Madrid, Spain) [BIO2006-14853-C02-01 (CONSOLIDER)] and by the European Union through an ERA-IB (PIM2010EEI-00677) international cooperation project. M. Martínez-Castro received a PFU fellowship of the Ministry of Education and Science. C. Barreiro was supported by the European Union program ERA-IB [BioProChemBB project (EIB.08.008)]. We acknowledge the scientific assistance of S. Albillos and the excellent technical support of B. Martín, J. Merino, A. Casenave, and A. Mulero (INBIOTEC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Castro, M., Salehi-Najafabadi, Z., Romero, F. et al. Taxonomy and chemically semi-defined media for the analysis of the tacrolimus producer ‘Streptomyces tsukubaensis’ . Appl Microbiol Biotechnol 97, 2139–2152 (2013). https://doi.org/10.1007/s00253-012-4364-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4364-x