Abstract
Heparin/heparan sulphate glycosaminoglycans (HSGAGs) are composed of linear chains of 20–100 disaccharide units of N-acetylated d-glucosamine α (1–4) linked to glucuronic acid. HSGAGs are widely distributed on the cell surface and extracellular cell matrix of virtually every mammalian cell type and play critical role in regulating numerous functions of blood vessel wall, blood coagulation, inflammation response and cell differentiation. These glycosaminoglycans present in this extracellular environment very significantly influence the blood coagulation system and cardiovascular functions. Recent studies have investigated the mechanism by which cancer causes thrombosis and emphasizes the importance of the coagulation system in angiogenesis and tumour metastasis. Heparan sulphate/heparin lyases or heparinases are a class of enzymes that are capable of specifically cleaving the (1–4) glycosidic linkages in heparin and heparan sulphate to generate biologically active oligosaccharides with substantially significant and distinct clinical, pharmaceutical and prophylactic/therapeutic applications. Bioavailability and pharmacokinetic behaviour and characteristics of these oligosaccharides vary significantly depending on the origin/nature of the substrate (heparin or heparan sulphate-like glycosaminoglycans), the source of enzyme and method of preparation. Various microorganisms are reported/patented to produce these enzymes with different properties. Heparinases are commercially used for the depolymerization of unfractionated heparin to produce low molecular weight heparins (LMWHs), an effective anticoagulant. Individual LMWHs are chemically different and unique and thus cannot be interchanged therapeutically. Heparinases and LMWHs are reported to control angiogenesis and metastasis also. This review catalogues the degradation of HSGAGs by microbial heparin/heparan sulphate lyases and their potential either specific to the enzymes or with the dual role for generation of oligosaccharides for a new generation of compounds, as shown by various laboratory or clinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heparin and its structural analogue, heparan sulphate (HS), are member of a family of polyanionic, polydisperse, linear polysaccharides called glycosaminoglycans (GAGs), which perform a variety of crucial biological functions in a number of physiological and pathological processes and have been extensively employed as therapeutic agents (Castelli et al. 2004; Casu 2005). Despite the widespread use of heparin as an anticoagulant, several aspects of the structure and physiological function of HSGAGs remained obscure for years. The molecular level understanding of glycosaminoglycans fine structure, for characterization of functionally active domains of heparin, controlling the diverse biological processes was possible by depolymerization of HSGAGs (Loganathan et al. 1990). The development of better defined heparin in terms of more or less uniform mass has been achieved in part by the generation of low molecular weight heparin (LMWH; M W 3,000–6,000 Da) by either gel filtration (Pangrazzi et al. 1985) or by partial enzymatic or chemical depolymerization of heparin (Guo and Conrad 1988; Linhardt 1992; Linhardt et al. 1986).
A variety of enzymes, both mammalian and bacterial in origin, are known to degrade HSGAGs (Ernst et al. 1995; Hopwood 1989; Linhardt et al. 1987). These mammalian and bacterial endolytic enzymes (endoglycosidases) cleave the glycosidic linkage between heparin and/or heparan sulphate residues at susceptible points within the polysaccharide chain. Mammalian endoglycosidases (or heparanases) cleave at the reducing end of glucuronic acid by a hydrolytic mechanism (Hopwood 1989; Pikas et al. 1998) whereas heparinases, from microbial sources, distinctively differ from heparanases as they depolymerise heparin and HS by β-eliminative cleavage. Heparanase cleaves the glucuronidic linkage between a non-sulphated glucuronic acid (GlcA) and N-sulpho-glucosamine (GlcN(S)) of heparin/HS. A highly sulphated glucosamine residue either 3-O-sulphated or 6-O-sulphated is critical for the enzyme action (Pikas et al. 1998). The GlcN(2-N-sulphate) structure on the reducing side and GlcN(6-O-sulphate) structure on the nonreducing side of the cleavage site are considerably important for the substrate recognition by the enzyme (Okada et al. 2002). The additional 2-N-sulphate group on the nonreducing GlcN or 6-O-sulphate group on the reducing GlcN appears to have a promoting effect on the heparanase action (Fig. 1).
Heparanases are found in a variety of normal and malignant cells and tissues, among which are cytotrophoblasts, endothelial cells (ECs), platelets, mast cells, neutrophils, macrophages, T and B lymphocytes and lymphoma, melanoma and carcinoma (Dempsey et al. 2000a, b, c; Gonzalez-Stawinski et al. 1999; Nakajima et al. 1986; Rosenberg 1989). The recent studies of cloning of a single gene by several groups (Bernard et al. 2001; Fairbanks et al. 1999; Hulett et al. 1999; Kussie et al. 1999; Toyoshima and Nakajima 1999; Vlodavsky et al. 1999), together with biochemical studies (Baker et al. 1999; Hulett et al. 2000; Levy-Adam et al. 2005), suggests that various normal and malignant mammalian cells express primarily identical or highly homologous sequences of a single 65-kDa latent heparanase enzyme (Parish et al. 2001). The human heparanase gene maps to chromosome 4 at band 4q21.3, contains 14 exons and encodes a 65-kDa polypeptide. This proenzyme undergoes proteolytic cleavage to yield active heparanase, a heterodimer of 50- and 8-kDa polypeptides (Vlodavsky et al. 1999).
Heparanase is an important modulator of the extracellular matrix and associated factors, specifically by releasing angiogenic factors and accessory fragments of HS from the tumour microenvironment to induce an angiogenic response (Hulett et al. 2000). Indeed, the heparanase enzyme is preferentially expressed in human tumours, and its overexpression in low-metastatic tumour cells is reported to facilitate tumour cell invasion and vascularization, events leading to cancer progression. However, heparanase expression in non-invasive and non-immune tissue suggests a role for heparanase in tissue morphogenesis, regeneration and repair during embryonic development and in the adult human phase. Both zymogen and active forms of heparanase have been shown to play biological functions which include osteoblastogenesis (Smith et al. 2010), nervous system development and neural cellular differentiation (Navarro et al. 2008; Takahashi et al. 2007).
Distinction of heparanases from heparinases is multifaceted which includes, but is not limited to, the working mechanism, substrate specificity, molecular properties, expression pattern, cellular activation and localization of these enzymes. Heparinases from various microbial sources and their commercial, pharmaceutical and clinical applications are the main focus of this review.
Heparin/heparan sulphate lyases: the HSGAG degrading enzymes
Heparin lyases—sources and properties
Heparinases or heparin lyases are a class of enzymes that are capable of specifically cleaving the major glycosidic linkages in heparin and heparan sulphate. Three heparin lyases have been identified in Pedobacter heparinus (formerly known as Flavobacterium heparinum), a heparin-utilising organism that also produces exoglycuronidases, sulphoesterases and sulphamidases that further act on the lyase-generated oligosaccharide products (Galliher et al. 1981, 1982; Sasisekharan et al. 1995; Yang et al. 1985). These lyases are designated as heparin lyase I (heparinase, EC 4.2.2.7), heparin lyase II (heparinase II, no EC number) and heparin lyase III (heparitinase, EC 4.2.2.8). The three purified heparin lyases differ in their capacity to cleave heparin and heparan sulphate: Heparin lyase I primarily cleaves heparin, heparin lyase III specifically cleaves heparan sulphate and heparin lyase II acts equally on both heparin and heparan sulphate (Linhardt et al. 1986, 1990).
The heparin lyases of P. heparinus are the most widely used and the best studied heparin degrading enzymes. Linker and Hovingh (1965) first reported the production of a crude lyase enzyme from P. heparinus, which had shown characteristic cleavage of heparin and heparitin sulphate to produce unsaturated oligosaccharide. Later, the same group separated this crude lyase fraction into a heparinase (heparin lyase I) and a heparitinase (heparin lyase III). Both activities were purified by 50–100-fold, but no physical characterization of these enzymes was performed. Dietrich et al. (1973), Silva and Dietrich (1974), Silva et al. (1976), Ototani and Yosizawa (1978) and Ototani et al. (1981) isolated three lyases, a heparinase (heparin lyase I) and two heparitinases, from the same microorganism (P. heparinus). The heparinase acted on heparin to produce mainly tri-sulphated disaccharides (Dietrich and Nader 1974; Dietrich et al. 1971).
Linhardt et al. (1984) reported the purification of heparinase (heparin lyase I) to a single band on sodium dodecyl sulphate–polyacrylamide gel electrophoresis (PAGE). Affinity purification of heparin lyase I on heparin-Sepharose failed, apparently due to degradation of the column matrix. Sufficient quantities of pure heparin lyase I for detailed characterization studies and amino acid analysis were first prepared by Yang et al. (1985). Heparin lyase I was used to prepare polyclonal antibodies in rabbits for affinity purification of heparin lyase I, but excessively harsh conditions required to elute the enzyme resulted in substantial loss of activity (Linhardt et al. 1985). By a combination of hydroxylapatite chromatography and negative adsorption on QAE-Sephadex at pH 8.3, Yang et al. (1987) also described a large-scale method to prepare heparin lyase I.
McLean and Williamson (1985) described the specificity of a partially purified heparinase II. Although no evidence of homogeneity or any physical properties for heparinase II were presented, the broad specificity on various polymeric substrates identified the enzyme as heparin lyase II (Linhardt et al. 1990; Moffat et al. 1991). Nader et al. (1990) purified two heparitinases, called heparitinase I and II, possibly corresponding to heparin lyases II and III and characterized their substrate specificity towards heparin and heparan sulphate, although no physical properties of these enzymes were presented. Heparitinase I degraded both N-acetylated and N-sulphated heparan sulphate. A single purification method to purify all the three heparinases was developed and used to purify these enzymes to homogeneity by Lohse and Linhardt (1992). All the three heparinases from P. heparinus are periplasmic proteins and have been isolated either by osmotic shock or sonication (Lohse and Linhardt 1992; Zimmermann et al. 1991). Their physical and kinetic characteristics were studied thoroughly, and optimal reaction conditions were established and these are summarized in Table 1.
Some Bacteroides spp., Bacillus sp. and Sphingobacterium sp. (Nakamura et al. 1976, 1988; Salyers et al. 1977) are also reported to produce heparinases (Table 1). Most of the heparinase producing bacteria were isolated from soil, although it is not known why a soil bacterium would produce an acidic polysaccharide degrading lyase. It is possible that these bacteria could use these enzymes to degrade the GAGs from carcasses. Heparinases were purified to apparent homogeneity from Bacteroides stercoris HJ-15, isolated from human intestine (Kim et al. 2000, 2004). A heparinase has also been purified to apparent homogeneity from an unidentified soil bacterium (Bohmer et al. 1990a, b).
All the heparinases are positively charged at neutral pH (pI, 8.5–10), which may be attributed to the basic nature of heparinases required to degrade highly polyanionic substrates. These enzymes differ from those isolated from P. heparinus in its molecular weight, pI, amino acid composition and kinetic properties (Table 1). Furthermore, antibody assays, amino acid analysis/sequencing, peptide mass fingerprinting and Southern blotting between heparinases from different bacteria have shown no cross-reactivity (Bellamy and Horikoshi 1992; Sasisekharan et al. 1993), suggesting a low degree of sequence and structural homology among heparinases from different species. However, Yoshida et al. (2002a) have reported that Bacillus circulans heparinase gene and deduced amino acid sequence have partial similarity with enzymes belonging to the family of acidic polysaccharide lyases that degrade chondroitin sulphate and hyaluronic acid. Recently, Hyun et al. (2010) have reported that recombinant heparinase III from B. stercoris HJ-15 has 70 % homology to heparinase II from P. heparinus.
All the three heparinases from P. heparinus have been sequenced, cloned and expressed in Escherichia coli (Godavarti et al. 1996; Sasisekharan et al. 1993; Shaya et al. 2004). The heparinase genes were expressed in E. coli with intact biological function (Sasisekharan et al. 1993; Su et al. 1996). Molecular analysis of the three heparinases revealed no significant homology either at the DNA or protein levels, nor were they closely linked on the P. heparinus chromosome (Su et al. 1996).
Elucidation of the catalytically critical amino acids in the heparinases active site and substrate binding domains was useful for understanding their mechanism of action and for their development as molecular tools for heparin or heparan sulphate-like glycosaminoglycan (HLGAG) analysis. The heparin-binding site in heparinase I contains two Cardin–Weintraub heparin-binding consensus sequences, a calcium coordinating motif, a cysteine and a histidine residue (Godavarti and Sasisekharan 1996). The primary sequence of heparinase II reveals three cysteine residues (cysteine-164, cysteine-189 and cysteine-348) in contrast to heparinase I, which has two cysteine residues and heparinase III which does not have cysteines. Similarly, calcium is required for the enzymatic activity of heparinases I and III, but heparinase II is not only active in the absence of calcium but the presence of calcium inhibits enzyme activity (Lohse and Linhardt 1992; Sasisekharan et al. 1996b).
Heparinases—clinical and diagnostic applications
The HLGAGs have chemical heterogeneity and structural complexity, which has limited the development of effective tools and methods for a rapid sequencing. Sequencing methodology of HLGAGs is mainly dependent on the chemical or enzymatic degradation of the polysaccharide in a sequence-specific manner (Venkataraman et al. 1999). An important enzymatic tool in this sequencing process is the heparinases, including heparinases I, II and III, produced by P. heparinus. Each of the heparinases has its own unique HLGAG sequence at which it cleaves, making these enzymes valuable tools in obtaining sequence-specific information (Dongfang et al. 2002). Heparinases I, II and III from P. heparinus cleave heparin/HS with a high degree of substrate specificity, at the α-1–4-glucosaminidic linkages within HS/heparin and produce disaccharides with a nonreducing 4,5-unsaturated uronic acid residue (Godavarti and Sasisekharan 1996). Heparin lyase I cleaves the glucosaminidic linkage in GlcN(N-sulphate) α1-4 IdceA(2-sulphate) and tolerates C-6 sulphation of the hexosamine unit (Fig. 1). Conversely, heparinase III requires primarily an unsulphated uronic acid moiety and cleaves the glucosaminidic linkage in GlcN(N-sulphate or N-acetylate) α1-4 GlcA and tolerates C-6 sulphation of the hexosamine unit (Desai et al. 1993; Sugahara et al. 1995; Yamada and Sugahara 1998). Heparinase II displays a broader substrate specificity, possessing the ability to cleave all glycosaminidic linkages independent of O- and/or N-sulphation as well as the type of the uronic acid residue of heparin/HS (Shriver et al. 1998b) (Fig. 1). Structure determination of heparin or heparin sulphate involves depolymerisation of their chains into constituent disaccharide components by a detailed degradation with a single or a cocktail of heparinases. The structural information with regard to the hexuronic acid epimer present at the nonreducing end is lost by the creation of a C=C bond between C4 and C5 (Stringer et al. 2003). Accurate sequence assignment relies on the highly pure activity of GAG degrading enzymes as well as a detailed understanding of their action towards specific linkages. The substrate specificity and action pattern of the enzymes provide important constraints to reconstruct the GAG sequence.
The preparation of structurally defined pure heparin-derived oligosaccharides is crucial in understanding heparin’s interaction with proteins and in determining the precise structural requirements essential for the binding of heparin with the proteins. Structurally defined heparin-derived oligosaccharides can be obtained from the mixtures of heparin-derived oligosaccharides produced by depolymerization of heparin. The oligosaccharides prepared from heparin are purified and analysed by fractionation on the basis of molecular weight, charge density, solubility characteristics and affinity (usually to antithrombin) (Hook et al. 1976). The oligosaccharide mixtures are first fractionated by low-pressure gel permeation chromatography into size-uniform mixtures of disaccharides, tetrasaccharides, hexasaccharides and higher oligosaccharides. Each size-fractionated mixture is then separated further by gradient PAGE (Edens et al. 1992; Rice et al. 1987), capillary electrophoresis (CE) (Linhardt et al. 1993), strong anion exchange (Hileman et al. 1997) and reversed-phase ion-pairing high-performance liquid chromatography (Guo and Conrad 1988; Linhardt et al. 1989; Thanawiroon and Linhardt 2003). These provide important data on composition and domain structure but generally yield indirect and incomplete sequence information. Chromatographic and electrophoretic approaches share several limitations. The enzymatic cleavage is characterized based purely on gel mobility or HPLC elution, which are not completely predictable. Elution of GAGs from SAX or amine columns using salt could potentially bias the analysis towards species that have relatively higher sulphation and may not be sensitive to the separation of species with low sulphation. Both methodologies rely on end-labelling of saccharides requiring the introduction of excess labelling reagent. The ladder of peaks or gel shifts caused by treatment with exolytic enzymes is not fully comprehensible, as these substrate specificities have not been thoroughly characterized.
Sensitive methodologies based on CE and mass spectrometry (MS) have been developed to detect the femto-/picomolar amounts of GAG. MS has also been applied to the analysis of oligosaccharides. Fast-atom bombardment MS, electrospray ionization MS and matrix-assisted laser desorption/ionization MS are capable of determining the molecular weight of oligosaccharides (Mallis et al. 1989). However, one- and two-dimensional nuclear magnetic resonance spectroscopy provides for the accurate determination of the structure of small oligosaccharides (Desai and Linhardt 1994; Linhardt et al. 1986; Mikhailov et al. 1996).
The heparinases have also been used to investigate in great detail the role of HSGAGs in aspects of heparin-binding proteins naming a few interaction of growth factors, including the fibroblast growth factors (FGFs) involved in cell proliferation, differentiation and angiogenesis (Bernfield et al. 1999; Tumova et al. 2000); interaction of HSGAGs as coreceptor for intracellular pathogens entry into host cells (Ascencio et al. 1993; Bugatti et al. 2007; Chen et al. 1997; Crublet et al. 2008; Urbinati et al. 2009); chemokines, a family of over 40 structurally related glycoproteins that facilitate leukocyte migration, angiogenesis and breast cancer metastasis (Lantz et al. 1991; Nelson et al. 1993); inhibition of smooth muscle cell growth for antiproliferative properties (Benitz et al. 1986; Castellot et al. 1982, 1985a, b; Jackson et al. 1991; Reilly et al. 1989); anticomplementary activity through interaction of heparin with complement proteins (Edens et al. 1993; Yu et al. 2005); cell adhesion (Edens et al. 2001) and lipid metabolism (Engelberg 1996).
The most thoroughly studied heparin-binding protein is the serine protease inhibitor antithrombin III (AT III) that interacts with thrombin and factor Xa in the blood coagulation cascade. The molecular basis for the anticoagulant function of heparin was elucidated in the early 1980s when a distinct pentasaccharide sequence within the heparin chain was identified as being crucial for binding and activating antithrombin, leading to accelerated inhibition of the coagulation cascade (Lindahl et al. 1980; Petitou et al. 2003). These studies were triggered by the finding that only a fraction of heparin molecules were capable of binding with high affinity to antithrombin and further that this fraction essentially accounted for the anticoagulant activity of the unfractionated material. Oligosaccharides obtained by selective, partial depolymerization of heparin were fractionated on immobilized antithrombin, and the smallest high-affinity molecules recovered were subjected to structural analysis, in conjunction with various modification steps (Petitou et al. 2003). This region is composed of one glucuronic acid unit, one iduronic acid unit and three glucosamine units, two of which are invariably N-sulphated, whereas the remaining one may be either N-acetylated or N-sulphated (Bourin and Lindahl 1993).
Heparinases are certainly essential agents in studying structural, biochemical, physiological and pathological roles of HLGAGs. Further, heparinases have shown potential for several diagnostic as well as therapeutic applications.
Low molecular weight heparin production
Although heparin is highly efficacious in a variety of clinical situations and has the potential to be used in many others, the side effects associated with heparin therapy are many and varied. Side effects such as heparin-induced thrombocytopenia are primarily associated with the long chain of unfractionated heparin (UFH), which provides binding domains for various proteins. This has led to the explosion in the generation and utilisation of low molecular weight heparin as an efficacious alternative to UFH. Although attention has been focussed on LMWH as heparin substitutes due to their more predictable pharmacological action, reduced side effects, sustained antithrombotic activity and better bioavailability, there is at present limited ability to standardize the LMWH manufacturing process. Because the LMWH are derived from heparins and hence are polydisperse and microheterogenous, with undefined structure, they possess inherent variability, which currently prevents an efficient process for their manufacture. It would be of value both medically and scientifically to have a consistent, quality controlled, time efficient, concentration independent and highly reproducible method for producing heparin and other glycosaminoglycans (Sundaram et al. 2003).
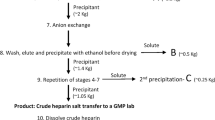
The development of better defined heparin in terms of more or less uniform mass has been achieved in part by the generation of LMWH (M W 3,000–6,000 Da) by either gel filtration (Pangrazzi et al. 1985) or by partial enzymatic or chemical depolymerization of heparin (Guo and Conrad 1988; Linhardt 1992; Linhardt et al. 1986). LMWHs possess distinct pharmacologic profile that is largely determined by their composition and thus on their preparative method (Fareed et al. 2000). The oxidative instability of heparin, as observed during pharmaceutical grade heparin preparation, suggested the possibility of preparation of LMWHs by oxidative methods, oxygen radical processes and oxidative depolymerization through nitrous acid deamination. Besides oxidative breakdown, two β-eliminative methods, one enzymatic and the other chemical, are used to commercially prepare LMWHs (Table 2) (Linhardt 1994; Linhardt and Toida 1997). Enzymatic breakdown of UFH for generation of LMWHs is reported by heparin lyases (or heparinases I, II, III) of P. heparinus, specifically heparinases I and II (Langer et al. 1983; Linhardt et al. 1992; Shriver et al. 2000; Viskov and Mourier 2007). The extent of heparinase reaction is monitored by measuring the change in absorbance associated with the unsaturated uronic acid residue formed in each product molecule (Linhardt 1994; Lohse and Linhardt 1992). The depolymerization is stopped by removing or inactivating the enzyme. After recovery of the GAG from the enzyme and removal of very low molecular weight by-products (i.e. disaccharides and tetrasaccharides), a LMWH is obtained that has the desired molecular weight and activity properties (Linhardt and Gunay 1999). This method is used to prepare the clinically used LMWH product, tinzaparin sodium (Table 2).
Heparin antagonist
Patients undergoing surgery with cardiopulmonary bypass (CPB) must receive systemic anticoagulation with intense antithrombin activity to prevent activation of the coagulation system by the artificial surfaces of the CPB apparatus. Heparin has long been used to temporarily render the blood incoagulable during extracorporeal circulation, cardiovascular surgery and other arterial interventions. But bleeding complications are especially common when the arterial tree is violated, occurring in as many as 10–15 % of cases. After the patient is separated from CPB, heparin’s anticoagulant effect must be neutralized to halt substantial bleeding. For cardiovascular surgery and many related interventions, protamine has long been the standard antagonist when acute and complete neutralization of heparin anticoagulant effect is necessary. Protamine is the only currently approved drug in the USA with antiheparin activity. Protamine’s efficacy is related in part to its total net cationic charge, but unfortunately so is its toxicity, including systemic hypotension, pulmonary vasoconstriction and anaphylactic reactions. For these reasons, there is renewed interest in developing heparin antagonists which will replace the use of protamine (Shenoy et al. 1999).
Heparinase I (Neutralase™; IBEX Technologies, Montreal, QC, Canada) that specifically inactivates heparin is a possible alternative to protamine. Heparinase neutralizes heparin by enzymatic cleavage of alpha–glycosidic linkages at the AT III binding site (Ammar and Fisher 1997). Several studies have examined the efficacy of heparinase I to reverse heparin-induced anticoagulation in vitro and compared heparinase I to protamine as an antagonist of heparin-induced anticoagulation in animal models like dogs and rabbits (Michelsen et al. 1996; Silver et al. 1998). Animal investigations demonstrated that heparinase I reverses heparin-prolonged activated clotting time (ACT) without significant hemodynamic changes. When given in doses up to 30 μg/kg, heparinase I successfully neutralized heparin’s anticoagulant effect in a dose-dependent fashion without significant adverse sequelae. Heres et al. (2001) assessed the heparin-neutralizing activity and safety profile of different doses of heparinase I in 49 patients undergoing coronary artery surgery. They found that heparinase I, 7 or 10 μg/kg, effectively restored the ACT after unfractionated heparin was given to patients undergoing CPB for coronary artery surgery. Heparinase I caused no clinically significant hemodynamic or other adverse responses. In addition, because heparinase I totally eliminated the antithrombin activity of heparin but only partially eliminated the anti-Xa activity, anti-Xa activity increased as additional heparin was metabolized by heparinase I. Heparinase I displayed a half-life of 12 min in patients with coronary artery disease. Its activity decayed accordingly, so that after approximately 36 min, little activity remained. However, another study examining the efficacy and safety of heparinase I as an alternative to protamine in patients undergoing aortocoronary bypass graft surgery found that though heparinase I reversed heparin anticoagulation but is not equivalent to protamine because of its inferior safety profile (Stafford-Smith et al. 2005). Further studies are required to compare heparinase I and protamine for clinically relevant outcome variables.
Anti-angiogenic activity of heparinase
Cancer development is characterized by uncontrolled tumour proliferation and secondary metastasis. The tumour tissue can be divided into three compartments, namely the tumour cell compartment, the endothelial cell compartment and the extracellular matrix compartment. ECM compartment interfaces with both tumour and endothelial cell compartments and regulates the overall development of cellular compartments. Heparan sulphate proteoglycans (HSPGs) along with structural proteins are key components of the cell surface–ECM interface. The strategic location of HSPGs facilitates regulation of cell proliferation and migration, the key components for the tumour growth and angiogenesis (Sanderson 2001). HSPGs on endothelial cell surfaces act as co-receptors for a variety of pro-angiogenic growth factors such as FGF and vascular endothelial growth factor (VEGF) and anti-angiogenic factors such as endostatin (Bikfalvi et al. 1997). There is sufficient experimental data to suggest that heparins may interfere with various aspects of cancer proliferation, angiogenesis and metastasis formation (Collen et al. 2000; Hasan et al. 2005; Weidner et al. 1991).
Vascularisation is the hallmark of malignant tumours without which solid tissue cannot grow beyond 1–2 mm (Pluda 1997). Tumour-associated neovascularization is a central pathogenic step in the process of tumour growth, invasion and metastasis. These complex processes involve multiple steps and pathways dependent on the local balance between positive and negative regulatory factors, as well as interactions among the tumour, its vasculature and the surrounding extracellular tissue matrix. Neovascularization is the process of generating new blood vessels mediated primarily by progenitor and/or endothelial cells leading to tube formation, resulting in a stabilized neovascular channel. Angiogenesis, the predominant form of neovascularization in carcinogenesis, is mediated by endothelial cells sprouting from postcapillary venules, leading primarily to new capillaries (Risau 1997).
The genetic background of the angiogenic switch during tumour progression is not fully understood, but discoveries of the endothelial mitogenic growth factors (VEGF, FGF, platelet-derived growth factor, hepatocyte growth factor) as the main angiogenic factors suggested that the switch is able to turn on the expression of the genes of these factors in tumours. The molecular interactions between endothelial mitogenic growth factors with their signalling or accessory receptors have become a major pharmacological target for development of anti-angiogenic drugs. The recognition of the common chemical nature of these endothelial growth factors, i.e. their heparin-binding potential, provides an easy though non-specific anti-angiogenic target. Besides other anti-angiogenic factors (Timar et al. 2001), heparinases I–III have been reported to reduce neovascularization by altering the action of FGF at the level of receptor binding (Dongfang et al. 2002; Raman and Kuberan 2010; Sasisekharan et al. 1994). It was found that heparinase treatment of capillary EC caused a significant loss in FGF binding capacity in these cells. Treatment with 125 nM heparinase I resulted in greater that 95 % loss in HSPG binding sites on ECs accompanied by an over 80 % decrease in receptor binding. The half maximal concentrations required to remove HSPG sites and reduce receptor binding were 0.7 and 1.5 nM, respectively. The concentrations of heparinase I required to reduce FGF binding to heparan sulphate and receptor sites by 50 % were 0.5 and 1.5 nM, respectively. For heparinase II, IC50 concentrations were 2 and 8 nM, respectively, for the heparan sulphate and the receptor binding sites. On the other hand, heparinase III was most potent in inhibiting the FGF binding, with an IC50 of 0.15 and 0.2 nM for the heparan sulphate and the receptor binding, respectively (Sasisekharan et al. 1996a).
Concluding remarks
The role of heparin as an anticoagulant was established much earlier to the discovery of heparinases. Although it is still in its infancy, clinical and diagnostic application of heparinases holds promise for unravelling the HLGAGs microstructure and biological activity along with a range of diverse applications. Deciphering their physiologic and clinical role will establish the evolutionary relationship of these proteins from various microbial sources but also having potential to improve and develop the role of heparinases in various clinical manifestations.
References
Ammar T, Fisher CF (1997) The effects of heparinase I and protamine on platelet reactivity. Anesthesiology 86:1382–1386
Ascencio F, Fransson LA, Wadstrom T (1993) Affinity of the gastric pathogen Helicobacter pylori for the N-sulphated glycosaminoglycan heparan sulphate. J Med Microbiol 38:240–244
Baker E, Crawford J, Sutherland GR, Freeman C, Parish CR, Hulett MD (1999) Human HPA endoglycosidase heparanase. Map position 4q21.3. Chromosome Res 7:319
Banga J, Tripathi CKM (2009) Rapid purification and characterization of a novel heparin degrading enzyme from Acinetobacter calcoaceticus. New Biotechnol 26:99–104
Banga J, Tripathi CKM (2010) Purification and characterization of a novel heparin degrading enzyme from Aspergillus flavus (MTCC-8654). Appl Biochem Biotechnol 160:1004–1016
Bellamy R, Horikoshi K (1992) Heparinase produced by microorganism belonging to the genus Bacillus. US Patent 5,145,778
Benitz WE, Lessler DS, Coulson JD, Bernfield M (1986) Heparin inhibits proliferation of fetal vascular smooth muscle cells in the absence of platelet-derived growth factor. J Cell Physiol 127:1–7
Bergqvist D (1996) Low molecular weight heparins. J Intern Med 240:63–72
Bernard D, Mehul B, Delattre C, Simonetti L, Thomas-Collignon A, Schmidt R (2001) Purification and characterization of the endoglycosidase heparanase 1 from human plantar stratum corneum: a key enzyme in epidermal physiology? J Invest Dermatol 117:1266–1273
Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M (1999) Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68:729–777
Bikfalvi A, Javerzat S, Perollet C, Savona C (1997) Angiogenesis and cancer. Bull Cancer 84:885–890
Bohmer LH, Pitout MJ, Allcock JL, Visser L (1990a) Heparin degradation by a novel microbial heparinase. Thromb Res 60:331–335
Bohmer LH, Pitout MJ, Steyn PL, Visser L (1990b) Purification and characterization of a novel heparinase. J Biol Chem 265:13609–13617
Bourin MC, Lindahl U (1993) Glycosaminoglycans and the regulation of blood coagulation. Biochem J 289(Pt 2):313–330
Bugatti A, Urbinati C, Ravelli C, De Clercq E, Liekens S, Rusnati M (2007) Heparin-mimicking sulfonic acid polymers as multitarget inhibitors of human immunodeficiency virus type 1 Tat and gp120 proteins. Antimicrob Agents Chemother 51:2337–2345
Castelli R, Porro F, Tarsia P (2004) The heparins and cancer: review of clinical trials and biological properties. Vasc Med 9:205
Castellot JJ Jr, Favreau LV, Karnovsky MJ, Rosenberg RD (1982) Inhibition of vascular smooth muscle cell growth by endothelial cell-derived heparin. Possible role of a platelet endoglycosidase. J Biol Chem 257:11256–11260
Castellot JJ Jr, Cochran DL, Karnovsky MJ (1985a) Effect of heparin on vascular smooth muscle cells. I. Cell metabolism. J Cell Physiol 124:21–28
Castellot JJ Jr, Wong K, Herman B, Hoover RL, Albertini DF, Wright TC, Caleb BL, Karnovsky MJ (1985b) Binding and internalization of heparin by vascular smooth muscle cells. J Cell Physiol 124:13–20
Casu B (2005) Structure and active domains of heparin. In: Garg HG, Linhardt RJ, Hales CA (eds) Chemistry and biology of heparin and heparan sulfate. Elsevier, London, pp 1–28
Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM (1997) Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med 3:866–871
Collen A, Smorenburg SM, Peters E, Lupu F, Koolwijk P, Van Noorden C, van Hinsbergh VW (2000) Unfractionated and low molecular weight heparin affect fibrin structure and angiogenesis in vitro. Cancer Res 60:6196–6200
Crublet E, Andrieu JP, Vives RR, Lortat-Jacob H (2008) The HIV-1 envelope glycoprotein gp120 features four heparan sulfate binding domains, including the co-receptor binding site. J Biol Chem 283:15193–15200
Dempsey LA, Brunn GJ, Platt JL (2000a) Heparanase, a potential regulator of cell–matrix interactions. Trends Biochem Sci 25:349–351
Dempsey LA, Plummer TB, Coombes S, Platt JL (2000b) Platelet heparanase in vascular responses to xenotransplantation. Transplant Proc 32:972
Dempsey LA, Plummer TB, Coombes SL, Platt JL (2000c) Heparanase expression in invasive trophoblasts and acute vascular damage. Glycobiology 10:467–475
Desai UR, Linhardt RJ (1994) Molecular-weight of low-molecular-weight heparins by C-13 nuclear-magnetic-resonance spectroscopy. Carbohydr Res 255:193–212
Desai UR, Wang HM, Linhardt RJ (1993) Specificity studies on the heparin lyases from Flavobacterium heparinum. Biochemistry 32:8140–8145
Dietrich CP, Nader HB (1974) Fractionation and properties of four heparitin sulfates from beef lung tissue. Isolation and partial characterization of a hemogeneous species of heparitin sulfate. Biochim Biophys Acta 343:34–44
Dietrich CP, Nader HB, Britto LR, Silva ME (1971) Chemical composition of heparitin sulfate. Fractionation and characterization of four acidic mucopolysaccharides in heparitin sulfate from beef lung tissue. Biochim Biophys Acta 237:430–441
Dietrich CP, Silva ME, Michelacci YM (1973) Sequential degradation of heparin in Flavobacterium heparinum. Purification and properties of five enzymes involved in heparin degradation. J Biol Chem 248:6408–6415
Dongfang L, Pojasek K, Shriver Z, Holley K, El-shabrawi Y, Venkataraman G, Sasisekharan R (2002) Heparinase III and uses thereof. EP Patent 1,266,013
Edens RE, al-Hakim A, Weiler JM, Rethwisch DG, Fareed J, Linhardt RJ (1992) Gradient polyacrylamide gel electrophoresis for determination of molecular weights of heparin preparations and low-molecular-weight heparin derivatives. J Pharm Sci 81:823–827
Edens R, Linhardtb R, Weiler J (1993) Heparin is not just an anticoagulant anymore: six and one-half decades of studies on the ability of heparin to regulate complement activity. In: Cruise JM, Lewis RE (eds) Complement today. Karger, Basel, pp 96–120
Edens RE, LeBrun LA, Linhardt RJ, Kaul PR, Weiler JM (2001) Certain high molecular weight heparin chains have high affinity for vitronectin. Arch Biochem Biophys 391:278–285
Engelberg H (1996) Actions of heparin in the atherosclerotic process. Pharmacol Rev 48:327–352
Ernst S, Langer R, Cooney CL, Sasisekharan R (1995) Enzymatic degradation of glycosaminoglycans. Crit Rev Biochem Mol Biol 30:387–444
Fairbanks MB, Mildner AM, Leone JW, Cavey GS, Mathews WR, Drong RF, Slightom JL, Bienkowski MJ, Smith CW, Bannow CA, Heinrikson RL (1999) Processing of the human heparanase precursor and evidence that the active enzyme is a heterodimer. J Biol Chem 274:29587–29590
Fareed J, Hoppensteadt DA, Bick RL (2000) An update on heparins at the beginning of the new millennium. Semin Thromb Hemost 26(Suppl 1):5–21
Galliher PM, Cooney CL, Langer R, Linhardt RJ (1981) Heparinase production by Flavobacterium heparinum. Appl Environ Microbiol 41:360–365
Galliher P, Linhardt R, Conway L, Langer R, Cooney C (1982) Regulation of heparinase synthesis in Flavobacterium heparinum. Appl Microbiol Biotechnol 15:252–257
Gao N, Cheng X, Yang J, Zhang S (2003) Production of a novel heparinase from Sphingobacterium sp. Wei Sheng Wu Xue Bao 43:813–816
Gesner BM, Jenkin CR (1961) Production of heparinase by Bacteroides. J Bacteriol 81:595–604
Godavarti R, Sasisekharan R (1996) A comparative analysis of the primary sequences and characteristics of heparinases I, II, and III from Flavobacterium heparinum. Biochem Biophys Res Commun 229:770–777
Godavarti R, Davis M, Venkataraman G, Cooney C, Langer R, Sasisekharan R (1996) Heparinase III from Flavobacterium heparinum: cloning and recombinant expression in Escherichia coli. Biochem Biophys Res Commun 225:751–758
Gonzalez-Stawinski GV, Parker W, Holzknecht ZE, Huber NS, Platt JL (1999) Partial sequence of human platelet heparitinase and evidence of its ability to polymerize. Biochim Biophys Acta 1429:431–438
Guo YC, Conrad HE (1988) Analysis of oligosaccharides from heparin by reversed-phase ion-pairing high-performance liquid chromatography. Anal Biochem 168:54–62
Han C, Spring S, Lapidus A, Glavina Del Rio T, Tice H, Copeland A, Cheng JF, Lucas S, Chen F, Nolan M (2009) Complete genome sequence of Pedobacter heparinus type strain (HIM 762-3T). Stand Genomic Sci 1:54
Hasan J, Shnyder SD, Clamp AR, McGown AT, Bicknell R, Presta M, Bibby M, Double J, Craig S, Leeming D, Stevenson K, Gallagher JT, Jayson GC (2005) Heparin octasaccharides inhibit angiogenesis in vivo. Clin Cancer Res 11:8172–8179
Heres EK, Horrow JC, Gravlee GP, Tardiff BE, Luber J Jr, Schneider J, Barragry T, Broughton R (2001) A dose-determining trial of heparinase-I (Neutralase) for heparin neutralization in coronary artery surgery. Anesth Analg 93:1446–1452
Hileman RE, Smith AE, Toida T, Linhardt RJ (1997) Preparation and structure of heparin lyase-derived heparan sulfate oligosaccharides. Glycobiology 7:231–239
Hook M, Bjork I, Hopwood J, Lindahl U (1976) Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett 66:90–93
Hopwood JJ (1989) Enzymes that degrade heparin and heparan sulphate. In: Lane DW, Lindahl U (eds) Heparin: chemical and biological properties, clinical applications. Edward Arnold, London, pp 190–229
Hulett MD, Freeman C, Hamdorf BJ, Baker RT, Harris MJ, Parish CR (1999) Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nat Med 5:803–809
Hulett MD, Hornby JR, Ohms SJ, Zuegg J, Freeman C, Gready JE, Parish CR (2000) Identification of active-site residues of the pro-metastatic endoglycosidase heparanase. Biochemistry 39:15659–15667
Hyun YJ, Lee JH, Kim DH (2010) Cloning, overexpression, and characterization of recombinant heparinase III from Bacteroides stercoris HJ-15. Appl Microbiol Biotechnol 86:879–890
Jackson RL, Busch SJ, Cardin AD (1991) Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev 71:481–539
Kim DH, Kim BT, Park SY, Kim NY, Han MJ, Shin KH, Kim WS, Kim YS (1998) Degradation of acharan sulfate and heparin by Bacteroides stercoris HJ-15, a human intestinal bacterium. Arch Pharm Res 21:576–580
Kim BT, Kim WS, Kim YS, Linhardt RJ, Kim DH (2000) Purification and characterization of a novel heparinase from Bacteroides stercoris HJ-15. J Biochem 128:323–328
Kim WS, Kim BT, Kim DH, Kim YS (2004) Purification and characterization of heparin lyase I from Bacteroides stercoris HJ-15. J Biochem Mol Biol 37:684–690
Kussie PH, Hulmes JD, Ludwig DL, Patel S, Navarro EC, Seddon AP, Giorgio NA, Bohlen P (1999) Cloning and functional expression of a human heparanase gene. Biochem Biophys Res Commun 261:183–187
Langer RS, Linhardt RJ, Cooney CL, Fitzgerald G, Grant A (1983) Heparinase derived anticoagulants. US patent 4396762
Lantz M, Thysell H, Nilsson E, Olsson I (1991) On the binding of tumor necrosis factor (TNF) to heparin and the release in vivo of the TNF-binding protein I by heparin. J Clin Invest 88:2026–2031
Levy-Adam F, Abboud-Jarrous G, Guerrini M, Beccati D, Vlodavsky I, Ilan N (2005) Identification and characterization of heparin/heparan sulfate binding domains of the endoglycosidase heparanase. J Biol Chem 280:20457–20466
Lindahl U, Backstrom G, Thunberg L, Leder IG (1980) Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci U S A 77:6551–6555
Linhardt RJ (1992) Chemical and enzymatic methods for the depolymerization and modification of heparin. In: Ogura H, Hasegawa A, Suami T (eds) Carbohydrates synthetic methods and applications in medicinal chemistry. Kodansha, Tokyo, pp 385–401
Linhardt RJ (1994) Analysis of glycosaminoglycans with polysaccharide lyases. In: Varki A (ed) Current protocols in molecular biology, analysis of glycoconjugates, 2008/02/12th edn. Wiley Interscience, Boston, pp 17.13.17–17.13.32
Linhardt RJ, Gunay NS (1999) Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost 25(Suppl 3):5–16
Linhardt RJ, Toida T (1997) Heparin oligosaccharides: new analogues development and applications. In: Witczak ZJ, Nieforth KA (eds) Carbohydrates in drug design. Marcel Dekker, New York, pp 277–341
Linhardt RJ, Cooney CL, Tapper D, Zannetos CA, Larsen AK, Langer R (1984) An immobilized microbial heparinase for blood deheparinization. Appl Biochem Biotechnol 9:41–55
Linhardt RJ, Merchant ZM, Persinger DW (1985) Immuno-affinity purification of heparinase. Int J Biochem 17:1179–1183
Linhardt RJ, Rice KG, Merchant ZM, Kim YS, Lohse DL (1986) Structure and activity of a unique heparin-derived hexasaccharide. J Biol Chem 261:14448–14454
Linhardt RJ, Galliher PM, Cooney CL (1987) Polysaccharide lyases. Appl Biochem Biotechnol 12:135–176
Linhardt RJ, Gu KN, Loganathan D, Carter SR (1989) Analysis of glycosaminoglycan-derived oligosaccharides using reversed-phase ion-pairing and ion-exchange chromatography with suppressed conductivity detection. Anal Biochem 181:288–296
Linhardt RJ, Turnbull JE, Wang HM, Loganathan D, Gallagher JT (1990) Examination of the substrate specificity of heparin and heparan sulfate lyases. Biochemistry 29:2611–2617
Linhardt RJ, Wang HM, Ampofo SA (1992) New methodologies in heparin structure analysis and the generation of LMW heparins. Adv Exp Med Biol 313:37–47
Linhardt R, Liu J, Han X (1993) Mapping and sequencing of oligosaccharides by electrophoresis. Trends Glycosci Glycotechnol 5:181–192
Linker A, Hovingh P (1965) The enzymatic degradation of heparin and heparitin sulfate: the fractionation of a crude heparinase from Flavobacteria. J Biol Chem 240:3724–3728
Loganathan D, Wang HM, Mallis LM, Linhardt RJ (1990) Structural variation in the antithrombin III binding site region and its occurrence in heparin from different sources. Biochemistry 29:4362–4368
Lohse DL, Linhardt RJ (1992) Purification and characterization of heparin lyases from Flavobacterium heparinum. J Biol Chem 267:24347–24355
Mallis LM, Wang HM, Loganathan D, Linhardt RJ (1989) Sequence analysis of highly sulfated, heparin-derived oligosaccharides using fast atom bombardment mass spectrometry. Anal Chem 61:1453–1458
McLean MW, F. LW, Williamson FR (1985) Proceeding of the VIIIth International Symposium on Glycoconjugates. Houston Paeger, New York
Michelsen LG, Kikura M, Levy JH, Lee MK, Lee KC, Zimmermann JJ, Szlam F (1996) Heparinase I (neutralase) reversal of systemic anticoagulation. Anesthesiology 85:339–346
Mikhailov D, Mayo KH, Vlahov IR, Toida T, Pervin A, Linhardt RJ (1996) NMR solution conformation of heparin-derived tetrasaccharide. Biochem J 318(Pt 1):93–102
Moffat CF, McLean MW, Long WF, Williamson FB (1991) Heparinase II from Flavobacterium heparinum. Action on chemically modified heparins. Eur J Biochem 197:449–459
Nader HB, Porcionatto MA, Tersariol IL, Pinhal MA, Oliveira FW, Moraes CT, Dietrich CP (1990) Purification and substrate specificity of heparitinase I and heparitinase II from Flavobacterium heparinum. Analyses of the heparin and heparan sulfate degradation products by 13C NMR spectroscopy. J Biol Chem 265:16807–16813
Nakajima M, Irimura T, Nicolson GL (1986) Tumor metastasis-associated heparanase (heparan sulfate endoglycosidase) activity in human melanoma cells. Cancer Lett 31:277–283
Nakamura T, Suginaka Y, Takazoe I (1976) Heparinase activity in lesion of periodontal diseases. Bull Tokyo Dent Coll 17:147–155
Nakamura T, Shibata Y, Fujimura S (1988) Purification and properties of Bacteroides heparinolyticus heparinase (heparin lyase, EC 4.2.2.7). J Clin Microbiol 26:1070–1071
Navarro FP, Fares RP, Sanchez PE, Nadam J, Georges B, Moulin C, Morales A, Pequignot JM, Bezin L (2008) Brain heparanase expression is up-regulated during postnatal development and hypoxia-induced neovascularization in adult rats. J Neurochem 105:34–45
Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, Bevilacqua MP (1993) Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood 82:3253–3258
Okada Y, Yamada S, Toyoshima M, Dong J, Nakajima M, Sugahara K (2002) Structural recognition by recombinant human heparanase that plays critical roles in tumor metastasis. Hierarchical sulfate groups with different effects and the essential target disulfated trisaccharide sequence. J Biol Chem 277:42488–42495
Okuda K, Kato T, Shiozu J, Takazoe I, Nakamura T (1985) Bacteroides heparinolyticus sp. nov. isolated from humans with periodontitis. Int J Syst Evol Microbiol 35:438
Ototani N, Yosizawa Z (1978) Interaction of mucopolysaccharides with glycosaminoglycans on glycosaminoglycan-bound AH-Sepharose 4B. J Biochem 84:1005–1008
Ototani N, Kikuchi M, Yosizawa Z (1981) Purification of heparinase and heparitinase by affinity chromatography on glycosaminoglycan bound AH-Sepharose 4B. Carbohydr Res 88:291–303
Pangrazzi J, Abbadini M, Zametta M, Naggi A, Torri G, Casu B, Donati MB (1985) Antithrombotic and bleeding effects of a low molecular weight heparin fraction. Biochem Pharmacol 34:3305–3308
Parish CR, Freeman C, Hulett MD (2001) Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta 1471:M99–M108
Petitou M, Casu B, Lindahl U (2003) 1976–1983, a critical period in the history of heparin: the discovery of the antithrombin binding site. Biochimie 85:83–89
Pikas DS, Li JP, Vlodavsky I, Lindahl U (1998) Substrate specificity of heparanases from human hepatoma and platelets. J Biol Chem 273:18770–18777
Pluda JM (1997) Tumor-associated angiogenesis: mechanisms, clinical implications, and therapeutic strategies. Semin Oncol 24:203–218
Raman K, Kuberan B (2010) Differential effects of heparitinase I and heparitinase III on endothelial tube formation in vitro. Biochem Biophys Res Commun 398:191–193
Reilly CF, Kindy MS, Brown KE, Rosenberg RD, Sonenshein GE (1989) Heparin prevents vascular smooth muscle cell progression through the G1 phase of the cell cycle. J Biol Chem 264:6990–6995
Rice KG, Rottink MK, Linhardt RJ (1987) Fractionation of heparin-derived oligosaccharides by gradient polyacrylamide-gel electrophoresis. Biochem J 244:515–522
Risau W (1997) Mechanisms of angiogenesis. Nature 386:671–674
Rosenberg RD (1989) Platelet heparitinase. Methods Enzymol 169:342–351
Salyers AA, Vercellotti JR, West SE, Wilkins TD (1977) Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol 33:319–322
Sanderson RD (2001) Heparan sulfate proteoglycans in invasion and metastasis. Semin Cell Dev Biol 12:89–98
Sasisekharan R, Bulmer M, Moremen KW, Cooney CL, Langer R (1993) Cloning and expression of heparinase I gene from Flavobacterium heparinum. Proc Natl Acad Sci USA 90:3660–3664
Sasisekharan R, Moses MA, Nugent MA, Cooney CL, Langer R (1994) Heparinase inhibits neovascularization. Proc Natl Acad Sci USA 91:1524–1528
Sasisekharan R, Lohse D, Cooney C, Linhardt R, Langer R (1995) Purification of heparinase I, II, and III from Flavobacterium heparinum. United States Patent 5389539
Sasisekharan R, Moses MA, Nugent MA, Cooney CL, Langer RS (1996a) Method for inhibiting angiogenesis using heparinase. US Patent 5567417
Sasisekharan R, Venkataraman G, Godavarti R, Ernst S, Cooney CL, Langer R (1996b) Heparinase I from Flavobacterium heparinum. Mapping and characterization of the heparin binding domain. J Biol Chem 271:3124–3131
Shaya D, Li Y, Cygler M (2004) Crystallization and preliminary X-ray analysis of heparinase II from Pedobacter heparinus. Acta Crystallogr D Biol Crystallogr 60:1644–1646
Shaya D, Tocilj A, Li Y, Myette J, Venkataraman G, Sasisekharan R, Cygler M (2006) Crystal structure of heparinase II from Pedobacter heparinus and its complex with a disaccharide product. J Biol Chem 281:15525–15535
Shenoy S, Harris RB, Sobel M (1999) Development of heparin antagonists with focused biological activity. Curr Pharm Des 5:965–986
Shriver Z, Hu Y, Pojasek K, Sasisekharan R (1998a) Heparinase II from Flavobacterium heparinum. Role of cysteine in enzymatic activity as probed by chemical modification and site-directed mutagenesis. J Biol Chem 273:22904–22912
Shriver Z, Hu Y, Sasisekharan R (1998b) Heparinase II from Flavobacterium heparinum. Role of histidine residues in enzymatic activity as probed by chemical modification and site-directed mutagenesis. J Biol Chem 273:10160–10167
Shriver Z, Sundaram M, Venkataraman G, Fareed J, Linhardt R, Biemann K, Sasisekharan R (2000) Cleavage of the antithrombin III binding site in heparin by heparinases and its implication in the generation of low molecular weight heparin. Proc Natl Acad Sci USA 97:10365–10370
Silva ME, Dietrich CP (1974) Isolation and partial characterization of three induced enzymes from Flavobacterium heparinum involved in the degradation of heparin and heparitin sulfates. Biochem Biophys Res Commun 56:965–972
Silva M, Dietrich CP, Nader HB (1976) On the structure of heparitin sulfates. Analyses of the products formed from heparitin sulfates by two heparitinases and a heparinase from Flavobacterium heparinum. Biochim Biophys Acta 437:129–141
Silver PJ, Broughton R, Bouthillier J, Quinn TA, Wallace AM, Weishaar RE (1998) Neutralase reverses the anti-coagulant but not the anti-thrombotic activity of heparin in a rabbit model of venous thrombosis. Thromb Res 91:143–150
Smith PN, Freeman C, Yu D, Chen M, Gatenby PA, Parish CR, Li RW (2010) Heparanase in primary human osteoblasts. J Orthop Res 28:1315–1322
Stafford-Smith M, Lefrak EA, Qazi AG, Welsby IJ, Barber L, Hoeft A, Dorenbaum A, Mathias J, Rochon JJ, Newman MF (2005) Efficacy and safety of heparinase I versus protamine in patients undergoing coronary artery bypass grafting with and without cardiopulmonary bypass. Anesthesiology 103:229–240
Stringer SE, Kandola BS, Pye DA, Gallagher JT (2003) Heparin sequencing. Glycobiology 13:97–107
Su H, Blain F, Musil RA, Zimmermann JJ, Gu K, Bennett DC (1996) Isolation and expression in Escherichia coli of hepB and hepC, genes coding for the glycosaminoglycan-degrading enzymes heparinase II and heparinase III, respectively, from Flavobacterium heparinum. Appl Environ Microbiol 62:2723–2734
Sugahara K, Tsuda H, Yoshida K, Yamada S, de Beer T, Vliegenthart JF (1995) Structure determination of the octa- and decasaccharide sequences isolated from the carbohydrate–protein linkage region of porcine intestinal heparin. J Biol Chem 270:22914–22923
Sundaram M, Venkataraman G, Shriver Z, Liu D, Qi Y, Sasisekharan R (2003) Methods and products related to low molecular weight heparin. EP Patent 1,319,183
Takahashi H, Matsumoto H, Kumon Y, Ohnishi T, Freeman C, Imai Y, Tanaka J (2007) Expression of heparanase in nestin-positive reactive astrocytes in ischemic lesions of rat brain after transient middle cerebral artery occlusion. Neurosci Lett 417:250–254
Thanawiroon C, Linhardt RJ (2003) Separation of a complex mixture of heparin-derived oligosaccharides using reversed-phase high-performance liquid chromatography. J Chromatogr A 1014:215–223
Timar J, Dome B, Fazekas K, Janovics A, Paku S (2001) Angiogenesis-dependent diseases and angiogenesis therapy. Pathol Oncol Res 7:85–94
Toyoshima M, Nakajima M (1999) Human heparanase. Purification, characterization, cloning, and expression. J Biol Chem 274:24153–24160
Tumova S, Woods A, Couchman JR (2000) Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int J Biochem Cell Biol 32:269–288
Urbinati C, Nicoli S, Giacca M, David G, Fiorentini S, Caruso A, Alfano M, Cassetta L, Presta M, Rusnati M (2009) HIV-1 Tat and heparan sulfate proteoglycan interaction: a novel mechanism of lymphocyte adhesion and migration across the endothelium. Blood 114:3335–3342
Venkataraman G, Shriver Z, Raman R, Sasisekharan R (1999) Sequencing complex polysaccharides. Science 286:537–542
Viskov C, Mourier P (2007) Oligosaccharides, preparation method and use thereof, and pharmaceutical compositions containing same. US patent 0,142,323
Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I (1999) Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med 5:793–802
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis correlation in invasive breast carcinoma. N Engl J Med 324:1–8
Yamada SH, Sugahara K (1998) Structure of oligosaccharides isolated from heparan sulfate heparin and substrate specificities of the degrading enzymes of bacterial origin. Trends Glycosci Glycotechnol 10:95–123
Yang VC, Linhardt RJ, Bernstein H, Cooney CL, Langer R (1985) Purification and characterization of heparinase from Flavobacterium heparinum. J Biol Chem 260:1849–1857
Yang VC, Bernstein H, Cooney CL, Langer R (1987) Large scale preparation and characterization of mucopolysaccharase contamination free heparinase. Appl Biochem Biotechnol 16:35–50
Yapeng C, Ningguo G, Xiulan C, Jing Y, Shijun Q, Shuzheng Z (2003) Rapid purification, characterization and substrate specificity of heparinase from a novel species of Sphingobacterium. J Biochem 134:365–371
Yoshida E, Arakawa S, Matsunaga T, Toriumi S, Tokuyama S, Morikawa K, Tahara Y (2002a) Cloning, sequencing, and expression of the gene from Bacillus circulans that codes for a heparinase that degrades both heparin and heparan sulfate. Biosci Biotechnol Biochem 66:1873–1879
Yoshida E, Sakai K, Tokuyama S, Miyazono H, Maruyama H, Morikawa K, Yoshida K, Tahara Y (2002b) Purification and characterization of heparinase that degrades both heparin and heparan sulfate from Bacillus circulans. Biosci Biotechnol Biochem 66:1181–1184
Yu H, Munoz EM, Edens RE, Linhardt RJ (2005) Kinetic studies on the interactions of heparin and complement proteins using surface plasmon resonance. Biochim Biophys Acta 1726:168–176
Zhang F, Yoder PG, Linhardt RJ (2004) Synthetic and natural polysaccharides with anticoagulant properties. In: Dumitriu S (ed) Polysaccharides: structural diversity and functional versatility, 2nd edn. Marcel Dekker, New York, pp 773–794
Zimmermann JJ, Oddie K, Langer R, Cooney CL (1991) The release of heparinase from the periplasmic space of Flavobacterium heparinum by three-step osmotic shock. Appl Biochem Biotechnol 30:137–148
Acknowledgements
This research was financially supported by grant from University Grants Commission, South Campus, Delhi University, Benito Juarez Marg, New Delhi-110021.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tripathi, C.K.M., Banga, J. & Mishra, V. Microbial heparin/heparan sulphate lyases: potential and applications. Appl Microbiol Biotechnol 94, 307–321 (2012). https://doi.org/10.1007/s00253-012-3967-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-3967-6