Abstract
Although the use of TNF-α in the treatment of cancer is restricted due to its non-specific cytotoxicity and narrow range of applications to different cancers in clinical trials, we investigated a safe anti-cancer drug by the use of engineered bacterial capsule harboring TNF-α. The engineered bacterial capsule was designed to target cancer cells, promote a tumor-suppressive environment, and increase the efficacy of existing cancer treatments, including chemotherapy, radiotherapy, and cell therapy. The engineered bacterial capsule was constructed with Salmonella capsulizing TNF-α protein, which was produced and capsulized by Salmonella to reduce side effects of the protein. This bacterial capsule induced a tumor-suppressive environment through the activation of natural killer cells. Engineered bacterial capsule invaded tumor cells, released TNF-α, and induced apoptosis of tumor cells without apparent side effects. In a murine melanoma model, the bacterial capsule of TNF-α significantly inhibited tumor growth by 80–100% and prolonged the survival of the mice. When tested in combination with chemotherapy (cisplatin), antibiotics, and vaccine, recombinant microbial treatment increased the anti-tumor effects of existing therapies. The anti-tumor effects of the bacterial capsule of TNF-α were also observed in cervical cancer, melanoma, breast cancer, colon cancer, and renal carcinoma. These results suggest that the bacterial capsule of TNF-α is a promising strategy for TNF-α treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor treatment currently focuses primarily on surgical excision, chemotherapy, radiation therapy, and immunotherapy (Jansen et al. 2009; Lissoni et al. 2009; Stupp et al. 2009). Although the effectiveness of these therapies has increased remarkably, their use is still restricted due to non-specific cell cytotoxicity, a narrow range of applications to different tumors, and the side effects of high-dose treatment (Le Pechoux et al. 2009).
In melanoma, surgical excision is the principal treatment for early-stage tumors. The use of chemotherapy and radiation therapy to treat melanoma is limited due to their side effects (Goren et al. 1986; Stewart et al. 1986; Stewart et al. 1988), but adjuvant therapies, such as interferon treatment, have been shown to promote survival in patients (Chen et al. 1992; Pajkos et al. 1998; Pisters and Evans 2008).
The goal of our work is to design tumor-specific treatments using TNF-α for chemotherapy, radiotherapy and immunotherapy. Toward this end, we have engineered a microbe that specifically targets various tumors, delivers drugs safely, and does not interfere with existing cancer therapies.
Salmonella, a facultative anaerobe, has been exploited as an antitumor agent that is capable of preferentially amplifying within tumors and inhibiting their growth (Saltzman et al. 1996; Bermudes et al. 2000; Zheng et al. 2000; Luo et al. 2001). In an effort to enhance therapeutic efficacy, this type of approach has been combined with gene-directed enzyme/prodrug therapy (King et al. 2002). For example, auxotrophic Salmonella typhimurium expressing prodrug-converting enzymes has been generated by transformation with a prokaryotic expression vector encoding herpes simplex virus thymidine kinase (Pawelek et al. 1997) or by chromosomal insertion of the Escherichia coli cytosine deaminase gene (Beck et al. 1972). Salmonella has also been exploited for the transfer of eukaryotic and prokaryotic expression vectors into mammalian cells in vitro and in vivo (Weiss 2003). Oral administration of attenuated S. typhimurium carrying an eukaryotic expression vector encoding interferon-gamma (IFN-γ) restores the production of this cytokine in the macrophages of IFN-γ-deficient mice (Paglia et al. 2000). When delivered orally to mice, S. typhimurium carrying eukaryotic expression vectors for cytokines (i.e., interleukin-12 or GM-CSF) mediates cytokine expression and exerts antitumor effects (Yuhua et al. 2001). Thus, it should be feasible to use Salmonella strains transformed with eukaryotic expression vectors to deliver various effector molecules to tumors, with the goal of enhancing antitumor activity.
The aim of our current work is to engineer Salmonella that produces tumor suppressor molecules without secreting into normal cells. We designed Salmonella to harbor a therapeutic protein, to be non-toxic to normal cells, and to be used to bombard tumors with suppressor proteins.
We focused on the suppressor protein TNF-α, since it is a major component of both innate and specific acquired immunity, and has the ability to induce apoptosis of tumor-associated cells, which can result in complete destruction of tumor cells (Green et al. 1979; Moriya et al. 1984; Ortaldo et al. 1986; Ziegler-Heitbrock et al. 1986; Lejeune et al. 2006).
However, studies in humans have shown that administration of high-dose recombinant cytokine is associated with serious side effects (Fichtner et al. 1990; Terlikowski 2002; Lejeune et al. 2006).
We postulated that local low-dose release of cytokine might overcome systemic toxicity without impairing immune recognition of malignant cells by the immune system (Terlikowski 2002). Because of its tumor-targeting and tumoricidal effects, we used S. typhimurium engineered to express TNF-α as a strategy for the effective administration of cytokine. A plasmid encoding the fusion protein SipB-TNF-α was used to create recombinant TNF-α-expressing S. typhimurium. SipB, a Salmonella invasion protein, induces apoptosis and localizes to the outer membrane during mammalian cell entry and in late exponential phase bacterial cultures (Hersh et al. 1999).
In the current study, we investigated the activity of S. typhimurium carrying a prokaryotic expression vector encoding TNF-α as a tumor-targeting anticancer agent and adjuvant to existing tumor therapies in syngeneic murine tumor models.
Material and method
Bacterial strains and growth conditions
We used the S. typhimurium BRD509 strain, which is an aroA aroD mutant of SL1344 (Hoiseth and Stoker 1981). Strains were grown on L-agar or in L-broth supplemented with 0.3 M sodium chloride. This strain was contributed by IS Lee (Hannam University, Korea).
Mouse
Balb/c and C57BL/6 mice were obtained from DaeHan BIOLINK (Korea). CD8−/−C57Bl/6 (H-2b) mice and NK−T+ C57Bl/6 transgenic mice lacking natural killer (NK) cells on C57BL/6 background were contributed by CW Hong, SH Park (Korea University, Korea). Mice were treated according to institutional animal care and use guidelines.
Tumor cell and challenge
We used various tumor cells for tumor model in mice. Tumors used are cervical tumor (TC-1), renal cell carcinoma (RENCA), colon cancer (CT-26), breast carcinoma (4T-1), EL4, and B16F10 cells. Syngeneic C57BL6 mice were injected subcutaneously with 104 tumor cells (TC-1, EL4, B16F10) in the thigh of 7-week-old female mice and BALB/c mice were injected (4T-1, CT-26, and RENCA). Tumor diameter was measured daily with a digital caliper. All animal experiments were performed in accordance with national animal care regulations.
Plasmid constructions
For the expression of the SipB 160 a.a-TNF-alpha fusion, pSSIM was used as vector. TNF-alpha fragment was amplified by primers (TNFL, 5′-agatctatgagcacagaaagcat-3′; TNFR, 5′-ctcgagtcacagagcaatgactcc-3′) with Top10P (TNF-alpha encoding plasmid). pSSIM was digested by BglII and XhoI and ligased with this TNF-alpha fragment. This construct was named as pSST (Plac-sipB160-tnf alpha). Plasmid constructions in this study were transformed into E. coli DH5α. From the transformed DH5α, these plasmids were extracted and again transformed into S. typhimurium SF586. And the plasmids from the transformed SF586 were transformed again to S. typhimurium BRD509. All resulting protein fusions were checked by DNA sequencing.
Preparation of culture supernatant proteins and immunoblotting
Culture supernatant proteins were prepared as follows. In brief, 10 ml of bacterial supernatant were briefly passed briefly through a 0.45-μm pore-size syringe filter to remove bacteria, and was saved for analysis of secretory proteins. The cell pellets were resuspended in phosphate buffered saline (PBS) buffer (pH 8.6). Culture supernatant and pellets were separated in a 12% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. SipB/TNF-alpha chimeric proteins were detected by immunoblot analysis. Western blots were treated with a polyclonal antibody against SipB, followed by incubation with a horseradish peroxidase-labeled anti-rabbit antibody. Blots were developed using a chemiluminescense detection kit.
Protocols for subcutaneous administration protocols
Bacteria were grown overnight until they reached mid-log phase. They were then harvested by centrifugation (3,000×g) and resuspended in a 10% sodium bicarbonate buffer. C57BL/6 mice were subcutaneously inoculated with the bacterial suspension (108 cfu/mouse) in a volume of approximate 100 μl. Each group of mice received the corresponding strain of recombinant Salmonella. Control mice received the buffer only.
Cytokine assay
Blood was collected from all mice before and after subcutaneous administration. The blood samples were stored at 5 °C for 12 h. After coagulation, sera were collected by centrifugation (5 min, 2,000×g at 4 °C). At the end of the experiment, samples of the same group that had been collected at the same time were pooled and tested by TNF-α sandwich ELISA kits (Bender Medsystems Inc., USA) with triplicate wells. Cytokine serum levels were measured by a powerful multiplexed assay combined with flow cytometry using commercially available kits BD™ Mouse TH1/TH2 Cytokine Cytometric Bead Array (CBA) according to the kit procedure (BD Biosciences Immunocytometry Systems and BD Bioscience Pharmigen, USA).
Histological analysis
Each mouse tissue were collected and sequentially immersion-fixed in 10% paraformaldehyde/0.1 M phosphate buffer (PB) pH 7.4, 12.5% glucose/0.1 M PB pH 7.4, and 25% glucose/0.1 M PB pH 7.4 for each 2 days. After the toes were decalcified using 5% ethylenediaminetetraacetic acid/25% glucose/0.1 M PB pH 7.4 for 2 days, they were routinely processed and embedded in 4% carboxymethyl cellulose. Standard sagittal sections measuring 5 μm in thickness were prepared from the frozen and fixed toe using a cryostat CM3050S (LIECA, Tokyo, Japan). The inflammation was histologically assessed on the sections, which were stained with Mayer’s hematoxylin and eosin, toluidine blue pH 7.0, and tartrate-resistant acid phosphatase.
Invasion assay
Murine melanoma cells were infected with Salmonella strains, at an MOI of 100, for 1 h. After infection, cells were washed three times with PBS and then treated with 100 μg/mL of gentamicin (Invitrogen Corporation), for 1 h at 37 °C. After antibiotic treatment, cells were washed again with PBS and then incubated with 1% Triton X-100 for 5 min at 37 °C. The number of internalized bacteria was determined by plating tenfold serial dilutions of the cell lysates on LB plates. Invasion rate (%) were determined using the formula: \( {\hbox{Invasion rate}}\left( \% \right) = {\hbox{number}}\,{\hbox{of}}\,{\hbox{internalized}}\,{\hbox{recombinant}}\,{\hbox{bacteria}}/{\hbox{number}}\,{\hbox{of}}\,{\hbox{internalized}}\,{\hbox{wild}} - {\hbox{type}}\,{\hbox{salmonella}} \times {1}00 \)
Cytotoxicity assay and apoptosis assay
For the analysis of cytotoxicity, we cultured tumor cells with sample after 24–48 h, and collected the culture supernatants. The supernatant was analyzed with CytoTox 96 non-radioactive cytotoxicity assay (Promega, USA). In the initial phase of apoptosis, the caspases became activated and the FLICA bound to these activated caspases. Mouse melanoma cells were treated with different microbe for 24 h. After 24 h, the cells were stained with FLICA Apoptosis detection kit caspase assay (Immunochemistry Technologies, LLC) according to the manufacturer’s instructions.
Tumor implantation and evaluation of antitumor effects
For tumor implantation, 6- to 8-week-old female, C57BL/6 mice were implanted subcutaneously on the mid-right side with 105 B16F10 cells in 100 μl PBS. Tumors were allowed to grow for 7 days before treatment. Bacterial strains administered next to the tumor. Mice were examined daily until tumors became palpable, and then their diameters were measured every other day in two dimensions, with a microcaliper. The antitumor activity of treatments was evaluated by measuring tumor growth inhibition. Tumors were measured individually with a caliper. Tumor volumes were determined using the formula: \( {\hbox{tumor}}\,{\hbox{volume}} = {\hbox{length}} \times {\hbox{widt}}{{\hbox{h}}^{{2}}} \times 0.{52} \). Survival time was used as one of the criteria for tumor inhibition. Mice surviving over 50 days and tumor-free animals were denoted as 50-day survivors. All animal experiments were approved by the institutional animal care committee in Korea University.
Chemotherapy and tumor regrowth delay assay
Each of microbe-treated mice received a single dose of the cisplatin. The mice received the dose of cisplatin during the same time window. Control experiments consisted of the injection of microbe. After treatment, the tumor growth was determined daily by measuring tumor diameter
Direct immunization with Salmonella
C57BL/6 mice were immunized twice with microbe, by intraperintoneal injection on day 0 and by subcutaneously injection on day 7. On day 30, animals were challenged with 1 × 104 B16F10 cells in PBS, were challenged with microbe, and were then analyzed of tumor growth and survival. Animals were monitored for tumor growth every 2–3 days for 60 days.
Statistical analysis
Statistical significance of the experimental results was determined by the Student’s t test. For all analyses, p < 0.05 was accepted as a significant probability level.
Results
Construction of a SipB/TNF-α secretion plasmid and expression of recombinant protein
To generate S. typhimurium harboring TNF-α, we constructed a SipB/TNF-α secretion plasmid (Fig. 1a). An amplified DNA fragment encoding TNF-α was fused to sequences encoding SipB to generate pSSTM (Plac-sipB160 TNF-α). E. coli DH5αE were transformed with pSSTM, and then the plasmid was extracted and used to transform S. typhimurium SF586. Plasmid from transformed SF586 cells was used to transform S. typhimurium BRD509. All resulting plasmid sequences were verified by DNA sequencing.
Construction of recombinant Salmonella-harboring TNF-α. a Schematic presentation of plasmid harboring recombinant TNF-α protein. Carboxyl-terminal ends of these hybrid proteins were encoded by the designated plasmids. b The expression of recombinant TNF-α fusion proteins into culture supernatants by S. typhimurium. Recombinant TNF-α proteins were detected with a mAb to TNF-α. All recombinant TNF-α proteins were not secreted into the culture supernatant at comparable concentrations. Expression was analyzed by immunoblotting with whole bacterial lysates. 1 Supernatants of S. typhimurium, 2 supernatants of S. typhimurium with secretion plasmid vector alone, 3 supernatants of S. typhimurium with TNF-α secretion plasmid, 4 pellets of S. typhimurium, 5 pellets of S. typhimurium with secretion plasmid vector alone, 6 pellets of S. typhimurium with TNF-α secretion plasmid. c Quantification of harbored TNF-α proteins of bacteria. Bacterial lyates were examined with TNF-α specific ELISA. d S. typhimurium-harboring TNF-α were cultivated without antibiotics. Bacteria were collected daily, each bacterial lysates were immunobloted by anti-TNF-α protein antibody. 1 PBS, 2 24 h-cultivation, 3 24–48 h-cultivation, 4 48–64 h-cultivation, 5 64–88 h-cultivation, 6 112–136 h-cultivation, 7 160–184 h-cultivation
We examined the protein expression of TNF-α from engineered S. typhimurium by immunoblot analysis of cell lysates and culture supernatants. We detected proteins of the expected molecular mass (44 kDa) of recombinant TNF-α in the cell lysates of all transformants, but not in the supernatants of cells cultivated in LB (Fig. 1b). To identify harbored proteins of recombinant bacteria, we examined bacterial lysates with ELISA. The quantitative yield of recombinant protein by bacterial transformants was about 100 pg of recombinant protein per 109 cells. In addition, recombinant bacteria expressed TNF-α during 24 h (Fig. 1c) and the expression of protein were only observed in 24 h (Fig. 1d). And recombinant Salmonella were reduced about 90% in tumor-bearing mouse after 24 h, this result suggested that the loss of bacteria induced releasing of TNF-α in tumor region (Electronic supplementary Fig. S1).
Engineered Salmonella induce tumor cell lysis by caspase activation
To determine the cytotoxic effects of engineered Salmonella, we treated B16F10 melanoma cells with recombinant bacteria. Recombinant Salmonella invaded tumor cells at a 50% higher invasion rate than normal Salmonella (Fig. 2a), and were able to kill the tumor cells. The results suggested that recombinant Salmonella-harboring TNF-α specifically kill B16F10 melanoma cells as compared to nornal strains (Fig. 2b, c), and that the cytotoxic effects of recombinant bacteria on tumor cells are due to the induction of caspase activation (Fig. 2d)
Cytotoxicity of recombinant Salmonella-harboring TNF-α. a Bacterial invasion rates were examined. Bacterial invasion were tested in B16F10 melanoma cells. The relative percent value against normal S. typhimurium strains were calculated by Cytotox 96 cytotoxicity kit. b Suppression of tumor cell growth by the recombinant TNF-α proteins of Salmonella. Mouse melanoma B16.F10 cells were treated with PBS-A, and S. typhimurium alone-B, S. typhimurium with a control vector-C and S. typhimurium harboring TNF-α-D as shown in the methods section. c Direct melanoma cell killing activities of the recombinant TNF-α proteins of Salmonella in vitro. Mouse melanoma B16F10 cells were treated with PBS, and S. typhimurium alone, S. typhimurium with control vectors and S. typhimurium harboring TNF-α. d detection of apoptosis in tumor cells by the recombinant TNF-α proteins of Salmonella. Mouse melanoma B16.F10 cells were treated with PBS-A, and S. typhimurium alone-B, S. typhimurium with a control vector-C and S. typhimurium harboring TNF-α-D as shown in the FLICA apoptosis detection assay
Safety of Salmonella-harboring TNF-α in sera and tissue
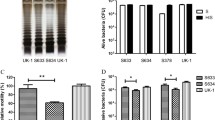
We analyzed bacterial existence in mice after treatments for safety. In mice with subcutaneous inoculation of recombinant microbe, we did not detect microbe from spleen, liver, and blood (Fig. 3a). We analyzed TNF-α secretion in mice inoculated with engineered S. typhimurium harboring TNF-α. Blood was collected from mice before and after subcutaneous administration of recombinant bacteria. Samples from the same group were pooled, and TNF-α levels were analyzed by CBA method. The levels of TNF-α in C57BL/6 mice with Salmonella-harboring TNF-α were similar to those of mice inoculated with normal S. typhimurium (Fig. 3b). To verify the effect of recombinant bacteria on tissue, we examined histological changes in various tissues after administration of recombinant Salmonella. We found that recombinant Salmonella did not induce significant histological side effects, such as severe inflammations (Fig. 3c)
Analysis of inflammation after subcutaneous-inoculation with recombinant bacteria. a Bacterial distribution was examined in mice after sc inoculations with 1 × 108 bacterial cells. At 5 days, bacteria were not cultivated from tissue in mice with subcutaneous recombinant salmonella inoculation. But in mice with wild-type Salmonella as positive control, bacteria were cultivated from tissue cultivated in medium. b Cytokine levels in sera of animals by delivery of S. typhimurium with and without vector by CBA assay. Serum was collected at 7 day after subcutaneous inoculations with 1 × 108 bacterial cells. c To examine severe inflammation by the recombinant TNF-α proteins of Salmonella. Histological sample were collected at 7 day after subcutaneous inoculations with 1 × 108 bacterial cells. Mice were treated with PBS, and S. typhimurium alone, S. typhimurium with a control vector (vec) and S. typhimurium harboring TNF-α. As shown in the H/E staining, all the samples were not shown severe inflammation
Engineered bacteria inhibit tumor cell growth and prolong survival
The tumor inhibitory activity of recombinant Salmonella was analyzed in C57BL/6 mice inoculated subcutaneously with B16F10 cells (104). Mice were inoculated at day 0; and at day 8, visible nodules had developed at all injection sites. Mice were inoculated subcutaneously with Salmonella on days 7 and 14, and were then examined daily until tumors became palpable, at which point, tumor diameter was measured in two dimensions with a micro-caliper every other day. Normal Salmonella and Salmonella carrying a control vector alone uniformly failed to elicit a protective response against lethal subcutaneous tumor cell challenge, and tumor growth in these mice was rapid and uniform. In contrast, there was a substantial decrease in tumor volume in mice treated with recombinant Salmonella-harboring TNF-α, with complete inhibition of tumor cell growth evident in all (90%) animals (Fig. 4a). Nine of ten mice that received Salmonella-harboring TNF-α exhibited a dramatic suppression of tumor growth and were cured of melanoma. In addition, mice with Salmonella and TNF-α recombinant protein were reduced tumor growth like mice with Salmonella-harboring TNF-α (data not shown).
Effect of S. typhimurium-harboring TNF-α in melanoma-bearing mouse. a Growth prevention of B16F10 tumors by a subcutaneous inoculation of S. typhimurium harboring TNF-α. Tumor-bearing mice were inoculated with S. typhimurium harboring TNF-α on day 7 and 14. Experimental animals in groups were each subcutaneously inoculated two times at 1 week intervals with 1 × 108 S. typhimurium alone, S. typhimurium with a control vector (vec) and S. typhimurium-harboring TNF-α. Animals were examined daily until the tumor became palpable, after which its diameter was measured with microcalipers in two dimensions every other day. b Survival was checked every day after inoculation. * P < 0.05 as compared with the control groups. To examine immunity against tumor challenge with S. typhimurium harboring TNF-α, cured mice were lethal challenged with 1 × 105 B16F10 melanoma cells. Tumor growth c and survival d were checked every day after inoculation. * P < 0.05 as compared with the control group (PBS)
We next assessed whether recombinant Salmonella prolonged survival time. Eight days after inoculation with 104 B16F10 cancer cells, all C57BL/6 mice developed tumors. Seven days after inoculation, mice were inoculated with Salmonella-harboring TNF-α, control vector, or no vector (blank control). The survival time of the TNF-α-treated group was much longer than that of the control vector and blank control groups (Fig. 4b). The cytokine-treated group tended to have a higher survival rate. To test whether the mice developed immunity against the tumor cells, eoght tumor-free mice received a second challenge (104 B16F10 cells). All of the formerly tumor-free mice developed tumors. Inhibition of tumor cell growth was not evident in any of the mice that received a second inoculation (data not shown). Furthermore, there was no significant difference in tumor growth inhibition (Fig. 4c) and survival between mice that received Salmonella-harboring TNF-α and the control groups following a second challenge (Fig. 4d). These results suggested that recombinant Salmonella alone does not elicit an immune response, but does induce direct protection against tumors.
Engineered Salmonella inhibit tumor cell growth by direct killing with TNF-α and by creating a tumor-suppressive environment
To investigate the mechanism of inhibition of tumor growth by engineered Salmonella, we implanted tumor cells and inoculated with recombinant Salmonella and TNF-α in mice. The result showed that combinatory effects with Salmonella and TNF-α were like those of recombinant Salmonella-harboring TNF-α (Fig. 5a). In further study of mechanism, we examined the efficacy of recombinant bacteria in various knockout mice. The efficacies of recombinant microbe treatment were not changed in CD8 T cell knockout mice (Fig. 5b). However, in mice that lacked NK cells, the efficacies of recombinant microbe treatment were particularly altered, reduced to a level below that of normal mice (Fig. 5c). NK cells are known as tumor suppressive cells. To confirm the role of NK cells in the tumor suppressive action of recombinant Salmonella, we isolated NK cells and treated them with recombinant Salmonella-harboring TNF-α. The NK cells were activated and secreted increased amounts of IFN-γ (Fig. 5d), and the activated NK cells induced cell lysis (Fig. 5e) and inhibited tumor growth in tumor-bearing mice (Fig. 5f). These results suggested that the efficacy of recombinant Salmonella is due to the dual effects of direct killing by the microbe harboring TNF-α and the induction of tumor-suppressive effects through activation of host NK cells.
NK cell activation by S. typhimurium harboring TNF-α induced tumor inhibition. a To test efficacy of TNF-α in treatment with S. typhimurium harboring TNF-α in mice, Salmonella strains were inoculated with 10 ng TNF-α protein in 100 μl PBS in C57BL6 mice, and the combinatory treatments with 1 × 108 Salmonella and TNF-α induced tumor suppression like S. typhimurium harboring TNF-α. b Growth prevention of B16F10 tumors in CD8 T cell-knockout mice by a subcutaneous inoculation. Tumor bearing mice were inoculated with 1 × 108 S. typhimurium harboring TNF-α on day 0 and 7. c Growth prevention of B16F10 tumors in NK-knock our mice by a subcutaneous inoculation of S. typhimurium harboring TNF-α. Tumor bearing mice were inoculated with S. typhimurium harboring TNF-α on day 0 and 7. * P < 0.05 as compared with the control groups. d Interferon gamma expressions were induced in NK cells by S. typhimurium harboring TNF-α in vitro. Isolated normal 1 × 106 murine NK cells were treated with PBS, and 1 × 108 bacterial groups. After incubation 24 h in RPMI medium with gentamycin, medium were analyzed with ELISA. e Direct melanoma cell killing activities of the NK cells activated by recombinant Salmonella in vitro. After NK cells cultured with PBS, and 1 × 108 bacterial groups for 24 h, isolated NK cells were co cultured with 1 × 105 B16F10 melanoma cells, after 24 h cultivation, tumor cell cytotoxicity were analyzed by CytoTox 96 non-radioactive cytotoxicity assay. * P < 0.05 as compared with the control groups. f Growth prevention of B16F10 tumors by a subcutaneous inoculation of NK cells activated with S. typhimurium-harboring TNF-α. Mice were inoculated with 1 × 105 B16F10 melanoma. Experimental animals in groups were each subcutaneously inoculated two times at 1 week intervals with 1 × 106 NK Cells with stimulated with S. typhimurium without secreting plasmid, S. typhimurium only (vec) and S. typhimurium-harboring TNF-α (TNF). Animals were examined daily until the tumor became palpable, after which its diameter was measured with microcalipers in two dimensions every other day. * P < 0.05 as compared with the control groups
Engineered Salmonella are induced anti-tumor effects in mice with antibiotics or vaccine or cisplatin
Elimination of bacterial infection by antibiotics and host immune responses would be disadvantageous to recombinant microbial treatment. To investigate the impact of antibiotics and immune responses on microbial tumor therapy, we examined the effect of antibiotics on bacterial treatments and tumor growth in mice that were vaccinated with normal Salmonella before inoculation with recombinant microbe. In vitro assay, antibiotics did not interfere with bacterial tumor therapy in cells (Fig 6a). In mice, treatment of microbe with antibiotics also reduced tumor growth (Fig. 6b). In mice that were vaccinated with normal Salmonella, host immune responses did not significantly affect tumor inhibition by recombinant Salmonella (Fig. 6c). These results suggested that antibiotics and host defense responses to subcutaneous inoculation do not interfere with the anti-tumor activity of recombinant Salmonella.
Tumor inhibition of S. typhimurium harboring TNF-α with antibiotics or vaccine or cisplatin. a Suppression of tumor cell growth by the recombinant TNF-α proteins of Salmonella with antibiotics. Mouse melanoma B16F10 cells were treated with PBS (A), and S. typhimurium alone and S. typhimurium with a control vector (B), and S. typhimurium with pSSTM (C) in medium with Salmonella-specific antibiotics (gentamycin). b Growth prevention of B16F10 tumors by a subcutaneous inoculation of S. typhimurium harboring TNF-α with gentamycin. c Growth prevention of B16F10 tumors by a subcutaneous inoculation of S. typhimurium harboring TNF-α after pretreatment of Salmonella-vaccine. d To examine growth prevention of B16F10 tumors by a subcutaneous inoculation of S. typhimurium harboring TNF-α with existent therapeutic agents, bacterial administration with cisplatin was examined. Each cisplatins were inoculated with S. typhimurium harboring TNF-α on day 0 and 7. Experimental animals in groups were each subcutaneously inoculated two times at 1 week intervals with 108 attenuated S. typhimurium without secreting plasmid and S. typhimurium only. Animals were examined daily until the tumor became palpable, after which its diameter was measured with microcalipers in two dimensions every other day. * P < 0.05 as compared with the control groups
To test the efficacy of recombinant bacterial treatment in combination with existing therapies, we co-treated mice with chemotherapy (cisplatin) along with Salmonella. In mice, treatment with cisplatin and microbe was associated with a better effect than single treatment with cisplatin (Fig. 6d). These results suggested that microbial therapy could be used as an adjuvant to existing cancer therapies for more effective treatments of cancers.
Engineered Salmonella reduce growth of different types of tumors
To determine whether microbial therapy effected different tumor types, we transplanted TC-1, 4T-1, CT-26, and RENCA cells into mice. Although there was some variation in efficacy, microbial treatment reduced tumor growth and was associated with prolonged survival in all types of tumor models. These results suggested that, unlike existing treatments, microbial therapy can be applied to different types of tumors (Fig. 7)
Comparision of growth prevention of various tumors by a subcutaneous inoculation of S. typhimurium harboring TNF-α. Cervical tumor (TC-1), brest carcinoma (4T-1), colon cancer (CT-26), renal carcinoma (RENCA) cells were each transplanted in mice. Tumor-bearing mice were inoculated with S. typhimurium harboring TNF-α on day 0 and 7. Experimental animals in groups were each subcutaneously inoculated two times at 1 week intervals with 1 × 108 S. typhimurium alone, S. typhimurium with a control vector (vec) and S. typhimurium harboring TNF-α (TNF). Animals were examined daily until the tumor became palpable, after which its diameter was measured with microcalipers in two dimensions every other day. * P < 0.05 as compared with the control groups
Discussion
In the development of cancer treatments, safe, low-cost, and versatile agents are needed, not only as existing treatments but also as new therapeutics. Biological agents such as cell-based therapies, gene therapy, and immunotherapy have all been developed as potential cancer therapeutics. Recently, bacterial cell-based tumor treatments have been considered as a potential technology for tumor treatments and tumor vaccines.
Salmonella in particular has been shown to possess tumor-targeting properties and has been used as an agent and diagnostic tool (He et al. 2003). Tumor-targeted Salmonella exhibited tumor accumulation ratios in excess of 1,000:1 as compared to normal tissues (Bermudes et al. 2000), and many groups have used Salmonella strains in the development of anticancer agents (Toso et al. 2002). In the current study, we created a genetically engineered bacterial strain of S. typhimurium that harbored TNF-α as a potential anticancer agent. One of the requirements of this bacterial strain is that it has to be safe in normal cells, while localizing to various tumor cells, invading the tumor cells, and bombarding them with tumor suppressor proteins to induce tumor cell lysis. Examples of tumor suppressor proteins include VEGF, p53, p19, interferon, and other cytokines. Among these proteins, cytokines are of particular importance for their role in the regulation of the immune system. Limitations associated with systemic administration of cytokines include the short half-life of many cytokines and the severe side-effects are commonly observed with direct administration (Bocci 1988; Lissoni et al. 1996). TNF-α, for example, showed early promise as a tumor inhibitory molecule, with limitation in clinical trial because of its side effects, but local immunotherapy with this protein were promising (Terlikowski 2002). Our goal is to use bioengineering to reduce the side effects of TNF-α and increase its use an anti-cancer agent.
In the current study, Salmonella that was engineered to express TNF-α did not secrete cytokine in normal cells, but released the protein in tumor cells to reduce side effects of TNF-α. Our recombinant microbe specifically attached to and destroyed tumor cells without harmful cytokine variation and histological changes. These results suggest that Salmonella-harboring TNF-α would be a good alternative to TNF-α treatment.
Our results indicate that cytokine-expressing S. typhimurium could act as a good biological anticancer agent without the cytotoxicity of high-dose cytokine administration. The production of recombinant Salmonella would be convenient and easy, with a low cost and a short production time; and biological anticancer agents would be expected to have synergistic effects (bacterial cytotoxicity and immune induction of anticancer cytokines). We showed that engineered Salmonella induces NK cell activation and activated NK cells induce tumor inhibition.
Previous reports have shown that bacteria can function as gene delivery shuttles for transporting recombinant gene vectors (Pawelek et al. 1997; Dietrich et al. 2000; Huang et al. 2000). In the current study, recombinant Salmonella produced 100 pg of TNF-α per 109 cells. TNF-α was not secreted into the normal environment, but was released in tumor cells after bacterial invasion of the tumor cells.
We examined whole-cell lysates and culture supernatants by Western blot for the expression and secretion of the fusion protein. Salmonella-harboring TNF-α produced TNF-α in bacterial cell cultures, and did not secrete the protein into culture supernatants. Thus, bioengineering resulted in an agent that was safer and with potentially fewer side effects than the direct administration of TNF-α. TNF-α secretion was not detected in the blood of inoculated mice and histological side effects were not observed.
We tested the effects of recombinant Salmonella on tumor growth and survival of tumor-bearing mice following subcutaneous inoculation of 106 B16F10 melanoma cells. After 7 days, 108 bacterial cells were inoculated subcutaneously at the site of tumor transplantation. The inhibition of tumor growth was higher in mice that received recombinant TNF-α-expressing Salmonella as compared to bacterial strains that did not express cytokine.
When we examined whether cured mice possessed memory T-cells to counteract the tumor cells, we found that all mice developed tumors following second inoculation of tumor cells. Thus, the effects of bioengineered Salmonella were due to the temporary killing of tumor cells, and additional approaches are needed to induce tumor immune responses, such as the development of TNF-α fused to a tumor antigen.
Previous research showed that TNF-α activates the immature NK-free subset (Jewett and Bonavida 1993, 1994). The antitumor activity generated by selective activation of NK cells was studied in vitro and in vivo, and activation of NK cells induced inhibition of tumor growth (Hanna 1982). Other researchers reported that activation of NK cells retains the ability to inhibit hematogenous tumor metastasis (Hanna 1983). These results are applied in our recombinant microbe. We examined whether bioengineered Salmonella induced a tumor-suppressive environment through immune cell activation using knockout mice. We repeated the tumor cell transplantation experiments in NK cell-, MHC I-, MHC II-, CD8-, CD4-, IL-4-, IL-12-, and TNF-α-knockout mice (data not shown), and observed a significant effect in NK cell-knockout mice. In NK cell-knockout mice, the effects of the engineered bacteria against tumor growth were reduced as compared to the control groups. NK cells suppress tumor cells, and have been used as a cell-based therapy. Recombinant Salmonella-harboring TNF-α activated NK cells, and these activated NK cells induced tumor suppression. These results provided evidence that the effects of engineered bacteria are due to bacterial cytotoxicity and the induction of host immune responses with NK cell activation.
We were also interested in the efficacy of bioengineered Salmonella as an adjuvant to existing therapies, such as chemotherapy with cisplatin. Generally, anticancer agents like cisplatin and radiation do not interfere with bacterial activities (Salles and Calsou 1992; Salles et al. 1994; Bouayadi and Salles 1995). Thus, the possibility of using recombinant Salmonella as an adjuvant to tumor treatments was explored. If the microbe was synergistic with existing therapies, many factors would have to be considered, such as immune rejection, interference by the chemical agent, and/or biological interference.
We demonstrated that the efficacy of engineered Salmonella was not reduced by antibiotics and anti-bacterial immune responses following subcutaneous inoculation of the microbe. Our results suggested that the anti-tumor activity of recombinant Salmonella could be not restricted by host immunity and chemotreatment.
To examine the effect of combined treatment with engineered Salmonella and existing therapies, we demonstrated that the engineered microbe did not interfere with the efficacy of cisplatin and, in fact, significantly increased the therapeutic effect of these therapies on survival and tumor growth. Finally, we showed that bioengineered Salmonella was effective against various types of tumors. This might be related to the ability of the microbe to target tumors, not through a tumor-specific antigen, but by targeting the tumor environment. These results showed that our microbe could be used as a universal tumor treatment.
For more predictable clinical results, new drugs are prescribed along with existing drugs for patients (Braybrooke et al. 2005; Freytag et al. 2007; Saif et al. 2007; Baselga et al. 2009). Parallel development of new drugs is important, as is the development of adjuvants to increase the capabilities of existing anti-cancer drugs and improve outcomes for patients. Bacterial agents can be produced easily and cheaply, and engineered bacteria can be applied to a variety of tumors and in combination with different treatments.
In conclusion, we demonstrated that recombinant Salmonella harboring a cytokine expression vector (S. typhimurium-harboring TNF-α) mediates cytokine gene expression in vitro and exerts antitumor effects in mice. This microbe could be used as a versatile agent against many tumors and as an adjuvant to existing therapies. Thus, S. typhimurium-harboring TNF-α could serve as an anticancer agent and provide a new, safe, and efficient way to treat cancer.
References
Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R, Bianchi G, Steinseifer J, Molloy B, Tokaji E, Gardner H, Phillips P, Stumm M, Lane HA, Dixon JM, Jonat W, Rugo HS (2009) Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27:2630–2637
Beck CF, Ingraham JL, Neuhard J (1972) Location on the chromosome of Salmonella typhimurium of genes governing pyrimidine metabolism. II. Uridine kinase, cytosine deaminase and thymidine kinase. Mol Gen Genet 115:208–215
Bermudes D, Low B, Pawelek J (2000) Tumor-targeted Salmonella. Highly selective delivery vectors. Adv Exp Med Biol 465:57–63. doi:10.1007/0-306-46817-4_6
Bocci V (1988) Central nervous system toxicity of interferons and other cytokines. J Biol Regul Homeost Agents 2:107–118
Bouayadi K, Salles B (1995) Influence of DNA supercoiling on cisplatin toxicity in Escherichia coli K-12. Mutat Res 348:25–31
Braybrooke JP, Slade A, Deplanque G, Harrop R, Madhusudan S, Forster MD, Gibson R, Makris A, Talbot DC, Steiner J, White L, Kan O, Naylor S, Carroll MW, Kingsman SM, Harris AL (2005) Phase I study of MetXia-P450 gene therapy and oral cyclophosphamide for patients with advanced breast cancer or melanoma. Clin Cancer Res 11:1512–1520
Chen JT, Hasumi K, Masubuchi K (1992) Interferon-alpha, interferon-gamma and sizofiran in the adjuvant therapy in ovarian cancer—a preliminary trial. Biotherapy 5:275–280
Dietrich G, Spreng S, Gentschev I, Goebel W (2000) Bacterial systems for the delivery of eukaryotic antigen expression vectors. Antisense Nucleic Acid Drug Dev 10:391–399
Fichtner I, Lemm M, Becker M, Tanneberger S (1990) Determination of antineoplastic activity and toxicity of tumor necrosis factor (TNF) in animal experiments. Correlation to clinical findings. Neoplasma 37:301–315
Freytag SO, Movsas B, Aref I, Stricker H, Peabody J, Pegg J, Zhang Y, Barton KN, Brown SL, Lu M, Savera A, Kim JH (2007) Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther 15:1016–1023
Goren MP, Wright RK, Horowitz ME (1986) Cumulative renal tubular damage associated with cisplatin nephrotoxicity. Cancer Chemother Pharmacol 18:69–73
Green S, Chiasson MA, Shah RG (1979) Evidence for the presence of an antitumor factor in serum of normal animals. Cancer Lett 6:235–240
Hanna N (1982) Inhibition of experimental tumor metastasis by selective activation of natural killer cells. Cancer Res 42:1337–1342
Hanna N (1983) Regulation of natural killer cell activation: implementation for the control of tumor metastasis. Nat Immun Cell Growth Regul 3:22–33
He Q, Xu RZ, Shkarin P, Pizzorno G, Lee-French CH, Rothman DL, Shungu DC, Shim H (2003) Magnetic resonance spectroscopic imaging of tumor metabolic markers for cancer diagnosis, metabolic phenotyping, and characterization of tumor microenvironment. Dis Markers 19:69–94
Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A (1999) The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA 96:2396–2401
Hoiseth SK, Stoker BAD (1981) Aromatic-dependent S. typhimunum are non-virulent and are effective as live vaccines. Nature 291:238–239
Huang Y, Hajishengallis G, Michalek SM (2000) Construction and characterization of a Salmonella enterica serovar typhimurium clone expressing a salivary adhesin of Streptococcus mutans under control of the anaerobically inducible nirB promoter. Infect Immun 68:1549–1556
Jansen EP, Boot H, Dubbelman R, Verheij M, Cats A (2009) Postoperative chemoradiotherapy in gastric cancer—a phase I–II study of radiotherapy with dose escalation of weekly cisplatin and daily capecitabine chemotherapy. Ann Oncol 21:530–534
Jewett A, Bonavida B (1993) Pivotal role of endogenous TNF-alpha in the IL-2-driven activation and proliferation of the functionally immature NK free subset. Cell Immunol 151:257–269
Jewett A, Bonavida B (1994) Activation of the human immature natural killer cell subset by IL-12 and its regulation by endogenous TNF-alpha and IFN-gamma secretion. Cell Immunol 154:273–286
King I, Bermudes D, Lin S, Belcourt M, Pike J, Troy K, Le T, Ittensohn M, Mao J, Lang W, Runyan JD, Luo X, Li Z, Zheng LM (2002) Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum Gene Ther 13:1225–1233
Le Pechoux C, Dunant A, Senan S, Wolfson A, Quoix E, Faivre-Finn C, Ciuleanu T, Arriagada R, Jones R, Wanders R, Lerouge D, Laplanche A (2009) Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 10:467–474
Lejeune FJ, Lienard D, Matter M, Ruegg C (2006) Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun 6:6
Lissoni P, Pittalis S, Ardizzoia A, Brivio F, Barni S, Tancini G, Pelizzoni F, Maestroni GJ, Zubelewicz B, Braczkowski R (1996) Prevention of cytokine-induced hypotension in cancer patients by the pineal hormone melatonin. Support Care Cancer 4:313–316
Lissoni P, Brivio F, Fumagalli L, Messina G, Meregalli S, Porro G, Rovelli F, Vigore L, Tisi E, D’Amico G (2009) Effects of the conventional antitumor therapies surgery, chemotherapy, radiotherapy and immunotherapy on regulatory T lymphocytes in cancer patients. Anticancer Res 29:1847–1852
Luo X, Li Z, Lin S, Le T, Ittensohn M, Bermudes D, Runyab JD, Shen SY, Chen J, King IC, Zheng LM (2001) Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models. Oncol Res 12:501–508
Moriya N, Miwa H, Orita K (1984) Antitumor effects of bacterial lipopolysaccharide and tumor necrosis factor in mice. Jpn J Surg 14:163–166
Ortaldo JR, Mason LH, Mathieson BJ, Liang SM, Flick DA, Herberman RB (1986) Mediation of mouse natural cytotoxic activity by tumour necrosis factor. Nature 321:700–702
Paglia P, Terrazzini N, Schulze K, Guzman CA, Colombo MP (2000) In vivo correction of genetic defects of monocyte/macrophages using attenuated Salmonella as oral vectors for targeted gene delivery. Gene Ther 7:1725–1730
Pajkos G, Bodoky G, Padi E, Izso J, Szanto J (1998) Low-dose leucovorin and interferon-alpha as modulators of 5-fluorouracil for adjuvant chemotherapy of colorectal cancer. Orv Hetil 139:1571–1575
Pawelek JM, Low KB, Bermudes D (1997) Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res 57:4537–4544
Pisters PW, Evans DB (2008) Cisplatin, fluorouracil, interferon-alpha, and radiation as adjuvant therapy for resected pancreatic cancer: is there a future for this regimen and/or should we change our approach to research and treatment of patients with pancreatic cancer? Ann Surg 248:152–153
Saif MW, Black G, Roy S, Bell D, Russo S, Eloubeidi MA, Steg A, Johnson MR, Zelterman D, Diasio RB (2007) Phase II study of capecitabine with concomitant radiotherapy for patients with locally advanced pancreatic cancer: up-regulation of thymidine phosphorylase. Cancer J 13:247–256
Salles B, Calsou P (1992) Involvement of glutathione in cis-platinum toxicity in Escherichia coli K12. Toxicology 72:341–350
Salles B, Calsou P, Bouayadi K, Vinial H (1994) Multiple mechanisms of resistance to cisplatin toxicity in an Escherichia coli K12 mutant. Toxicology 93:235–247
Saltzman DA, Heise CP, Hasz DE, Zebede M, Kelly SM, Curtiss R 3rd, Leonard AS, Anderson PM (1996) Attenuated Salmonella typhimurium containing interleukin-2 decreases MC-38 hepatic metastases: a novel anti-tumor agent. Cancer Biother Radiopharm 11:145–153
Stewart F, Bohlken S, Begg A, Bartelink H (1986) Renal damage in mice after treatment with cisplatinum alone or in combination with X-irradiation. Int J Radiat Oncol Biol Phys 12:927–933
Stewart FA, Luts A, Oussoren Y, Begg AC, Dewit L, Bartelink H (1988) Renal damage in mice after treatment with cisplatin and X-rays: comparison of fractionated and single-dose studies. NCI Monogr 6:23–27
Stupp R, Mayer M, Kann R, Weder W, Zouhair A, Betticher DC, Roth AD, Stahel RA, Majno SB, Peters S, Jost L, Furrer M, Thierstein S, Schmid RA, Hsu-Schmitz SF, Mirimanoff RO, Ris HB, Pless M (2009) Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trial. Lancet Oncol 10:785–793
Terlikowski SJ (2002) Local immunotherapy with rhTNF-alpha mutein induces strong antitumor activity without overt toxicity—a review. Toxicology 174:143–152
Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F, Freezer LJ, Morton KE, Seipp C, Haworth L, Mavroukakis S, White D, MacDonald S, Mao J, Sznol M, Rosenberg SA (2002) Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol 20:142–152
Weiss S (2003) Transfer of eukaryotic expression plasmids to mammalian hosts by attenuated Salmonella spp. Int J Med Microbiol 293:95–106
Yuhua L, Kunyuan G, Hui C, Yongmei X, Chaoyang S, Xun T, Daming R (2001) Oral cytokine gene therapy against murine tumor using attenuated Salmonella typhimurium. Int J Cancer 94:438–443
Zheng LM, Luo X, Feng M, Li Z, Le T, Ittensohn M, Trailsmith M, Bermudes D, Lin SL, King IC (2000) Tumor amplified protein expression therapy: Salmonella as a tumor-selective protein delivery vector. Oncol Res 12:127–135
Ziegler-Heitbrock HW, Moller A, Linke RP, Haas JG, Rieber EP, Riethmuller G (1986) Tumor necrosis factor as effector molecule in monocyte mediated cytotoxicity. Cancer Res 46:5947–5952
Acknowledgments
This work was supported by KFDA and Korea Science & Engineering Foundation (Grant No. E00156).
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea. (A090228)
We are grateful to YuChang Park, YeSu Ju, and HaNa Nim for their expert, technical assistance.
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Survival of recombinant bacteria in tumor-bearing mice. B16F10 tumor-bearing C57BL6 mice were subcutaneously inoculated with 1 × 108 S. typhimurium harboring TNF-α next to tumor. At 1, 12, and 24 h after treatment, the tumor region was homogenized. Tumor cell lysates were cultivated and the percent survival of recombinant bacteria was calculated. (DOC 71 kb)
Rights and permissions
About this article
Cite this article
Yoon, W.S., Chae, Y.S., Hong, J. et al. Antitumor therapeutic effects of a genetically engineered Salmonella typhimurium harboring TNF-α in mice. Appl Microbiol Biotechnol 89, 1807–1819 (2011). https://doi.org/10.1007/s00253-010-3006-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-3006-4