Abstract
Increasingly, winemakers are looking for ways to introduce aroma and flavour diversity to their wines as a means of improving style and increasing product differentiation. While currently available commercial yeast strains produce consistently sound fermentations, there are indications that sensory complexity and improved palate structure are obtained when other species of yeast are active during fermentation. In this study, we explore a strategy to increase the impact of non-Saccharomyces cerevisiae inputs without the risks associated with spontaneous fermentations, through generating interspecific hybrids between a S. cerevisiae wine strain and a second species. For our experiments, we used rare mating to produce hybrids between S. cerevisiae and other closely related yeast of the Saccharomyces sensu stricto complex. These hybrid yeast strains display desirable properties of both parents and produce wines with concentrations of aromatic fermentation products that are different to what is found in wine made using the commercial wine yeast parent. Our results demonstrate, for the first time, that the introduction of genetic material from a non-S. cerevisiae parent into a wine yeast background can impact favourably on the wine flavour and aroma profile of a commercial S. cerevisiae wine yeast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Saccharomyces sensu stricto complex consists of a number of closely related species (Naumov 1987; Vaughan-Martini and Martini 1987; Naumov et al. 2010). Of these, Saccharomyces cerevisiae has been utilised by humans down through the ages, culminating in recent decades in a large number of industrial S. cerevisiae wine yeast strains being available to commercial winemaking. These strains show robust growth characteristics in grape juice, tolerating both the initial high sugar concentration at the onset of fermentation and high ethanol concentrations towards the end. In contrast, other Saccharomyces species generally ferment more slowly than S. cerevisiae and are often unable to tolerate the high alcohol concentrations encountered. However, there are indications that sensory complexity and more rounded palate structure is obtained when other species of yeast are active during fermentation, as in the case of traditional, spontaneous fermentations. Spontaneous fermentations allow the many different species of indigenous microorganisms that populate the vineyard, grape-picking equipment and winery to contribute to vinification. Studies on spontaneous ferments have identified a number of non-Saccharomyces species present at the early stages of fermentation (Fleet and Heard 1993), and products of the metabolism of these species are thought to contribute to more complex aroma and flavour profiles in the wine. Nonetheless, because of their unpredictable nature, the desirability of spontaneous fermentations is a source of debate, many winemakers preferring to inoculate with a proven S. cerevisiae industrial strain.
Experiments using inoculations of mixtures of S. cerevisiae strains in a grape juice show dynamic population fluctuations between strains (Howell et al. 2004; King et al. 2008), with unpredictable fermentation outcomes. The situation is even worse when less robust non-S. cerevisiae strains are used. Fermentations using co-inocula of S. cerevisiae and non-Saccharomyces strains typically have limited success, with the non-Saccharomyces strain having only a minor impact on wine aroma and composition (Soden et al. 2000). The dominance of S. cerevisiae over other species in spontaneous fermentations is due mainly to their tolerance of high sugar and high ethanol concentrations (Pretorius 2000) and perhaps for some S. cerevisiae strains the capacity to produce ‘killer’ compounds that trigger cellular death of non-Saccharomyces strains (Heard and Fleet 1987; Perez-Navzdo et al. 2006).
An alternative to co-ferment that avoids growth competition between species is to use an interspecific hybrid strain, where the genomes of different species are contained within the one cell. Species of the Saccharomyces sensu stricto clade are able to mate with each other to form interspecific hybrids, but the hybrids formed are sterile, having non-viable ascospores (Naumov et al. 2000). This occurs in nature and, in fact, one member of the Saccharomyces sensu stricto complex, Saccharomyces pastorianus, the lager making yeast, has been identified as a stable, natural hybrid from an evolutionary timeframe (Groth et al. 1999) resulting from a cross between S. cerevisiae and a Saccharomyces bayanus-type yeast (Masneuf et al. 1998; Marinomi et al. 1999; Dunn and Sherlock 2008).
We have used a rare-mating strategy (Spencer and Spencer 1996) to generate interspecific hybrids between a robust diploid S. cerevisiae commercial wine strain, AWRI 838, and strains of either Saccharomyces paradoxus or Saccharomyces kudriavzevii. AWRI 838 is an isolate of EC 1118 and genomic sequencing has revealed that it is a diploid (Novo et al. 2009).
In nature, yeast mating is activated by the presence of pheromones produced by haploid yeast, but sporulation of the wine yeast parent to generate haploid spores might lead to the loss of important wine fermentation traits. Rare mating relies upon an infrequent event (1 × 10−6 cells) whereby mating type switching within the diploid genome leads to a cell homozygous at the mating type loci, either a/a or α/α (Gunge and Nakatomi 1972). These homozygotes are able to enter the mating pathway and can conjugate with a cell of the opposite mating type, leading to an interspecific hybrid.
In order to establish an experimental precedent and for ease of selection, the diploid wine yeast strain was first mated with a haploid, auxotrophic S. paradoxus strain. Metabolite analysis was performed on the resultant interspecific hybrid to confirm that the addition of the S. paradoxus genome had an impact on the parental wine yeast metabolome. Additional interspecific hybrids were then generated using random spores of wild-type strains of either S. paradoxus or S. kudriavzevii. Hybrids resulting from each of the wild-type crosses were chosen for grape juice fermentation and the wine analysed for important wine fermentation compounds.
Materials and methods
Yeast strains and media
Parental strains are S. cerevisiae AWRI 838 (an isolate of the commercial wine yeast strain EC 1118), S. paradoxus strains N17–78–Mat a ho ura3 lys2 met13 provided by Rhona Borts (Hunter et al. 1996) and 52–153 (Herman J. Phaff Yeast Culture Collection, University of California Davis) and S. kudriavzevii type strain NCYC 2889. Strains generated from this study are listed in Table 1. All yeasts were grown in YEPD medium (1% (w/v) yeast extract, 2% (w/v) peptone, 2% (w/v) glucose) with shaking (100 rpm) at 25°C. Mitochondrial mutants of AWRI 838 were isolated by treating cells for 8 h in synthetic complete medium containing 10 μg mL−1 ethidium bromide. Cells were then diluted in water, and due to their inability to utilise glycerol as a carbohydrate source, the mitochondrial mutants were revealed by their petite colony growth on YPDG (1% (w/v) yeast extract, 2% (w/v) peptone, 3% (w/v) glycerol and 0.1% (w/v) glucose; Sherman et al. 1986).
Generation of interspecific hybrid yeast
Rare-mating, essentially as described by Spencer and Spencer (1996), was used throughout. Strains were grown to stationary phase in YEPD at 27°C. Spores of strains 52–153 and NCYC 2889 were generated by inoculating the equivalent of 2 mL of a washed YEPD culture into 5 mL of sporulation medium (1%, w/v potassium acetate). After sporulation, cells were washed and re-suspended in sterile water. In a 250-mL conical flask, 1 mL of each parent strain was added to 20 mL of fresh YEPD and incubated for 7 days at 27°C. Appropriate numbers of cells were washed in sterile water and plated onto selective plates. Wild-type strains were assayed under several phenotypic conditions to determine selection criteria for hybridisation. Selection in mating experiments was performed on YNB–glycerol–ethanol plates (0.67% (w/v) yeast nitrogen base without amino acids, 3% (w/v) glycerol, 3% (v/v) ethanol, 2% (w/v) agar) for the auxotrophic strain cross and YEP–glycerol–ethanol plates (1% (w/v) yeast extract, 2% (w/v) peptone, 3% (w/v) glycerol, 14% (v/v) ethanol, 2% (w/v) agar) for wild-type strain crosses.
PCR confirmation of hybrids
For all strains, DNA was purified using mechanical breakage with glass beads (Ausubel et al. 1994). Yeast cells were disrupted using a Mini-Beadbeater® (BioSpec) for 3 min with glass beads. Genomic DNA was used as template for PCR analyses, with amplification using the δ transposon primer set MLD1 5′-CAAAATTCACCTAAA/TTCTCA-3′ and MLD2 5′-GTGGATTTTTATTCCAACA-3′ (Ness et al. 1993) and the intron primer set EI1 5′-CTGGCTTGGTGTATGT-3′ and LA2 5′-CGTGCAGGTGTTAGTA-3′ (de Barros Lopes et al. 1996). PCR fragments were resolved on a 1.5% (w/v) agarose gel. rDNA PCR-RFLP was conducted using the rDNA Internal Transcribed Spacer unit primer pair ITS1 5′-TCCGTAGGTGAACCTGCGG-3′ and ITS4 5′-TCCTCCGCTTATTGATATGC-3′ and the Restriction Enzyme HaeIII and fragments were resolved on a 2% (w/v) agarose gel (Esteve-Zarzosa et al. 1999).
Fermentation stress assay plates
Assay plates containing 14% (w/v) ethanol were produced by addition of a requisite volume of 99% (w/v) ethanol to cooled YEPD. The plates were wrapped in parafilm during storage and after plating were incubated at 22°C. Glucose assay plates of YEP plus 25% (w/v) glucose were also incubated after plating at 22°C. Strains were grown to stationary in liquid YPD (2 days), and 5 μL of 10-fold serial dilutions was spotted to plates.
Chemical profiling of volatile metabolite products
Hybrid strains generated from the S. cerevisiae × S. paradoxus N17–78 cross were screened for robust growth in YEPD, and a single strain, AWRI 1519, was selected for further study. Parent and hybrid strains were inoculated in triplicate at 1 × 106 cells from a pre-culture (2 days growth in YEPD) into 50 mL of Synthetic Complete medium with 4× amino acid mix (Sambrook and Russel 2001) and 8% (w/v) glucose. On completion of fermentation (<0.25% residual sugar as determined with Clinitest® tablets, Bayer, Switzerland), duplicate samples were analysed for volatile metabolites using gas chromatography–mass spectroscopy (GC-MS; Eglinton et al. 2002).
Fermentation product analysis of hybrid-generated wines compared to their commercial yeast parent
Small-scale industrial ferments were carried out at a commercial winery in 240 L barrels. Chardonnay grapes were machine harvested with no sulphur dioxide added. Fruit was tank-pressed, homogenised and transferred to barrels. Triplicate fermentations were conducted at 11–17°C using either the commercial wine yeast parent AWRI 838, the S. cerevisiae × S. paradoxus hybrid strain, AWRI 1501 or the S. cerevisiae × S. kudriavzevii hybrid strain, AWRI 1503. Wines were not produced by the non-S. cerevisiae parents, as neither was able to grow in the Chardonnay grape juice. At completion of fermentation (determined with Clinitest® tablets), wines were settled with sulphur dioxide and ascorbic acid added and treated with 250 μg/L copper. Triplicate wines were then pooled, filtered and bottled. Chemical analysis of target compounds, previously identified as important for wine flavour and aroma, was undertaken from duplicate samples of the resultant wines using GC-MS preceded by a headspace solid-phase micro-extraction (HS-SPME), with polydeuterated internal standards for stable isotope dilution analysis (Siebert et al. 2005).
Statistical analysis
A one-way analysis of variance and Student’s t test (<0.05) were used to determine significant differences of compound concentrations between media and wines fermented by each yeast strain.
Results
Rare matings
Colonies formed on selection plates following interspecific matings were scored (Table 2) and subsequently picked onto new selection plates. Mating efficiency in the AWRI 838 × N17–78 cross was 30-fold greater than the AWRI 838 × 52–153 cross, while the less closely related S. kudriavzevii strain had the lowest mating frequency of the crosses at 100-fold less than the S. cerevisiae × S. paradoxus haploid cross. The differences in mating efficiency between the crosses could be due to a number of factors; for example, S. paradoxus 52–153 spores may have mated with each other reducing the pool of spores available to mate with S. cerevisiae whereas N17–78 is a stable haploid; there may be inherent differences in sporulation efficiency between the two non-S. cerevisiae species; and the greater evolutionary distance between S. cerevisiae and S. kudriavzevii.
Confirmation of hybrid status of mating products
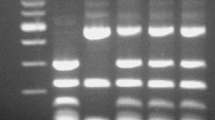
The hybrid nature of colonies from the S. cerevisiae × S. paradoxus crosses was confirmed by PCR analysis of genomic DNA utilising amplification with δ transposon primers and intron primers (Figs. 1a, b and 2a, b). A control PCR using DNA from both parents revealed that the transposon primers showed a bias towards S. cerevisiae targets, while the intron primers showed a bias towards S. paradoxus targets.
The AWRI 838 × N17–78 hybrids showed a transposon PCR pattern with specific bands from both parents, however, not all of the parent-specific bands were observed in all hybrids. For instance, hybrid strains A1, A2 A3 and A4 contain all five major bands amplified from the S. cerevisiae parent, while hybrid strain A5 is missing the lowest S. cerevisiae specific band (Fig. 1a). The intron PCR pattern for this cross showed a bias towards the S. paradoxus genome, with all hybrids having the complete set of S. paradoxus bands but only faint S. cerevisiae specific bands (Fig. 1b).
The AWRI 838 × 52–153 hybrids showed a transposon PCR pattern with mainly S. cerevisiae specific bands, but, again, not all bands were amplified in each hybrid (Fig. 2a), as hybrid strains B1 and B3 are missing both of the two lowest S. cerevisiae specific bands whereas B5 is missing only the lowest band. Three S. paradoxus specific bands were amplified strongly in the intron PCR (Fig. 2b), with all five hybrid strains amplifying the middle band, but not the top band. Hybrid B1 alone amplified the lowest S. paradoxus specific band. Collectively, PCR analyses confirmed the hybrid nature of the putative hybrid strains.
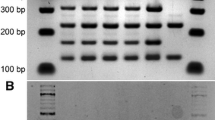
The hybrid nature of products from the S. cerevisiae × S. kudriavzevii cross was unable to be confirmed by transposon or intron PCR, as both analyses showed a fragment pattern attributed to the AWRI 838 parent only (Fig. 3a, b). ITS PCR-RFLP targeting the rDNA tandem repeat loci, however, revealed the existence of rDNA from both species within these hybrid strains (Fig. 3c).
Interspecific hybrids inherited wine-relevant traits from the wine yeast parent
Two confirmed interspecific hybrids were chosen for grape juice fermentation studies: AWRI 1501 from the S. cerevisiae × S. paradoxus (wild-type) cross and AWRI 1503 from the S. cerevisiae × S. kudriavzevii cross. Assay plates were designed to test the tolerance of hybrids to two major stresses encountered during fermentations: high sugar and high ethanol concentrations. Medium incorporating a high concentration of glucose (25%, w/v) allowed robust growth of both hybrids and the S. cerevisiae parent, while the S. kudriavzevii parent showed less robust growth and the S. paradoxus parent no growth at all (Fig. 4). Neither the S. paradoxus nor the S. kudriavzevii parent was able to grow on high ethanol (14%, v/v) plates, but both hybrid strains grew well, although AWRI 1503 showed slightly weaker growth than the S. cerevisiae parent.
Fermentation stress assay plates. Assay plates left to right: YEPD control, YEP-25% glucose, YEPD-14% ethanol. Strains, left to right, are AWRI 838 (S. cerevisiae parent), 52–153 (S. paradoxus parent), NCYC 2889 (S. kudriavzevii parent), AWRI 1501 (S. cerevisiae × S. paradoxus interspecific hybrid) and AWRI 1503 (S. cerevisiae × S. kudriavzevii interspecific hybrid). Spotted cultures are in 10-fold serial dilutions from top to bottom
Chemical analysis of volatile metabolites from hybrid AWRI 1519 and parent strains in defined medium
After the completion of fermentation (<0.25% residual sugar), GC-MS analysis of defined medium fermented by AWRI 1519 and its parent strains identified 32 compounds (Table 3), 13 of which showed a significant changed concentration for the hybrid relative to the S. cerevisiae wine yeast parent. The chemical concentration profile of the hybrid volatile metabolites followed the gamut of all possible outcomes. In some cases, the hybrid strain produced a compound at the higher-producing parent level, but on other occasions produced a compound at the lower-producing parent level. For example, in the case of benzaldehyde, the hybrid generated 13.0 μg/L, an amount equivalent to 85% of the S. paradoxus parent (14.9 μg/L), while the S. cerevisiae parent generated considerably less (2.44 μg/L). Conversely, for dodecalactone, the hybrid generated 3.51 μg/L, a level similar to the S. cerevisiae parent (6.5 μg/L), whereas the S. paradoxus parent generated a far greater amount (30.9 μg/L). Some compounds were produced by the hybrid at an intermediate level between the two parental levels (e.g. 2-phenylethyl acetate), while two compounds were produced by the hybrid at remarkably lower levels than for either parent (cis-4-hydroxymethyl-2-methyl-1,3-dioxolane and cis-5-hydroxy-2-methyl-1,3-dioxane). A third compound, ethyl hexanoate, was produced by the hybrid at a concentration much higher than the cumulative total of the parents.
Chemical analysis of fermentation products of hybrids AWRI 1501 and AWRI 1503 from small-scale grape juice industrial ferments
Small-scale (240 L) industrial ferments of Chardonnay juice were carried out using interspecific hybrid strains AWRI 1501 and AWRI 1503 and the wine yeast parent AWRI 838 alone, as the non-S. cerevisiae parents were unable to grow in the juice. The resultant wines (all having fermented to completion with <0.25% residual sugar) were analysed for volatile compounds using HS-SPME-GC-MS targeting 31 compounds (Table 4) previously established as important flavour and aroma compounds in wine (Siebert et al. 2005).
Relative to the S. cerevisiae parent strain, AWRI 1501 showed noteworthy differences in the concentration levels of 17 of the compounds analysed, with six compounds increasing and 11 compounds decreasing. Similarly, AWRI 1503 produced considerable differences in 20 compounds relative to the S. cerevisiae parent; seven showed an increase and 13 showed a decrease in concentration. A number of compounds that can have a negative effect on wine aroma and flavour were produced at much lower concentrations by the hybrid yeasts: acetic acid (vinegar), 3-methylbutanoic acid (blue cheese) and ethyl acetate (nail polish) decreased to 35%, 50% and 60%, respectively, of the concentrations produced by the S. cerevisiae parent. On the other hand, a number of compounds that contribute to fruity aromas had increased levels in the hybrid yeast wines. Ethyl hexanoate (green apple) levels increased to 120% for both hybrid yeast wines while the fruity aroma compounds, ethyl butanoate and ethyl propanoate, also showed increases, 117% and 160% (AWRI 1501) and 123% and 124% (AWRI 1503), respectively. 2-Methylpropyl acetate (banana) was produced in higher amounts by hybrid strain AWRI 1503. Compounds associated with more complex characters were also produced at increased levels to the parent wine yeast: Hexanoic acid (sweaty) levels were 137% (AWRI 1501) and 135% (AWRI 1503) and butanol (fusel) were 122% and 110%, respectively.
Discussion
Rapid and consistent fermentations are essential in large-scale, commercial wine production, and the majority of wineries worldwide rely upon inoculating their ferments with active dried yeast products from a yeast-manufacturing company. These ADY products are commonly strains of S. cerevisiae, although a small number have been identified as natural hybrids between members of the Saccharomyces sensu stricto species (Masneuf et al. 1998; Groth et al. 1999; Gonzalez et al. 2006; Bradbury et al. 2006). Different wine yeasts vary in their efficiency and reliability when fermenting grape juice (Pretorius 2000) and can impart different sensory properties to wine (King et al. 2008). This variation in yeast strain performance and delivery of product quality gives winemakers options when attempting to tailor their products to the preferences of different market segments. Development of new yeast strains with improved and/or desirable novel flavours is of growing importance for winemakers needing to produce wines that are differentiated from others in a competitive, over-crowded market.
Traditional breeding techniques are commonly used for yeast strain improvement (Winge and Lausten 1938; Pretorius 2000), and typically, these strain development programmes involve hybridising yeast of the same Saccharomyces species (i.e. S. cerevisiae), to produce intraspecific hybrids. This manuscript describes, for the first time, laboratory-based interspecific matings between a S. cerevisiae wine yeast strain and strains from two other Saccharomyces sensu stricto species—S. paradoxus and S. kudriavzevii—in order to generate wine yeast that produce novel wine and flavour aroma profiles.
Initially, for ease of selection, to optimise mating conditions, and for proof of concept experiments, S. cerevisiae wine yeast hybrids were generated using a genetically modified (GM) laboratory S. paradoxus haploid strain carrying auxotrophic markers. However, as only non-GM yeast are used by the Australian wine industry, interspecific hybrids were subsequently generated using non-genetically modified, natural isolates of S. paradoxus and S. kudriavzevii. Yeast mating is activated by the presence of pheromones normally produced by haploid yeast, and so the parent S. paradoxus and S. kudriavzevii strains were sporulated to generate haploid spores. To minimise the risk of potential loss of important wine yeast fermentation properties, the wine yeast parent was not sporulated; rare matings (Spencer and Spencer 1996) were used to form presumptive triploid interspecific hybrids.
Strain-specific and species-specific banding patterns generated using primers that target δ-transposon regions, introns and rDNA regions were used as markers to confirm the presence of each parental input in the resultant hybrid strains. Although a degree of preferential amplification of the S. cerevisiae parent genome was observed with the transposon primers, the intron primers showed a preference for S. paradoxus genomic sequences in the S. cerevisiae × S. paradoxus hybrids. However, both δ-transposon and intron primer sets showed a preference for S. cerevisiae DNA sequences in the S. cerevisiae × S. kudriavzevii hybrid. Nonetheless, the hybrid status of all progeny used in fermentative work was confirmed using the above primer sets, or by targeting the rDNA ITS region that, upon restriction digestion, generated species-specific banding patterns.
Interestingly, different hybrids generated from the same cross did not give identical banding patterns. This may be due to genome loss or rearrangement during the incipient stages of interspecific hybrid evolution. In previous work, genomic analyses of natural interspecific yeast hybrids have identified loss of varying portions of parental genomes (Dunn and Sherlock 2008; Belloch et al. 2009), and plant studies have shown that changes within newly formed interspecific hybrid genomes occur rapidly leading to extensive inter- and intra-genome rearrangements and gene loss (Song et al. 1995; Kashkush et al. 2002).
Genetic stability analysis (using the same PCR approach as for confirmation of hybridisation) was carried out on each hybrid strain generated in this study. Twenty individual isolates from each hybrid strain were assessed after 50 generations in YEPD and at the end of model medium and grape juice fermentations. No further change in fingerprint profile was identified (results not shown).
Genome loss and rearrangement in newly formed wine yeast hybrids might lead to loss of industrially important traits, such as stress tolerance. Two stresses common to grape juice fermentations are high sugar concentration experienced at the beginning of fermentation and high ethanol concentration that builds towards the end of fermentation. Hence, assay plates designed to select for tolerances to these stresses were used to confirm that hybrids chosen for further investigation at least retained these traits.
All hybrids generated for this work clearly produce different volatile fermentation product profiles to the wine strain parent. Chemical analysis of volatile metabolites in spent minimal medium for AWRI 1519 showed this yeast to be very different to its parental strains. In some cases, levels of metabolites for the hybrid followed closely that of the ‘highest-producing’ parent, but, on other occasions, a compound was produced at the level of the ‘lower-producing’ parent. Moderating effects (where hybrid levels are midway between parents) were also noted. Intriguingly, a small number of compounds were produced by the hybrid in a considerably reduced concentration relative to either parent, or at a level much higher than a cumulative amount. It is possible that flavour-active metabolites of interspecific hybrids, at concentrations not predicted by their parental metabolite profiles, could lead the generation of new yeast strains capable of creating unique wine styles from conventional grape varieties.
Chardonnay wines produced by hybrids AWRI 1501 and AWRI 1503 again showed compound concentrations that were greater or less than produced by the wine yeast parent. Interestingly, the magnitude of differences varied between the two interspecific hybrids, highlighting the potential for different hybrid strains to tailor wines towards different consumer groups (Lattey et al. 2007). The specific contribution of the non-S. cerevisiae parents was not assessed, as neither was able to grow in the Chardonnay grape juice. The compounds that were present at altered levels in the hybrid-made wines contribute flavours such as fruits (banana, strawberry and green apple), perfumes and flowers, and compounds with the more pungent attributes of blue cheese, rancid cheese and fusel. High concentrations of flavour or aroma compounds in wine result in a greater sensory impact but may also lead to the masking of less obvious flavours and aromas. Conversely, lowering the level of a particular compound may result in the unmasking of other flavours and aromas within the wine (Saison et al. 2009).
It is important to note that the number of differences in fermentation products between hybrid-made wine and S. cerevisiae-made wine will be a conservative estimate, as the fermentation product analysis targeted only compounds that have previously been identified as important contributors to flavour and aroma in wines, wines typically produced by a single industrial S. cerevisiae strain (Siebert et al. 2005). Thus, there may be other important flavour and aroma compounds produced by the input of the non-S. cerevisiae genome component of the hybrid strains that were not considered in this study.
Metabolite differences between hybrid and parental strain(s) were identified in both model medium and grape juice fermentations. These differences in metabolite levels may be the direct result of polyploidy (Hull-Sanders et al. 2009); the additive effect of an extra genome; synergistic genetic interactions (Mani et al. 2008); heterosis, whereby the hybrid displays superior growth and yield over both parents (Lippman and Zamir 2006); or differences in gene expression. Differences in gene expression could be explained by the observations that divergence of transcription factor binding sites across the Saccharomyces species far exceeds the interspecies variation in orthologous genes (Borneman et al. 2007); alterations in transcription factor binding within the hybrid genome could lead to differences in gene regulation effecting metabolite production. All, or any, of the above genomic effects would potentially contribute to the novel wine flavour and aroma profiles produced by interspecific wine yeast hybrids.
Performance of interspecific wine yeast hybrids in an industrial setting
Informal blind tastings on wines made using interspecific wine yeast hybrids described in this manuscript concluded that the hybrid yeast wines were more complex, with a wider range of flavour and aroma attributes (results not shown). The above hybrids have since been used to produce award winning wines and are now available commercially having been adopted by winemakers internationally.
In conclusion, this manuscript describes a new strategy for developing wines with greater complexity. By combining the genomes of a commercial S. cerevisiae wine yeast strain and other Saccharomyces sensu stricto yeast, we have successfully bred new commercial wine yeast strains capable of producing novel wine aroma and flavour profiles. These new hybrid yeasts can assist winemakers in their search for tools that introduce flavour and aroma diversity to their wines.
References
Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994) Current protocols in molecular biology. Wiley, New York
Belloch C, Pérez-Torrado R, González S, Pérez-Ortín J, Garcia-Martinez J, Querol A, Barrio E (2009) The chimeric genomes of natural hybrids of Saccharomyces cerevisiae and Saccharomyces kudriavzevii. Appl Environ Microbiol 75:2534–2544
Borneman AR, Gianoulis TA, Zhang ZD, Yu H, Rozowaky J, Seringhaus MR, Wang LY, Gerstein M, Snyder M (2007) Divergence of transcription factor binding sites across related yeast species. Science 317:815–819
Bradbury J, Richards K, Niederer H, Soon L, Dunbar R, Gardner R (2006) A homozygous diploid subset of commercial wine yeast strains. Antonie Leeuwenhoek 89:27–37
de Barros Lopes M, Soden A, Henschke P, Langridge P (1996) PCR differentiation of commercial yeast strains using intron splice site primers. Appl Environ Microbiol 62:4514–4520
Dunn B, Sherlock G (2008) Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res 18:1610–1623
Eglinton JM, Heinrich AJ, Pollnitz AP, Langridge P, Henschke PA, de Barros Lopes M (2002) Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALDP6 aldehyde dehydrogenase gene. Yeast 19:295–301
Esteve-Zarzosa B, Belloch C, Uruburu F, Querol A (1999) Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int J Syst Bacteriol 49:329–337
Fleet GH, Heard GM (1993) The growth of yeasts during wine fermentations. In: Fleet GH (ed) Wine microbiology and biotechnology. Harwood Academic, Chur, pp 27–54
Gonzalez S, Barrio E, Gafner J, Querol A (2006) Natural hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus and Saccharomyces kudriavzevii in wine fermentations. FEMS Yeast Res 6:1221–1234
Groth C, Hansen J, Piskur J (1999) A natural chimeric yeast containing genetic material from three species. Int J Syst Bacteriol 49:1933–1938
Gunge N, Nakatomi Y (1972) Genetic mechanisms of rare mating of Saccharomyces cerevisiae heterozygous for mating type. Genetics 70:41–58
Heard GM, Fleet GH (1987) Occurrence and growth of killer yeasts during wine fermentation. Appl Environ Microbiol 53:2171–2174
Howell KS, Bartowsky EJ, Fleet GH, Henschke PA (2004) Microsatellite PCR profiling of Saccharomyces cerevisiae strains during wine fermentation. Lett Appl Microbiol 38:315–320
Hull-Sanders HM, Johnson RH, Owen HA, Meyer GA (2009) Effects of polyploidy on secondary chemistry, physiology, and performance of native and invasive genotypes of Solidago gigantae (Asteraceae). Am J Bot 96:762–770
Hunter N, Chambers SR, Louis EJ, Borts RH (1996) The mismatch repair system contributes to meiotic sterility in an interspecific yeast hybrid. EMBO J 15:1726–1733
Kashkush K, Feldman M, Levy AA (2002) Gene loss, silencing and activation in a newly synthesised wheat allotetraploid. Genetics 160:1651–1659
King ES, Swiegers JH, Travis B, Francis IL, Bastian SEP, Pretorius IS (2008) Coinoculated fermentations using Saccharomyces yeast affect the volatile composition and sensory properties of Vitis vinifera L. cv. Sauvignon Blanc wines. J Agric Chem 56:10829–10837
Lattey KA, Bramley BR, Francis IL, Herderich MJ, Pretorius IS (2007) Wine quality and consumer preferences: understanding consumer needs. Aust New Zealand Wine Ind J 22:31–39
Lippman ZB, Zamir D (2006) Heterosis; revisiting the magic. Trends Genet 33:60–66
Mani R, St Onge RP, Hartman JL IV, Giaever G, Roth FP (2008) Defining genetic interaction. Proc Natl Acad Sci USA 105:3461–3466
Marinomi G, Manuel M, Peterson AF, Hvidtfeldt J, Sulo P, Piskur J (1999) Horizontal transfer of genetic material among Saccharomyces yeasts. J Bacteriol 181:6488–6496
Masneuf I, Hansen J, Groth C, Piskur J, Dubourdieu D (1998) New hybrids between Saccharomyces sensu stricto species found among wine and cider production strains. Appl Environ Microbiol 64:3887–3892
Naumov G (1987) Genetic basis for classification and identification of the ascomyceteous yeasts. Stud Mycol 30:469–475
Naumov GI, James SA, Naumova ES, Louis EJ, Roberts IN (2000) Three new species in the Saccharomyces sensu stricto complex: Saccharomyces cariocanus, Saccharomyces kudriavzevii and Saccharomyces mikatae. Int J Syst Evol Microbiol 50:1931–1942
Naumov GI, Naumova ES, Masneuf-Pomarède I (2010) Genetic identification of new biological species Saccharomyces arboricolus Wang et Bai. Antonie Leeuwenhoek 98:1–7
Ness F, Lavallee F, Dubourdieu D, Aigle M, Dulau L (1993) Identification of yeast strains using the polymerase chain reaction. J Sci Food Agric 62:89–94
Novo M, Bigey F, Beyne E, Galeote V, Gavory F, Mallet S, Cambon B, Legras J, Wincher P, Casaregola S, Dequin S (2009) Eukaryote-to-eukaryote gene transfer events revealed by the genome sequence of the wine yeast Saccharomyces cerevisiae EC1118. Proc Natl Acad Sci USA 106:16333–16338
Perez-Navzdo F, Albergaria H, Hogg T (2006) Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int J Food Microbiol 108(3):336–345
Pretorius IS (2000) Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16:675–729
Saison D, De Schutter DP, Uyttenhove B, Delvaux F, Delvaux FR (2009) Contribution of staling compounds to the aged flavor of lager beer by studying their flavor thresholds. Food Chem 114:1206–1215
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, New York
Sherman F, Fink G, Hicks J (1986) Laboratory course manual for methods in yeast genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor
Siebert TE, Smyth HE, Capone DL, Neuwöhner C, Pardon KH, Skouroumounis GK, Herderich MJ, Sefton MA, Pollnitz AP (2005) Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal Bioanal Chem 381:937–947
Soden A, Francis IL, Oakey H, Henschke PA (2000) Effects of co-fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of Chardonnay wine. Aust J Grape Wine Res 6:21–30
Song K, Lu P, Tang K, Osborn TC (1995) Rapid genome change in synthetic polyploids of Brassica and its implications for polyploidy evolution. Proc Natl Acad Sci USA 92:7719–7723
Spencer JFT, Spencer DM (1996) Rare-mating and cytoduction in Saccharomyces cerevisiae. In: Evans I (ed) Methods in molecular biology, vol 53. Humana, Totowa, pp 39–44
Vaughan-Martini A, Martini A (1987) Three new delimited species of Saccharomyces sensu stricto. Antonie Leeuwenhoek 53:77–84
Winge O, Lausten O (1938) Artificial species hybridization in yeast. Comp Rend Trav Lab Carslberg Sér Physiol 22:235–244
Acknowledgements
This work was financially supported by Australia’s grapegrowers and winemakers through their investment body the Grape and Wine Research Development Corporation, with matching funds from the Australian Government. The AWRI is part of the Wine Innovation Cluster.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bellon, J.R., Eglinton, J.M., Siebert, T.E. et al. Newly generated interspecific wine yeast hybrids introduce flavour and aroma diversity to wines. Appl Microbiol Biotechnol 91, 603–612 (2011). https://doi.org/10.1007/s00253-011-3294-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3294-3