Abstract
In order to achieve high butanol production by Clostridium saccharoperbutylacetonicum N1-4, the effect of lactic acid on acetone–butanol–ethanol fermentation and several fed-batch cultures in which lactic acid is fed have been investigated. When a medium containing 20 g/l glucose was supplemented with 5 g/l of closely racemic lactic acid, both the concentration and yield of butanol increased; however, supplementation with more than 10 g/l lactic acid did not increase the butanol concentration. It was found that when fed a mixture of lactic acid and glucose, the final concentration of butanol produced by a fed-batch culture was greater than that produced by a batch culture. In addition, a pH-controlled fed-batch culture resulted in not only acceleration of lactic acid consumption but also a further increase in butanol production. Finally, we obtained 15.5 g/l butanol at a production rate of 1.76 g/l/h using a fed-batch culture with a pH-stat continuous lactic acid and glucose feeding method. To confirm whether lactic acid was converted to butanol by the N1-4 strain, we performed gas chromatography–mass spectroscopy (GC-MS) analysis of butanol produced by a batch culture during fermentation in a medium containing [1,2,3-13C3] lactic acid as the initial substrate. The results of the GC-MS analysis confirmed the bioconversion of lactic acid to butanol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increase in the price of oil and awareness of global warming, the production of biofuels from renewable resources has gained importance (Demain 2009; Ezeji et al. 2005; Thang et al. 2010). Butanol, one of the valuable biofuels, is produced by acetone–butanol–ethanol (ABE)-producing clostridia through fermentation (Lee et al. 2008). Compared with the traditional biofuel ethanol, butanol has a lot of advantages because it shows non-hygroscopicity and low vapor pressure and can be used in any concentration with gasoline (Dürre 2007). Therefore, fermentative butanol production has drawn much attention in recent years.
The metabolism of ABE-producing clostridia can be divided into the following two distinct phases: acidogenesis and solventogenesis (Jones and Woods 1986). During acidogenesis, ABE-producing clostridia form organic acids such as lactic acid, acetic acid, and butyric acid. Accumulation of these acids results in the induction of solventogenesis, during which these acids are reutilized for the production of solvents. Because of this reutilization pathway of ABE-producing clostridia, these organic acids have great potential as substrates in the production of butanol. In particular, considerable research has been conducted on the reutilization pathways of acetic acid and butyric acid using ABE-producing clostridia in the area of metabolic engineering (Green et al. 1996; Harris et al. 2000), metabolic flux analysis (Desai et al. 1999, Harris et al. 2000), DNA array-based transcriptional analysis (Zhao et al. 2005), kinetics of enzyme activity (Hartmanis et al. 1984; Hartmanis and Gatenbeck 1984), and fermentation technology (Bahl et al. 1982; Chen and Blaschek 1999; Hüsemann and Papoutsakis 1990; Matta-el-Ammouri et al. 1987; Tashiro et al. 2004; Tashiro et al. 2007). The effect of acetic acid and butyric acid on fermentative characteristics has been investigated in detail to develop ABE fermentation systems. It has been reported that the addition of acetic acid not only increased solvent concentration in batch cultures (Chen and Blaschek 1999) and fed-batch cultures (Fond et al. 1985) but also stabilized solvent production (Chen and Blaschek 1999). On the other hand, the addition of butyric acid inhibited its own formation and increased butanol concentration (Matta-el-Ammouri et al. 1987). Furthermore, several ABE fermentation techniques involving the use of butyric acid have been developed, including batch culture (Matta-el-Ammouri et al. 1987), fed-batch culture (Fond et al. 1985; Tashiro et al. 2004), continuous culture (Bahl et al. 1982), and the butanol production system by resting cells (Tashiro et al. 2007). Nevertheless, there is no report describing butanol production when using lactic acid as the substrate, and effects of lactic acid on ABE fermentation have not yet been investigated.
This study aims to establish a promising fermentation system in which lactic acid is used as a substrate for butanol production. Here, we investigated the effect of lactic acid on ABE fermentation and developed a method for producing high concentrations of butanol using lactic acid as a substrate. As a result, the addition of 5 g/l lactic acid to a medium containing 20 g/l glucose increased butanol concentration and the yield of butanol to glucose. By applying the pH-stat fed-batch culture, 15.5 g/l butanol was produced at a production rate of 1.76 g/l/h. Finally, we confirmed the bioconversion of lactic acid to butanol by gas chromatography–mass spectroscopy (GC-MS) analysis.
Materials and methods
Bacterial strain
Clostridium saccharoperbutylacetonicum N1-4 ATCC 13564 was used in this study (Lee et al. 1995). The cells were maintained as spores in fresh potato glucose (PG) medium at 4°C. The spore suspension (1 ml) was transferred aseptically to 9 ml of PG medium, subjected to heat shock in boiling water for 1 min, and cultured at 30°C for 24 h (Lee et al. 1995).
Culture conditions
A pre-culture of the N1-4 strain was anaerobically grown without agitation at 30°C for 15 h in tryptone-yeast extract-acetate (TYA) medium (Ishizaki et al. 1999). TYA medium for the main culture contained 50 g/l glucose. An inoculum of 10% (v/v) was transferred into the medium. After 19 h of main culture, the cells were harvested by centrifugation and suspended in the modified TYA medium (hereafter, referred to as TY medium) containing 0–20 g/l glucose, 0–20 g/l dl-lactic acid, 2 g/l of yeast extract, 6 g/l of tryptone, 2.57 g/l of ammonium sulphate, 0.3 g/l of MgSO4·7H2O, 0.5 g/l of KH2PO4, and 10 mg/l of FeSO4·7H2O at the equivalent of 10 g/l dry cell weight (DCW). In all the experiments, the initial pH of the medium was adjusted to 5.5 by using 3 M KOH, and all media were sterilized at 115°C for 15 min. All cultures were maintained at 30°C. Samples were periodically withdrawn for analysis of products.
Batch cultures were carried out statically in a test tube with a 10-ml working volume. To investigate the effect of lactic acid, lactic acid was first sterilized by passing it through a 0.45-µm membrane filter, and was then added to sterilized TY medium at concentrations of 5, 10, and 20 g/l.
Batch and fed-batch cultures were carried out statically without pH control in 300-ml Erlenmeyer flasks with a 100-ml working volume of TY medium initially containing 20 g/l glucose and 5 g/l lactic acid. After inoculation, the broth was sparged with filtered oxygen-free nitrogen gas to maintain strictly anaerobic conditions. In the fed-batch culture, the pH of a 10-ml mixed solution of 50 g/l lactic acid and 200 g/l glucose was adjusted to 5.5. This solution was then added to the flask at 6 h of cultivation so that the lactic acid and glucose could serve as substrates.
pH-controlled batch and fed-batch cultures and pH-stat fed-batch cultures were performed in TY medium containing glucose (20 g/l) and lactic acid (5 g/l) as the initial substrates in a working volume of 400 ml in 1–l jar fermentors. The three cultures were maintained anaerobically by sparging nitrogen gas as described above, and the pH was automatically maintained at 5.5 by a pH controller (PHC-2201; Able, Tokyo, Japan). In the pH-controlled fed-batch culture, 3 M HCl was used for pH control, and a 40-ml mixed solution of 50 g/l lactic acid and 200 g/l glucose (pH 5.5) was added all at once at 6 h of cultivation. On the other hand, a solution of 50 g/l lactic acid and 200 g/l glucose (pH approximately 2.0) was used in the pH-stat fed-batch culture and fed intermittently to maintain the pH value at 5.5. In total, 103.5 ml of the solution was added to fermentor during the whole feeding period of 48 h.
To confirm that lactic acid was converted to butanol, a batch culture without pH control was carried out in a working volume of 3 ml in a test tube containing 20 g/l 12C6-glucose and 6.2 g/l sodium [1,2,3-13C3] l-lactate (Sigma-Aldrich, St. Louis, MO). After cultivation for 48 h, the supernatant was collected and used for GC-MS analysis by the method described below.
Analytical procedures
DCW was calculated using a predetermined correlation between optical density (OD) at 562 nm by a spectrophotometer (V-530; JASCO, Tokyo, Japan) and DCW. An OD of 1.0 was equivalent to 0.301 g of DCW per liter. The concentrations of acetic acid, butyric acid, and solvents in culture supernatant were determined according to a method described previously (Tashiro et al. 2004). The concentrations of glucose and lactic acid were determined by high-performance liquid chromatography (LC10AD coupled with RID10A; Shimadzu, Kyoto, Japan) using an RSpak KC-811 column (Showdex, Tokyo, Japan). In order to find out whether butanol was converted from [1,2,3-13C3] lactic acid, the supernatant was analyzed using a GCMS-QP2010 instrument (Shimadzu). Butanol was separated on an HP-INNOWAX column 19091 N-233 (Agilent Technologies, Palo Alto, CA). The split ratio was set at 25. The oven temperature was programmed to increase from 50°C to 170°C at the rate of 10°C/min. The injector temperature and ion source temperatures were 250°C and 200°C, respectively. Helium was used as the carrier gas with a flow rate of 1 ml/min. The ionizing energy was 70 eV. All data were obtained by collecting the mass spectra within the scan range 50–80 amu.
Results
Solvent production in the presence of lactic acid
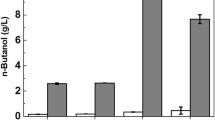
To investigate the effect of lactic acid on ABE fermentation, batch cultures with the N1-4 strain were carried out in TY medium containing various concentrations of lactic acid (0–20 g/l) with or without 20 g/l glucose. As shown in Table 1, very little butanol (0.129 g/l) was produced in the absence of glucose in spite of lactic acid consumption (3.58 g/l). In the presence of glucose, butanol production was accompanied by the consumption of lactic acid and glucose in TY medium supplemented with 5 and 10 g/l lactic acid (G20L5 and G20L10), whereas lactic acid concentration was not changed in TY medium lacking lactic acid (G20), which indicated neither production nor consumption of lactic acid (data not shown). When 5 g/l lactic acid was added with 20 g/l glucose (G20L5), 3.46 g/l lactic acid was consumed, and the butanol concentration increased to 5.98 g/l from 4.95 g/l in the absence of lactic acid (G20). Moreover, G20L5 also gave a higher yield of butanol to glucose than that in the absence of lactic acid (G20), although the yields of butanol, solvents, and all products to the total substrates did not change significantly. These results suggested that lactic acid is a useful substrate and can be converted to butanol. However, addition of over 10 g/l lactic acid (G20L10 and G20L20) sharply decreased not only butanol concentration but also consumption of lactic acid and glucose. On the basis of the above results, we decided to use 5 g/l lactic acid with 20 g/l glucose as the initial substrates in all the following experiments.
Fed-batch culture without pH control
To reduce a negative effect of high concentration of lactic acid on butanol production and to obtain a greater amount of butanol from lactic acid and glucose, a fed-batch culture was carried out by feeding a mixed solution of lactic acid and glucose at 6 h of cultivation. Figure 1 shows temporal profile of butanol, lactic acid, and glucose in fed-batch and batch cultures not subjected to pH control. The butanol concentration increased on adding the mixed solution; however, both substrates were not consumed completely within 48 h. The highest butanol concentration in the fed-batch culture was 10.2 g/l, whereas that in the batch culture was 6.62 g/l (Table 2). The maximum rate of butanol production or substrate consumption and the yield of butanol to total substrates did not vary significantly between fed-batch and batch cultures. These data indicated that a method of feeding substrate could boost the final butanol concentration of butanol by the N1-4 strain.
Time course of batch and fed-batch cultures not subjected to pH control. The cultures contained 20 g/l glucose and 5 g/l lactic acid as initial substrates. a Batch culture. b Fed-batch culture in which the mixture of glucose and lactic acid was fed at 6 h of cultivation. The data represent the average of duplicate experiments. Filled circles, butanol concentration; filled triangles, lactic acid concentration; filled squares, glucose concentration; empty triangles, pH
Effect of pH control on batch or fed-batch culture with lactic acid and glucose
We found that a culture under low and controlled pH conditions (pH 5.5) stimulated consumption of organic acids such as butyric acid and boosted the production of butanol by the N1-4 strain, compared to that in culture not subjected to pH control or under high and controlled pH conditions (pH 6.5, unpublished data). In addition, it is assumed that a conversion of organic acid to neutral butanol consumes a proton by the reduction of carboxyl group to alcoholic group, which results in an increase in pH. As well as butyric acid (unpublished data), the pH in batch and fed-batch cultures not subjected to pH control gradually increased up to approximately 6.6 after 48 h of cultivation and was accompanied by lactic acid consumption (Fig. 1), although a similar increase in pH was not observed in the batch culture lacking lactic acid (data not shown). Therefore, we investigated the effect of maintaining the pH at 5.5 (to prevent an increase in pH during cultivation) on butanol production or substrate consumption using lactic acid as the substrate.
The N1-4 strain could consume most of the lactic acid in the pH-controlled batch and fed-batch cultures (Fig. 2); however, lactic acid was found in both cultures not subjected to pH controlled, where the rise in pH appeared to inhibit lactic acid consumption (Fig. 1). pH-controlled batch and fed-batch cultures showed the maximum consumption rate of lactic acid (0.976 and 1.07 g/l/h) and glucose (3.92 and 3.73 g/l/h); these rates were much higher than the corresponding rates in cultures not subjected to pH control (lactic acid, 0.201 and 0.357 g/l/h; glucose, 1.45 and 1.80 g/l/h) (Table 2). Furthermore, butanol production (concentration and maximum production rate) by pH-controlled batch and fed-batch cultures also increased significantly as compared to production in cultures not subjected to pH control. In particular, the butanol concentration increased up to 12.6 g/l when the pH was kept constant at 5.5 in the fed-batch culture by feeding of substrates at 6 h of cultivation (Fig. 2, Table 2), although differences in the yield of butanol to glucose or total substrates were not observed between batch and fed-batch cultures. These results suggested that pH maintained at 5.5 could stimulate not only substrate consumption (particularly consumption of lactic acid) but also butanol production; this result indicated that the method is a good strategy for achieving high butanol production from lactic acid.
Time course of pH-controlled batch and fed-batch cultures. The cultures contained 20 g/l glucose and 5 g/l lactic acid as initial substrates. The pH was maintained at 5.5 using 3 M HCl. a Batch culture. b Fed-batch culture in which the mixture of glucose and lactic acid was fed at 6 h of cultivation. Data of the fed-batch culture represent the average of duplicate experiments. Filled circles, butanol concentration; filled triangles, lactic acid concentration; filled squares, glucose concentration
pH-stat fed-batch culture with lactic acid and glucose and comparison of butanol production between different culture modes
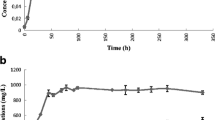
It is suggested that a high or drastic increase in lactic acid, as shown on transient feeding of lactic acid (Figs. 1 and 2), causes the N1-4 strain to be under stress, and results in low butanol production. To develop a more efficient butanol production system, a pH-stat fed-batch culture at a set pH value of 5.5 was carried out by continuous feeding of lactic acid and glucose to maintain the lactic acid concentration at a low level. We succeeded in a stable concentration of lactic acid of below 5 g/l (Fig. 3) at a constant pH of 5.5 in the broth by applying the pH-stat feeding method.
Time course of pH-stat fed-batch culture in which the mixture of glucose and lactic acid was fed. The culture contained 20 g/l glucose and 5 g/l lactic acid as initial substrates. The pH was maintained at 5.5 using a solution of 50 g/l lactic acid and 200 g/l glucose (pH approximately 2.0). Data represent the average of duplicate experiments. Filled circles, butanol concentration; filled triangles, lactic acid concentration; filled squares, glucose concentration
Table 2 summarizes the results of different culture modes analyzed in this study. Among these, the pH-stat fed-batch culture by feeding lactic acid and glucose showed optimal values: butanol concentration, 15.5 g/l; maximum butanol production rate, 1.76 g/l/h; butanol yield to glucose, 0.555 C-mol/C-mol; maximum glucose consumption rate, 5.23 g/l/h; and maximum lactic acid consumption rate, 1.49 g/l/h. The highest consumption ratio of lactic acid to glucose (0.265 C-mol/C-mol) was also obtained in the pH-stat fed-batch culture; this result indicated that the metabolic flux of lactic acid consumption was enhanced. Thus, we established a high-producing and highly efficient butanol production system with lactic acid and glucose using a pH-stat fed-batch culture.
Verification of lactic acid conversion to butanol
On the basis of the results of the various culture experiments described above, it was assumed that consumed lactic acid was converted to butanol as a result of metabolism in the N1-4 strain. GC-MS analysis of the batch culture broth with [1,2,3-13C3] lactic acid was performed to confirm whether the N1-4 strain converted lactic acid to butanol during cultivation. When 12C4-butanol was used as the standard substance in GC-MS, we observed a peak (peak 1) corresponding to 56.05 of the molecular weight, which was suggested to show a mass shift of −18.0 because of the dehydration reaction of butanol (Fig. 4a). GC-MS analysis results of the culture broth, which was used to cultivate the N1-4 strain using [1,2,3-13C3] lactic acid and 12C6-glucose as substrates, showed 3 peaks with different relative intensities, i.e., peaks 1, 2, and 3 (Fig. 4b). Similar to peak 1, peak 2 (m/z 58.05), and peak 3 (m/z 60.05) corresponded to dehydrated 13C-butanol of which two and four carbons were substituted by 13C-atoms derived from [1,2,3-13C3] lactic acid, respectively. Thus, we confirmed the bioconversion of lactic acid to butanol by the N1-4 strain.
Mass spectra of 12C4-butanol and 13C-butanol by GC-MS analysis. a 12C4-butanol solution as a standard substance. b Culture broth of the N1-4 strain with 12C6-glucose and [1,2,3-13C3] lactic acid. Peaks 1, 2, and 3, indicated by arrows, were derived from three types of butanol: 12C4-butanol, 13C-butanol in which two out of four carbons were replaced by 13C-atoms, and 13C4-butanol, respectively
Discussion
Some researchers have reported an improvement in solvent production after the addition of acetic acid or butyric acid to cultures using several species of ABE-producing clostridia, including the N1-4 strain (Chen and Blaschek 1999; Fond et al. 1985; Matta-el-ammouri et al. 1987; Tashiro et al. 2004). In our previous study using the N1-4 strain, solvent production increased after supplementing the medium with acetic acid, whereas the addition of up to 5 g/l butyric acid also enhanced the production of both butanol and acetone giving a greater yield of solvents to glucose (Tashiro et al. 2004). However, since there are no reports describing butanol production by the N1-4 strain using lactic acid as substrate, the present study aimed at gaining a better understanding of the characteristics of lactic acid and establishing a high butanol production system using the N1-4 strain.
In this study, we revealed that the addition of 5 g/l lactic acid to a medium containing glucose increased butanol concentration and the yield of butanol to glucose without significantly increasing acetone production (Table 1). Therefore, these results indicated that lactic acid as well as acetic acid or butyric acid could be used as a substrate for efficient ABE fermentation. Furthermore, abundant lactic acid (i.e. more than 100 g/l) can be obtained as fermentative feedstock from renewable resources by lactic acid fermentation, which produces different forms of lactic acid (d-, l-, or racemic) depending on the species of lactic acid bacteria (Hofvendahl and Hahn-Hägerdal 1997; Linko and Javanainen 1996; Yun et al. 2004). Because the N1-4 strain consumed both d- and l-lactic acids simultaneously in a batch culture (data not shown), we can infer that all forms of lactic acid can be used as a substrate of butanol.
We have indicated the increase in the production of butanol and acetone, and consumption of lactic acid under low and controlled pH conditions (pH 5.5) in both batch and fed-batch cultures (Figs. 1 and 2, Table 2). In ABE fermentation, the pH of the broth is reported to be an essential regulatory factor in distinguishing between acidogenesis and solventogenesis, high pH indicating the former and low pH, the latter (Jones and Woods 1986). Furthermore, a pH of 5.5 is shown to be the transition point for the N1-4 strain marking the end of acidogenesis and beginning of solventogenesis (Tashiro et al. 2004). In addition, the high activity of enzymes involved in butanol and acetone production, namely butanol dehydrogenase, butyraldehyde dehydrogenase, and acetoacetate decarboxylase, has been described using glucose as the sole substrate in solventogenesis at low pH control (Andersch et al. 1983; Hüsemann and Papoutsakis 1989). It has also been reported that low pH enhanced acetone production and glucose consumption (Monot et al. 1984). These fermentative characteristics also support the results of our study. Thus, the production of acetone using lactic acid as the substrate increased with an increase in the glucose consumption rate in the cultures subjected to pH control (Fig. 2, Table 2).
The inhibitory effect of several organic acids, used as substrates, on microorganisms can be reduced by maintaining the organic acids at low concentrations in the medium throughout the culture. A pH-stat continuous feeding of organic acid is one of the most successful methods for achieving this task (Tashiro et al. 2004; Tsuge et al. 2001). As expected, we were able to maintain the lactic acid concentration in the broth below 10 g/l that was suggested to cause the decay of butanol concentration with the N1-4 strain (Table 1) throughout the pH-stat fed-batch culture by using lactic acid and glucose (Fig. 3); this experiment also produced optimal results (in terms of product concentration, rate of production, and substrate consumption) from among all other experiments performed in this study (Table 2). Surprisingly, the pH-stat fed-batch culture also showed the highest consumption ratio of lactic acid to glucose and yield of butanol to glucose (Table 2). Furthermore, a gradual accumulation of glucose fed to the broth was observed after 3 h of cultivation (Fig. 3) when the volume of the feeding solution was relatively high (data not shown). These data suggested that the metabolism of lactic acid by the N1-4 strain was activated to some extent in pH-stat fed-batch cultures with lactic acid and glucose, which will result in efficient butanol production from lactic acid.
There have been several reports on the conversion of acetic acid or butyric acid to butanol, confirmed by isotope analysis (Hartmanis et al. 1984; Wood et al. 1945). However, to the best of our knowledge, this is the first study describing the conversion of lactic acid to butanol using ABE-producing clostridia, confirmed by GC-MS analysis. In the metabolic pathways, both glucose and lactic acid are converted to pyruvate by glycolysis enzyme and lactate dehydrogenase, respectively, and then to acetyl-CoA (Jones and Woods 1986). Two molecules of acetyl-CoA are condensed to synthesize one molecule of acetoacetyl-CoA; thereafter, butanol results from acetoacetyl-CoA by several reductive reactions without carbon loss. On the basis of this knowledge, we could explain why three types of 12C- and 13C-butanol were observed on GC-MS analysis with [1,2,3-13C3] lactic acid and 12C6-glucose (Fig. 4b). Two types of acetyl-CoA, i.e., 12C2- and 13C2-acetyl-CoA, can be produced from 12C6-glucose and [1,2,3-13C3] lactic acid, respectively. Furthermore, these two types of acetyl-CoA can yield three types of acetoacetyl-CoA by the condensation of each molecule: (1) 12C4-acetoacetyl-CoA, (2) 13C-acetoacetyl-CoA in which two carbons are replaced by 13C-atoms, and (3) 13C4-acetoacetyl-CoA. As shown in Fig. 4b, three mass spectra of butanol strongly indicated three corresponding types of acetoacetyl-CoA. In addition, we indicated that 54.3% of lactic acid was converted to butanol by the results of GC-MS and HPLC analyses with [1,2,3-13C3] lactic acid (data not shown). Unfortunately, we could not detect other products such as acetone, ethanol, and CO2 under the measurement conditions of GC-MS in this study. Given that 33.3% of lactic acid being converted to CO2 by judging from the metabolic pathways, the N1-4 strain would convert 12.4% lactic acid to acetone and ethanol.
In this study, we achieved butanol production from lactic acid by adding glucose to medium as a co-substrate although it was presumed that lactic acid could be converted to butanol without glucose metabolism by judging from the metabolic pathways of ABE-producing clostridia (Jones and Woods 1986). In the pathways, ATP formed by glucose metabolism should not be necessary for the conversion. Nevertheless, the N1-4 strain maybe requires it for keeping them metabolically active. In addition, lactic acid was actually consumed (ca 3.6 g/l) but little butanol (0.13 g/l) was produced in the absence of glucose (L20; Table 1), which suggested that an intermediate metabolite derived from the lactic acid metabolism was accumulated in the N1-4 strain. To elucidate the above phenomena and assumption, a metabolome analysis would be required further. At least the cultures with added lactic acid resulted in approximately 10–20% increase in yield of butanol to glucose compared with that without lactic acid (Tables 1 and 2). Glucose, which was used as co-substrates of lactic acid in this study, is considered as an expensive substrate. To become more economically and technically feasible, further research is planned to investigate substrates other than glucose such as xylo-oligosaccharide or cello-oligosaccharide obtained by a hydrolysis of abundant plant biomass.
In conclusion, we are the first to report that lactic acid enhances butanol production by the N1-4 strain in ABE fermentation. Furthermore, we developed a system that is capable of producing high concentrations of butanol using a pH-stat continuous lactic acid feeding method and obtained 15.5 g/l butanol at a production rate of 1.76 g/l/h. Finally, by GC-MS analysis, we confirmed the bioconversion of lactic acid to butanol.
References
Andersch W, Bahl H, Gottschalk G (1983) Level of enzymes involved in acetate, butyrate, acetone and butanol formation by Clostridium acetobutylicum. Eur J Appl Microbiol Biotechnol 18:327–332
Bahl H, Andersch W, Braun K, Gottschalk G (1982) Effect of pH and butyrate concentration on the production of acetone and butanol by Clostridium acetobutylicum grown in continuous culture. Eur J Appl Microbiol Biotechnol 14:17–20
Chen CK, Blaschek HP (1999) Effect of acetate on molecular and physiological aspects of Clostridium beijerinckii NCIMB 8052 solvent production and strain degeneration. Appl Environ Microbiol 65:499–505
Demain AL (2009) Biosolutions to the energy problem. J Ind Microbiol Biotechnol 36:319–332
Desai RP, Harris LM, Welker NE, Papoutsakis ET (1999) Metabolic flux analysis elucidates the importance of the acid-formation pathways in regulating solvent production by Clostridium acetobutylicum. Metab Eng 1:206–213
Dürre P (2007) Biobutanol an attractive biofuel. Biotechnol J 2:1525–1534
Ezeji TC, Qureshi N, Blaschek HP (2005) Continuous butanol fermentation and feed starch retrogradation: butanol fermentation sustainability using Clostridium beijerinckii BA101. J Biotechnol 115:179–187
Fond O, Matta-Ammouri G, Petitdemange H, Engasser JM (1985) The role of acids on the production of acetone and butanol by Clostridium acetobutylicum. Appl Microbiol Biotechnol 22:195–200
Green EM, Boynton ZL, Harris LM, Rudolph FB, Papoutsakis ET, Bennett GN (1996) Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142:2079–2086
Harris LM, Desai RP, Welker NE, Papoutsakis ET (2000) Characterization of recombinant strains of the Clostridium acetobutylicum butyrate kinase inactivation mutant: need for new phenomenological models for solventogenesis and butanol inhibition? Biotechnol Bioeng 67:1–11
Hartmanis MGN, Gatenbeck S (1984) Intermediary metabolism in Clostridium acetobutylicum: levels of enzymes involved in the formation of acetate and butyrate. Appl Environ Microbiol 47:1277–1283
Hartmanis MGN, Klason T, Gatenbeck S (1984) Uptake and activation of acetate and butyrate in Clostridium acetobutylicum. Appl Microbiol Biotechnol 20:66–71
Hofvendahl K, Hahn-Hägerdal B (1997) l-lactic acid production from whole wheat flour hydrolysate using strains of Lactobacilli and Lactococci. Enzyme Microb Technol 20:301–307
Hüsemann MHW, Papoutsakis ET (1989) Enzymes limiting butanol and acetone formation in continuous and batch cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 31:435–444
Hüsemann MH, Papoutsakis ET (1990) Effects of propionate and acetate additions on solvent production in batch cultures of Clostridium acetobutylicum. Appl Environ Microbiol 56:1497–1500
Ishizaki A, Michiwaki S, Crabbe E, Kobayashi G, Sonomoto K, Yoshino S (1999) Extractive acetone-butanol-ethanol fermentation using methylated crude palm oil as extractant in batch culture of Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564). J Biosci Bioeng 87:352–356
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microbiol Rev 50:484–524
Lee TM, Ishizaki A, Yoshino S, Furukawa K (1995) Production of acetone, butanol and ethanol from palm oil waste by Clostridium saccharoperbutylacetonicum N1-4. Biotechnol Lett 17:649–654
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by clostridia. Biotechnol Bioeng 101:209–228
Linko YY, Javanainen P (1996) Simultaneous liquefaction, saccharification, and lactic acid fermentation on barley starch. Enzyme Microb Technol 19:118–123
Matta-el-Ammouri G, Janati-Idrissi R, Junelles AM, Petitdemange H, Gay R (1987) Effects of butyric and acetic acids on acetone-butanol formation by Clostridium acetobutylicum. Biochimie 69:109–115
Monot F, Engasser JM, Petitdemange H (1984) Influence of pH and undissociated butyric acid on the production of acetone and butanol in batch cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 19:422–426
Tashiro Y, Takeda K, Kobayashi G, Sonomoto K, Ishizaki A, Yoshino S (2004) High butanol production by Clostridium saccharoperbutylacetonicum N1-4 in fed-batch culture with pH-stat continuous butyric acid and glucose feeding method. J Biosci Bioeng 98:263–268
Tashiro Y, Shinto H, Hayashi M, Baba S, Kobayashi G, Sonomoto K (2007) Novel high-efficient butanol production from butyrate by non-growing Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) with methyl viologen. J Biosci Bioeng 104:238–240
Thang VH, Kanda K, Kobayashi G (2010) Production of acetone–butanol–ethanol (ABE) in direct fermentation of cassava by Clostridium saccharoperbutylacetonicum N1–4. Appl Biochem Biotechnol 161:157–170. doi:10.1007/s12010-009-8770-1
Tsuge T, Tanaka K, Ishizaki A (2001) Development of a novel method for feeding a mixture of l-lactic acid and acetic acid in fed-batch culture of Ralstonia eutropha for poly-d-3-hydroxybutyrate production. J Biosci Bioeng 91:545–550
Wood HG, Brown RW, Werkman CH (1945) Mechanism of the butyl alcohol fermentation with heavy carbon acetic and butyric acids and acetone. Arch Biochem 6:243–260
Yun JS, Wee YJ, Kim JN, Ryu HW (2004) Fermentative production of dl-lactic acid from amylase-treated rice and wheat brans hydrolyzate by a novel lactic acid bacterium, Lactobacillus sp. Biotechnol Lett 26:1613–1616
Zhao Y, Tomas CA, Rudolph FB, Papoutsakis ET, Bennett GN (2005) Intracellular butyryl phosphate and acetyl phosphate concentrations in Clostridium acetobutylicum and their implications for solvent formation. Appl Environ Microbiol 71:530–537
Acknowledgement
We are deeply grateful to Daisuke Miura (Innovation center for medical redox navigation, Kyushu University, Fukuoka, Japan) for GC-MS analysis. This research was in-part financially supported by the Sumitomo Corporation (Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oshiro, M., Hanada, K., Tashiro, Y. et al. Efficient conversion of lactic acid to butanol with pH-stat continuous lactic acid and glucose feeding method by Clostridium saccharoperbutylacetonicum . Appl Microbiol Biotechnol 87, 1177–1185 (2010). https://doi.org/10.1007/s00253-010-2673-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2673-5