Abstract
To remain competitive in increasingly overcrowded markets, yeast strain development programmes are crucial for fermentation-based food and beverage industries. In a winemaking context, there are many yeast phenotypes that stand to be improved. For example, winemakers endeavouring to produce sweet dessert wines wrestle with fermentation challenges particular to fermenting high-sugar juices, which can lead to elevated volatile acidity levels and extended fermentation times. In the current study, we used natural yeast breeding techniques to generate Saccharomyces spp. interspecific hybrids as a non-genetically modified (GM) strategy to introduce targeted improvements in important, wine-relevant traits. The hybrids were generated by mating a robust wine strain of Saccharomyces cerevisiae with a wine isolate of Saccharomyces bayanus, a species previously reported to produce wines with low concentrations of acetic acid. Two hybrids generated from the cross showed robust fermentation properties in high-sugar grape juice and produced botrytised Riesling wines with much lower concentrations of acetic acid relative to the industrial wine yeast parent. The hybrids also displayed suitability for icewine production when bench-marked against an industry standard icewine yeast, by delivering icewines with lower levels of acetic acid. Additionally, the hybrid yeast produced wines with novel aroma and flavour profiles and established that choice of yeast strain impacts on wine colour. These new hybrid yeasts display the desired targeted fermentation phenotypes from both parents, robust fermentation in high-sugar juice and the production of wines with low volatile acidity, thus establishing their suitability for wine styles that are traditionally troubled by excessive volatile acidity levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Developing improved strains of yeast is crucial for fermentation industries in the food and beverage sectors. There are many yeast phenotypes that stand to be improved (e.g., stress tolerance) and others that could be introduced (e.g., novel metabolic pathways for desirable flavour production) into existing strains. Whilst genetic engineering approaches provide a means of achieving this for a wide range of phenotypes, with the potential to deliver precise genetic changes and optimal quality assurance, there is reluctance by consumers in some market segments to accept genetically modified organisms (GMOs) in the human food chain. Thus, traditional approaches and variations thereof remain the only options in most food and beverage industries.
Fortunately, there are many non-GMO approaches that can be used for industrial yeast strain development. In recent years, our laboratory has used interspecific hybridization as a non-GMO strategy to produce novel desirable phenotypes in wine yeast. The hybrids were generated by mating a wine strain of Saccharomyces cerevisiae with Saccharomyces paradoxus (Bellon et al. 2011) and Saccharomyces mikatae (Bellon et al. 2013). The Saccharomyces genus comprises several species of yeast that are evolutionarily closely related and have the same highly conserved mating system, enabling them to interbreed (see Morales and Dujon 2012 for a detailed review of interspecific hybridization in yeasts). Whilst the diploid progeny of Saccharomyces spp. interspecific hybrids is largely sterile, they can reproduce (and therefore grow) asexually (Naumov 1996). However, converting sterile diploid hybrids to allotetraploids restores fertility and allows the production of viable diploid spores (Greig et al. 2002). On the other hand, triploid interspecific hybrids have been shown to have poor spore viability (Sebastini et al. 2002), which is consistent with observations by Bellon et al. (2013) in which it was found that allotriploid interspecific hybrids were relatively stable.
The above hybrids were generated largely to see what was achievable in the context of their application to winemaking and to wine quality. For both hybrids, wine quality parameters were improved, but not in predictable ways. Building on this foundational work, the current study aimed to test interspecific hybridization as means of introducing targeted phenotypic changes to meet a particular challenge, namely the generation of a wine yeast strain capable of fermenting high-sugar must to make sweet dessert wines without excessive amounts of acetic acid or ethyl acetate.
Sweet dessert wines (e.g., icewine and botrytised wine) are made from grape juices with extremely high sugar content. When S. cerevisiae is in an environment with a high-sugar concentration, it produces increased levels of glycerol as a compatible solute. This process utilises NADH, which has to be regenerated to maintain redox balance. This is largely achieved through oxidation of acetaldehyde, leading to the production of acetic acid (Blomberg and Adler 1989).
Whilst acetic acid concentrations in Canadian commercial icewine range from 0.49 to 2.29 g/L (Nurgel et al. 2004), well below the sensory threshold of 3.185 g/L (Cliff and Pickering 2006), the production of high acetic acid levels during fermentation can lead to the esterification of acetic acid by ethanol to form another volatile metabolite, ethyl acetate, with a characteristic solvent or nail polish aroma. Nurgel et al. (2004) found that ethyl acetate concentrations in icewine ranged from 0.086 to 0.369 g/L, with some wines well above the sensory threshold of ethyl acetate of 0.198 g/L (Cliff and Pickering 2006).
In the case of botrytised wine, Botrytis cinerea not only concentrates sugar content in grapes, it also generates considerable amounts of acetic acid; as much as 1 g/L can be found in juice from infected grapes (Zoecklein et al. 1995a). Thus, there is a high level of acetic acid even before wine yeast begins fermentation.
In addition to the negative impacts of high osmolarity on acetic acid production, a high concentration of sugar also causes significant stress for yeast cells (Kontkanen et al. 2004) which can potentially lead to a suboptimal (stuck or sluggish) fermentation. When fermentation is compromised in this way, there is a disproportionate impact on the two major sugars in must. Grape juice contains approximately equal quantities of glucose and fructose. Uptake of these sugars by yeast is mediated by specific transporters, encoded by HXT genes. There are up to 20 HXT genes and these have varying substrate affinities (Wieczorke et al. 1999), but all have a higher affinity for glucose than fructose (Reifenberger et al. 1997). Consequently, as fermentation progresses, the ratio of fructose/glucose increases. At the same time, membrane transporters become compromised due to the increasing concentration of ethanol (Walker 1998). Extremely low glucose/fructose ratios can impact negatively on fermentation completion and result in slow or stuck fermentation (Gafner and Schütz 1996).
Unlike S. cerevisiae, Saccharomyces bayanus carries the FSY1 gene, which encodes an active transporter with high affinity for fructose (Rodrigues de Sousa et al. 2004), thus offering the potential to reduce the risk of a suboptimal fermentation associated with accumulation of this sugar. In addition, studies have shown that some strains of S. bayanus contribute less acetic acid to wines than S. cerevisiae (Castellari et al. 1994) whilst contributing more savoury wine sensory attributes such as ‘cooked orange peel’, ‘honey’, ‘yeasty’, ‘nutty’ and ‘aldehydic’ (Eglinton et al. 2000). All-in-all, this Saccharomyces yeast has a great deal to offer in the context of high-sugar wine fermentations.
However, whilst phenotypic studies of S. bayanus grape juice isolates have shown reasonable sugar tolerance, this species has poor ethanol tolerance compared to S. cerevisiae wine strains (Belloch et al. 2008), which limits its usefulness in industrial wine production. Nonetheless, the combined traits of S. bayanus and wine strains of S. cerevisiae suggest progeny of a cross involving these yeasts would have the potential to efficiently ferment high-sugar juice and produce quality wine. However, the genetic basis of desirable winemaking properties in industrial yeasts is largely unknown. In this context, heterozygosities in the wine yeast parent used in this study may contribute to a wide range of wine-related phenotypes. Spore-spore hybridisations between these species have been undertaken by researchers previously (see for example: Zambonelli et al. 1997; Rainieri et al. 1998) and the assortment of chromosomes during meiosis led to the resultant hybrids displaying a diverse range of fermentation traits. For this reason, our approach has been to use diploid S. cerevisiae wine yeast for hybridisation relying upon a rare mating type switching event to produce mating-competent diploid cells.
In the current study, interspecific wine yeast hybrids were generated by rare mating a commercial S. cerevisiae wine yeast with a S. bayanus grape juice isolate. (The S. bayanus parent of the hybrids generated for this study has been molecularly typed as S. bayanus var uvarum; a subgroup of the S. bayanus species. Recent studies in other laboratories indicate that this subgroup should constitute a separate species, Saccharomyces uvarum (Pérez-Través et al. 2014). At this time, however, there is not a single, agreed, classification so the authors have retained the existing name of S. bayanus.) Two progeny from the cross (AWRI 1571 and AWRI 1572) were investigated for their suitability to produce wines from high-sugar grape juices. The two strains were compared with their parents in a series of fermentations using Chardonnay juice with varying additional sugar supplementations and botrytised Riesling. Subsequently, the same two interspecific hybrid strains were assessed for icewine fermentation suitability by benchmarking against a S. cerevisiae industry standard yeast K1-V1116. Strains were evaluated for fermentation rates, sugar consumption patterns and production of ethanol, glycerol and acetic acid. Organic acid analyses were performed on the Chardonnay and botrytised Riesling wines, whilst ethyl acetate levels were quantified in botrytised Riesling and icewines. In addition, botrytised Riesling wines were analysed for targeted volatile flavour-active fermentation products and wine colour differences. Finally, in order to establish their potential for commercialisation, the hybrid strains were evaluated for genetic stability over 200 generations of mitotic growth.
Material and methods
Yeast strains
S. cerevisiae AWRI838 (an isolate of the commercial wine yeast strain EC1118), S. bayanus AWRI 1176 (isolated from fermenting grape juice) and standard commercial S. cerevisiae wine yeast K1-V1116 (supplied by Lallemand Inc. Montreal, QB, Canada) yeast strains were used. Control yeast strains for ploidy determinations using fluorescence flow cytometry analysis were BY4741 MAT a, haploid and BY4743, diploid, (Euroscarf®, Frankfurt, Germany) and 53-7 tetraploid (Salmon 1997). AWRI strains are available from the Australian Wine Research Institute Microorganism Culture Collection (WDCM 22).
Generation of interspecific hybrid yeast
Rare mating was used for interspecific hybridizations between the diploid wine yeast S. cerevisiae AWRI 838 and haploid spores of a wine isolate of S. bayanus as described previously (Bellon et al. 2011).
PCR confirmation of hybrids
PCR-RFLP analysis on genomic DNA (Ausubel et al. 1994) of the rDNA internal transcribed spacer using the restriction enzyme HaeIII (Esteve-Zarzoso et al. 1999) was undertaken to establish the presence of both parent species.
Genetic stability of interspecific hybrid isolates
To determine the genetic stability of interspecific hybrids over many rounds of mitotic growth, the hybrid strains were sub-cultured daily using nutritional liquid medium, YEPD (1 % w/v yeast extract, 2 % w/v peptone, 2 % w/v glucose) for 200 generations. Subsequently, 20 isolates from each hybrid were investigated using PCR-RFLP targeting each arm of the individual 16 chromosomes (Supplemental Table S1). Primer design and molecular analysis were performed as previously described (Bellon et al. 2013) using S. cerevisiae S288c and S. bayanus MCYC 623 sequences.
Fluorescence flow cytometry analysis to determine ploidy of interspecific hybrids
Ploidy analyses on newly formed hybrids were undertaken using the fluorescent dye propidium iodide as previously described (Bellon et al. 2013). Cells harvested following 200 generations of mitotic growth were analysed using a SYBR Green 1-based staining protocol which includes a protein removal step using 40 U/mL Proteinase K (Fortuna et al. 2001). SYBR Green 1-stained cells were detected at 530/30 nm (FL1) using BD FACSFlow™ (Becton Dickinson, Sydney, Australia) sheath fluid and fluorescence plotted to a linear scale. Twenty-five thousand cells per sample were analysed to obtain cell DNA intensities.
High-sugar Chardonnay fermentations
Fermentations were performed in filter sterilised Chardonnay juice: total sugars (glucose and fructose) 145 g/L, yeast assimilable nitrogen 269 mg/L, titratable acid 6.8 g/L, pH 3.01, acetic acid <0.05 g/L sourced from The Yalumba Wine Company (Angaston, South Australia) with the addition of 300 mg/L di-ammonium phosphate. High-sugar juices were prepared to 195, 250 and 355 g/L sugar levels by the addition of equal amounts of glucose and fructose.
All strains were initially grown in YEPD medium for 2 days and then acclimatised by 2 days growth in ½ X Chardonnay grape juice medium (diluted with sterile water), shaking (100 rpm), for 2 days, with the exception of the 355 g/L sugar fermentation that underwent a two-step acclimatisation over 4 days starting with a ¼ X Chardonnay grape juice medium for 2 days.
Triplicate 100 ml fermentations were carried out at 22 °C as described previously (Bellon et al. 2013) and sampled in duplicate for chemical analyses.
Botrytised Riesling fermentation
Riesling juice: total sugars (glucose and fructose) 315 g/L, yeast assimilable nitrogen 312 mg/L, titratable acid 10.5 g/L, pH 3.11 sourced from The Yalumba Wine Company (Angaston, South Australia), was filter sterilised, and fermentations were performed as previously described, using the two-step acclimitisation. Triplicate fermentations were sampled in duplicate for chemical analyses.
Wine chemical analysis
Concentrations of residual sugars (glucose and fructose), ethanol, glycerol, and acetic, succinic, lactic and citric acids, were determined by Agilent 1200 Series HPLC (Agilent, Melbourne, Australia) using a Bio-Rad HPX-87 column (Bio-Rad Laboratories, Sydney, Australia) (Nissen et al. 1997).
Targeted volatile fermentation products analysis
Stable isotope dilution combined with gas chromatography/mass spectroscopy (GC/MS) (Siebert et al. 2005) was used to analyse target compounds previously identified as important for wine flavour and aroma. Wine samples were prepared in two dilutions, 1/20 and 3/10, with model wine (11 % ethanol, 10 % potassium hydrogen tartrate, pH adjusted with tartaric acid to 3.1). Analysis was performed on an Agilent 7890A gas chromatograph equipped with Gerstel MPS2 multi-purpose sampler and coupled to an Agilent 5975C VL mass selective detector (Agilent, Melbourne, Australia). Instrument control and data analysis were performed with Agilent ChemStation software.
Wine colour
Wines were analysed spectrally to obtain the CIELab parameters L*, a*, b* (Bakker et al. 1986) by measuring the transmittance of the wine every 1 nm over the visible spectrum from 360 to 830 nm using a Varian Cary 300 spectrophotometer (Varian Australia, Melbourne, Australia) with a 10-mm quartz cuvette, a D65 illuminant and a 10° standard observer.
ΔEab values (colour difference) were determined by the Hunter-Scotfield equation (Damasceno et al. 2008)
Statistical analyses
A one-way analysis of variance (ANOVA) and Student’s t test (p < 0.05) were used to determine differences between wines produced by different yeast strains.
Icewine fermentation
Riesling icewine juice: sugars (glucose and fructose) 473 g/L, yeast assimilable nitrogen 359 mg/L, titratable acid 6.1 g/L, pH 3.48), was kindly provided by Niagara Vintage Harvesters Ltd (Virgil, ON, Canada) and filter sterilised. Three yeast strains (K1-V1116, AWRI 1571 and AWRI 1572) were inoculated from starter cultures in YEPD medium into ¼ X Riesling icewine juice (with the addition of 2 g/L di-ammonium phosphate) and grown aerobically at 25 °C with shaking at 130 rpm until cell density reached 2 × 108 cells per millilitre after which 25 ml were diluted with 25 ml of ½ X Riesling icewine juice, respectively, and held for 1 h without shaking at room temperature. A half volume (25 ml) of undiluted Riesling icewine juice was added into these 50-ml cultures, which were then held for 2 h without shaking at room temperature.
Following this acclimatisation procedure, each 75-ml starter cultures was inoculated into 425 ml of 1 X Riesling icewine juice to achieve a yeast inoculum rate of 1 × 107 cells per millitre in a final volume of 500 ml. Fermentations were carried out at 17 °C in triplicate and continued until the yeast stopped consuming sugar, signaled by no further change in sugar concentration for 3 days. Sugar concentration was measured by the Lane-Eynon method (Zoecklein et al. 1995b).
Yeast cell densities were determined by cell counting in a haemocytometer. Acetic acid, glycerol, ammonia nitrogen and primary amino nitrogen were determined using enzymatic kits (Megazyme International Ireland Ltd, Bray, Ireland). Ethanol and ethyl acetate were measured using gas chromatography (Agilent, Santa Clara, USA) using an Agilent 6890 system equipped with flame ionisation detector and DB Wax (30 m × 0.23 mm × 0.25 μm) column. The carrier gas was helium. For ethanol measurement, samples were diluted 10-fold and 1.0 μl was injected into the injection port heated to 225 °C. The column head pressure was set as 24.4 psig, and the flow rate of helium gas was 2.5 ml/min. The oven temperature was programmed to start at 60 °C, increase to 95 °C at 15 °C ml/min and then increase to 225 °C at 75 ml/min and hold for 1 min. The detector temperature was 225 °C, and 2 % 1-butanol was used as an internal standard. For ethyl acetate measurement, 1.0 μl of sample was injected and heated to 230 °C. The column head pressure was 15.4 psig, and helium flow rate was 1.5 ml/min. The oven temperature was hold at 35 °C for 2 min, and then increased to 230 °C at 10 ml/min and hold for 2 min. The detector temperature was 230 °C, and 5 % 4-methyl-2-pentanol (2 g/L) was used as an internal standard.
Differences between variables were determined by XLSTAT statistical software package released by Addinsoft (Version 7.1; Paris, France). Analysis of variance (ANOVA) with mean separation by Fisher’s Least Significant Difference (LSD, p < 0.05) was used for statistical analysis.
Results
Generation and genetic stability of interspecific hybrids
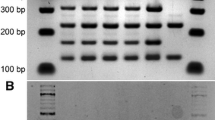
Two interspecific hybrid colonies (AWRI 1571 and AWRI 1572) were generated through the rare mating of the diploid S. cerevisiae wine yeast strain AWRI838 with spores of S. bayanus AWRI 1176. The existence of both parental genomes in these hybrids was confirmed by species-specific PCR-RFLP of target rDNA (Fig. 1).
Fluorescence flow cytometry analysis with linear plots of cell fluorescence was performed to determine ploidy levels of hybrid strains. Although cells were grown to late stationary phase, all cultures produced dual peaks of fluorescence and this could be attributed to some cells undergoing DNA synthesis, or perhaps more likely, it reflects cell pairs where mother-daughter cells have not yet completely separated. Diploid and tetraploid control strains gave non-dividing (G0/G1) fluorescent peaks in the region of double or quadruple fluorescent levels of the control haploid strain, respectively. Parental yeast strains, AWRI 838 and AWRI 1176, gave fluorescent peaks equivalent to the diploid control strain whilst both hybrid strains appear to have a triploid genome complement, with non-dividing peak levels approximately midway between diploid and tetraploid peaks (Fig. 2).
In addition, the genome of each hybrid strain was assessed for genetic stability after 200 mitotic generations. Species-specific PCR-RFLP markers to each arm of all 16 chromosomes revealed that the incipient hybrids were stable as no loss of chromosome from either parent species was identified in any of the twenty isolates from each hybrid investigated (Supplemental Data Fig. S1). Also, the hybrids remained stable triploids as no reduction in DNA fluorescence levels was observed in any 200-generation hybrid isolates (Supplemental data Fig. S2).
Chardonnay wines
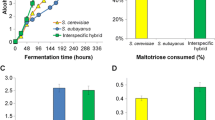
Four sets of replicate Chardonnay grape juice containing a range of reducing sugar concentrations were fermented with either a commercial S. cerevisiae strain AWRI 838, S. bayanus AWRI 1176 or their hybrid progeny AWRI 1571 or 1572. Analysis of the final wines revealed that all strains were capable of completing fermentation at the lowest sugar concentration of 145 mg/L and all were challenged, to varying degrees, by juices with 355 g/L reducing sugar (Fig. 3a, b).
Generally, the least robust yeast was the bayanus strain AWRI 1176, in most cases producing wines with higher levels of residual glucose (Fig. 3a) and fructose (Fig. 3b). AWRI1176 wines made from juice with 355 g/L had not only higher fructose concentrations, but more than double the concentration of residual glucose than wines made by S. cerevisiae AWRI 838 (57 g/L compared to 26 g/L). In contrast, hybrid strains AWRI 1571 and AWRI 1572 produced wines with the lowest residual sugars in all Chardonnay juices, but most notable in 355 g/L sugar Chardonnay juice (17.4 and 19.5 g/L glucose compared to 25.9 g/L produced by AWRI 838, and 65 and 67 g/L fructose compared to 78 g/L produced by AWRI 838).
From Fig. 3c, it is evident that at lower levels of reducing sugar (145 and 195 g/L), S. cerevisiae AWRI 838 produced wines with more than double the amount of acetic acid than S. bayanus AWRI 1176 and hybrids AWRI 1571 and 1572. However, whilst S. cerevisiae AWRI 838 still produced almost double the amount of acetic acid relative to the hybrid strains in fermentations with higher concentrations of reducing sugar (0.38 g/L compared to 0.15 g/L for hybrid strains in 250 g/L reducing sugar and 1.11 g/L compared to 0.65 g/L for hybrid strains in 355 g/L reducing sugar), S. bayanus AWRI 1176 produced wines with excessively high concentrations of acetic acid, 0.61 and 1.42 g/L in 250 g/L reducing sugar and 355 g/L reducing sugar, respectively.
Glycerol content in final wines is shown in Fig. 3d. Whereas S. bayanus AWRI 1176 generally produced wines with glycerol concentration 2 g/L higher than S. cerevisiae AWRI 838 in each juice, at lower concentrations of reducing sugar the hybrid strains produced wines with glycerol levels similar to their S. cerevisiae parent, AWRI 838, but in 355 g/ L reducing sugar the production of glycerol by the hybrid strains increased dramatically to levels higher than their S. bayanus parent, reaching 17.8 and 15.7 g/L for AWRI 1571 and 1572, respectively, compared to 14.8 g/L for AWRI 1176 and 12.7 g/L for AWRI 838.
Little difference was observed in ethanol production between S. cerevisiae AWRI 838 and hybrid strains AWRI 1571 and 1572 (Fig. 3e). S. bayanus AWRI 1176 made wine with reduced concentrations of ethanol in each juice, particularly in 250 and 355 g/L reducing sugar, but this result is attributable to the much higher residual sugar levels in the final wines.
Measurements of cell growth (optical density) and sugar utilisation (refractive index) during all Chardonnay fermentations are provided in supplementary data (Supplemental data Fig. S3 and S4 respectively). S. cerevisiae strain AWRI 838 showed the most robust fermentation properties of all strains, evident for cell growth and final cell density following fermentation of the juice with the highest sugar concentration (Supplemental data Fig. S3d). Although little difference in sugar utilisation was observed between strains at lower concentrations of juice sugar, S. bayanus strain AWRI 1176 displayed a slower rate of sugar utilisation at higher juice sugar levels (Supplemental data Fig. S4c and S4d).
Botrytised Riesling wines
All four yeast strains started well in botrytised Riesling juice. However, after day 2, S. bayanus strain AWRI 1176 grew at a slower rate than the other three strains, which had similar increases in cell densities (Fig. 4a) and sugar utilisation profiles (Fig. 4b). The lower cell density of AWRI 1176 was reflected in a reduced level of sugar utilisation during fermentation with the finished wine having higher residual sugars, glucose 69 g/L and fructose 139 g/L compared to glucose concentrations of 47–52 g/L and fructose concentrations of 119–124 g/L in S. cerevisiae and hybrid-made wines (Table 1).
AWRI 1176 also produced wines with a lower ethanol concentration, 9.5 % v/v compared to 12.1–12.5 % v/v for S. cerevisiae and hybrid-made wines. All strains produced wines with similar levels of glycerol (23.4 to 24.5 g/L) and little difference was observed in succinic, lactic and citric acids levels. However, acetic acid concentrations varied considerably between wines (Table 1), with AWRI 1176 (S. bayanus) and AWRI 838 (S. cerevisiae) producing considerably more than the hybrid strains AWRI 1571 and AWRI 1572, (1.1 and 0.9 g/L compared to 0.55 and 0.67 g/L, respectively).
Analysis of volatile fermentation products (Table 2) revealed that there were clear differences between the two ‘parental wines’ (there were significant differences for 13 of the 16 compounds analysed), and these differed from the hybrid-made wines. In general, hybrid AWRI 1571 produced wines with volatile fermentation product concentrations similar to that of the S. cerevisiae parent, including similar levels of ethyl acetate. Only two compounds followed solely the S. bayanus parental profile, 2-methyl propyl acetate (banana) and 2-methyl propanol (fusel), whilst two compounds were produced at an intermediate level, ethyl 3-methyl butanoate (berry) and 2-phenyl ethyl acetate. Ethyl 2-methyl butanoate (sweet fruit) was produced at a higher concentration than for either parent.
Wines produced by hybrid AWRI 1572 were less similar to S. cerevisiae parent-made wine, with only seven of the sixteen compounds analysed following the S. cerevisiae parent wine profile. Four compounds were produced in much lower concentrations than either parent, ethyl acetate (nail polish), ethyl 2-methyl propanoate (fruity), 2-methyl propyl acetate (banana) and 2-methyl propanol (fusel). Conversely, butanol (fusel) was produced in much higher concentrations than either parent, 721 μg/L compared to 630 μg/L for AWRI 838 (S. cerevisiae) and 445 μg/L AWRI 1176 (S. bayanus).
Wine colour was analysed spectrally by measuring absorbance across the visible range of the spectrum from 360 to 830 nm, using CIELab parameters, L* (a measure of intensity, the higher the value the lighter the colour), a* (positive values relate to redness, negative values to greenness) and b* (positive values relate to yellowness, negative values to blueness). Browness of wines is attributed to absorbance at 420 nm.
Botrytised Riesling wines made by S. bayanus AWRI 1176 had the strongest browness (A420 58.36) with S. cerevisiae AWRI 838 having the weakest (A420 42.91), whilst wines made by hybrids AWRI 1571 and 1572 showed an intermediate level of brownness (A420 53.09 and A420 48.77, respectively) (Table 3). The CIELab L* parameter revealed that the intensity of colour was in the reverse order to browness, AWRI 1176 wines being lightest and AWRI 838 wines being darkest. The hybrid-made wines also showed intermediate levels of red/green and yellow/blue hues, AWRI 838-produced wines with both higher a* values, (less negative value relates to less green colour) and higher b* values (more yellow) whilst AWRI 1176 produced wines with the greatest green colour and the least yellow colour. The obvious visual differences in colour intensity and hue between the wines was confirmed with calculated ΔEab values of 11.1 (AWRI 838/AWRI 1176), 8.6 (AWRI 838/AWRI 1571) and 6.0 (AWRI 838/AWRI 1572). Note that ΔEab >1 indicates that samples are just readily perceived visually as different to each other in colour.
Icewines
Riesling icewine fermentations were conducted with three yeast strains; commercial S. cerevisiae icewine standard yeast K1-V1116, and two S. cerevisiae x S. bayanus hybrid strains AWRI 1571 and AWRI 1572. Although growth appeared slower and reached a lower cell density for hybrid strains AWRI 1571 and 1572 relative to the commercial strain K1-V1116 (Supplemental data Fig. S5), all three strains showed similar rates of sugar consumption throughout fermentation (Fig. 5a) with final residual sugar values being virtually identical (ranging from 263 to 267 g/L) (Table 4)
Commercial wine yeast K1-V1116 showed the highest production of acetic acid during the fermentation (Fig. 5b) with a final wine concentration of 2.13 g/L, whilst hybrid strains AWRI 1571 and 1572 produced significantly lower levels at 1.72 and 1.57 g/L, respectively (Table 4).
Interestingly, analysis of ethanol, glycerol and ethyl acetate concentrations of wines made by standard yeast K1-V1116 and wines made by hybrids AWRI 1571 and AWRI 1572 showed no significant differences with average concentrations of 10.5 % v/w ethanol, 10.1 g/L glycerol and 73.7 mg/L ethyl acetate (Table 4).
Discussion
Microbial strain development for the food and beverage sector is hindered by consumer reluctance to accept genetically modified organisms (GMOs) in the human food chain. This has led researchers to return to traditional approaches including targeted breeding. Whilst such strategies lack the precision of genetically modified (GM) techniques and quality assurance is more of a challenge, there are many non-GMO methods available, particularly when working with highly tractable microbes such as Saccharomyces spp.
Building on prior research and development that tested the feasibility of using interspecific breeding of yeasts in the Saccharomyces genus to generate novel phenotypes for application in winemaking (Bellon et al. 2011, 2013), the current manuscript describes a proof of concept trial to generate interspecific Saccharomyces spp. hybrids to introduce targeted improvements in important, wine-relevant traits. Specifically, the aim was to generate novel wine yeast that can tolerate the many challenges of growing in and fermenting high-sugar grape juice without generating excessive volatile acidity in the form of acetic acid and ethyl acetate.
Winemakers endeavouring to produce sweet dessert wines from high-sugar juices require wine yeasts that can conduct efficient fermentation in reasonable time whilst reaching a target ethanol concentration (10 and 13 % v/v ethanol for Canadian icewine and French Sauternes, respectively, for instance). However, fermentations involving high-sugar juices commonly suffer from elevated volatile acidity levels and extended fermentation times.
S. bayanus has been reported to produce reduced levels of volatile acidity in wine fermentations (Eglinton et al. 2000), and has some potential advantages in dealing one of the problems associated with suboptimal (slow or sluggish) fermentation, namely the production of heightened ratios of fructose/glucose in the latter stage of fermentation. This becomes particularly pronounced in suboptimal fermentations and is due to S. cerevisiae lacking a high affinity fructose transporter. An active fructose-specific transport system (FSY1) has been identified in S. bayanus (Rodrigues de Sousa et al. 2004),
Whilst S. bayanus may seem like a good option to trial in high-sugar fermentations, it is not sufficiently robust to deal with the harsh conditions of grape must fermentation; it is highly affected by high glucose content and high ethanol content in culture media (Belloch et al. 2008). However, it’s potential when mated with a robust S. cerevisiae wine yeast is clearly worth exploring.
In the current study, laboratory-scale fermentations in high-sugar juice (Chardonnay with sugar additions, botrytised Riesling and Riesling icewine) were used to investigate the suitability of two S. cerevisiae × S. bayanus hybrid strains (AWRI 1571 and AWRI 1572) for high-sugar grape juice wine production. The strategy of rare mating (where a diploid cell becomes homozygous for mating type and can mate without sporulation) was adopted to ensure that no loss of important fermentation traits from the wine yeast parent would occur due to the assortment of chromosomes that would occur if it was sporulated (Zambonelli et al. 1997). Previous reports have identified stable natural allotriploid yeast from the wine industry including the wine yeast VIN7 (Borneman et al. 2012) and the spoilage yeast Brettanomyces bruxellensis (Curtin et al. 2012).
The putative hybrids from successful rare mating events between a diploid S. cerevisiae commercial wine yeast AWRI 838 and haploid spores of a S. bayanus Australian grape juice isolate, AWRI 1176, were confirmed using PCR-RFLP analysis of the ITS region within the rDNA tandem repeat on chromosome XII. Fluorescence flow cytometry analysis showed that hybrid ploidy content was consistent with fluorescent levels of a triploid genome with peaks midway between that of the diploid and tetraploid genome control strains. Genome instability and ploidy reduction have previously been reported in S. cerevisiae polyploids (Mayer and Aguilera 1990) and Saccharomyces interspecific hybrids (Kunicka-Styczyńska and Rajkowska 2011; Kumaran et al. 2013). Thus it was important to evaluate stability of the hybrids generated for the current study. Thirty-two species-specific genetic markers were designed to monitor the presence of each arm of every parental chromosome. None of these markers were lost from either hybrid after two hundred mitotic generations. Furthermore, fluorescence flow cytometry analyses confirmed that each hybrid remained triploid. In addition, it is important to note that wine yeast is not re-pitched as in the brewing industry. Instead, inocula are generated fresh from stock cultures each vintage and go through no more than 8–10 generations during a wine fermentation. Thus, it is unlikely that genomic instability will prove to be an issue in the application of the yeast strains generated in the current study.
An assessment of fermentation ability showed that, generally, the interspecific hybrids fermented the various high-sugar juices at least as well as the S. cerevisiae wine yeast parent, with similar levels of residual sugar, similar growth curves and similar rates of sugar utilisation. In contrast, and as expected, the S. bayanus parent performed poorly except at the lowest concentrations of sugar in the Chardonnay juice fermentations. In addition, the hybrid yeasts wines had reduced levels of volatile acidity for all three musts.
Other wine quality parameters (glycerol, ethanol, succinate, etc. concentrations) were favourable, largely being similar to AWRI 838 wine yeast or falling somewhere between the two parents. In the Chardonnay juice fermentations at high-sugar levels, the two hybrid strains outperformed the S. cerevisiae wine yeast in sugar utilisation and were similar to this wine yeast in all other respects apart from acetic acid and glycerol production. Acetic acid levels for both hybrids were about 60 % of what was found in the S. cerevisiae-made wines and glycerol levels were slightly greater. Interestingly, whilst the S. bayanus parent, as expected, produced wines with lower levels of acetic acid compared to the S. cerevisiae parent at lower sugar levels, this was reversed in juices with 250 g/L and above. Whilst the reason for this is unknown, the phenotype did not carry over into the hybrids.
Botrytised Riesling wines produced by hybrid yeast strains showed differences in concentrations in a number of the volatile secondary metabolites relative to wines made by S. cerevisiae parent AWRI 838. Both hybrids produced acetic acid at levels at about 65 % of the S. cerevisiae parent but in the case of ethyl acetate only AWRI 1572 produced significantly lower levels. Nonetheless, ethyl acetate has been shown to have a suppressive effect on the formation of other fruity-aroma compounds, even at concentrations below the sensory threshold for this compound (Etiévant 1991). In addition, ethyl acetate levels below the sensory threshold can impart an added richness and sweetness, whereas levels above convey a characteristic solvent or nail polish remover aroma. Importantly, both hybrids produced less volatile acidity overall than the S. cerevisiae parent.
Desirable (from a winemaking perspective) flavour-active compounds were produced in higher concentrations by one or both hybrid strains relative to their S. cerevisiae wine yeast parent; ethyl 2-methyl butanoate (‘sweet fruit’) and 2-phenyl ethyl acetate (‘floral’). Increasing the concentration of a flavour or aroma compound can lead to an increased sensory impact of that particular compound, and can also lead to the masking of other (potentially non-desirable) flavours or aromas (Saison et al. 2009).
Production of volatiles in the hybrids was moderated relative to the S. cerevisiae parent: a number of metabolites were produced in mid-range concentrations by both hybrids relative to the parental strains. Generally, wine made by hybrid AWRI 1571 followed the highest metabolite concentration produced by S. cerevisiae parent AWRI 838 more often than hybrid AWRI 1572, although, on a small number of occasions, metabolites were produced at lower concentrations similar to S. bayanus parent yeast AWRI 1176. Interestingly, transgressive phenotypes were more apparent in hybrid AWRI 1572 than hybrid AWRI 1571, in the form of both increased and decreased concentrations in a number of secondary metabolites. A positive aspect to the production of lower metabolite concentrations is that this hybrid yeast produced much lower concentrations of two compounds with negative sensory attributes, ethyl acetate (‘nail polish’) and 2-methyl propanol (‘fusel’).
It is important to note that differences between the phenoypes of the two hybrids are not unexpected. Whilst they have the same S. cerevisiae genomic inputs, the S. bayanus parent was sporulated prior to mating to produce haploids. The meiotic events in this process would have generated genetic variants with differing phenotypic traits (Zambonelli et al. 1997).
Colour is one of the first wine sensory properties evaluated in the glass and invariably one of the first descriptors used in assessing a botrytised wine, with colour terms ranging from ‘glowing yellow-green’ to ‘pale gold’, ‘deep gold’ and ‘amber’. The easily visually discernible colour differences between the botrytised wines were confirmed by high ΔE*ab values recorded for each assessment of S. cerevisiae wines relative to S. bayanus and hybrid wines; 11.1 (S. cerevisiae AWRI 838/S. bayanus AWRI 1176), 8.6 (S. cerevisiae AWRI 838/hybrid AWRI 1571) and 6.0 (S. cerevisiae AWRI 838/hybrid AWRI 1572).
B. cinerea-infected vines have a grey, powdery appearance with the berries developing a light brown colour in white cultivars, resulting in a distinctly brown-coloured grape juice. B. cinerea produces a powerful oxidative enzyme (laccase) that can oxidise hydroxycinnamic acids (grape juice phenolic compounds caftaric and coutaric acid) to caftaric acid o-quinone. Condensation reactions of cafteric acid o-quinone generate brown polymeric pigments (Salgues et al. 1986). Glutathione can interfere with this process by trapping caftaric acid quinones in the form of 2-S-glutathionyl caftaric acid, also referred to as grape reaction product (GRP). The formation of GRP is believed to limit juice browning (Cheynier et al. 1986). As all botrytised Riesling fermentations were conducted in the same juice and under the same conditions, the differences in wine colour can be attributed to differences in yeast metabolism between the different strains. Studies have shown that the ratio of grape hydroxycinnamic acids to glutathione molar ratio alone does not correlate well with oxidative browning and that the presence of other compounds capable of trapping free o-quinones may be involved (Cheynier et al. 1990). Both hybrid strains produced wine colour attributes intermediate to the individual parent-made wines, indicating that genetic material inherited from both parents impact on yeast fermentation metabolites involved in the development of wine colour. Descriptor marketing plays an important role for wine companies wanting an edge in tight economical times and yeast that can deliver colour variations to the commonly industry-used S. cerevisiae strains could assist winemakers in developing novel wine styles.
The hybrid strains also displayed suitability for icewine production. Whilst the hybrid strains consumed equivalent amounts of sugar as S. cerevisiae industry standard yeast K1-V1116 during fermentation, their wines contained acetic acid levels of approximately 75 % relative to the S. cerevisiae-made wine. No difference in ethanol, glycerol and ethyl acetate concentrations were seen between hybrid-made wines and S. cerevisiae-made wine. Growth curves for the three strains in icewine indicate that the hybrids had a lower growth rate and reached a lower final cell number than K1-V1116. This however appears not to have impacted on their fermentation performance.
In conclusion, this manuscript demonstrates that interspecific hybridization can be used in rational wine yeast development to introduce targeted phenotypic outcomes. The introduction of genetic material from non-cerevisiae Saccharomyces species to traditional S. cerevisiae wine yeast can impact positively on wine yeast metabolite production during fermentation of high-sugar musts to deliver wines with low volatile acidity. Novel interspecific hybrids generated from a cross between a robust S. cerevisiae wine yeast and a S. bayanus grape juice isolate produce botrytised Riesling wines with much lower concentrations of acetic acid relative to the industrial wine yeast parent and lower levels of acetic acid when benchmarked against an industry standard icewine yeast. Additionally, the hybrid yeast produce wines with novel aroma and flavour profiles and establish that yeast strain choice can impact on wine colour. These new wine yeast provide an opportunity for winemakers wishing to minimise acetic acid levels in wine styles that are traditionally fraught with volatile acidity issues.
This study was performed at laboratory-scale as ‘proof of concept’. Future industry-scale winemaking trials will determine the commercial potential of these strains for application in the wine industry.
References
Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994) Current protocols in molecular biology. Wiley, New York
Bakker J, Bridle P, Timberlake CF (1986) Tristimulus measurements (CIELAB 76) of port wine colour. Vitis 25:67–78
Belloch C, Orlic S, Barrio E, Querol A (2008) Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int J Food Microbiol 122:188–195
Bellon JR, Eglinton JM, Siebert TE, Pollnitz AP, Rose L, de Barros LM, Chambers PJ (2011) Newly generated interspecific wine yeast hybrids introduce flavour and aroma diversity to wines. Appl Microbiol Biotechnol 91:603–612
Bellon JR, Schmid F, Capone DL, Chambers PJ (2013) Introducing a new breed of wine yeast: interspecific hybridization between a commercial Saccharomyces cerevisiae wine yeast and Saccharomyces mikatae. PLoS ONE 8(4):e62053. doi:10.1371/journal.pone0062053
Blomberg A, Adler L (1989) Roles of glycerol and glycerol-3-phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae. J Bacteriol 171:1087–1092
Borneman AR, Desany BA, Riches R, Affourtit JP, Forgan AH, Pretorius IS, Egholm M, Chambers PJ (2012) The genome sequence of the wine yeast Vin7 reveals an allotriploid genome with Saccharomyces cerevisiae and Saccharomyces kudriavzevii origins. FEMS Yeast Res 12:88–96
Castellari L, Ferruzzi M, Magrini A, Giudici P, Passarelli P, Zambonelli C (1994) Unbalanced wine fermentation by cryotolerant vs. non-cryotolerant Saccharomyces strains. Vitis 33:49–52
Cheynier V, Trousdale E, Singleton VL, Salgues M, Wdlde R (1986) Characterisation of 2-S-glutathionyl caftaric acid and its hydrolysis in relation to grape wines. J Agric Food Chem 34:217–221
Cheynier V, Rigaud J, Souquet J-M, Duprat F, Moutounet M (1990) Must browning in relation to the behavior of phenolic compounds during oxidation. Am J Enol Vitic 41:346–349
Cliff MA, Pickering GJ (2006) Determination of odour detection thresholds for acetic acid and ethyl acetate in ice wine. J Wine Res 17:45–52
Curtin CD, Borneman AR, Chambers PC, Pretorius IS (2012) De-novo assembly and analysis of the heterozygous triploid genome of the wine spoilage yeast Dekkera bruxellensis AWRI 1499. PLoS ONE 7(3):e33840. doi:10.1371/journal.pone0033840
Damasceno LF, Fernandes FA, Magalhães MM, Brito ES (2008) Non-enzymatic browning in clarified apple juice during thermal treatment: kinetics and process control. Food Chem 106:172–179
Eglinton JM, McWilliam SJ, Fogarty MW, Francis IL, Kwiatkowski MJ, Høj PB, Henschke PA (2000) The effect of Saccharomyces bayanus-mediated fermentation on the chemical composition and aroma profile of Chardonnay wine. Aust J Grape Wine Res 6:190–196
Esteve-Zarzoso B, Belloch C, Uruburu F, Querol A (1999) Identification of yeasts by Saccharomyces cerevisiae heterozygous for mating type. Genetics 70:41–58
Etiévant PX (1991) Wine. In: Maarse H (ed) Volatile compounds in food. Food Science and Technology. Marcel Dekker Inc, New York, pp 362–371
Fortuna M, Sousa MJ, Corte-Real M, Leao C, Salvador A, Sansonetty F (2001) Cell cycle analysis of yeasts. Curr Protocol Cytom 11.13.1–11.13.9
Gafner J, Schütz M (1996) Impact of glucose-fructose-ratio on stuck fermentations; practical experiences to restart stuck fermentations. Vitic Enol Sci 51:214–218
Greig D, Borts RH, Louis EJ, Travisano M (2002) Epistasis and hybrid sterility in Saccharomyces. Proc R Soc Lond 269:1167–1171
Kontkanen D, Inglis D, Pickering G, Reynolds A (2004) Effect of yeast inoculation rate, acclimatization, and nutrient addition on Icewine fermentation. Am J Enol Vitic 55:363–370
Kumaran R, Yang SY, Leu JY (2013) Characterization of chromosome stability in diploid, polyploidy and hybrid yeast cells. PLoS One 8:e68094
Kunicka-Styczyńska A, Rajkowska K (2011) Physiological and genetic stability of hybrids of industrial wine yeasts Saccharomyces sensu stricto complex. J Appl Microbiol 110:1538–1549
Mayer VW, Aguilera A (1990) High levels of chromosome instability in polyploids of Saccharomyces cerevisiae. Mutat Res 231:177–186
Morales L, Dujon B (2012) Evolutionary role of interspecies hybridization and genetic exchanges in yeasts. Microbiol Mol Biol Rev 76:721–739
Naumov GI (1996) Genetic identification of biological species in the Saccharomyces sensu stricto complex. J Ind Microbiol 17:295–302
Nissen TL, Schulze U, Nielsen J, Villadsen J (1997) Flux distributions in anaerobic, glucose-limited cultures of Saccharomyces cerevisiae. Microbiology 143:203–218
Nurgel C, Pickering GJ, Inglis DL (2004) Sensory and chemical characteristics of Canadian ice wines. J Sci Food Agric 84:1675–1684
Pérez-Través L, Lopes CA, Querol A, Barrio E (2014) On the complexity of the Saccharomyces bayanus taxon: hybridisation and potential hybrid speciation. PLoS One 9:e93729
Rainieri S, Zambonelli C, Giudici P, Castellari L (1998) Characterisation of thermotolerant Saccharomyces cerevisiae hybrids. Biotechnol Lett 20:543–547
Reifenberger E, Boles E, Ciracy M (1997) Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem 245:324–333
Rodrigues de Sousa H, Spencer-Martins I, Gonçalves P (2004) Differential regulation by glucose and fructose of a gene encoding a specific fructose/H+ symporter in Saccharomyces sensu stricto yeasts. Yeast 21:519–530
Saison D, De Schutter DP, Uyttenhove B, Delvaux F, Delvaux FR (2009) Contribution of staling compounds to the aged flavor of lager beer by studying their flavor thresholds. Food Chem 114:1206–1215
Salgues M, Cheynier V, Gunata Z, Wylde R (1986) Oxidation of grape juice 2-S-glutathionyl caffeoyl tartaric acid by Botrytis cinerea laccase and characterization of a new substance: 2,5-di-S-glutathionyl caffeoyl tartaric acid. J Food Sci 51:1191–1194
Salmon JM (1997) Enological fermentation kinetics of an isogenic ploidy series derived from an industrial Saccharomyces cerevisiae strain. J Ferment Bioeng 83:253–260
Sebastini F, Barberio Casalone E, Cavalieri D, Polsinelli M (2002) Crosses between Saccharomyces cerevisiae and Saccharomyces bayanus generate fertile hybrids. Res Microbiol 153:53–58
Siebert TE, Smyth HE, Capone DL, Neuwöhner C, Pardon KH, Skouroumounis GK, Herderich MJ, Sefton MA, Pollnitz AP (2005) Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal Bioanal Chem 381:937–947
Walker GM (1998) Yeast physiology and biotechnology. Wiley, London
Wieczorke R, Krampe S, Weierstall T, Friedel K, Hollenberg CP, Boles E (1999) Concurrent knockout of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett 464:123–128
Zambonelli C, Passarelli P, Rainieri S, Bertolini L (1997) Technological properties and temperature response of interspecific Saccharomyces hybrids. J Sci Food Agric 74:7–12
Zoecklein BW, Fugelsang KC, Gump BH, Nury FS (1995a) Wine analysis and production. Chapter: volatile acidity. Chapman and Hall, New York, pp 192–198
Zoecklein BW, Fugelsang KC, Gump BH, Nury FS (1995b) Wine analysis and production. Chapter: laboratory procedures. Chapman and Hall, New York, pp 474–477
Acknowledgments
This work was financially supported by Australia’s grapegrowers and winemakers through their investment body the Australian Grape and Wine Authority, with matching funds from the Australian Government. The AWRI is part of the Wine Innovation Cluster.
The icewine component of this research was financed by the Natural Sciences and Engineering Research Council of Canada.
The authors would like to thank Jean-Michel Salmon (INRA, France) for his generous gift of tetraploid yeast strain 53–7 and Nick Van Holst Pellekaan for his assistance with the fluorescence flow cytometry analyses.
Funding
This study was funded by the Australian Grape and Wine Authority (project number AWR 1301) and the Natural Sciences and Engineering Research Council of Canada (Discovery grant number 238872).
Conflict of interest
The authors state that they have no conflicts of interest to disclose.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1175 kb)
Rights and permissions
About this article
Cite this article
Bellon, J.R., Yang, F., Day, M.P. et al. Designing and creating Saccharomyces interspecific hybrids for improved, industry relevant, phenotypes. Appl Microbiol Biotechnol 99, 8597–8609 (2015). https://doi.org/10.1007/s00253-015-6737-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6737-4