Abstract
Insect killing fungi have high potential in controlling agriculturally harmful pest, but their slow progress and high variation in killing insect are major impediments to successful industrialization. The present work describes the use of supernatant from the liquid culture of Beauveria bassiana SFB-205 to surmount this problem, particularly efficient production of thermotolerant chitinase, which is one of the major pathogenesis-related enzymes in the supernatant. The chitinase was precipitated using varying mineral precipitants and followed by lyophilization, which was compared with a salting out method using ammonium sulfate in effectiveness. Incorporating of the supernatant fraction of the Beauveria preparation with attagel at 0.5% (w/v) as a precipitant enabled this treatment to show the greatest chitinase-precipitation efficiency (93.4%), followed up with excellent insecticidal activity against cotton aphids when it was mixed with 0.01% (v/v) polyoxyethylene-(3)-isotridecyl ether (TDE-3) as a spreading agent in laboratory conditions. Consequently, lyophilized attagel-mediated precipitation pellet was superior to lyophilized salting out pellet in maintaining chitinase activity against a thermal stress at 50°C. This finding provides that the attagel-mediated precipitation can be exploited to improve the thermotolerance of B. bassiana SFB-205 chitinase as a novel strategy for biopesticide production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insect-killing (entomopathogenic) fungi, in common with other natural enemies of insects, are widely available biological control agents (BCAs) for controlling agriculturally harmful pests (Burges 1998; Roberts and Hajek 1992; Wraight et al. 2001). Several Hypocreal species from the genera Beauveria, Metarhizium, Isaria, and Lecanicillium and some others have been registered in the USA by the Environmental Protection Agency (EPA) and commercialized (EPA 2009). Such entomopathogenic fungal products, however, constitute a small percentage of a total insecticide market (Yatin et al. 2006) although they are still among the practical BCAs against agricultural pests that feed on crops by sucking (Roberts and Hajek 1992; Vandenberg 1996). In general, the commercialized products, based on spores (conidia), still have some limitations to successful commercialization in terms of phyllospheric applications. One is their slow progress in killing insect, compared to chemical pesticides (Wraight et al. 2001). Peculiarly, some insects, which have very short life cycle, may discard conidia attached on their cuticles before fungal conidia germinate and penetrate into insect cuticles. Another is their high variation in control effectiveness, possibly affected by environmental abiotic factors during pathogenesis (Bateman and Alves 2000; Burges 1998; Inglis et al. 1997). Germination of conidia, initiation of vegetative growth, and succeeding enzymatic activations, mainly involved in pathogenesis, are deeply influenced by abiotic factors such as humidity, temperature, sunlight, etc.
Alternatively, fungal culture broth can enhance fungus-induced mortality soon after application, less influenced by such abiotic factors (Cliquet and Jackson 2005; Fargues et al. 2001; Jackson et al. 2004; Vidal et al. 1998). Culture broth of entomopathogenic fungi contains various pathogenesis-related components such as blastospores, hyphal bodies, and metabolites contributing in multiple ways to the insect-killing process. Enzymes, among the metabolites, have been reported to play important roles in pathogenesis (Charnley 2003) and studied intensively to improve the insecticidal activities of original isolates or to determine the function of enzymes (Donatti et al. 2008; Fan et al. 2007a, b; Kaur and Padmaja 2008; Pava-Ripoll et al. 2008). In particular, chitinase [EC 3.2.1.14] plays an indispensable role in penetrating insect cuticles (Kim et al. 2009, 2010; St. Leger et al. 1998; St. Leger and Screen 2001) though there are many other important pathogenesis-related factors such as Pr1 [EC 3.4.21] and Pr2 [EC 3.4.21] proteases relevant to the penetration (Campos et al. 2005; Donatti et al. 2008; Goettel et al. 1989; Kaur and Padmaja 2008; Moraes et al. 2003; St. Leger et al. 1996), Mcl1(Metarhizium collagen-like proteins), MAD1, MPL1, and MOS1, which are involved in host immune response, adhesion of conidia, appressorial turgor pressure, and recognition of host cuticles (Wang and St. Leger 2006, 2007a, b, 2008). In Beauveria bassiana (Bals.-Criv.) Vuill. (Ascomycota: Hypocreales), a widely used biological control agent, several chitinolytic enzymes have been investigated as follows: 45 and 110 kDa (Havukkala et al. 1993); 28 kDa (Fuguet et al. 2004); 33 kDa (Fang et al. 2005); and 55 kDa (Murad et al. 2007).
Stability problems of such enzymes, however, often arise in a liquid state due to the complex native structure of the protein molecules (Fan et al. 2007a, b; Hou et al. 1998). In general, such instability is followed up with serious defects in manufacturing and storing protein products (Arakawa et al 1993; Kim et al. 2008a; Pikal 1994). Thus proteins are frequently formulated in a dry state through lyophilization (Kumar et al. 2009; Pikal 1994; Wang 2000). In the beginning of the downstream processing of proteins, precipitation is one of the typical processes before drying and usually applied to the large scale recovery and purification of proteins (Chen and Berg 1993). Many precipitants have been developed as follows: salts (Czok and Bucher 1960; Scopes and Stoter 1982); organic solvents (Askonas 1951; Englards and Seifter 1990); non-ionic polymers (Juckes 1971); polyeletrolytes (Burgess 1991; Kim and Kim 2000; Sternberg and Hershberger 1974); and metals (Jeyarajah and Allen 1994; Kawahara et al. 1994; Satoh et al. 1990). Despite varying precipitation methods using these precipitants, ammonium sulfate-mediated precipitation (salting out) at low temperature conditions is a widely used method for preparative protein precipitation because the salting out method is simple and easy to use even when large volume of materials should be processed in laboratory conditions (Hilbrig and Freitag 2003).
However, the salting out using ammonium sulfate is hardly applied to the precipitation of proteins for pest control because the process needs a considerable amount of ammonium sulfate (>55% (w/v)) and low temperatures (<10°C) for ca. 5–12 h for maximum protein precipitation efficiency (Bollag et al. 1996). Besides, ammonia, which is a byproduct of the process, is very reactive with stainless steel, a primary building material for purification facilities. Other salts, such as sodium sulfate, are sometimes used to surmount this problem, but none are as good as ammonium sulfate in protein precipitation efficiency. In the present work, we first describe the precipitation of chitinase from the supernatnat, i.e., cultured medium, of B. bassiana SFB-205 (KCCM 10892P) (Kim et al. 2008b; Kim et al. 2009, 2010) using varying mineral precipitants for improving the thermotolerance of chitinase, compared to the salting out method. Silica gel, pyrophillite, kaolin, and attagel were used as precipitants, and processed supernatants with the precipitants were incubated at room temperature for 1 h before precipitation. This novel approach for biopesticide production provides not only (1) precipitation efficiency of chitinase using attagel (selected among the precipitants), precipitation of proteins confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and improved thermotolerance of chitinase by attagel-mediated precipitation but also (2) aphicidal activity of the attagel-mediated precipitation pellet against cotton aphids, followed by the degradation of cotton aphid cuticles by the pellet to place value in the novel approach.
Materials and methods
Fungal isolate
B. bassiana SFB-205 (Kim et al. 2008b), deposited at Korean Culture Center of Microorganisms (KCCM; www.kccm.or.kr) (KCCM 10892P), was also stored at –80°C in 10% (v/v) glycerol at the Dongbu Advanced Research Institute of Dongbu HiTeK. It was propagated on Sabouraud dextrose agar medium supplemented with 0.5% (w/v) yeast extract (SDAY, pH 6) in Petri dishes in darkness at 25 ± 1°C for 14 days (Humber 1997).
Liquid culture
A liquid culture was produced in a soluble starch-yeast extract-glycerol medium (SYG, pH 6; per liter): 10 g soluble starch (Gibco), 5 g yeast extract (Gibco), and 5 ml glycerol (Fisher Scientific). A conidial suspension was aseptically inoculated into SYG medium, ending up to 1 × 106 conidia/ml of final concentration in a 200-ml culture broth. The inoculated broth was held on a rotary shaker at 150 rpm and 27 ± 1°C for 3 days (Humber 1997).
Salting out
A supernatant, i.e., cultured medium, was prepared by the centrifugation of the cultured broth, and the supernatant was incorporated with ammonium sulfate to obtain protein pellet (salting out) (Bollag et al. 1996). Centrifugation of the cultured broth was conducted at 16,000×g and 4°C for 10 min and followed by filtration (0.2 μm). Chitinase activity of the supernatant was assayed to evaluate the precipitation efficiency of chitinase in subsequent experiments, the detail of which is described later. A protein pellet was prepared by slowly adding crystalline ammonium sulfate into a 10-ml supernatant with gentle stirring until 70% (w/v) saturation and held at 4°C for 12 h (Bollag et al. 1996). A precipitate was collected by centrifugation of the processed supernatant at 16,000×g and 4°C for 10 min and either lyophilized or dissolved in 0.1 M citrate-phosphate buffer (pH 6) to adjust to the original volume (10 ml) of intact supernatant.
Mineral-mediated precipitation
As another approach to obtain protein pellet, a supernatant was incorporated with a mineral precipitant and centrifuged. A 10-ml supernatant was mixed with the following precipitant at 0.5% (w/v) in a 15-ml conical polypropylene centrifuge tube (Falcon) with three replications (three tubes): silica gel (230–400 mesh; Merck), pyrophillite (300–400 mesh; Samshin CM), kaolin (300–400 mesh; Ju-Hyun Chemical), attagel (320–400 mesh; Engelhard-BASF), or ammonium sulfate (Fisher Scientific) as a control. pH of each mixture was measured at 25 ± 1°C to characterize the precipitation condition. All mixtures were held on a rotary shaker at 150 rpm and 25 ± 1°C for 1 h and centrifuged at 16,000×g and 4°C for 10 min. After separation of solutions from the tubes, all pellets were suspended in the citrate-phosphate buffer (pH 6) to adjust to the original volume (10 ml) of intact supernatant. The salting out method, described above (70% (w/v) saturation and 12 h of incubation at 4°C), served as a positive control. All pellet suspensions and separated solutions after the centrifugation were subjected to pH measurement at 25 ± 1°C, followed by chitinase assays. Chitinase precipitation efficiency was defined as follow: \( \left( {{\hbox{chitinase activity of pellet suspension}}/{\hbox{chitinase activity of intact supernatant}}} \right) \times {1}00\% \). Each treatment was replicated three times in an experimental replicate, and the entire experiment was repeated twice using different batches of supernatant on different days.
Thermotolerance of chitinase
Supernatant and lyophilized pellets made by salting out or attagel-mediated precipitation, which showed the greatest chitinase precipitation, were exposed to a high temperature to compare the thermotolerance of chitinase between (1) supernatant vs. lyophilized salting out pellet; and (2) lyophilized salting-out pellet vs. lyophilized attagel-mediated precipitation pellet. Firstly, a 10-ml supernatant and a lyophilized salting out pellet from the same amount (10 ml) of supernatant were held in capped 15-ml conical polypropylene centrifuge tubes (Falcon; 15 tubes/treatment) and exposed to 50 ± 1°C of an incubator, except three tubes from each treatment as controls (non-exposed; 0 h). Every three tubes in each treatment were taken out of the incubator at 1 h of interval for 4 h. After the exposure, all lyophilized salting out pellets were suspended into the citrate-phosphate buffer (pH 6) to make 10 ml suspensions. All the supernatants and pellet suspensions were subjected to chitinase assays. Secondly, a 0.01-g lyophilized attagel-mediated precipitation pellet and a 0.01-g lyophilized salting out pellet were held in capped 15-ml conical tubes (15 tubes/treatment) and exposed to the same thermal stress described above. After the exposure, all pellets were suspended into the citrate-phosphate buffer (pH 6) to make 10 ml suspensions and followed by chitinase assays. This entire experiment was repeated twice using different batches of products on different days.
Chitinase assay
All processed products, such as supernatants, pellet suspensions, and separated solutions after the precipitation, were assayed on the level of chitinase, by determining the release of p-nitrophenol from p-nitrophenyl-β-d-acetylglucosaminide (pNG) (Roberts and Selitrennikoff 1988). A 100-μl sample was mixed with 100 μl of 10 mM pNG (Sigma-Aldrich) and 300 μl of 0.1 M citrate-phosphate buffer (pH 6). After incubation at 37°C for 1 h, 500 μl of 1 M sodium carbonate was added into the solution. The kinetic assay was performed using a spectrophotometer (UV-260, Shimadzu) at 405 nm. One unit of chitinase activity was defined as the amount of enzyme needed to release one millimole of p-nitrophenol per hour per ml. Each treatment was replicated three times within an experimental replicate, and the entire assay was repeated twice using different batches of products.
SDS-PAGE
The size of proteins, which were placed in salting out pellet and attagel-mediated precipitation pellet, was investigated by SDS- PAGE. All pellets made from the same amount of intact supernatant (10 ml) were subjected to 12% SDS-PAGE, followed by Coomassie Blue R-250 staining (Laemmli 1970).
Aphicidal activity
Bioassays against cotton aphids, as a step to explore the aphicidal activity of attagel-mediated precipitation pellet, were conducted using a leaf-disc method in laboratory conditions. Cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae), adults were obtained from the insectary of the Institute of Dongbu HiTeK Co. Ltd. (Daejeon, Republic of Korea). They were maintained on hot peppers, Capsicum annuum L. var. grossum, at 25 ± 1°C and a 16:8 (L/D) photoperiod with 40–50% relative humidity (RH) in acrylic chambers (45 × 30 × 30 cm) with aphid-proof meshes. Hot pepper leaves were taken from 5-leaf stage plants, which had been infested with adult cotton aphids. All leaves were cut to make leaf discs (6 mm diameter) having ca. 270–300 adults per disc. Every three leaf discs (three replications/treatment) were dipped into the following solutions or suspensions for 10 s and dried at room temperature for 30 min: supernatant (100×); attagel-mediated pellet suspension (adjusted to the original volume of intact supernatant) (100×); separated solution (100×) after the precipitation using attagel; and intact attagel suspension (0.5% (w/v)) (100×). All dilutions were conducted using 0.1 M citrate-phosphate buffer (pH 6). As a wetting agent, 0.01% (v/v) polyoxyethylene-(3)-isotridecyl ether (TDE-3; CAS 24938-91-8; Coseal Co.) was mixed with all dilutions and also served as a control treatment, which was based on the citrate phosphate buffer. Overall, the loss of cotton aphid adults from leaf discs due to the dipping was <3%. All leaf discs were placed in Petri dishes with dampened filter papers (0.5 ml sterile water/plate), and initial aphid population per leaf disc was counted. All plates were covered with lids having aphid-proof meshes, sealed with Parafilm, and incubated at 25 ± 1°C and a 16:8 (L/D) photoperiod with 40–50% RH. Control efficacy at 2 days post-treatment was evaluated as the percentage of the non-treated control aphid population, assuming the non-treated population was 100%, by counting the number of living cotton aphids per leaf disc and correcting for control mortality using the Abbott's formula (1925). Each treatment was replicated three times within an experimental replicate, and the entire experiment was repeated twice using different batches of samples on different days.
Transmission electron microscopy of aphid cuticles
Cuticle structure of cotton aphids treated with an attagel-mediated pellet suspension was observed using a transmission electron microscopy, which was compared with supernatant and non-treated control treatments. Adult cotton aphids, obtained from the insectary, were surface-sterilized with 1% (v/v) sodium hypochlorite for 1 min and subsequently washed with sterile water. Ten surface-sterilized aphid adults were placed in 1 ml of supernatant (100×) and attagel-mediated pellet suspension (100×), respectively and incubated at room temperature for 12 h. The pellet suspension was prepared by adding lyophilized pellet—originated from a 10-ml of supernatant—into 0.1 M citrate-phosphate buffer (pH 6) to make 10 ml of suspension. All dilutions were conducted using the same method that was used in the bioassay. TDE-3 (0.01%) based on the citrate phosphate buffer was served as a control treatment. After incubation, all adults were taken out of the tubes, washed with excess amount of the citrate phosphate buffer twice, and dried at room temperature for 2 h. All adults were fixed in 20% (v/v) paraformaldehyde and 2% (v/v) glutaraldehyde in 0.05 M sodium cacodylate buffer (pH 7.2) for 2 h. After post-fixation in 1% (w/v) OsO4 in the same buffer, the samples were dehydrated in an ethanol/propylene oxide series and embedded in an Epon–Araldite mixture (Ibarra and Fedrici 1986). Ultrathin sections were cut with a RMC MT-X ultramicrotome and examined in a JEM-1010 (JEOL) transmission electron microscope.
Data analysis
Data on the chitinase activity, the pH, and the percentage of aphid population were analyzed by the analysis of variance or the general linear model, considering possible block effects caused by experimental repetitions. Theses analyses were followed with Tukey's honestly significant difference (HSD) for multiple comparisons. In addition, linear regressions of the data on the chitinase activity based on exposure time were carried out to compare the overall thermotolerance between treatments. All the analyses were conducted using SPSS ver. 17.0 (SPSS Inc. 2009) at the 0.05 (α) level.
Results
Thermotolerance of chitinase: supernatant vs. lyophilized salting out pellet
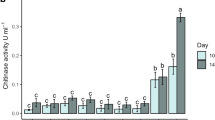
The lyophilized salting out pellet was superior to the supernatant in maintaining the chitinase activity against the thermal stress (Fig. 1). The chitinase activity of supernatant reduced by 97.7% (12.8 to 0.3 units/ml) at 4 h post-exposure. In contrast, a suspension made with the lyophilized salting out pellet that was exposed to the same thermal stress showed only 20.6% reduction (12.6 to 10.0 units/ml) in chitinase activity. From the linear regressions, the lyophilized salting out pellet treatment showed –0.614 of slope (\( {\hbox{R}}_{{\rm{adj}}{.}}^2 = 0.{923} \), p < 0.001), which was lower than the slope of the supernatant treatment (–3.244) (\( {\hbox{R}}_{{\rm{adj}}{.}}^2 = 0.831 \), p < 0.001).
Chitinase activities (mean ± SE) of supernatant and lyophilized salting-out pellet prior to (0 h) and after exposure to 50°C for 1, 2, 3, and 4 h (n = 90). Bars labeled with the same lower case letter within the same exposure time or the same upper case letters throughout the exposure times within the same product do not significantly differ according to Tukey's HSD test (p < 0.05)
Precipitation of chitinase by mineral precipitants
The attagel treatment showed the highest chitinase precipitation efficiency among the several precipitant treatments (0.5% minerals and ammonium sulfate), which was comparable to the salting out method at 70% saturation with ammonium sulfate (Table 1). At 1 h post-incubation at 25 ± 1°C using 0.5% dosage of precipitants, the attagel treatment exhibited 93.3% chitinase precipitation efficiency, which was significantly higher than the other treatments as follows: silica gel (78.4%); pyrophillite (80.3%); kaolin (59.9%); and ammonium sulfate (6.6%). Unusually, in the kaolin treatment, the sum of chitinase activity of pellet suspension and separated solution did not correspond to the chitinase activity of intact supernatant. Most of supernatants that were mixed with the mineral precipitants showed ca. pH 6–7, similar to the pH of intact supernatant (pH 6.6). However, adding kaolin into the supernatant resulted in the change of the pH into more alkali condition (pH 8.4). Even after the precipitation in the kaolin treatment, the separated solution still showed such alkali pH. The attagel-mediated precipitation (93.3% efficiency) was similar to the salting out (94.8% efficiency) in the precipitation of chitinase. In the SDS-PAGE, similar pattern of protein bands were detected between attagel-mediated pellet and salting out pellet treatments (Fig. 2). Among several protein bands, ca. 55 kDa of protein band was most observable in the two treatments with a similar level of intensity.
Aphicidal activity of attagel-mediated pellet
The attagel-mediated pellet suspension showed high insecticidal activity against cotton aphids in laboratory conditions, though a little less than the supernatant (Fig. 3). The attagel-mediated pellet suspension showed 81.2% control efficacy, significantly higher than the separated solution (8.1%) after precipitation at 2 d post-treatment. However, the control efficacy of the pellet suspension was lower than that of the supernatant (91.9%; p < 0.001). Intact attagel suspension (4.4%) showed very low control efficacy, similar to the non-treated control (p > 0.05). Dead black- and brown-colored cotton aphids, mostly deformed and flattened, were observed in the supernatant and attagel-mediated pellet treatments, as compared to the dark green living aphids in the non-treated control. The mortality of the non-treated control treatment, natural mortality, was <2% at 2 days post-treatment.
Percentage of cotton aphid population (mean ± SE) in hot pepper leaf discs treated with supernatant or attagel-mediated pellet suspension amended with 0.01% (v/v) TDE-3 in laboratory conditions at 2 days post-treatment (n = 45). Bars labeled with the same lower case letter do not significantly differ by the Tukey's HSD test (p < 0.05)
Degradation of aphid cuticles by attagel-mediated pellet
The attagel-mediated pellet suspension remarkably degraded cotton aphid cuticles as much as the supernatant did (Fig. 4). In the two treatments, all cotton aphid cuticles were substantially degraded, and the structures of the aphid haemocoel were deformed, as compared to the 0.01% TDE-3 treatment as a control. No normal cuticular laminations were observed in the supernatant and pellet suspension treatments. Difference in the level of cuticle degradation was not observed between the two treatments. In contrast, such laminations were apparently observed in the control treatment (TDE-3 only).
Thermotolerance of chitinase: lyophilized attagel-mediated pellet vs. salting out pellet
The lyophilized attagel-mediated precipitation pellet was superior in maintaining chitinase activity against the high temperature condition, as compared to the lyophilized salting out pellet (Fig. 5). The lyophilized attagel-mediated precipitation pellet showed 91.5% of the original chitinase activity (unexposed) at 4 h post-exposure. However, the lyophilized salting out pellet showed 78.7% of the original (unexposed). The linear regressions showed that the slope (–8.417) of the lyophilized attagel-mediated precipitation pellet treatment (\( {\hbox{R}}_{{\rm{adj}}{.}}^2 = 0.{865} \), p < 0.001) was lower than the slope (–18.988) of the lyophilized salting out pellet treatment (\( {\hbox{R}}_{{\rm{adj}}{.}}^2 = 0.{935} \), p < 0.001).
Chitinase activities (mean ± SE) of lyophilized pellets made by salting out or attagel-mediated precipitation prior to (0 h) and after exposure to 50 °C for 1, 2, 3, and 4 h (n = 90). Bars labeled with the same lower case letter within the same exposure time or the same upper case letters throughout the exposure times within the same product do not significantly differ according to Tukey's HSD test (p < 0.05)
Discussion
Lyophilizing of salting out pellet served as a background for the present achievement by indicating a significant improvement in thermotolerance of chitinase (Fig. 1). Among the mineral precipitants, finally attagel was selected as the best precipitant, which did not require as long incubation time at low temperature or large amount of precipitant as salting out method (Table 1). The attagel-mediated precipitation achieved 93.3% chitinase precipitation efficiency, similar to the salting out (94.8%). It was demonstrated by the same pattern of protein bands between the two treatments in the SDS-PAGE (Fig. 2). The most observable protein band, ca. 55 kDa, possibly involves the chitinase of B. bassiana SFB-205 (Kim et al. 2010; Murad et al. 2007).
The metal ions of the mineral precipitants may positively affect the chitinase precipitation efficiency. Pyrophillite and kaolin have 2 moles of Al3+ ions, but attagel has 5 moles of Mg2+ ions. Furthermore, silica gel, pyrophillite, and kaolin have 1, 4, and 2 moles of Si4+ ions, respectively, whereas attagel has 8 moles of Si4+ ions. However, the four hydroxyl groups (OH−) of kaolin, which increased the pH of the supernatant after incorporation, may be followed up with the inhibition of chitinase activity. In fact, in the kaolin treatment, some of chitinase activity was not detected, given the chitinase activity of the intact supernatant.
Attagel (Mg5Si8O2(HO)2(OH2)4·4H2O) is a crystalloid hydrous magnesium-aluminum silicate with a laminated chain structure including a crystalline lattice displacement (Ying et al. 1995). Such structure causes the crystals to possess uncertain quantities of Na+, Ca2+, Fe3+ and Al3+ ions, which are present in the shape of needles, fibers or fibrous clusters of attagel. It has good colloidal properties such as specific features in dispersion, high temperature endurance, salt and alkali resistance, and high adsorbing and de-coloring capabilities. These characteristics may enable the chitinase of the lyophilized attagel-mediated pellet to achieve higher thermotolerance than the lyophilized salting out pellet (Fig. 5). Attagel is enough safe to be used as a food additive (Engelhard-BASF).
The high level of aphicidal activity of the attagel-mediated precipitation pellet (81.2% control efficacy) can be explained by the degradation of cotton aphid cuticles by the pellet though it was a little less than the efficacy of supernatant. The cotton aphid cuticles, comprising epicuticles and procuticles, were mostly degraded by the attagel-mediated pellet treatment, which was comparable to the supernatant treatment. Not only the chitinase but also other enzymes, such as proteases, lipases, aminopeptidases, etc., may be involved in the cuticular degradation by the pellet, given the existence of Pr1 and Pr2 proteases in the supernatant (Kim et al. 2009, 2010) and the characteristics of attagel such as high adsorbing capacity, described above. The pellet possibly degrades cuticle proteins, chitin-binding proteins, and chitins. Consequently, many pathogenesis-related enzymes including the chitinase may be precipitated by adding attagel into the supernatant; their identities remain to be determined in a future study. However, some insecticidal metabolites, such as beauvericin (CAS No. 26048-05-5), oxolinic acid (CAS No. 14698-29-4), etc, may still exist in the supernatant even after the separation of pellet. The background of this assumption is the lower insecticidal activity of the attagel-mediated pellet than the supernatant (91.9% control efficacy) and the physical and chemical properties of attagel. These metabolites cannot easily bind attagel, given the nature of silicate in a chromatography technology. As a proof of evidence, oxolinic acid is not being detected in the lyophilized attagel-mediated pellet, but it is in the supernatant.
Furthermore, TDE-3 as a wetting agent for cotton aphids can improve the aphicidal activity of the attagel-mediated precipitation pellet. Kim and Je (2010) reported that application of SFB-205 supernatant incorporated with TDE-3 more significantly reduces cotton aphid population, as compared to supernatant incorporated with Tween 80, which is correlated with the degradation of cotton aphid cuticles by the two treatments, confirmed by SDS-PAGE. The synergistic activity of TDE-3 can be explained by the smaller hydrophile-lipophile balance (HLB) value—representing a surfactant activity—of TDE-3 (HLB = 9.0) than Tween80 (HLB = 15.0). A smaller HLB value means more lipophilic and penetrable into lipids compounds, main components of the most outer part of insect cuticles. Consequently, within cotton aphid cuticles, TDE-3 may serve as a cuticle destroyer to facilitate the enzyme activity of the attagel-mediated precipitation pellet.
In an industrial perspective, the attagel-mediated precipitation can be used to produce a technical material as a biological control agent within ca. 2 weeks. The process comprises liquid cultures (seed and main cultures), precipitation using attagel at 0.5% (w/v) at the end of the main culture, and lyophilization of the precipitate. In particular when the attagel-mediated precipitation pellet is compared with the supernatant in the light of handling, the pellet production method has advantages in moving and storing of final products.
In conclusion, the attagel-mediated precipitation, similar to salting out in chitinase precipitation efficiency, enabled B. bassiana SFB-205 chitinase to have more improved thermotolerance than salting out. This method requires neither as long incubation time at low temperature or large amount of precipitant as salting out, finally suggesting high potential of the attagel-mediated precipitation in industrial use. Successfully, the attagel-induced precipitation method has been exploited as a main process to produce a fungal enzyme product as a novel biopesticide which has been commercialized in the Korean agricultural market as the commercial name of New-Chungzigi® Suspension Concentrate (http://www.dongbuhitek.co.kr/agri/), under the guideline on the registration of biopesticides in the Republic of Korea since 2009.
References
Arakawa T, Prestrelski S, Kenney WC, Carpenter JF (1993) Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev 10:1–28
Askonas BA (1951) The use of organic solvents at low temperature for the separation of enzymes. Biochem J 48:42–48
Bateman RP, Alves RT (2000) Delivery systems for mycoinsecticides using oil-based formulations. Aspect Appl Biol 57:163–170
Bollag DM, Rozycki MD, Edelstein SJ (1996) Protein methods, 2nd edn. Wiley-Liss, New York
Burges HD (1998) Formulation of mycopesticides. In: Burges HD (ed) Formulation of microbial biopesticies: beneficial microorganisms, nematodes and seed treatment. Kluwer Academic Publishers, Dordrecht, pp 131–186
Burgess RR (1991) Use of polyethyleneimine in purification of DNA binding proteins. Methods Enzymol 208:3–10
Campos RA, Arruda W, Boldo JT, Silvia MV, Barros NM, Azedevo JL, Schrank A, Vainstein MH (2005) Boophilus microplus infection by Beauveria amorpha and Beauveria bassiana: SEM analysis and regulation of subtilisin-like proteases and chitinases. Curr Microbiol 50:257–261
Charnley AK (2003) Fungal pathogens of insects: cuticle degrading enzymes and toxins. Adv Bot Res 40:241–321
Chen W, Berg JC (1993) The effect of polyelectrolyte dosage on floc formation in protein precipitation by polyelectrolytes. Chem Eng Sci 48:1775–1784
Cliquet S, Jackson MA (2005) Impact of carbon and nitrogen nutrition on the quality, yield and composition of blastospores of the bioinsecticidal fungus Paecilomyces fumosoroseus. J Indus Microbiol Biotechnol 32:204–210
Czok R, Bucher T (1960) Crystallized enzymes from the myogen of rabbit skeletal. Adv Protein Chem 15:315–415
Donatti AC, Furlaneto-Maia L, Fungaro MH, Furlaneto MC (2008) Production and regulation of cuticle-degrading proteases from Beauveria bassiana in the presence of Rhammatocerus schistocercoides cuticle. Curr Microbiol 56:256–260
Englards S, Seifter S (1990) Precipitation techniques. Methods Enzymol 182:285–300
Environmental Protection Agency (2009) Biopesticide Active Ingredient Fact Sheets. EPA US Web. Available at http://www.epa.gov/pesticides/biopesticides/ingredients/. Accessed 17 Dec 2009
Fan Y, Zhang Y, Yang X, Pei X, Guo S, Pei Y (2007a) Expression of a Beauveria bassiana chitinase (Bbchit1) in Escherichia coli and Pichia pastoris. Protein Expr Purif 56:93–99
Fan Y, Fang W, Guo S, Pei X, Zhang Y, Xiao Y, Li D, Jin K, Bidochka MJ, Pei Y (2007b) Increased insect virulence in Beauveria bassiana strains overexpressing an engineered chitinase. Appl Environ Microbiol 73:295–302
Fang W, Leng B, Xiao Y, Jin K, Ma J, Fan Y, Feng J, Yang X, Zhang Y, Pei Y (2005) Cloning of Beauveria bassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl Environ Microbiol 71:363–370
Fargues J, Smits N, Vidal C, Vey A, Vega F, Mercadier G, Quimby P (2001) Effect of liquid culture media on morphology, growth, propagule production, and pathogenic activity of the Hyphomycete, Metarhizium flavoviride. Mycopathologia 154:127–138
Fuguet R, Théraud M, Vey A (2004) Production in vitro of toxic macromolecules by strains of Beauveria bassiana, and purification of a chitosanase-like protein secreted by a melanizing isolate. Comp Biochem Physiol C Toxicol Pharmacol 138:149–161
Goettel MS, St. Leger RJ, Rizzo NW, Staples RC, Roberts DW (1989) Ultrastructural localization of a cuticle degrading protease produced by the entomopathogenic fungus, Metarhizium anisopliae during penetration of host cuticle. J Gen Microbiol 135:2223–2239
Havukkala I, Mitamura C, Hara S, Hirayae K, Nishizawa Y, Hibi T (1993) Induction and purification of Beauveria bassiana chitinolytic enzymes. J Invertebr Pathol 61:97–102
Hilbrig F, Freitag R (2003) Protein purification by affinity precipitation. J Chromatogr B 790:79–90
Hou WC, Chen YC, Lin YH (1998) Chitinase activity of sweet potato (Ipomoea batatas [L.] Lam var. Tainong 57. Bot Bull Acad Sin 39:93–97
Humber RA (1997) Fungi: preservation of cultures. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, San Diego, pp 269–279
Ibarra JE, Federici BA (1986) Isolation of a relatively nontoxic 65-kilodalton protein inclusion from the parasporal body of Bacillus thuringiensis subsp. israelensis. J Bacteriol 165:527–533
Inglis GD, Johnson DL, Goettel MS (1997) Effects of temperature and sunlight on mycosis of Beauveria bassiana (Hyphomycetes: Sympodulosporae) of grasshoppers under field conditions. Environ Entomol 26:400–409
Jackson MA, Payne AR, Odelson DA (2004) Liquid-culture production of blastospores of the bioinsecticidal fungus Paecilomyces fumosoroseus using portable fermentation equipment. J Ind Microbiol Biotech 31:149–154
Jeyarajah S, Allen JC (1994) Calcium binding and salt-induced structural changes of native and preheated β-lactoglobulin. J Agric Food Chem 42:80–85
Juckes IR (1971) Fractionation of proteins and viruses with PEG. Biochim Biophys Acta 82:463–475
Kaur G, Padmaja V (2008) Relationships among activities of extracellular enzyme production and virulence against Helicoverpa armigera in Beauveria bassiana. J Basic Microbiol 48:1–10
Kawahara M, Muramoto K, Kobayashi K, Mori H, Kuroda Y (1994) Aluminum promotes the aggregation of Alzheimer's amyloid beta-protein in vitro. Biochem Biophys Res Commun 198:531
Kim JS, Lee HY, Chung BJ, Je YH (2008a) Method for enhancing the thermal tolerance of entomopathogenic fungal spores, blastospores and enzymes. PCT Patent Publication No. WO/2008/063011 (Korean Patent No. 10-0835689-0000).
Kim JS, Roh JY, Choi JY, Shin SC, Jeon MJ, Je YH (2008b) Identification of an entomopathogenic fungus, Beauveria bassiana SFB-205 toxic to the green peach aphid, Myzus persicae. Int J Indust Entomol 17:211–215
Kim JS, Roh JY, Choi JY, Wang Y, Shim HJ, Je YH (2009) Correlation of the aphicidal activity of Beauveria bassiana SFB-205 supernatant with enzymes. Mycol Res 114:120–128
Kim JS, Je YH (2010) Relation of aphicidal activity with cuticular degradation by Beauveria bassiana SFB-205 supernatant incorporated with polyoxyethylene-(3)-isotridecyl ether. J Microbiol Biotechnol 20:506–509
Kim JS, Roh JY, Choi JY, Je YH (2010) Influence of two FPLC fractions from Beauveria bassiana SFB-205 supernatant on the insecticidal activity against cotton aphid. Biocon Sci Technol 20:77–81
Kim WS, Kim WS (2000) Lysozyme precipitation with polyacrylic acid in semi-batch reactor. HWAHAK KONGHAK 38:236–243
Kumar V, Sharma VK, Kalonia DS (2009) In situ precipitation and vacuum drying of interferon alpha-2a: development of a single-step process for obtaining dry, stable protein formulation. Int J Pham 366:88–98
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Moraes CK, Schrank A, Vainstein MH (2003) Regulation of extracellular chitinase and proteases in the entomopathogen and acaricide Metarhizium anisopliae. Curr Microbiol 46:205–210
Murad AM, Laumann RA, Mehta A, Noronha EF, Franco OL (2007) Screening and secretomic analysis of enthomopathogenic Beauveria bassiana isolates in response to cowpea weevil (Callosobruchus maculates) exoskeleton. Comp Biochem Physiol C Toxicol Pharmacol 145:333–338
Pava-Ripoll M, Posada FJ, Momen B, Wang C, St. Leger RJ (2008) Increased pathogenicity against coffee berry borer, Hypothenemus hampei (Coleoptera: Curculionidae) by Metarhizium anisopliae expressing the scorpion toxin (AaIT) gene. J Invertebr Pathol 99:220–226
Pikal MJ (1994) Freeze drying of proteins: process, formulation, and stability. In: Cleland JL, Langer R (eds) Formulation and delivery of peptides and proteins, vol 1. American Chemical Society, Washington DC, pp 120–133
Roberts DW, Hajek AE (1992) Entomopathogenic fungi as bioinsecticides. In: Leatham GF (ed) Frontiers of industrial mycology. Chapman and Hall, New York, pp 114–159
Roberts WK, Selitrennikoff CP (1988) Plant and bacterial chitinases differ in antifungal activity. J Gen Microbiol 134:169–176
Satoh K, Adachi H, Yamashita S, Hirano H, Yasin Y, Ueda Y (1990) Calcium-induced aggregation of the urea-deaggregated human lens insoluble protein. Exp Eye Res 50:719–723
Scopes RK, Stoter A (1982) All the glycolytic enzymes from one muscle extract. Methods Enzymol 90:82–93
SPSS version 17.0 for Window (2009) SPSS Inc., Chicago, IL
St. Leger RJ, Joshi L, Roberts DW (1998) Ambient pH is a major determinant in the expression of cuticle-degrading enzymes and hydrophobin by Metarhizium anisopliae. Appl Environ Microbiol 64:709–713
St. Leger RJ, Screen SE (2001) Prospects for strain improvement of fungal pathogens of insects and weeds. In: Butt TM, Jackson C, Magan N (eds) Fungi as biocontrol agents. CABI, Wallingford, pp 219–237
St. Leger RJ, Joshi L, Bidochka MJ, Roberts DW (1996) Construction of an improved mycoinsecticide overexpressing a toxic protease. Proc Natl Acad Sci USA 93:6349–6354
Sternberg M, Hershberger P (1974) Separation of proteins with polyacrylic acids. Biochim Biophys Acta 34:195–206
Vandenberg JD (1996) Standardized bioassay and screening of Beauveria bassiana and Paecilomyces fumosoroseus against the Russian wheat aphid (Homoptera: Aphididiae). J Econ Entomol 89:1418–1423
Vidal C, Fargues J, Lacey L, Jackson M (1998) Effect of various liquid culture media on morphology, growth, propagule production, and pathogenic activity to Bemisia argentifolii of the entomopathogenic Hyphomycete, Paecilomyces fumosoroseus. Mycopathologia 143:33–46
Wang C, St. Leger RJ (2006) A collagenous protective coat enables Metarhizium anisopliae to evade insect immune responses. Proc Natl Acad Sci USA 103:6647–6652
Wang C, St. Leger RJ (2007a) The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects: the MAD2 adhesin enables attachment to plants. Eukaryot Cell 6:808–816
Wang C, St. Leger RJ (2007b) Metarhizium anisopliae perilipin homolog MPL1 regulates lipid metabolism, appressorial turgor pressure and virulence. J Biol Chem 282:21110–21115
Wang C, St. Leger RJ (2008) MOS1 osmosensor of Metarhizium anisopliae is required for adaptation to insect host hemolymph. Eukaryot Cell 7:302–309
Wang W (2000) Lyophilization and development of protein pharmaceuticals. Int J Pharm 203:1–60
Wraight SP, Jackson MA, de Kock SL (2001) Production, stabilization and formulation of fungal biological agents. In: Butt TM, Jackson C, Magan N (eds) Fungi as biocontrol agents. CABI, Wallingford, pp 253–287
Yatin BT, Venkataraman NS, Parija TK, Panneerselvam D, Govindanayagi P, Geetha K (2006) The new biopesticide market. Business Communications Research, Denver
Ying Z, Gevert B, Otterstedt J, Sterte J (1995) Large-pore catalysts for hydro processing of residual oils. Ind Eng Chem Res 34:1566–1571
Acknowledgements
This work was supported by the Biopesticide Research and Development Project of Dongbu HiTek Co., Ltd. as well as by the “Specific Joint Agricultural Research-promoting Projects (Project No. 20090101030080),” RDA, Republic of Korea. Many thanks go to Dr. Bong Jin Chung and Dr. Tae Joon Kim (Dongbu HiTek, Korea), Dr. Jong Ryul Roh (Seoul National University), Dr. Dong Soo Yang (Abson BCL Inc., Korea), Dr. Dave Moore (CABI, UK), Dr. Richard A. Humber (USDA-ARS), and Dr. Robert W. Jones (University of Vermont) for their helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.S., Je, Y.H. A novel biopesticide production: attagel-mediated precipitation of chitinase from Beauveria bassiana SFB-205 supernatant for thermotolerance. Appl Microbiol Biotechnol 87, 1639–1648 (2010). https://doi.org/10.1007/s00253-010-2543-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2543-1