Abstract
Numerous studies have been published on the antimicrobial and antioxidant properties of various plant components. However, there is relatively little information on the impact of such components on the enhancement of probiotics and production of antimicrobial compounds from these probiotics. Hence, this paper focuses on the influence of plant-derived components against pathogens, enhancement of cell viability and functionality of probiotics, and potential applications of such components in food safety and human health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are rich in several functional compounds including phytochemicals, phenols, polyphenols, essential oils, and micronutrients (Cowan 1999; Tajkarimi et al. 2010). These compounds have been reported to have antimicrobial and antioxidant activity. These natural compounds have shown potential for food preservation and quality applications (Olasupo et al. 2003; Tajkarimi et al. 2010). In addition, these natural compounds play a major role in the functionality of many biological systems including microbes. Microbes including natural microflora (gut microbiota) in human require several mineral nutrients for their survival and growth. Thus, the aforementioned natural compounds, as a good source of nutrients, could enhance the microbial and enzyme activities (Bomba et al. 2006; Sutherland et al. 2009; Yadav et al. 2011). These natural compounds contain a number of antioxidants, which make the compounds a functional food. Several antioxidants have shown to improve a population of beneficial intestinal flora especially Lactobacillus. Duda-Chodak et al. (2008) have shown that antioxidants such as catechin and cholorogenic acids in the range of 100–400 μM act as a stimulatory effect on the growth of Lactobacillus casei (DSM 20011). These antioxidants, major components of certain fruits, and green tea serve as an oxygen scavenger and reduce the redox potential of the media. As probiotics including lactic acid bacteria grow well in low oxygen environment, catechol or other simple phenols produced from catechin and cholorogenic acid metabolism could be related to the stimulatory effect of probiotic bacteria. Phytochemical components have been also shown to stimulate the growth of lactic acid bacteria (Bomba et al. 2006). Gut microflora including probiotics is anaerobic or facultative anaerobic. Since oxygen is toxic to these microorganisms, the presence of antioxidant helps scavenge oxygen and thus reduces the availability of oxygen from the environment and promotes growth. In addition, antioxidants increase the activities of antioxidant enzymes such as superoxide dismutase and catalase. These enzymes require minerals (Mn2+, Ca2+) as cofactors for various biological activities of microorganisms (Alberto et al. 2001). The normal intestinal flora with these enzymes is able to neutralize reactive oxygen species and could thus contribute to prevent oxidative epithelial damages (Lin and Yen 1999; Wijeratne et al. 2005; Spyropoulos et al. 2011; del Carmen et al. 2011). Higher enzyme production could ultimately provide treatment of inflammatory diseases or post-cancer drug treatments. These enzymes also enhance the production of organic acids such as lactic and acetic acids, which act as antimicrobial agents and suppressed the growth of pathogenic bacteria (Ibrahim and Bezkorovainy 1994; Nakashima 1997; Ibrahim and Salameh 2001; Ibrahim et al. 2003; Ibrahim 2005).
Furthermore, plants rich in micronutrients such as manganese and zinc also have shown to enhance the ability of probiotics to produce organic acids. These organic acids offer the potential as a natural antimicrobial, thus improving the safety of foods and human health (Nakashima 1997; Ibrahim et al. 2003; Bomba et al. 2006; Kang and Fung 2000). In addition, plant components indirectly induce probiotics activity to promote the production of antimicrobial compounds (Ibrahim and Bezkorovainy 1994; Calomme et al. 1995; Bomba et al. 2002; Wishon et al. 2010). To date, little information is available on the indirect influence of mineral nutrients on the production of antimicrobial agents by probiotics including bifidobacteria. Kang and Fung (2000) and Zaika and Kissinger (1984) found that manganese ions (Mn2+) present in spices are strong stimulants for starter cultures. Several microorganisms require iron for growth (O'Sullivan 2001; Ibrahim 2005; Yadav et al. 2011). Bifidobacteria tend to be better at iron scavenging than other intestinal flora. Pathogens must be able to adapt to this iron-limiting environment in order to infect. Hence, growth inhibition of these competing organisms by depriving them of iron (O'Sullivan 2001) is an excellent example of the indirect effect of plant components on microorganisms. Since the early 1990s, our research group has focused on understanding the concept and mechanism to establish how these functional compounds from plants could indirectly influence the production of antimicrobial compounds contributing to improve human health (Wishon et al. 2010; Ibrahim 2005; O'Sullivan 2001; Ibrahim and Salameh 2001; Ibrahim and Bezkorovainy 1994). However, the actual mechanism for this influence has not been demonstrated comprehensively yet.
Our literature review showed that different plant components and their derivatives exhibit antibacterial activity against different pathogens or spoilage microorganisms. Most previous studies on plant products have focused mainly on antimicrobial and antioxidant properties. However, there is relatively little information pertaining to the dual impact of plant functional components on probiotics and pathogens. Plant components rich in antioxidants have not only shown to inhibit the growth of pathogens but also proven to favor the growth of probiotics. Therefore, in this review, we aimed to provide an overview on the impact of plant derivatives on the growth of foodborne pathogens and the functionality of probiotics.
Antimicrobial properties against pathogenic bacteria
The antimicrobial properties of natural compounds derived from plants have been recognized for centuries, but only scientifically confirmed in the last 30 years (Olasupo et al. 2003). There has been increasing interest in finding new natural antimicrobials for application in food production to prevent or inhibit microbial growth and extend shelf life (Fattouch et al. 2007; Lanciotti et al. 2004). Some of the studies on antimicrobial properties of plant products against foodborne pathogens are discussed below.
Garlic (Allium sativum) extract has a broad range of antimicrobial activity. Allicin, an organosulfur compound present in garlic, acts as a growth inhibitor for both Gram-positive and Gram-negative bacteria including Escherichia, Salmonella, Streptococcus, Staphylococcus, Klebsiella, Proteus, and Helicobacter pylori (Belguith et al. 2009; Ankri and Mirelman 1999). Avato et al. (2000) have also reported that the antibacterial activity of garlic is due to the action of allicin, diallyl thiosulfinic acid, or diallyl disulfide. A recent study showed that organosulfur compounds present in garlic have higher antimicrobial activity than those of garlic phenolic compounds. Lv et al. (2011) have reported that a bactericidal property of garlic-derived organosulfur compound was much greater against Campylobacter jejuni compared to phenolic compound. Garlic extract has excellent antibacterial activity against Escherichia coli, Salmonella, and Aeromonas hydrophila. Indu et al. (2006) reported that garlic extract showed antibacterial activity against all serogroups of E. coli. However, enterohemorrhagic E. coli (serogroup O157) and enterotoxigenic E. coli (serogroup O8) were highly sensitive to garlic extract. Similarly, Indu et al. (2006) have also reported high antibacterial activity against Salmonella serotypes and A. hydrophila at 75 and 100 % concentrations of garlic extracts.
Antimicrobial activity of Capsicum annuum extract against Salmonella Typhimurium (ATCC 14028) in beef meat was reported by Careaga et al. (2003). The minimum inhibitory concentration of C. annuum extract to prevent the growth of Salmonella Typhimurium in minced beef meat was 1.5 ml/100 g. Similarly, Dorantes et al. (2000) have reported the inhibitory effect of three varieties of C. annuum extracts against Listeria monocytogenes (Scott A), Staphylococcus aureus (FRI-S6), Salmonella Typhimurium (ATCC 13311), and Bacillus cereus (wild type). The phenolic compound, 3-hydroxycinnamic acid (coumaric acid), is responsible for antimicrobial activity in Capsicum (Dorantes et al. 2000).
Chinese chive that belongs to the same family as garlic is an important ingredient in Asian cooking. Ibrahim et al. (2009) have shown that crude chive extract containing sulfur compounds can be effective against the growth of Salmonella and could be used in food products to prevent the growth of this pathogen. The antimicrobial effect of Chinese chive against E. coli and yeast (Pichia membranaefaciens CCRC 20859) has been also reported (Mau et al. 2001).
Caffeine (1,3,7-trimethylexanthine) is a methylated xanthine alkaloid derivative present in plant species. It has shown significant growth inhibition against E. coli O157:H7 at a concentration of 0.5 % (Ibrahim et al. 2006). Scientific literature suggests that antibacterial properties in coffee have been reported to arise from caffeic acid, chlorogenic acid, and protocatechnic acid (Dogasaki et al. 2002). Similarly, polyphenols (epicatechin, catechin, caffeine, chlorogenic acid, gallic acid, theobromine, theophylline, gallocatechin, epigallocatechin gallate, catechin gallate, epicatechin gallate, and theaflavin) from tea have also been found to have antimicrobial activity against Salmonella aureus and L. monocytogenes in laboratory medium (Kim et al. 2004). Kim and Fung (2004) have reported the antimicrobial activity of crude water-soluble arrowroot (Puerariae radix) tea against E. coli O157:H7, Salmonella enterica, L. monocytogenes, and S. aureus. The antimicrobial activity of arrowroot tea was due to the presence of catechins. Catechins present in green tea extract, epigallocatechin gallate and epigallocatechin, showed strong antimicrobial activity due to the galloyl moiety present in their structures (Shimamura et al. 2007). Polyphenols extracted from green tea extract have shown inhibitory effects on Gram-positive as well as Gram-negative bacteria (Gadang et al. 2008; Perumalla and Hettiarachchy 2011). Ndi et al. (2007) have reported antimicrobial activity of the plant Eremophila (Myoporaceae) against Gram-positive organisms such as streptococci and staphylococci. It has been suggested that the antibacterial properties of Eremophila is due to the compound serrulatanes, which is structurally related to a diterpenoid quinone antibiotic called bioflorin (Ghisalberti 1994).
Essential oils (EOs) obtained from plants have been known to possess antimicrobial activity. EOs and their constituents have been used in numerous food applications and are classified as generally recognized as safe. Several previous studies have shown that EOs from cinnamon along with geraniol, lemongrass, and clove have antimicrobial effect (Aureli et al. 1992; Burt 2004; Chao et al. 2000; Kim et al. 1995). EOs of lemongrass, cinnamon, and geraniol were found to be the most effective in inhibiting the growth of Salmonella enteritidis, E. coli, and Listeria innocua (Raybaudi-Massilia et al. 2006). Turgis et al. (2009) have shown that mustard EOs can be used against E. coli O157:H7 and Salmonella Typhi. Allyl isothiocyanate, a non-phenolic volatile compound found in the crucifereae family at low concentration, effectively inhibits several pathogenic microorganisms (Turgis et al. 2008). In a recent study, oregano EOs showed antimicrobial effects against E. coli, S. aureus, Bacillus subtilis, and Saccharomyces cerevisiae (Lv et al. 2011). Phenols and terpenes are the main two groups of constituents responsible for antimicrobial effects in oregano EOs. The major constituents of oregano EO include carvacrol (30.17 %), p-cymene (15.20 %), γ-terpinen (12.44 %), and thymol (8.62 %) (Lv et al. 2011). The use of natural organic compounds as food additives could also be helpful in controlling Cronobacter spp. in various types of foods. In a study by Lee and Jin (2008), the order of inhibition of the natural organic compounds (EOs) against Cronobacter spp. was thymol > eugenol > diacetyl > cinnamic acid.
Nanasombat and Lohasupthawee (2005) have reported the degree of antibacterial property of various spices tested against Salmonella and other enterobacteria in the order of clove > kaffir lime peels > cumin > cardamom > coriander > nutmeg > mace > ginger > garlic > holy basil > kaffir lime leaves. The antimicrobial activity in the components of spice oils of the terpenoid family is due to the presence of terpene fraction of the oils (Davidson and Branden 1981). Different types of pepper, parsley, and dill have shown antibacterial activity against natural microflora, coliforms, yeast and molds, and S. aureus in Kareish cheese (Wahba et al. 2010). All chilli peppers contain phytochemicals known collectively as capsaicinoids (Antonious et al. 2009; Spiller et al. 2008). Shan et al. (2007) have suggested that the antibacterial activity of a total of 46 extracts from spices and herbs was closely associated with the presence of their phenolic constituents. The authors have reported that all the tested spices have a strong antibacterial effect against B. cereus, L. monocytogenes, S. aureus, E. coli, and Salmonella Anatum. Oregano and thyme EOs possess significant in vitro colicidal and colistatic properties, and this bactericidal concentration of oregano EOs irreversibly damaged E. coli O157:H7 cells within 1 min (Burt and Reinders 2003). The bulb extracts of Allium cepa and A. sativum exhibited activity against filamentous and non-filamentous fungi. A. sativum showed significant inhibition of all tested bacterial (Gram-positive and Gram-negative) and fungal pathogens (Srinivasan et al. 2001). The volatile oils of black pepper, clove, geranium, nutmeg, oregano, and thyme possessed antibacterial activity against 25 different genera of tested bacteria with various degrees of growth inhibition (Dorman and Deans 2000). Nutmeg showed good antimicrobial activity against L. monocytogenes, whereas its effect on E. coli and Salmonella were strain dependent (Indu et al. 2006).
Mechanisms of antimicrobial action
The possible mode of action for different plant-derived compounds as antimicrobial agents has been reviewed extensively (Burt 2004; Lanciotti et al. 2004; Smith-Palmer et al. 2001; Sofos et al. 1998; Lopez-Malo et al. 2005; Davidson 2001). The exact mechanism of action is not yet fully understood. However, there are number of proposed mechanisms of antimicrobial action of such compounds (Holley and Patel 2005; Lanciotti et al. 2004; Proestos et al. 2008; Lambert et al. 2001; Ultee et al. 1999; Raccach 1984).
Most of the plant species (fruits and vegetables) naturally synthesized organic acids such as acetic, citric, succinic, malic, tartaric, benzoic, and ascorbic. In addition, microorganisms including lactic acid bacteria and bifidobacteria also produce acids as a result of fermentation (Ibrahim and Bezkorovainy 1994). These organic acids inhibit the growth of both bacterial and fungal cells (Brul and Coote 1999). The inhibition of microorganisms by acetic acids is due to the decrease of intracellular pH by releasing protons from undissociated molecules in the cytoplasm causing metabolism inhibition (Olasupo et al. 2004; Hosein et al. 2011). Some organic acids such as lactic acid have been shown to alter the permeability levels of the bacterial outer membrane. The exposure to such acidic substances increases the outer membrane permeability of Gram-negative bacteria (Alakomi et al. 2000; Helander and Mattila–Sandholm 2000; Derrickson-Tharrington et al. 2005). Gill and Holley (2006) have reported the effect of trans-cinnamaldehyde (bark extract of cinnamon) on the bacterial plasma membrane causing changes in cell membrane composition and thereby deactivating bacterial growth. Helander et al. (1998) reported that the outer membranes of E. coli and Salmonella Typhimurium disintegrated when exposed to carvacrol and thymol. Similarly, Rasooli et al. (2006) have reported thickening, disruption of the cell wall, and increased roughness and lack of cytoplasm in L. monocytogenes following exposure to thyme essential oil. Fisher and Phillips (2008) have also mentioned the morphological changes in the Enterococcus species after treatment with citrus essential oils. Carvacrol and tea tree oil have been shown to increase membrane fluidity and cause the leakage of protons and potassium ions causing disruption of membrane and inhibition of adenosine triphosphate (ATP) synthesis (Ultee et al. 1999) against S. aureus (Halcón and Milkus 2004). Oussalah et al. (2006) have reported that the control of cellular processes such as DNA transcription, protein synthesis, and loss of enzymatic activity due to decrease in pH occurs during cell membrane disruption in the presence of various essential oils. Essential oils penetrate the cell membrane as well as the mitochondrial membrane, leading to greater cytoplasmic and K+ ion loss (Raybaudi-Massilia et al. 2006). Lambert and Hammond (1973) have likewise mentioned potassium leakage as an early indicator of membrane damage.
Phenols affect enzyme activity associated with energy production at low concentration, while they cause denaturation of protein at high concentration. Therefore, the modes of action of phenolic compounds (EO fractions) as antimicrobial agents can be concentration dependent (Davidson 2001; Nychas 1995; Lopez-Malo et al. 2005; Sofos et al. 1998; Juven et al. 1994). Bajpai et al. (2008) reported that the antimicrobial activity is due to phenols’ ability to alter microbial cell permeability, causing the loss of macromolecules, interfering with membrane function, and thereby causing deformation in structure and functionality (Rico-Munoz et al. 1987; Kabara and Eklund 1991). The antimicrobial activity of isothiocynates derived from onion and garlic is responsible for the inactivation of extracellular enzymes through oxidative cleavage of disulfide bonds. The formation of the reactive thiocyanate radical is believed to produce the antimicrobial effect (Delaquis and Mazza 1995). Similarly, Helander et al. (1998) have reported that carvacrol, thymol, and trans-cinnamaldehyde decrease the intracellular ATP content of E. coli O157:H7 cells and subsequently increase extracellular ATP, indicating the disruptive action on the cell membrane.
Phenolic compounds and their essential oils containing hydroxyl group (–OH) are more inhibitory against microorganisms compared to those compounds that contain the carbonyl group. The –OH group can easily bind the active site of enzymes altering cell metabolism of microorganisms (Farag et al. 1989). In addition, the position of the –OH group in the phenolic ring structure influences antimicrobial activity. Pauli and Knobloch (1987) have reported that the alkyl substitution (o- and p-alkyl) into phenolic compounds showed strong antifungal activity. The essential oils of eugenol and thymol contribute to the inhibitory action by membrane disruption in both Gram-negative and Gram-positive bacteria (Walsh et al. 2003). Gustafson et al. (1998) have demonstrated that tea tree oil acts on the cell membrane causing cytoplasmic leakage, cell lysis, and cell death. It has also been reported that there is a rapid inhibition of the energy metabolism of L. monocytogenes and Listeria sakei in the presence of high concentrations of eugenol and cinnamaldehyde. Polyphenol compounds known as catechins have shown strong antimicrobial effects by altering the membrane morphology and disrupting the cell membrane (Ikigai et al. 1993). Other phenolic compounds such as caffeic acid and chlorogenic acid can stimulate the DNA degradation induced by Fe (III) and bleomycin (Hiramoto et al. 1996; Moran et al. 1997). Moran et al. (1997) have also reported that some antioxidants with trace amount of metals show enough prooxidative activity to cause DNA damage. Hashimoto et al. (1999) have demonstrated that gallic acid esters in epicatechin and epigallocatechin gallate have an affinity for lipid bilayers and that the effect of this affinity causes damage to the membrane structure. The actual mechanism of inhibition by all phenolic compounds is primarily the compounds’ reacting with the cell membrane or inactivating essential cellular enzymes or a combination of both (Davidson and Branden 1981). Therefore, the damage to bacterial cells occurs with the physical disruption of the target site cell membrane, dissipation of the proton motive force, or by the inhibition of associated membrane enzyme activity as suggested by Maillard (2002).
Various authors in the past have hypothesized different modes of action of spices and plant derivatives. Juven et al. (1994) have reported hydrophobic and hydrogen bonding of phenolic compounds to membrane proteins, followed by partition in the lipid bilayer. Cox et al. (2000) have shown the perturbation of membrane permeability consequent to its expansion and increased fluidity resulting in the inhibition of membrane-embedded enzymes. Membrane disruption, cell wall perturbation, and destruction of the electron transport system are a few other antimicrobial mechanisms of plant spices and their derived products (Caccioni et al. 1998; Juglal et al. 2002; Tassou et al. 2000).
Previous studies have shown that Gram-positive bacteria are more sensitive to antimicrobial action compared to Gram-negative bacteria that possess a lipopolysaccharide outer membrane, which is relatively impermeable to phenolic compounds (Smith-Palmer et al. 2001; Burt 2004; Farag et al. 1989; Lemos et al. 1990). It can be inferred that phenolic compounds can sensitize the phospholipid bilayer of the cytoplasmic membrane causing increased permeability, unavailability of vital intracellular components, and impairment of bacterial enzyme systems (Juven et al. 1994; Kim et al. 1995; Farag et al. 1989; Wendakoon and Sakaguchi 1995). Further research to demonstrate the mechanisms by which these plant-derived products cause cell death is warranted before utilizing them in food as natural preservatives or as commercial applications in the pharmaceutical industry.
Plant components on viability and growth of probiotics
Plant species possess several functional components as growth-promoting factors for probiotics. Functional components such as phenolic compounds, antioxidants, and micronutrients have shown not only to activate the growth but also to increase bacterial populations. Some species of these probiotics are able to metabolize phenolic compounds, and these compounds also serve as oxygen scavenger, which could have increased the growth of certain probiotic bacteria (Alberto et al. 2001). The functional components derived from plant source when incorporated into the diet could lead to beneficial physiological changes in human microflora. Probiotics, the beneficial microorganism in the human gut, can therefore be potentiated by functional components of natural origin. This section summarizes the effect of such components on the enhancement of selected probiotics.
Phenolic compounds present in berries have shown to enhance the growth of lactobacilli and bifidobacteria (Tabasco et al. 2011). Alberto et al. (2001) reported that phenolic compounds such as gallic acid and catechin normally present in grapes have stimulated the growth rate and resulted in greater cell densities of Lactobacillus hilagardii 5W in the FT80 liquid medium. This growth stimulation could be related to the ability of Lactobacillus spp. to metabolize these phenolic compounds. These compounds serve as substrate for the bacteria and this could have provided the stimulatory effects. Anthocyanin pigments present in berries have also influenced the growth of Lactobacillus acidophilus (Pratt et al. 1960). Ávila et al. (2009) have mentioned that β-glucosidase present in Lactobacillus spp. (Lactobacillus plantarum IFPL722, L. casei LC-01) and Bifidobacterium lactis BB-12 can convert anthocyanin into other phenolic acid compounds with different bioavailability and bioactivity that could have contributed to the increase in growth of probiotics. Similarly, carbohydrates such as pectins and pectic-oligosaccharides have been reported to stimulate the growth of probiotic including Bifidobacterium spp. (Manderson et al. 2005; Olano–Martin et al. 2002).
The supplementation of probiotic food products containing L. casei with plant compounds containing antioxidant properties such as catechin at concentrations of 100–400 μM and chlorogenic acid at 400 μM has been shown to enhance the growth of L. casei (Duda-Chodak et al. 2008). Tea phenolic compounds including epicatechin, catechin, 3-O-methylgallic acid, gallic acid, and caffeic acid have been identified as inhibitory agents for Clostridium and Bacteroides spp. However, the growth of probiotics such as Lactobacillus spp. and Bifidobacterium spp. was less inhibited indicating the possible effect of phenolic compounds on the viability of probiotics (Lee et al. 2006). Similarly, Jaquet et al. (2009) reported an increase in bifidobacterial populations after drinking coffee in healthy volunteers with lower initial bifidobacterial numbers. This stimulating effect is mainly due to the presence of phenolic compounds (chlorogenic acids) and soluble fiber.
The consumption of tannin-rich pomegranate products by humans has shown the potential to improve the imbalance of intestinal bacteria caused by stress and other factors. Bialonska et al. (2009) reported that commercial extract of pomegranate byproduct at 0.01 % (v/v) significantly enhanced the growth of Bifidobacterium breve NRRL B-41408 and Bifidobacterium infantis NRRL B-41661, thereby promoting human gut health. Aqueous extraction of garlic and black peppercorns significantly enhance the growth of Lactobacillus reuteri. Similarly, according to Sutherland et al. (2009), the growth-promoting activities of aqueous extracts of banana, apple, and orange have been shown to enhance the growth of L. reuteri, Lactobacillus rhamnosus, and B. lactis. Components present in these plant extracts such as sugars and phytochemicals including phenolic and organic acids have been shown to enhance the growth of these probiotics. Soy germ rich in isoflavones had a positive effect on the viability of probiotics mainly due to the presence of oligosaccharides, which could act as prebiotics that help promote the growth and activity of these beneficial microorganisms. De Boever et al. (2000) have reported the viability of lactobacilli when the cultures were subjected to a 2-week treatment period by adding 2.5 g/day soy germ powder to the culture medium. Michael et al. (2010) studied the effect of several plant extracts on the viability of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus in nonfat yogurt. This study demonstrated that the addition of olive, garlic, onion, and citrus extracts at 0.5 and 0.1 % (w/v) maintained the viability of the yogurt cultures during refrigerated storage for 21 days. In vitro evaluation of almond skins during fermentation showed potential to be used as a novel source of prebiotics to enhance the populations of bifidobacteria (Mandalari et al. 2010).
The growth in bacterial population is attributed to the conversion of polyphenols to phenolic acids as one of the major group of phenolic metabolites by the colonic microbiota (Lafay and Gil-Izquierdo 2008). Since bifidobacteria are acetate and lactate producers, the increase in concentration of organic acids (acetate and propionate) during fermentation of almond skin might have increased the number of bifidobacteria (Mandalari et al. 2010). Roberfroid (2000) suggested fructooligosaccharides as prebiotics, as they stimulate the growth and metabolism of probiotic bacteria in the gut. Panesar and Shinde (2012) have shown good viability of L. acidophilus and Bifidobacterium bifidum in aloe vera and water blend juice (1:3)-fortified yogurt during a 28-day storage period. Germinated rough rice is another potential supplement for the growth of L. plantarum in food fermentation (Trachoo et al. 2006). High levels of nutrients and bioactive compounds such as proteins, amino acids, sugars, vitamins, gamma-oryzanol, gamma-amino butyric acid, tocotrienols, tocopherols, and other phytochemical substances may have promoted the growth of these bacteria (Hagiwara et al. 2004; Oh et al. 2003; Tian et al. 2004).
Impact of plant components on probiotics and pathogens
Plant contains several functional components such as phenolic compounds, antioxidants, and minerals. Such plant-derived components have been shown to influence colonic microflora (Kelly et al. 1994) either by promoting the growth of beneficial microorganisms or by acting against pathogens. This could be due to the effect of plant extracts, which might be used in potentiating the neutralization effect of beneficial microorganisms against enterotoxin-producing bacteria (Bomba et al. 2002). This section highlights the potential influence of plant components on probiotics and the mode of action of such probiotics against pathogens. Our main interest here is to identify the dual activity shown by these plant components. Table 1 shows the impact of various plant components on probiotics and harmful microorganisms. The impact of such functional components can be either direct or indirect (Fig. 1).
Direct effect
Plant components have a direct effect on the growth and viability of probiotics as well as the inhibition of harmful microorganisms. Some of the recognized functional components in selected plant species are summarized in Table 1, which provides an outline of functional components, the impact of these components on the growth and viability of probiotics, antimicrobial action against pathogens, and references. This direct effect can be attributed to the impact of functional components to enhance the growth of probiotics or antimicrobial action against pathogens (Fig. 1).
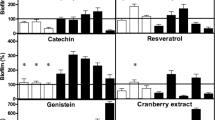
Sedighi et al. (2011) have demonstrated that almond pudding (10 % almond smash in 90 % rice extract, w/v) in infant’s diet may enhance the protection against E. coli O157:H7 by increasing the Bifidobacterium spp. populations in infant’s gastrointestinal system. Almond is a good source of oligosaccharide, which acts as a prebiotic to promote the growth of beneficial microorganisms (Bifidobacterium spp. and lactobacilli) and inhibit the growth of Clostridium hystolyticum (Mandalari et al. 2010). The presence of phytochemicals and nutrients such as vitamin E could have attributed to the growth enhancement and suppression of Bifidobacterium spp. and E. coli O157:H7, respectively. Some spices including cinnamon and origanum have been shown to enhance the growth of lactic acid bacteria and Bifidobacterium spp., which has resulted in higher acid production that acts as an antimicrobial compound against pathogens including E. coli O157:H7 and H. pylori (Shelef 1984; Ibrahim et al. 2003; Ali et al. 2005; Behrad et al. 2009). Polyphenols, phytic acid, and oxalic acid are some of the important classes of antioxidant present in plants. Oxalic acid showed growth-enhancing response for L. acidophilus. Similarly, plant products such as apple skins, onions, tea, red wine, leafy green vegetables, and berries are rich in quercetin. A recent study by Yadav et al. (2011) showed the enhancing effect of antioxidant quercetin at 0.5–2.0 mg/5 mL toward the growth of probiotics such as L. acidophilus and marketed consortium of eight probiotic cultures. Salem et al. (2009) also reported the potentiality of apple skins as a media source for the production of lactic acid using L. reuteri. The produced lactic acid may act as an antimicrobial agent against harmful microorganisms. The components like oxalic acid and phytic acid are also known to act as chelator for iron, thereby creating an environment to suppress the survival and growth of other microorganisms (Yadav et al. 2011). Hence, microorganisms especially pathogens that require iron for their growth cannot compete with other microorganisms without iron in the environment. Grape extract has shown that the active compounds present in grapes promote the growth of Bifidobacterium spp. as well as are effective against pathogenic microorganisms (Özkan et al. 2004; Tabasco et al. 2011). The antioxidant resveratrol (0.143 mg/kg/day) present in grapes has shown to enhance the growth of Bifidobacterium and Lactobacillus in a rat model study after 20 days of resveratrol intake (Larrosa et al. 2009). On the other hand, resveratrol at 60 μg/mL prevent the expression of virulence factors of Proteus mirabilis protecting human urothelial cell damage (Wang et al. 2006).
Tea phenolics and their derivatives have shown to inhibit the growth of pathogenic bacteria such as Clostridium perfringens, Clostridium difficile, Bacteroides spp., S. aureus, Salmonella Typhimurium, and L. monocytogenes, while probiotics such as Bifidobacterium spp. and Lactobacillus spp. were relatively unaffected, thus improving the intestinal microflora (Akahoshi and Takahashi 1996; Lee et al. 2006). Rosenthal et al. (1997, 1999) reported that tea catechins and ferulic acid also inhibit the growth of pathogenic bacteria (coliforms and Salmonella) but not the growth of lactic acid bacteria, indicating the role of fermentative metabolism of lactic acid bacteria. Similarly, Weisburger (1999, 2000) reported that the consumption of tea reduces the number of undesirable bacteria in the intestine but has the reverse effect on beneficial bacteria showing the dual activity of functional component catechins. Goto et al. (1999) demonstrated the effect of tea catechins (300 mg/day for 6 weeks) on fecal contents and metabolites of elderly residents and found that consumption of green tea promotes the growth of Bifidobacterium and Lactobacillus in the gut wall and decreases the survival of S. aureus and Streptococcus pyogenes. As shown in Table 1, plant-derived functional components increase the viability of probiotics such as lactic acid bacteria and thus enhance the metabolic activity and survivability of these probiotics. Lactic acid bacteria, in turn, enhance intestinal health by producing antimicrobial substances that inhibit epithelial invasion by pathogens (Servin and Coconnier 2003). Lactobacilli also produce antimicrobial proteins known as bacteriocins that have been shown to display a wide antibacterial spectrum against Gram-positive bacteria (Jack et al. 1995). Some strains of L. reuteri have the ability to convert glycerol into the broad spectrum antimicrobial substance reuterin. Reuterin can inhibit the growth of Gram-negative and Gram-positive bacteria, yeasts, fungi, and protozoa and also remain active at a wide range of pH (Talarico et al. 1988, 1990; Dobrogosz and Lindgren 1994). Reuterin also affect pathogens by producing stress response thereby taking longer time to adapt to the environment (Bian et al. 2011). Rasch et al. (2007) reported that reuterin elongates the lag phase of L. innocua ATCC 33090 and hinders cell division irrespective of the pH of the media. As shown in Table 1, some of the plant components enhance the growth of bifidobacteria. Some of the mechanisms that have been suggested for the inhibitory action of bifidobacteria towards Gram-negative pathogens include reduction of pH by the production of organic acids, the inhibitory action of undissociated organic acid molecules, and the production of specific antimicrobial substances (Fuller 1989; Ibrahim and Bezkorovainy 1994; Servin 2004). Makras and De Vuyst (2006) have reported strong antimicrobial activity of organic acids produced by Bifidobacterium strains against S. enterica Typhimurium and E. coli.
This dual effect is probably due to the inhibition of other bacteria in the intestinal tract that could have provided a niche for the enhancement of probiotics. Most of the plant components have been found to have strong antimicrobial properties against numerous pathogens. In addition to these strong antimicrobial properties, plant components also promote the growth and viability of probiotics, which produce organic acids, and other antimicrobial substances that inhibit a wide range of pathogenic microorganisms.
Indirect effect
Several mineral nutrients such as Mg2+, Mn2+, Fe2+, and Ca2+ have been demonstrated to bring an impact on growth and functionality of probiotics (Boyaval 1989; Ibrahim and Bezkorovainy 1994; Wishon et al. 2010). Magnesium (Mg2+) is considered as an essential nutrient for the growth of several lactic acid bacteria such as Lactobacillus helveticus, Lactobacillus lactis, and L. delbrueckii (Rogosa and Mitchell 1950). Aksu and Kutsal (1986) have reported 70 % increase in lactic acid production by L. delbrueckii NRRL B-445 when MgSO4 was added in molasses, yeast extract, and (NH4)2HPO4. Similarly, Mg2+ has shown to increase the survival of Streptococcus lactis ML3 in phosphate buffer (Thomas and Batt 1968). It has been also reported that many enzymes, which require Mg2+, may also be activated in the presence of manganese (Mn2+). Mn2+ present in tomato juice (0.011 %) and asparagus juice has been shown to enhance the growth and acid production by lactic acid bacteria (Stamer et al. 1964). Tiwari et al. (1980) and Tewari et al. (1985) have shown increased growth of Lactobacillus bulgaricus with Fe-EDTA complex and higher acid production with the addition of ferric chloride (9 × 10−5 mM) and cobalt chloride to paneer whey. McDonald (1957) suggested that Ca2+ and Mg2+ ions have stimulated the growth of Lactococcus lactis and Lactococcus cremoris. These enhancing effects could have resulted from an increase of free ionic form of minerals in growth media that act as an activator of different metabolic reactions such as cell division and stabilization of nucleic acids (Boyaval 1989). Therefore, the presence of these minerals has shown to create an environment that induces a new metabolic pathway. This metabolic pathway could enhance the growth-specific metabolites of probiotics that ultimately lead to the production of functional compounds. These functional compounds could have antimicrobial properties that act against pathogens (Fig. 1, indirect effect).
Some examples on how plant components such as iron (Fe2+) and manganese (Mn2+) indirectly influence the survivability of microorganisms have been described earlier (Ibrahim and Bezkorovainy 1994). Iron is an essential nutrient for all microorganisms; however, probiotics such as bifidobacteria and lactobacilli bind the iron and hence reduce the availability to pathogenic microrganisms (Bomba et al. 2002). Spices are rich in Mn2+ ions and have been reported to be strong stimulators of starter culture and rapid acid production (Zaika and Kissinger 1979, 1984; Kang and Fung 2000). Ibrahim et al. (2010) have reported that the addition of Mn2+ to the growth culture stimulated the production of α- and β-galactosidase activity of L. reuteri CF2-7F. Therefore, Mn2+ ions could be used for the growth of bacteria and high activity of these enzymes. Extracts of plant components such as clove, cardamom, ginger, celery seed, cinnamon, and turmeric contain Mn2+ that enhances acid production by L. plantarum (Zaika and Kissinger 1984). Stimulating the presence of Mn2+ has shown to stimulate the growth of L. casei YIT-9018 (Nakashima 1997). The production of lactic acid and other antimicrobial compounds from Lactobacillus spp. could act antagonistically against foodborne pathogens (Fig. 1). Ibrahim and Bezkorovainy (1994) reported that bifidobacteria have the ability to inhibit the growth of pathogenic bacteria by producing organic acids, and the antimicrobial activity of such probiotic (Bifidobacterium longum, NCFB 2259) is influenced by spices (Ibrahim et al. 2003). A diagram of the indirect stimulatory effect of Mn2+ ions (the mineral found in spices) on the production of bifidogenic compound by bifidobacteria is shown in Fig. 2. Novel functional compounds produced by bifidobacteria under low Fe2+ concentration have been reported by Ibrahim (2005) and O'Sullivan (2001). Iron is known to be a growth-promoting factor for bifidobacteria, and Mn2+ has been shown to have a strong inhibitory effect against Fe2+ uptake (Ibrahim and Bezkorovainy 1994; Bezkorovainy et al. 1996). When Fe2+ is limited in the growth environment, bifidobacteria tend to grow at a slower rate and start to produce a bifidogenic compound that has antimicrobial properties. This bifidogenic compound scavenges Fe2+ from the environment, thus making Fe2+ unavailable to pathogens (Bezkorovainy et al. 1996; Miller-Catchpole et al. 1997) and other competitive microorganisms. Ibrahim (2005) also reported that bifidobacteria could inhibit E. coli O157:H7. From Ibrahim’s study, it was confirmed that ferrous (Fe2+) plays an important role in the production of bifidogenic compounds, and when Fe2+ is not available or iron uptake is inhibited, bifidobacterial cells could also be stressed by several environmental and chemical factors that ultimately lead to the production of antimicrobial bifidogenic compound (Fig. 2) (Ibrahim 2005; Morrison et al. 2001). This is how the Mn2+ present in most of the spices inhibits the uptake of Fe2+ and ultimately suppressed the growth of pathogens by producing new antimicrobial compounds.
Almost all bacteria, including probiotics, require iron in their natural habitat as an essential element for growth except some lactobacilli (Weinberg 1997). For this reason, lactobacilli have an advantage over other microorganisms that depend on iron. Elli et al. (2000) reported that L. acidophilus and L. delbrueckii are able to bind ferric hydroxide on their cell surface making it unavailable to pathogenic microorganisms. Functional component tannins are capable of forming complexes with polymers and minerals, making nutrients unavailable to other pathogen (Smith et al. 2005). Thus, it can be inferred that the competition by these probiotic bacteria for limited resources is an indirect way of inhibiting the growth of pathogenic microorganisms. However, the level of stimulation and inhibition of such plant components on bacteria varies depending on the bacterial species and the chemical structure of the compounds. Our literature review has shown that plant products possess bioactive components that not only promote the growth of probiotics but also inhibit the growth of pathogens directly or indirectly with various antimicrobial compounds produced from probiotics.
Applications
Plants, their respective extracts, and functional compounds from them have been used as antibacterial agents and remedies for human illnesses. Various plant species have been known to contain naturally occurring compounds with antimicrobial activity (Kim et al. 2004; Beuchat and Golden 1989; Lopez-Malo et al. 2005). Major groups of chemicals present in plant components that are responsible for enhancing the safety and quality of food and drugs include polyphenols, quinines, flavanols/flavanoids, alkaloids, and lectins (Cowan 1999). Enhancement of food safety is one of the interests of the food industry, and the use of natural food preservatives from plant sources is one of the ways to control microbial growth and reduce the incidence of foodborne illnesses and spoilage (Eklund 1980). These plants contain large amounts of antioxidant compounds, such as ascorbic acid, vitamin E, and phenolic compounds, and are also potentially useful in reducing oxidative damage to human cells (Jayakumar et al. 2011). Many of these plant products have been studied in the past for different purposes as antimicrobials, antioxidants, medicinal, and food preservatives on a commercial basis. Therefore, plant products are of great interest in food safety and human health.
Food safety
Despite the use of various preservatives and methods to control food spoilage and foodborne pathogens, food safety continues to be a challenge to the entire food industry. Antibiotic-resistant foodborne pathogens have become a major health concern (Kiessling et al. 2002), as these pathogens are resistant to several food preservation techniques such as heat and acid treatment. There is a need to develop natural preservatives that could prevent the growth of such pathogens. Consumers are increasingly concerned about the safety of foods prepared with chemical preservatives. Therefore, plant-derived compounds as a natural alternative could be used to prolong the shelf life of foods and to enhance microbial safety.
The antibacterial activity of spices has attracted the interest of those who work in food safety occupations. For example, rosemary at 0.5 % w/v and its essential oil (1 % v/w) produced significant antilisterial effect on pork sausage during storage at 5 °C for 50 days (Pandit and Shelef 1994). Grohs and Kunz (2000) reported the inhibitory effect of a mixture of several aromatic herbs on the growth of meat spoiling microorganisms (B. subtilis, Enterococcus spp., Staphylococcus spp., E. coli K12, and Pseudomonas fluorescens) thus preserving the color and smell of fresh-portioned pork meat. Leuschner and Zamparini (2002) observed the bacteriostatic and bactericidal effect of garlic (1 % w/v) and clove (1 % w/v) against S. enterica and E. coli O157:H7, respectively, in mayonnaise. Skandamis et al. (2002) observed 1–2 log reduction of Salmonella Typhimurium in beef fillets stored under aerobic and modified atmosphere packing when treated with oregano essential oil. Growth of S. aureus was inhibited when 1 % of cloves, onion, ginger, and black and red pepper were added to meat homogenates (Nkanga and Uraih 1981). Caffeic acid, the most effective of the phenolic acids known to occur in apples, has been shown to inhibit E. coli O157:H7 and other foodborne pathogens (Reinders et al. 2001). Components of natural origin such as caffeic acid could be a better alternative to the currently used method for food preservation. Green tea components have been shown to enhance the shelf life of raw, frozen, and cooked meat patties (Jo et al. 2003; Tang et al. 2001). Baņķn et al. (2007) suggested that ascorbate, green tea extract, and grape seed extracts delayed microbial spoilage, redness loss, and lipid oxidation, in low sulfite beef patties, thereby increasing shelf life. Trans-cinnamaldehyde, a major component of bark extract of cinnamon (Holley and Patel 2005), could be used as an antimicrobial agent to inactivate E. coli O157:H7 in apple juice and apple cider (Baskaran et al. 2010). Extracts of pepper, parsley, and dill have been shown to exhibit antimicrobial activity against natural microflora, coliforms, yeasts and molds, and S. aureus in Kareish cheese to prevent spoilage (Wahba et al. 2010). The compound thiosulfinates (allicin) present in garlic extract have also been shown to prevent food contamination from pathogenic microorganisms (Durairaj et al. 2009).
A combination of spices and bifidobacteria could be used to control the growth of E. coli O157:H7 in ready-to-eat foods and to increase the bio-safety of many consumable food products (Ibrahim et al. 2003). Plant products such as ginger enhance the growth of L. reuteri (Sutherland et al. 2009), which produces the broad spectrum antimicrobial compound reuterin (Axelsson et al. 1989). The antimicrobial activity of reuterin has been studied against different foodborne Gram-positive and Gram-negative pathogens in milk. Results showed that reuterin exhibited bacteriostatic and bactericidal activity against L. monocytogenes Ohio serotype 4b and E. coli O157:H7 ATCC 43894, respectively (Arques et al. 2004). A variety of metabolic products produced by lactic acid bacteria have been shown to interfere with the growth of other microbes and have been applied to food systems to prevent the growth of harmful microorganisms (Vandenbergh 1993).
Consumers are increasingly demanding healthier and safer food products that are free of chemical additives. Natural preservatives may lower processing costs and extend product shelf life. Natural preservatives could also be exploited by small food industries that do not require advanced technology such as non-thermal processing methods. Therefore, these preservatives could maintain both quality and reduce processing cost. In addition, natural preservatives may help prevent the emergence of antibiotic resistance microorganisms (Gálvez et al. 2010). Therefore, the use of natural preservatives to increase the shelf life of food is a promising method, as these substances of plant origin have antioxidant and antimicrobial properties.
Human health
In the last few years, considerable attention has also been given to the study on how diet can impact gut microflora. The use of dietary components to increase the metabolic activity or the number of beneficial microorganisms (probiotics) without altering the existing gut ecosystem has been shown to be the best way to improve human health from a practical standpoint. Consumption of plant source products can have considerable potential for the prophylaxis and therapy of gut infections. Many studies in the past have shown methods to promote beneficial bacteria through the intake of dairy products incorporated with bifidobacteria (Poch and Bezkorovainy 1988; Collins and Hall 1984). Recently, attention to the impact of plant-derived components has focused largely on the beneficial physiological changes in the colonic microenvironment.
As discussed earlier, plant components can stimulate the growth of probiotics that can promote other health benefits in human health (Fukushima and Iino 2006). Parkar et al. (2008) have reported the potential influence of polyphenols on gut microecology with regard to inhibiting the growth and adhesion of the gut pathogens while at the same time enhancing the proliferation and adhesion of the probiotic, L. rhamnosus 299. This probiotic activity also helps restore the balance of the intestinal microflora in the host (Plummer et al. 2005). Shelef (1984) has mentioned that the consumption of spices may reduce cancer risk by producing a bacterial shift in the intestinal tract.
Up to now, studies have focused on the antibacterial effect or beneficial effect of probiotics mainly on the basis of selection of efficient strains of probiotics with regard to source, functionality, adhesion property, bile and acid resistance, enzyme activity, and their viability in food (Ouwehand et al. 2002; Parvez et al. 2006; Percival 1997). Other factors that may enhance the efficacy of probiotics include gene manipulation, the combination of number of strains of microorganisms, and the combination of probiotics and synergistically acting components (Bomba et al. 2002). However, the contribution of probiotics to the improvement of microflora depends not only on the survivability of probiotics during storage conditions but also on the survivability of probiotics during transit period of acidic conditions of the stomach and during degradation by hydrolytic enzymes and bile salts in the small intestine (Playne 1994). It is also believed that the ingested probiotics pass through the feces without having adhered or multiplied in the intestinal mucosal cells (Servin and Coconnier 2003). Orrhage et al. (1991) have also reported that intake of supplements with bifidobacteria does not cause any significant change in the intestinal microbiota as bifidobacteria do not easily adhered in the gut. Adherence of probiotics to intestinal mucosal cells is believed to provide maximum probiotic effect. However, it has been reported that exogenously administered probiotics seem to pass into feces instead of adhering to the mucosal cells. Thus, Bezkorovainy (2001) has suggested a continuous ingestion of probiotic culture to obtain a continuous exogenous probiotic effect. However, this may not seem as practical as getting a probiotic benefit from the consumed diet itself. Ouwehand et al. (2001) reported that resistance to low pH is not only important for the survival of probiotic but also for adhesion. The authors have also mentioned that the adhesion of most of the tested strains was significantly reduced once exposed to pepsin or low pH after passing through the stomach. These findings again indicate that probiotics obtained through dietary supplements may not be able to fulfill all probiotic properties as claimed. Furthermore, probiotics must survive and retain their functionality during storage as well as in the foods into which they are incorporated. Thus, increasing the number of natural microbiota including probiotic bacteria present in the human gut via consumption of natural ingredients of plant origin seemed to be the most practical approach for a healthy gut ecosystem.
However, people are not much aware of the impact of plant-based diet and its influence on existing microflora including probiotics in the human gut. The use of functional components of plant origin can modify the colonic microbiota by increasing the number of specific probiotics. This could help transform some of the phenolic compounds present in plant components and ultimately provide a wide range of implications for the health of the host, thereby producing beneficial effects. An increase in human consumption of natural ingredients of plant origin would provide a dual health benefit by (1) stimulating the growth of probiotics and (2) inhibiting the growth of harmful microorganisms. Consuming a diet rich in functional ingredients from plant sources has been shown to have a positive impact on human health. As a result, we strongly believe that such ingredients indirectly influence the functionality on the natural microbiota in the human gut, including bifidobacteria (Bialonska et al. 2009; Sutherland et al. 2009; Alberto et al. 2001; Ibrahim et al. 2010; Liu et al. 2011). Several reports have demonstrated that functional ingredients enhance the growth of lactobacillus and bifidobacteria, a major part of human microbiota. For instance, several polyphenols have been shown to enhance the proliferation and adhesion of probiotics and inhibit the growth and adhesion of gut pathogens in in vitro and in vivo studies. It is desirable to increase the number of probiotics and inhibit the growth of potential pathogens in the human gut. Therefore, consumption of a diet containing these plant-derived compounds has the potential to influence our gut microecology, improve the intestinal ecosystem, and thereby contribute to gut health benefits and the overall maintenance of human health.
Conclusions
Plant species have been known to contain a wide variety of functional components. The impact of these components on microorganisms can be either direct or indirect. Most of these compounds have shown antimicrobial properties against foodborne pathogens. Similarly, antioxidants present in plant compounds have also shown to stimulate the growth of probiotics. These probiotics in turn produce organic acids, which act as antimicrobial agents. On the other hand, mineral nutrients present in plants indirectly induce growth-specific metabolites of probiotics to promote the production of antimicrobial compounds. Hence, the production of these organic acids and antimicrobial compounds could contribute to improve food safety and human health.
Recently, scientists are showing interest in the relationship between nutrients and gene expression known as nutrigenomics. Understanding the impact of plant components on gene expression in probiotics such as lactobacilli and bifidobactaria could further help improve food safety and human health. Screening for the risk of various diseases and determining an individual's ideal health-promoting diet through genetic testing is a new area of research to understand human response to foods. Future research in the area of nutrigenomics could play a significant role to understand how diet affects gene expression to improve an individual’s entire health. Hence, there are many opportunities to use this information to accelerate progress toward a comprehensive understanding of the functionality of plant-derived components on neutrigenomics.
References
Agarry O, Olaleye M, Bello-Michael C (2005) Comparative antimicrobial activities of aloe vera gel and leaf. Afr J Biotechnol 4:1413–1414
Akahoshi R, Takahashi Y (1996) Yogurt containing bifidobacterium and process for producing the same. In: wo patent wo/1996/037,113
Aksu Z, Kutsal T (1986) Lactic acid production from molasses utilizing Lactobacillus delbrueckii and invertase together. Biotechnol Lett 8:157–160
Alakomi HL, Skytta E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander I (2000) Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol 66:2001–2005
Alberto MR, Farías ME, de Nadra MCM (2001) Effect of gallic acid and catechin on Lactobacillus hilgardii 5w growth and metabolism of organic compounds. J Agric Food Chem 49:4359–4363
Ali SM, Khan AA, Ahmed I, Musaddiq M, Ahmed KS, Polasa H, Rao LV, Habibullah CM, Sechi LA, Ahmed N (2005) Antimicrobial activities of eugenol and cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann Clin Microbiol Antimicrob 4:20
Ankri S, Mirelman D (1999) Antimicrobial properties of allicin from garlic. Microbes Infect 1:125–129
Antonious GF, Berke T, Jarret RL (2009) Pungency in Capsicum chinense: variation among countries of origin. J Environ Sci Health (B) 44:179–184
Arques JL, Fernandez J, Gaya P, Nunez M, Medina M (2004) Antimicrobial activity of reuterin in combination with nisin against foodborne pathogens. Int J Food Microbiol 95:225–229
Arunkumar S, Muthuselvam M (2009) Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J Agric Sci 5:572–576
Aureli P, Costantini A, Zolea S (1992) Antimicrobial activity of some plant essential oils against Listeria monocytogenes. J Food Protect 55:344–348
Avato P, Tursi F, Vitali C, Miccolis V, Candido V (2000) Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicine 7:239–243
Ávila M, Hidalgo M, Sánchez-Moreno C, Pelaez C, Requena T, Pascual-Teresa S (2009) Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus. Food Res Int 42:1453–1461
Axelsson L, Chung T, Dobrogosz W, Lindgren S (1989) Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb Ecol Health Dis 2:131–136
Bajpai V, Rahman A, Dung N, Huh M, Kang S (2008) In vitro inhibition of food spoilage and foodborne pathogenic bacteria by essential oil and leaf extracts of Magnolia liliflora desr. J Food Sci 73:M314–M320
Baņķn S, Díaz P, Rodríguez M, Garrido MD, Price A (2007) Ascorbate, green tea and grape seed extracts increase the shelf life of low sulphite beef patties. Meat Sci 77:626–633
Baskaran SA, Amalaradjou MAR, Hoagland T, Venkitanarayanan K (2010) Inactivation of Escherichia coli O157: H7 in apple juice and apple cider by trans-cinnamaldehyde. Int J Food Microbiol 141:126–129
Behrad S, Yusof M, Goh K, Baba A (2009) Manipulation of probiotics fermentation of yogurt by cinnamon and licorice: effects on yogurt formation and inhibition of Helicobacter pylori growth in vitro. World Acad Sci Eng Tech 60:590–594
Belguith H, Kthiri F, Ben Ammar A, Jaafoura H, Ben Hamida J, Landoulsi A (2009) Morphological and biochemical changes of Salmonella hadar exposed to aqueous garlic extract. Int J Morphol 27:705–713
Beuchat LR, Golden DA (1989) Antimicrobials occurring naturally in foods. Food Technol 43:134–142
Bezkorovainy A (2001) Probiotics: determinants of survival and growth in the gut. Am J Clin Nutr 73:399S
Bezkorovainy A, Kot E, Miller-Catchpole R, Haloftis G, Furmanov S (1996) Iron metabolism in bifidobacteria. Int Dairy J 6:905–919
Bialonska D, Kasimsetty SG, Schrader KK, Ferreira D (2009) The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J Agric Food Chem 57:8344–8349
Bian L, Molan AL, Maddox I, Shu Q (2011) Antimicrobial activity of Lactobacillus reuteri DPC16 supernatants against selected food borne pathogens. World J Microbiol Biotechnol 1–8
Bisignano G, Tomaino A, Cascio RL, Crisafi G, Uccella N, Saija A (1999) On the in–vitro antimicrobial activity of oleuropein and hydroxytyrosol. J Pharm Pharmacol 51:971–974
Bomba A, Nemcová R, Mudroová D, Guba P (2002) The possibilities of potentiating the efficacy of probiotics. Trends Food Sci Technol 13:121–126
Bomba A, Jonecova Z, Koscova J, Nemcova R, Gancarikova S, Mudronova D, Scirankova L, Buleca V, Lazar G, Posivak J, Kastel R, Marekova M (2006) The improvement of probiotics efficacy by synergistically acting components of natural origin: a review. Biologia 61:729–734
Boyaval P (1989) Lactic acid bacteria and metal ions. Le Lait 69:87–113
Brul S, Coote P (1999) Preservative agents in foods: Mode of action and microbial resistance mechanisms. Int J Food Microbiol 50:1–17
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253
Burt SA, Reinders RD (2003) Antibacterial activity of selected plant essential oils against Escherichia coli O157: H7. Lett Appl Microbiol 36:162–167
Caccioni DRL, Guizzardi M, Biondi DM (1998) Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. Int J Food Microbiol 43:73–79
Calomme M, Branden K, Berghe D (1995) Selenium and Lactobacillus species. J Appl Microbiol 79:331–340
Careaga M, Fernandez E, Dorantes L, Mota L, Jaramillo ME, Hernandez-Sanchez H (2003) Antibacterial activity of Capsicum extract against Salmonella Typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int J Food Microbiol 83:331–335
Chao SC, Young DG, Oberg CJ (2000) Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. J Essent Oil Res 12:639–649
Collins E, Hall B (1984) Growth of bifidobacteria in milk and preparation of Bifidobacterium infantis for a dietary adjunct. J Dairy Sci 67:1376–1380
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
Cox S, Mann C, Markham J, Bell H, Gustafson J, Warmington J, Wyllie S (2000) The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol 88:170–175
Davidson PM (2001) Chemical preservatives and naturally antimicrobial compounds. In: Doyle MP, Beuchat LR, Montville TJ (eds) Food microbiology: fundamentals and frontiers, 2nd edn. ASM, Washington, pp 593–628
Davidson P, Branden A (1981) Antimicrobial activity of non-halogenated phenolic compounds. J Food Protect 44:623–632
De Boever P, Deplancke B, Verstraete W (2000) Fermentation by gut microbiota cultured in a simulator of the human intestinal microbial ecosystem is improved by supplementing a soygerm powder. J Nutr 130:2599–2606
del Carmen S, de LeBlanc AM, Miyoshi A, Rocha CS, Azevedo V, LeBlanc JG (2011) Potential application of probiotics in the prevention and treatment of inflammatory bowel diseases. Ulcers. doi:10.1155/2011/841651
Delaquis P, Mazza G (1995) Antimicrobial properties of isothiocyanates in food preservation. Food Technol 49:73–84
Derrickson-Tharrington E, Kendall PA, Sofos JN (2005) Inactivation of Escherichia coli O157: H7 during storage or drying of apple slices pretreated with acidic solutions. Int J Food Microbiol 99:79–89
Dobrogosz WJ, Lindgren SE (1994) Method of determining the presence of an antibiotic produced by Lactobacillus reuteri. In: US Patent 5,352,586
Dogasaki C, Shindo T, Furuhata K, Fukuyama M (2002) Identification of chemical structure of antibacterial components against Legionella pneumophila in a coffee beverage. Yakugaku zasshi 122:487–494
Dorantes L, Colmenero R, Hernandez H, Mota L, Jaramillo ME, Fernandez E, Solano C (2000) Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annuum extracts. Int J Food Microbiol 57:125–128
Dorman H, Deans S (2000) Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 88:308–316
Duda-Chodak A, Tarko T, Statek M (2008) The effect of antioxidants on Lactobacillus casei cultures. Acta Sci Pol Technol Aliment 7:39–51
Durairaj S, Sangeetha S, Lakshmanaperumalsamy P (2009) In vitro antibacterial activity and stability of garlic extract at different pH and temperature. e J Bio 5:5–10
Eklund T (1980) Inhibition of growth and uptake processes in bacteria by some chemical food preservatives. J Appl Microbiol 48:423–432
Elli M, Zink R, Rytz A, Reniero R, Morelli L (2000) Iron requirement of Lactobacillus spp. in completely chemically defined growth media. J Appl Microbiol 88:695–703
Farag R, Daw Z, Hewedi F, El-Baroty G (1989) Antimicrobial activity of some Egyptian spice essential oils. J Food Protect 52:665–667
Fattouch S, Caboni P, Coroneo V, Tuberoso CIG, Angioni A, Dessi S, Marzouki N, Cabras P (2007) Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J Agric Food Chem 55:963–969
Fisher K, Phillips C (2008) Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Technol 19:156–164
Fukushima Y, Iino H (2006) Probiotics in food safety and human health: current status of regulations on the use of probiotics in foods in Japan. In: Goktepe I, Juneja VK, Ahmedna M (eds) Probiotics in food safety and human health. CRC /Taylor & Francis Group, Boca Raton, pp 431–463
Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66:365–378
Gadang V, Hettiarachchy N, Johnson M, Owens C (2008) Evaluation of antibacterial activity of whey protein isolate coating incorporated with nisin, grape seed extract, malic acid, and EDTA on a turkey Frankfurter system. J Food Sci 73:M389–M394
Gálvez A, Abriouel H, Benomar N, Lucas R (2010) Microbial antagonists to food-borne pathogens and biocontrol. Curr Opin Biotechnol 21:142–148
Ghisalberti E (1994) The ethnopharmacology and phytochemistry of Eremophila species (Myoporaceae). J Ethnopharmacol 44:1–9
Gill A, Holley R (2006) Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int J Food Microbiol 108:1–9
Goto K, Kanaya S, Ishigami T, Hara Y (1999) Effects of tea polyphenols on fecal conditions, part 2. The effects of tea catechins on fecal conditions of elderly residents in a long-term care facility. J Nutr Sci Vitaminol 45:135–141
Grohs BM, Kunz B (2000) Use of spice mixtures for the stabilization of fresh portioned pork. Food Control 11:433–436
Gustafson J, Liew Y, Chew S, Markham J, Bell H, Wyllie S, Warmington J (1998) Effects of tea tree oil on Escherichia coli. Lett Appl Microbiol 26:194–198
Haddadin M (2010) Effect of olive leaf extracts on the growth and metabolism of two probiotic bacteria of intestinal origin. Pak J Nutr 9:787–793
Hagiwara H, Seki T, Ariga T (2004) The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem 68:444–447
Halcón L, Milkus K (2004) Staphylococcus aureus and wounds: a review of tea tree oil as a promising antimicrobial. Am J Infect Control 32:402–408
Hashimoto T, Kumazawa S, Nanjo F, Hara Y, Nakayama T (1999) Interaction of tea catechins with lipid bilayers investigated with liposome systems. Biosci Biotechnol Biochem 63:2252–2255
Helander I, Mattila-Sandholm T (2000) Fluorometric assessment of gram–negative bacterial permeabilization. J Appl Microbiol 88:213–219
Helander IM, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LGM, Von Wright A (1998) Characterization of the action of selected essential oil components on Gram-negative bacteria. J Agric Food Chem 46:3590–3595
Hiramoto K, Ojima N, Sako KI, Kikugawa K (1996) Effect of plant phenolics on the formation of the spin-adduct of hydroxyl radical and the DNA strand breaking by hydroxyl radical. Biol Pharm Bull 19:558–563
Holley RA, Patel D (2005) Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol 22:273–292
Hosein AM, Breidt F Jr, Smith CE (2011) Modeling the effects of sodium chloride, acetic acid, and intracellular pH on survival of Escherichia coli O157: H7. Appl Environ Microbiol 77:889–895
Ibrahim SA (2005) Composition and method for inhibition of harmful bacteria. In: US Patent 6,932,992
Ibrahim SA, Bezkorovainy A (1994) Growth–promoting factors for Bifidobacterium longum. J Food Sci 59:189–191
Ibrahim SA, Salameh MM (2001) Simple and rapid method for screening antimicrobial activities of Bifidobacterium species of human isolates. J Rapid Meth Autom Microbiol 9:53–62
Ibrahim SA, Dharmavavaram S, Seo C, Shahbazi G (2003) Antimicrobial activity of Bifidobacterium longum (NCFB 2259) as influenced by spices. Int J Food Saf 2:6–8
Ibrahim SA, Salameh M, Phetsomphou S, Yang H, Seo C (2006) Application of caffeine, 1, 3, 7-trimethylxanthine, to control Escherichia coli O157: H7. Food Chem 99:645–650
Ibrahim SA, Tse T, Yang H, Fraser A (2009) Antibacterial activity of a crude chive extract against Salmonella in culture medium, beef broth and chicken broth. Food Prot Trends 29:155–160
Ibrahim SA, Alazzeh AY, Awaisheh SS, Song D, Shahbazi A, AbuGhazaleh AA (2010) Enhancement of α- and β-galactosidase activity in Lactobacillus reuteri by different metal ions. Biol Trace Elem Res 136:106–116
Ikigai H, Nakae T, Hara Y, Shimamura T (1993) Bactericidal catechins damage the lipid bilayer. BBA Biomembranes 1147:132–136
Indu M, Hatha A, Abirosh C, Harsha U, Vivekanandan G (2006) Antimicrobial activity of some of the south-Indian spices against serotypes of Escherichia coli, Salmonella, Listeria monocytogenes and Aeromonas hydrophila. Braz J Microbiol 37:153–158
Jack R, Tagg B, Ray J (1995) Bacteriocins of gram-positive bacteria. Microbiol Rev 59:171–200
Jaquet M, Rochat I, Moulin J, Cavin C, Bibiloni R (2009) Impact of coffee consumption on the gut microbiota: a human volunteer study. Int J Food Microbiol 130:117–121
Jayakumar T, Thomas P, Sheu J, Geraldine P (2011) In-vitro and in-vivo antioxidant effects of the oyster mushroom Pleurotus ostreatus. Food Res Int 44:851–861
Jo C, Son JH, Son CB, Byun MW (2003) Functional properties of raw and cooked pork patties with added irradiated, freeze-dried green tea leaf extract powder during storage at 4°C. Meat Sci 64:13–17
Juglal S, Govinden R, Odhav B (2002) Spice oils for the control of co-occurring mycotoxin-producing fungi. J Food Protect 65:683–687
Juven B, Kanner J, Schved F, Weisslowicz H (1994) Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J Appl Microbiol 76:626–631
Kabara J, Eklund T (1991) Organic acids and esters. In: Russell NJ, Gould GW (eds) Food preservatives, 1st edn. Blackie, Glasgow, pp 44–71
Kang DH, Fung DYC (2000) Stimulation of starter culture for further reduction of foodborne pathogens during salami fermentation. J Food Protect 63:1492–1495
Kelly D, Begbie R, King T (1994) Nutritional influences on interactions between bacteria and the small intestinal mucosa. Nutr Res Rev 7:233–258
Kiessling CR, Cutting JH, Loftis M, Kiessling WM, Datta AR, Sofos JN (2002) Antimicrobial resistance of food-related Salmonella isolates, 1999–2000. J Food Protect 65:603–608
Kim S, Fung D (2004) Antibacterial effect of crude water–soluble arrowroot (Puerariae radix) tea extracts on food–borne pathogens in liquid medium. Lett Appl Microbiol 39:319–325
Kim J, Marshall MR, Wei C (1995) Antibacterial activity of some essential oil components against five foodborne pathogens. J Agric Food Chem 43:2839–2845
Kim S, Ruengwilysup C, Fung D (2004) Antibacterial effect of water-soluble tea extracts on foodborne pathogens in laboratory medium and in a food model. J Food Protect 67:2608–2612
Kramer RP, Hindorf H, Jha HC, Kallage J, Zilliken F (1984) Antifungal activity of soybean and chickpea isoflavones and their reduced derivatives. Phytochemistry 23:2203–2205
Kristanti R, Punbusayakul N (2008) Antioxidant and antimicrobial activity of commercial green tea in Chiang Rai. Acta Horticulturae 53–58
Lafay S, Gil-Izquierdo A (2008) Bioavailability of phenolic acids. Phytochem Rev 7:301–311
Lambert P, Hammond S (1973) Potassium fluxes, first indications of membrane damage in microorganisms. Biochem Biophys Res Commun 54:796–799
Lambert R, Skandamis PN, Coote PJ, Nychas GJE (2001) A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 91:453–462
Lanciotti R, Gianotti A, Patrignani F, Belletti N, Guerzoni M, Gardini F (2004) Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci Technol 15:201–208
Larrosa M, Yañéz-Gascón MJ, Selma MV, González-Sarrías A, Toti S, Cerón JJ, Tomás-Barberán F, Dolara P, Espín JC (2009) Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J Agric Food Chem 57:2211–2220
Lee SY, Jin HH (2008) Inhibitory activity of natural antimicrobial compounds alone or in combination with nisin against Enterobacter sakazakii. Lett Appl Microbiol 47:315–321
Lee HC, Jenner AM, Low CS, Lee YK (2006) Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res Microbiol 157:876–884
Lemos T, Matos F, Alencar J, Craveiro A, Clark A, McChesney J (1990) Antimicrobial activity of essential oils of Brazilian plants. Phytother Res 4:82–84
Leuschner RGK, Zamparini J (2002) Effects of spices on growth and survival of Escherichia coli O157 and Salmonella enterica serovar Enteritidis in broth model systems and mayonnaise. Food Control 13:399–404
Lin MY, Yen CL (1999) Antioxidative ability of lactic acid bacteria. J Agric Food Chem 47:1460–1466
Liu Y, Cheng G, Han T, Yang H, Ibrahim S, Huang W (2011) Microbial transformation of tectoridin from Pueraria flos by Lactobacillus and bifidobacteria. Food Chem 131:149–154
Lopez-Malo A, Alzamora SM, Palou E (2005) Naturally occurring compounds: plant sources. In: Davidson PM, Sofos JN, Branen AL (eds) Antimicrobials in food, 3rd edn. CRC, New York, pp 429–251
Lv F, Liang H, Yuan Q, Li C (2011) In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res Int 44:3057–3064
Maillard JY (2002) Bacterial target sites for biocide action. J Appl Microbiol 92:16S–27S
Makras L, De Vuyst L (2006) The in vitro inhibition of Gram-negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int Dairy J 16:1049–1057
Mandalari G, Faulks RM, Bisignano C, Waldron KW, Narbad A, Wickham MSJ (2010) In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol Lett 304:116–122
Manderson K, Pinart M, Tuohy K, Grace W, Hotchkiss A, Widmer W, Yadhav M, Gibson G, Rastall R (2005) In vitro determination of prebiotic properties of oligosaccharides derived from an orange juice manufacturing by-product stream. Appl Environ Microbiol 71:8383–8389
Markin D, Duek L, Berdicevsky I (2003) In vitro antimicrobial activity of olive leaves. Mycoses 46:132–136
Mau JL, Chen CP, Hsieh PC (2001) Antimicrobial effect of extracts from Chinese chive, cinnamon, and corni fructus. J Agric Food Chem 49:183–188
McDonald I (1957) Effect of acetate, citrate, and divalent metal ions on utilization of sodium caseinate by lactic streptococci. Can J Microbiol 3:411–417
Michael M, Phebus RK, Schmidt KA (2010) Impact of a plant extract on the viability of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus in nonfat yogurt. Int Dairy J 20:665–672
Miller-Catchpole R, Kot E, Haloftis G, Furmanov S, Bezkorovainy A (1997) Lactoferrin can supply iron for the growth of Bifidobacterium breve. Nutr Res 17:205–213
Moran JF, Klucas RV, Grayer RJ, Abian J, Becana M (1997) Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radic Biol Med 22:861–870
Morrison Z, Ibrahim SA, Salameh A, Shahbazi A, Seo, CW (2001) Continuous production of antimicrobial compound(s) and organic acids by bifidobacteria cells entrapped. J Dairy Sci 84: Suppl 1. Abst 747.
Nakashima A (1997) Stimulatory effect of phytin and acid production by Lactobacillus casei. J Nutr Sci Vitamonol 43:419–424
Nanasombat S, Lohasupthawee P (2005) Antibacterial activity of crude ethanolic extracts and essential oils of spices against Salmonellae and other enterobacteria. KMITL Sci Tech J 5:527–538
Ndi CP, Semple SJ, Griesser HJ, Barton MD (2007) Antimicrobial activity of some Australian plant species from the genus Eremophila. J Basic Microbiol 47:158–164
Nkanga E, Uraih N (1981) Prevalence of Staphylococcus aureus in meat samples from traditional markets in Benin City, Nigeria and probable control by use condiments. J Food Protect 44:4–8
Nychas G (1995) Natural antimicrobials from plants. In: Gould GW (ed) New methods of food preservation. Black Academic & Professional, Glasgow, pp 58–89
Oh SH, Soh JR, Cha YS (2003) Germinated brown rice extract shows a nutraceutical effect in the recovery of chronic alcohol-related symptoms. J Med Food 6:115–121
Olano-Martin E, Gibson G, Rastall R (2002) Comparison of the in vitro bifidogenic properties of pectins and pectic–oligosaccharides. J Appl Microbiol 93:505–511
Olasupo N, Fitzgerald D, Gasson M, Narbad A (2003) Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar Typhimurium. Lett Appl Microbiol 37:448–451
Olasupo N, Fitzgerald D, Narbad A, Gasson M (2004) Inhibition of Bacillus subtilis and Listeria innocua by nisin in combination with some naturally occurring organic compounds. J Food Protect 67:596–600
Orrhage K, Lidbeck A, Nord C (1991) Effect of Bifidobacterium longum supplements on the human faecal microflora. Microb Ecol Health Dis 4:265–270
O'Sullivan DJ (2001) Screening of intestinal microflora for effective probiotic bacteria. J Agric Food Chem 49:1751–1760
Oussalah M, Caillet S, Lacroix M (2006) Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157: H7 and Listeria monocytogenes. J Food Protect 69:1046–1055
Ouwehand AC, Tuomola EM, Tolkko S, Salminen S (2001) Assessment of adhesion properties of novel probiotic strains to human intestinal mucus. Int J Food Microbiol 64:119–126
Ouwehand AC, Salminen S, Isolauri E (2002) Probiotics: an overview of beneficial effects. Anton Van Lee 82:279–289
Özkan G, Sagdic O, Göktürk Baydar N, Kurumahmutoglu Z (2004) Antibacterial activities and total phenolic contents of grape pomace extracts. J Sci Food Agric 84:1807–1811
Pandit V, Shelef L (1994) Sensitivity of Listeria monocytogenes to rosemary (Rosmarinus officinalis L.). Food Microbiol 11:57–63
Panesar PS, Shinde C (2012) Effect of storage on syneresis, pH, Lactobacillus acidophilus count. Bifidobacterium bifidum count of aloe vera fortified probiotic yoghurt. Curr Res Dairy Sci. doi:10.3923/crds.2011
Parkar SG, Stevenson DE, Skinner MA (2008) The potential influence of fruit polyphenols on colonic microflora and human gut health. Int J Food Microbiol 124:295–298
Parvez S, Malik K, Ah Kang S, Kim HY (2006) Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 100:1171–1185
Pauli A, Knobloch K (1987) Inhibitory effects of essential oil components on growth of food-contaminating fungi. Z Lebensm Unters Forsch 185:10–13
Percival M (1997) Choosing a probiotic supplement. Clin Nutr Insights 6:1–4
Perumalla A, Hettiarachchy N (2011) Green tea and grape seed extracts—potential applications in food safety and quality. Food Res Int 44:827–839
Playne M (1994) Probiotic foods. Food Aust 46:362–366
Plummer SF, Garaiova I, Sarvotham T, Cottrell S, Le Scouiller S, Weaver MA, Tang J, Dee P, Hunter J (2005) Effects of probiotics on the composition of the intestinal microbiota following antibiotic therapy. Int J Antimicrob Agents 26:69–74
Poch M, Bezkorovainy A (1988) Growth-enhancing supplements for various species of the genus Bifidobacterium. J Dairy Sci 71:3214–3221
Pratt D, Powers JJ, Somaatmadja D (1960) Anthocyanins. I. The influence of strawberry and grape anthocyanins on the growth of certain bacteria. J Food Sci 25:26–32
Proestos C, Boziaris IS, Kapsokefalou M, Komaitis M (2008) Natural antioxidant constituents from selected aromatic plants and their antimicrobial activity against selected pathogenic microorganisms. Food Tech Biotechnol 46:151–156
Puupponen-Pimiä R, Nohynek L, Meier C, Kähkönen M, Heinonen M, Hopia A, Oksman-Caldentey KM (2001) Antimicrobial properties of phenolic compounds from berries. J Appl Microbiol 90:494–507
Puupponen-Pimiä R, Nohynek L, Hartmann-Schmidlin S, Kähkönen M, Heinonen M, Määttä-Riihinen K, Oksman-Caldentey KM (2005) Berry phenolics selectively inhibit the growth of intestinal pathogens. J Appl Microbiol 98:991–1000
Raccach M (1984) The antimicrobial activity of phenolic antioxidants in foods: a review. J Food Saf 6:141–170
Rasch M, Metris A, Baranyi J, Bjorn Budde B (2007) The effect of reuterin on the lag time of single cells of Listeria innocua grown on a solid agar surface at different pH and NaCl concentrations. Int J Food Microbiol 113:35–40
Rasooli I, Rezaei MB, Allameh A (2006) Ultrastructural studies on antimicrobial efficacy of thyme essential oils on Listeria monocytogenes. Int J Infect Dis 10:236–241
Raybaudi-Massilia RM, Mosqueda-Melgar J, Martin-Belloso O (2006) Antimicrobial activity of essential oils on Salmonella Enteritidis, Escherichia coli, and Listeria innocua in fruit juices. J Food Protect 69:1579–1586
Reinders RD, Biesterveld S, Bijker PGH (2001) Survival of Escherichia coli O157: H7 ATCC 43895 in a model apple juice medium with different concentrations of proline and caffeic acid. Appl Environ Microbiol 67:2863–2866
Rico-Munoz E, Bargiota E, Davidson P (1987) Effect of selected phenolic compounds on the membrane-bound adenosine triphosphatase of Staphylococcus aureus. Food Microbiol 4:239–249
Roberfroid MB (2000) Prebiotics and probiotics: are they functional foods? Am J Clin Nutr 71:1682S
Rogosa M, Mitchell JA (1950) The indispensability of magnesium for Lactobacillus helveticus and its unavailability in a magnesium-azide complex. Bacteriol Proc 130
Rosenthal I, Rosen B, Bernstein S (1997) Phenols in milk. Evaluation of ferulic acid and other phenols as antifungal agents. Milchwissenschaft 52:134–138
Rosenthal I, Bernstein S, Nakimbugwe D (1999) Effects of tea solids on milk. Mil chwissenschaft 54:149–152
Salem MME, Ibrahim SA, Kim C, Seo CW, Shahbazi A, AbuGhazaleh A (2009) Lactic acid production from apple skin waste by immobilized cells of Lactobacillus reuteri. In: Uzoechokwu GA et al (eds) Proceedings of the 2007 National Conference on Environmental Science and Technology. Springer, New York, pp 31–37
Sedighi R, Tajkarimi M, Ibrahim SA (2011) Comparison between E. coli O157:H7 and Bifibobacterium spp. activity in almond pudding infant supplemental food. Food Nutr Sci 2:909–915
Servin AL (2004) Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28:405–440
Servin AL, Coconnier MH (2003) Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 17:741–754
Shan B, Cai YZ, Brooks JD, Corke H (2007) The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol 117:112–119
Shelef L (1984) Antimicrobial effects of spices. J Food Saf 6:29–44
Shimamura T, Zhao WH, Hu ZQ (2007) Mechanism of action and potential for use of tea catechin as an antiinfective agent. Anti-Infect Agents Med Chem (Formerly Curr Med Chem Anti-Infect Agents) 6:57–62
Skandamis P, Tsigarida E, Nychas G (2002) The effect of oregano essential oil on survival/death of Salmonella Typhimurium in meat stored at 5°C under aerobic, VP/MAP conditions. Food Microbiol 19:97–103
Sklenickova O, Flesar J, Kokoska L, Vlkova E, Halamova K, Malik J (2010) Selective growth inhibitory effect of biochanin A against intestinal tract colonizing bacteria. Molecules 15:1270–1279
Smith AH, Zoetendal E, Mackie RI (2005) Bacterial mechanisms to overcome inhibitory effects of dietary tannins. Microb Ecol 50:197–205
Smith-Palmer A, Stewart J, Fyfe L (2001) The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol 18:463–470
Sofos J, Beuchat L, Davidson P, Johnson E (1998) Naturally occurring antimicrobials in food: task force report no. 132. Council for Agricultural Science and Technology, Ames, Iowa, 103.
Spiller F, Alves MK, Vieira SM, Carvalho TA, Leite CE, Lunardelli A, Poloni JA, Cunha FQ, Oliveira JR (2008) Anti–inflammatory effects of red pepper (Capsicum baccatum) on carrageenan– and antigen–induced inflammation. J Pharm Pharmacol 60:473–478
Spyropoulos BG, Misiakos EP, Fotiadis C, Christos NS (2011) Antioxidant properties of probiotics and their protective effects in the pathogenesis of radiation-induced enteritis and colitis. Dig Dis Sci 56:285–294
Srinivasan D, Nathan S, Suresh T, Lakshmana Perumalsamy P (2001) Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J Ethnopharmacol 74:217–220
Stamer J, Albury MN, Pederson C (1964) Substitution of manganese for tomato juice in the cultivation of lactic acid bacteria. Appl Microbiol 12:165–168
Sutherland J, Miles M, Hedderley D, Li J, Devoy S, Sutton K, Lauren D (2009) In vitro effects of food extracts on selected probiotic and pathogenic bacteria. Int J Food Sci Nutr 60:717–727
Tabasco R, Sánchez-Patán F, Monagas M, Bartolomé B, Moreno-Arribas M, Peláez C, Requena T (2011) Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: resistance and metabolism. Food Microbiol 28:1345–1352
Tajkarimi M, Ibrahim SA, Cliver D (2010) Antimicrobial herb and spice compounds in food. Food Control 21:1199–1218
Talarico T, Casas I, Chung TC, Dobrogosz W (1988) Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob Agents Chemother 32:1854–1858
Talarico TL, Axelsson LT, Novotny J, Fiuzat M, Dobrogosz WJ (1990) Utilization of glycerol as a hydrogen acceptor by Lactobacillus reuteri: purification of 1, 3-propanediol: NAD+ oxidoreductase. Appl Environ Microbiol 56:943–948
Tang S, Kerry JP, Sheehan D, Buckley DJ, Morrissey PA (2001) Antioxidative effect of added tea catechins on susceptibility of cooked red meat, poultry and fish patties to lipid oxidation. Food Res Int 34:651–657
Tassou C, Koutsoumanis K, Nychas GJE (2000) Inhibition of Salmonella enteritidis and Staphylococcus aureus in nutrient broth by mint essential oil. Food Res Int 33:273–280
Tewari H, Sethi R, Sood A, Singh L (1985) Lactic acid production from paneer whey by Lactobacillus bulgaricus. J Res Punjab Agric Univ 22:89–98
Thomas T, Batt R (1968) Survival of Streptococcus lactis in starvation conditions. J Gen Microbiol 50:367–382
Tian S, Nakamura K, Kayahara H (2004) Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J Agric Food Chem 52:4808–4813
Tiwari K, Mishra N, Pandey A (1980) Influence of EDTA and its metal complexes on lactic acid fermentation. Zbl Bakt 135:223–225
Trachoo N, Boudreaux C, Moongngarm A, Samappito S, Gaensakoo R (2006) Effect of geminated rough rice media on growth of selected probiotic bacteria. Pak J Biol Sci 9:2657–2661
Turgis M, Borsa J, Millette M, Salmieri S, Lacroix M (2008) Effect of selected plant essential oils or their constituents and modified atmosphere packaging on the radiosensitivity of Escherichia coli O157: H7 and Salmonella Typhi in ground beef. J Food Protect 71:516–521
Turgis M, JaeJoon H, Caillet S, Lacroix M (2009) Antimicrobial activity of mustard essential oil against Escherichia coli O157: H7 and Salmonella Typhi. Food Control 20:1073–1079
Ultee A, Kets E, Smid E (1999) Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 65:46064610
Vandenbergh PA (1993) Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol Rev 12:221–237
Wahba M, Ahmed AS, Ebraheim ZZ (2010) Antimicrobial effects of pepper, parsley, and dill and their roles in the microbiological quality enhancement of traditional Egyptian kareish cheese. Foodborne Pathog Dis 7:411–418
Walsh SE, Maillard JY, Russell A, Catrenich C, Charbonneau D, Bartolo R (2003) Activity and mechanisms of action of selected biocidal agents on Gram–positive and–negative bacteria. J Appl Microbiol 94:240–247
Wang WB, Lai HC, Hsueh PR, Robin YYC, Lin SB, Liaw SJ (2006) Inhibition of swarming and virulence factor expression in Proteus mirabilis by resveratrol. J Med Microbiol 55:1313–1321
Weinberg ED (1997) The Lactobacillus anomaly: total iron abstinence. Perspect Biol Med 40:578–583
Weisburger JH (1999) Tea and health: the underlying mechanisms. Exp Biol Med 220:271–275
Weisburger JH (2000) Eat to live, not live to eat. Nutrition 16:767–773
Wendakoon CN, Sakaguchi M (1995) Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components in spices. J Food Protect 58:280–283
Wijeratne SSK, Cuppett SL, Schlegel V (2005) Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J Agric Food Chem 53:8768–8774
Wishon LM, Song D, Ibrahim S (2010) Effect of metals on growth and functionality of Lactobacillus and Bifidobacteria. Milchwissenschaft 65:369–372
Yadav S, Gite S, Nilegaonkar S, Agte V (2011) Effect of supplementation of micronutrients and phytochemicals to fructooligosaccharides on growth response of probiotics and E. coli. BioFactors, vol 37. Oxford, England, p 58
Zaika L, Kissinger J (1979) Effects of some spices on acid production by starter cultures. J Food Protect 42:572–576
Zaika LL, Kissinger JC (1984) Fermentation enhancement by spices: identification of active component. J Food Sci 49:5–9
Acknowledgments
This work was supported by USDA National Institute of Food and Agriculture, Hatch project number NC.X-234-5-09-170-1 in the Agricultural Research Program at North Carolina Agricultural and Technical State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gyawali, R., Ibrahim, S.A. Impact of plant derivatives on the growth of foodborne pathogens and the functionality of probiotics. Appl Microbiol Biotechnol 95, 29–45 (2012). https://doi.org/10.1007/s00253-012-4117-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4117-x