Abstract
We cloned and expressed the gene for an intracellular α-amylase, designated AmyB, from the hyperthermophilic bacterium Thermotoga neapolitana in Escherichia coli. The putative intracellular amylolytic enzyme contained four regions that are highly conserved among glycoside hydrolase family (GH) 13 α-amylases. AmyB exhibited maximum activity at pH 6.5 and 75°C, and its thermostability was slightly enhanced by Ca2+. However, Ca2+ was not required for the activity of AmyB as EDTA had no effect on enzyme activity. AmyB hydrolyzed the typical substrates for α-amylase, including soluble starch, amylose, amylopectin, and glycogen, to liberate maltose and minor amount of glucose. The hydrolytic pattern of AmyB is most similar to those of maltogenic amylases (EC 3.2.1.133) among GH 13 α-amylases; however, it can be distinguished by its inability to hydrolyze pullulan and β-cyclodextrin. AmyB enzymatic activity was negligible when acarbose, a maltotetraose analog in which a maltose residue at the nonreducing end was replaced by acarviosine, was present, indicating that AmyB cleaves maltose units from the nonreducing end of maltooligosaccharides. These results indicate that AmyB is a new type exo-acting intracellular α-amylase possessing distinct characteristics that distinguish it from typical α-amylase and cyclodextrin-/pullulan-hydrolyzing enzymes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thermotoga neapolitana, a marine hyperthermophile isolated from geothermally heated biotopes, and T. maritima belong to the order Thermotogales (Conners et al. 2006). Interestingly, members of the genus Thermotoga have the largest number of genes for carbohydrate-active enzymes in free-living prokaryotes known to date (Nelson et al. 1999). T. neapolitana and T. maritima have both been shown to catabolize a wide variety of α- and β-linked glucans via a fermentative metabolism (Bibel et al. 1998; Bok et al. 1998; Bronnenmeier et al. 1995; Chhabra et al. 2001, 2002, 2003; Conners et al. 2005; Duffaud et al. 1997; Gabelsberger et al. 1993; Liebl 2001; McCutchen et al. 1996; Miller et al. 2001; Nguyen et al. 2001; Parker et al. 2001a, b; Ruile et al. 1997; Veith et al. 2003; Wassenberg et al. 1997; Yernool et al. 2000).
Genomic and biochemical data suggest that T. maritima can catabolize α-1,4 linked maltose as well as pullulan and starch, which contain mixed α-1,4 and α-1,6 linkages (Bibel et al. 1998; Chhabra et al. 2003). Microarray analysis of T. maritima revealed apparent specificities for hydrolases with specific carbon sources and associated transcriptional regulators for the various pathways (Conners et al. 2005, 2006). Biochemical characterization of the specific enzymes that break down α-linked polysaccharides preceded the sequencing of the T. maritima genome, and these works also contributed to the reconstruction of these various pathways (Bibel et al. 1998; Liebl et al. 1997; Schumann et al. 1991).
In the amino acid sequence-based classification system for carbohydrate active enzymes the vast majority of α-amylases are sorted into the glycoside hydrolase family (GH) 13 (Henrissat 1991). All enzymes of this family share a (β/α)8-barrel as the common supersecondary structure. Some α-amylases are also classified into GH 57. This enzyme family is considerably smaller than GH 13 and less well investigated. Among the starch-degrading enzymes of T. maritima described in the literature thus far are three α-amylases; an extracellular lipoprotein Amy13A (KEGG Database entry TM1840) and two enzymes Amy13B (TM1650) and Amy57C (TM1438) located in the cytoplasm (Ballschmiter et al. 2006; Liebl et al. 1997; Lim et al. 2003). Amy13A, a Ca2+-requiring membrane-bound α-amylase, hydrolyzes extracellular starch in an endo-type fashion. However, it exhibits less activity in the context of more highly branched polysaccharides such as glycogen, amylopectin, and pullulan (Liebl et al. 1997; Schumann et al. 1991). The activity of intracellular α-amylases Amy13B and Amy57C appears to complement that of an α-amylase that hydrolyzes amylose and starch, but their catalytic properties have not been studied in details (Ballschmiter et al. 2006; Lim et al. 2003). It is believed that these two enzymes may be involved in the utilization of maltodextrin or storage polysaccharides like glycogen, but the physiological roles of the intracellular amylases are still unknown.
In this study, the gene coding for an Amy13B homolog from T. neapolitana was cloned and expressed in E. coli to study its biochemical and catalytic properties. The enzymatic characteristics of the T. neapolitana intracellular α-amylase (AmyB) provide evidence that AmyB is a new type exo-acting α-amylase that possesses distinct characteristics from typical α-amylases (EC 3.2.1.1) and cyclodextrin (CD)-hydrolyzing enzymes such as maltogenic amylase (MAase, EC 3.2.1.133), cyclodextrinase (CDase, EC 3.2.1.54), and neopullulanase (EC 3.2.1.135).
Materials and methods
Bacterial strains and culture conditions
Thermotoga neapolitana Korean Culture Center of Microorganisms (KCCM) 41025 was obtained from the KCCM and cultivated under anaerobic conditions as described previously (Park et al. 2005a, b). Escherichia coli BL21(DE3) was used as the heterologous host for protein overexpression. E. coli transformants were grown in Luria–Bertani broth [1% (w/v) Bacto-tryptone, 0.5% (w/v) yeast extract, 0.5% (w/v) NaCl] containing kanamycin (50 μg ml−1) at 37°C. Plasmid pET-29b(+) (Novagen, San Diego, CA) was used as the cloning and expression vector.
Cloning and expression of the T. neapolitana amyB gene
T. neapolitana genomic DNA was prepared using the GenomiPhi DNA Amplification Kit (GE Healthcare, Piscataway, NJ). The gene encoding AmyB (TM1650 homolog) was identified in the T. neapolitana genome sequence using two amylase-specific internal primers MA2 (5′-GAC GGY TGG CGB YTN GAT GT-3′) and Deg2 (5′- ACR CGM GGC WGR TCR TGG TT-3′), and protein blast searches. AmyB-specific oligonucleotide primers, TNamyB-F and TNamyB-R, were designed to flank the 5′ and 3′ ends of TM1650 sequence and the gene was amplified by PCR using Taq polymerase and T. neapolitana genomic DNA as a template. The amplified DNA fragment was cloned into the pGEM-T vector (Promega, Madison, WI). For the overexpression of AmyB protein in E. coli, the forward primer (amy-N, 5′- GGT ACC ATG AAC CTC AAA AAC CTG ATA ATA TAC-3′) and the reverse primer (amy-R, 5′-GAA TTC TCA GTA GAG CAC GAA AGA AAG TAT CAG AGG-3′) were designed containing KpnI and EcoRI restriction sites (underlined), respectively, for directional cloning into the expression vector pET-29b(+). The resulting plasmid was named pET-AmyB. The nucleotide sequence of the PCR-generated gene was determined using the BigDye terminator cycle sequencing kit and an ABI 377 Prism DNA Sequencer (AME Bioscience Ltd., Bedfordshire, UK). Detailed bioinformatic analyses of the gene and the deduced amino acid sequences of various genes and proteins were performed using DNASIS, PROSIS (v7.0, Hitachi Software, Tokyo, Japan) and CLUSTAL programs (Thompson et al. 1994).
Purification of the recombinant AmyB enzyme
The recombinant E. coli BL21(DE3) strain carrying pET-AmyB was grown in 2 l of liquid culture with kanamycin selection in a 5-l baffled flask at 37°C. The T7 promoter of the plasmid was induced with 0.2 mM isopropyl-1-thio-β-d-galactoside at an optical density (600 nm) of 0.6. After 12 h of induction, the cells were harvested by centrifugation (10,000 × g, 20 min, 4°C) and resuspended in 20 mM sodium phosphate buffer (pH 6.5). Cells were broken by a twofold passage through a French pressure cell (American Instruments, Silver Spring, MD). The crude cell extract was centrifuged at 12,000 × g (20 min, 4°C) to remove cell debris. The supernatant was then incubated at 80°C for 30 min to denature thermolabile host proteins and centrifuged again at 12,000 × g (20 min, 4°C) to remove denatured protein from the extract. The resulting supernatant was dialyzed against 20 mM sodium phosphate (pH 6.5) at 4°C for overnight and subjected to cation-exchange chromatography using a HiTrap SP HP column (GE Healthcare/Amersham, Freiburg, Germany) equilibrated with the same buffer. Elution was carried out with a 0 to 1.0 M NaCl gradient in the same buffer in 15 column volumes at a flow rate of 1.0 ml min−1.
The molecular mass and the purity of the recombinant AmyB protein was estimated by gel electrophoresis in a 12% (w/v) sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel. To determine the native molecular mass of the purified protein, analytical size exclusion chromatography was carried out using a Sephacryl S-200 column (GE Healthcare). An isocratic gradient of 20 mM Tris-HCl (pH 8.0) with the addition of 0.15 M NaCl was applied. The protein concentration was determined according to the Bradford method (Bradford 1976), with bovine serum albumin as a standard.
Enzyme assay
Enzyme activity was assayed at 75°C in 50 mM sodium phosphate buffer (pH 6.5) with 3,5-dintrosalicylic acid as described by Miller (Miller 1959). The reaction mixture (0.5 ml) was composed of 0.1 ml of 0.5% (wt/vol) soluble starch as a substrate and 0.1 ml of enzyme solution (43 U ml−1). The reaction mixture was incubated for 30 min at 75°C to facilitate the enzymatic reaction, and was terminated by quenching on ice. Color development was measured at 575 nm, and the specific activity was calculated using maltose as a standard. One unit of AmyB was defined as the amount of enzyme that released 1 nmol of reducing sugar equivalents per minute under the described test conditions.
Effects of pH and temperature on the activity and stability of AmyB
The influence of pH on the activity of AmyB was measured at 75°C in sodium acetate (pH 4.5 to 5.5), MES (pH 5.5 to 6.5), N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES; pH 6.5 to 8.0) and Tris-HCl (pH 8.0 to 9.0). The pH of each buffer system was adjusted at 75°C using the standard enzyme assay conditions described above. The influence of temperature on the activity of AmyB was determined in 50 mM sodium phosphate buffer (pH 6.5) ranging from 50°C to 95°C. The thermostability of the enzyme was analyzed by incubating the enzyme solution (0.1 mg ml−1 in 50 mM sodium phosphate buffer, pH 6.5) at 80°C, 90°C, 95°C, and 100°C. Aliquots were taken at every hour and placed immediately in an ice-water bath to halt enzymatic activity. The residual soluble starch-hydrolyzing activities of the aliquots were measured at the optimal temperature condition. The effect of various metal ions and chemical reagents on enzyme activity was also examined. The sodium phosphate buffer was replaced by HEPES buffer in order to avoid precipitation. The concentration of metal ions and chemical reagents was 1 mM except for urea (2 M) and SDS (2%).
Hydrolytic patterns of AmyB
To examine the hydrolytic patterns of AmyB, purified AmyB (43 U ml−1) was incubated with 0.5 ml of 0.5% (wt/vol) maltooligosaccharides (maltotriose to maltoheptaose), soluble starch, amylose, amylopectin, glycogen, pullulan, and β-cyclodextrin in 50 mM sodium phosphate buffer (pH 6.5). Each reaction was incubated at 75°C for 16 h, and subsequently placed immediately in an ice-water bath to stop the reaction. The resulting reaction products were analyzed by thin-layer chromatography (TLC) on Whatman K5F silica gel plates (Whatman, Madistone, UK) with 1-propanol/ethyl acetate/H2O (6:2:3, vol/vol/vol) as the solvent system. After irrigating twice, the TLC plate was dried and visualized by dipping it into a solution containing 0.3% (wt/vol) N-(1-naphthyl)-ethylenediamine and 5% (v/v) H2SO4 in methanol and then heating for 5 min at 120°C (Robyt and Mukerjea 1994). For the labeled maltohexaose reaction, maltohexaose labeled with [14C]-glucose at the reducing end was prepared as previously described (Park et al. 2005a, b). The enzymatic reaction was carried out at 0.5% [14C]-maltohexaose in a 50 mM sodium phosphate buffer (pH 6.5) at 75°C and stopped immediately in an ice-water bath. The reaction products were separated by TLC and developed as described above. To visualize radioactive intermediates, the TLC plate was placed on an imaging plate for 12 h and the radioactivity of each spot was measured using an image analyzer (Typhoon; Bio-Rad, Hercules, CA).
Kinetic parameters of AmyB
Kinetic parameters of AmyB for maltotriose, maltopentaose, maltoheptaose, and soluble starch were determined. Samples (0.5 ml) from the reaction mixture containing enzyme and substrate in 50 mM sodium phosphate buffer (pH 6.5) at 75°C were taken at timed intervals of 90 s, and the reaction was immediately stopped by the addition of an equal volume of 0.1 M HCl on ice. After neutralizing by adding an equal volume of 0.1 N NaOH, the amount of glucose and maltose released from maltooligosaccharides was assayed by the glucose oxidase–peroxidase method (Miwa et al. 1972) and HPLC, respectively. In brief, 0.1 ml of enzyme solution was added to 0.4 ml of substrate solution. The hydrolysis reaction was performed and then mixed with 1 ml of the glucose oxidase/peroxidase solution. After incubation for 30 min at 37°C, the reaction was stopped by adding 1 ml of 12 N H2SO4 and the product absorbance was measured at 540 nm. The amount of reducing sugars produced from soluble starch (average molecular weight, 10,000) was measured by the copper-bicinchoninate method (Fox and Robyt 1991). In the case of p-nitrophenyl-α-d-hexaoside (pNPG6), the absorbance was measured at 405 nm with p-nitrophenol as a standard.
Kinetic data were converted to Lineweaver–Burk plots with the SigmaPlot program (version 5.0; SPSS Inc., Chicago, IL). The K m values were calculated from the slopes of the curves, and the catalytic turnover values (k cat) were calculated by dividing the maximal reaction velocities by the total amount of enzyme in the reaction mixture.
Nucleotide sequence accession number
The nucleotide sequence of and the deduced amino acid sequence encoded by amyB gene in T. neapolitana have been submitted to GenBank under accession no. EU871663.
Results
Comparison of AmyB with various amylolytic enzymes
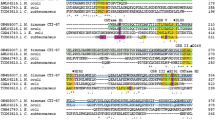
The T. neapolitana genome was screened for amylase genes using two amylase-specific internal primers. The sequence analysis of a 299-bp PCR fragment was attributed to the amy13B gene (KEGG database number TM1650) of T. maritima. Based on the genomic sequence of T. maritima, the entire T. neapolitana gene encoding AmyB was successfully amplified by PCR using AmyB-specific oligonucleotide primers (TNamyB-F and TNamyB-R) and sequenced. It was expected that the open reading frame of AmyB (1,269 bp) would encode a single polypeptide of 422 amino acids with an estimated molecular mass of 50,119 Da. The deduced amino acid sequence of the T. neapolitana AmyB shares 86% identity with that of Amy13B from T. maritima MSB8, 86% identity with an Amy13B homolog (TRQ2_1179) from Thermotoga sp. RQ2, and 82% identity with an Amy13B homolog (Tpet_1141) from T. petrophila RKU-1. However, it exhibited unexpectedly low sequence similarity (5−15% identity) with other GH 13 amylolytic enzymes, including α-glucosidase (EC 3.2.1.20; Peist et al. 1996), α-amylase (EC 3.2.1.1; Stam et al. 2006), oligo-1,6-glucosidase (EC 3.2.1.10; Oslancová and Janecek 2002), maltogenic amylase (EC 3.2.1.133; Park et al. 2000) and CD-/pullulan-hydrolyzing enzymes (Lee et al. 2002a, b; Park et al. 2000). Interestingly, a multiple sequence alignment of AmyB with closely related enzymes revealed that an extra N-terminal domain (Kim et al. 2001) known to exist in CD-hydrolyzing enzymes and is involved in the oligomerization of the enzyme, did not exist in AmyB from T. neapolitana. The structure/function and evolutionary relationships within GH 13 amylolytic enzymes have revealed the existence of the conserved sequence regions (Janecek 1994, 1995, 2002). Although four well-known conserved regions (I, II, III, and IV) and invariant catalytic residues of GH 13 were evident (Cha et al. 1998; Janecek 2002), additional three conserved sequence regions (V, VI, and VII) have also been known. These regions are thought to be important for the sequence features characteristic of certain enzyme specificities from the family. The examination of the variations of the residues in these three conserved regions among GH 13 enzymes indicates that AmyB is a new type of amylolytic enzyme(Fig. 1).
Comparison of AmyB and related other GH 13 amylolytic enzymes. The invariant sequences are highlighted in black and white inversion, and the catalytic triad is signified by asterisks. The highly conserved sequences are emphasized by boxes. Amy13A Thermotoga maritima extracellular α-amylase (CAA72194), Amy13B Thermotoga maritima intracellular α-amylase (AAD36717), AmyB T. neapolitana intracellular α-amylase (ACF75909), Aspergillus oryzae Taka_α-amylase A (BAA00336), Bacillus circulans strain 8, cyclodextrin glucanotransferase (CAA48401), Bacillus cereus, oligo-1,6-glucosidase (CAA37583), Pseudomonas stutzeri maltotetraohydrolase (AAA25707), Pseudomonas amyloderamosa isoamylase (AAA25854), Thermoactinomyces vulgaris R-47 neopullulanase (BAA02473), Thermus sp. IM6501 maltogenic amylase (AAC15072), Bacillus stearothermophilus maltogenic α-amylase (AAA22233). Multiple alignment was performed using Clustal W2 and visualized using ESPript (http://espript.ibcp.fr/ESPript/ESPript/)
Expression of AmyB in E. coli
The AmyB protein was highly expressed in E. coli BL21(DE3) harboring pET-AmyB (Fig. 2). Heat treatment (80°C for 30 min) was shown to efficiently exclude substantial amounts of heat-labile host proteins from cell-free extracts, and the protein was further purified using HiTrap SP HP column chromatography. The purified enzyme showed a single protein band on SDS-PAGE (Fig. 2), and the molecular mass of purified AmyB was estimated to be approximately 48 kDa. The same molecular mass was found via gel filtration chromatography, suggesting that the enzyme is monomeric.
Enzymatic properties of AmyB
The pH range at which the recombinant AmyB was active was determined using soluble starch as the substrate. As shown in Fig. 3a, the maximum activity was observed at pH 6.5. More than 50% of the maximum activity was obtained in the range between pH 5.0 and 8.5. The optimal temperature of AmyB was approximately 75°C, and relatively low activities were observed above 90°C (Fig. 3b). However, AmyB exhibited remarkable thermal stability, retaining its full activity after 7 h of incubation at 90°C (Fig. 3c). The half-life of AmyB was determined to be 50.2 and 28 h at 95°C and 100°C, respectively. This result implies that the recombinant enzyme was not only successfully expressed in E. coli but it also preserved its thermostability. The effect of various metal ions on enzyme activity was also examined. Ca2+ at a concentration of 1 mM had a positive effect on AmyB activity; however, Zn2+, Mg 2+, and Cu2+ reduced the activity to 70.4%, 69.4%, and 52.2%, respectively, while Co2+, Mn2+, and Hg2+ completely inactivated the enzyme. However, the addition of EDTA did not inhibit AmyB activity, indicating that Ca2+, although stimulating, was not required for activity of the recombinant enzyme. Urea (2 M) had a strong inhibitory effect, whereas DTT, 2-mercaptoethanol (1 mM), and SDS (2%) had no effect on activity (Table 1).
Effect of pH and temperature on the activity and stability of AmyB. a For the determination of optimal pH, the following buffers were used: pH 4.5 to 5.5, 50 mM sodium acetate (filled circle); pH 5.5 to 6.5, 50 mM MES (empty triangle); pH 6.5 to 8.0, 50 mM HEPES (filled square); and pH 8.0 to 9.0, 50 mM Tris-HCl (empty diamond). The values are shown as the specific activity of AmyB. b To determine optimal temperature, activity was measured at the indicated temperatures under the standard conditions of the assay. The values are shown as the specific activity of AmyB. c To determine the thermostability of AmyB, purified enzyme was incubated at 80°C (filled circle), 90°C (empty circle), 95°C (filled inverted triangle), and 100°C (empty triangle ) in 50 mM sodium phosphate buffer (pH 6.5). After various time intervals, samples were withdrawn and the residual activity was measured at 75°C under the standard conditions of the assay
The enzymatic activity of AmyB was examined with various substrates typically used for analyzing the hydrolytic activity of α-amylases, such as maltooligosaccharides, soluble starch, amylose, amylopectin, glycogen, pullulan, and β-cyclodextrin (Fig. 4). AmyB hydrolyzed soluble starch, amylose, amylopectin, and glycogen; typical substrates for α-amylases that produce mainly maltose with minor amounts of glucose (Fig. 4, lanes 3 to 6). In contrast, pullulan and β-cyclodextrin, substrates easily metabolized by CD-/pullulan-hydrolyzing enzymes such as MAase, was barely degraded by AmyB. This hydrolytic pattern was also confirmed by the measurement of AmyB specific activity. The specific activities of AmyB toward starch, amylose, and amylopectin were 18.8, 10.9, and 6.3 U mg−1, respectively. The degradation of pullulan and β-cyclodextrin was negligible, which was less than 10% of the specific activity of starch (data not shown). The hydrolysis of maltotriose, maltopentaose, and maltoheptaose by AmyB resulted in maltose and glucose products, whereas the hydrolysis of maltotetraose and maltohexaose yielded only maltose (Fig. 4, lanes 1 and 2, 9 to 13). The hydrolysis pattern of AmyB with these substrates is similar to that of MAase, but it can be distinguished from the MAase in that AmyB cannot hydrolyze pullulan and β-cyclodextrin (Table 2). The different charactersitics between AmyB and MAase (CD hydrolyzing enzyme) is supported by the structure of primary sequences which shows the separated branch by evolutionary tree analysis (see discussion).
Hydrolysis pattern of AmyB on various substrates. Lane M, maltooligosaccharide standards (glucose to maltopentaose); lanes 1 and 9, maltotriose; lanes 2 and 10, maltotetraose; lane 3, soluble starch; lane 4, amylose; lane 5, amylopectin; lane 6, glycogen; lane 7, pullulan; lane 8, β-cyclodextrin; lane 11, maltopentaose; lane 12, maltohexaose; lane 13, maltoheptaose. AmyB was incubated with the various substrates at a concentration of 0.5% (wt/vol) at 75°C for 16 h
Kinetic parameters of AmyB for maltooligosaccharides and other substrates
The kinetic parameters K m and k cat of AmyB were determined with starch, maltotriose, maltopentaose, maltoheptaose, and pNPG6 using Lineweaver-Burk plots (Table 3). The K m values of AmyB for maltotriose, maltopentaose, and maltoheptaose were 1.26, 1.69 and 3.37 mM, respectively. However, the k cat values for maltotriose and maltopentaose (809 and 283 s−1, respectively) are much faster than those observed for maltoheptaose, pNPG6, and starch. Moreover, the k cat value for maltotriose was the fastest among the maltooligosaccharides. The k cat value for starch was 0.02 s−1, suggesting that starch is not a favorable substrate for AmyB compared with other maltooligosaccharides. From the k cat/K m data, it can be inferred that AmyB efficiently hydrolyzes maltooligosaccharides that have short glucose unit chain lengths.
Catalytic pattern of the enzyme reveals that AmyB is a new type α-amylase
In order to understand the detailed reaction mode of AmyB, the hydrolysis pattern was further investigated as a function of time with maltopentaose, maltoheptaose, pNPG6, and acarbose as substrates. AmyB was incubated with maltoheptaose at 75°C for various reaction times ranging from 30 min to 12 h to investigate the change in reaction products (Fig. 5a). Maltopentaose and maltose appeared first; with maltotriose and glucose increasing gradually, indicating the AmyB enzyme is an exo-type enzyme similar to Thermus maltogenic amylase (ThMA), which liberates maltose residues from the maltooligosaccharide (Kim et al. 1999). However, it can be distinguished from the ThMA in that pullulan and β-cyclodextrin are favorable substrates for ThMA, but not for AmyB. pNPG6 was used as a substrate to scrutinize whether AmyB releases maltose from the reducing or nonreducing end. It was observed that pNPG6 was initially degraded into maltose and pNPG4, and later, maltose and p-nitrophenol increased gradually, suggesting that the enzyme recognizes the maltose moiety at the nonreducing end of maltooligosaccharides (Fig. 5b). The ability of AmyB to release maltose units from the nonreducing end was confirmed using [14C]-maltohexaose, in which a [14C]-glucose is attached to the maltopentaose by a α-1,4-glycosidic linkage at the reducing end. AmyB was incubated with [14C]-maltohexaose at 75°C for reaction times ranging from 30 min to 12 h to investigate the change in reaction products (Fig. 5b). TLC analysis showed that [14C]-maltotetraose appeared first and [14C]-maltose increased gradually, providing supporting evidence that AmyB cleaves maltooligosaccharides to maltose units at the nonreducing end. Interestingly, when acarbose, a maltotetraose analog in which the maltose residue at the nonreducing end is replaced by acarviosine, was used as a substrate, AmyB could not cleave this substrate (data not shown). This data supports the previous evidence that AmyB releases the maltose unit at the nonreducing end. The inability of AmyB to act on acarbose indicates that the enzyme is sensitive to the type of residue at the nonreducing end of the substrate.
AmyB hydrolysis products of maltopentaose (G5), maltoheptaose (G7), p-nitrophenyl-α-d-maltohexaoside (pNPG6), and [14C]-maltohexaose ([ 14 C]-G6) as a function of reaction time. a Lane M, maltooligosaccharide standards (glucose to maltoheptaose); lanes 1–6, hydrolysis product of maltopentaose at different reaction times (0, 0.5, 1, 1.5, 2, and 12 h, respectively); lanes 7–12, hydrolysis product of maltoheptaose at different reaction times (0, 0.5, 1, 2, 4, and 12 h, respectively). b Lane M, maltooligosaccharide standards (glucose to maltopentaose); lane 1, pNPG1; lanes 2–7, hydrolysis product of pNPG6 at different reaction times (0, 0.5, 1, 1.5, 2, and 12 h, respectively); lanes 8–14, hydrolysis product of [14C]-maltohexaose at different reaction times (0, 0.5, 1, 1.5, 2, 3, and 12 h, respectively). AmyB was incubated with 1% (wt/vol) maltoheptaose and pNPG6 or 0.5% (wt/vol) maltopentaose and [14C]-maltohexaose at 75°C
Discussion
The pathway for α-linked glucan utilization in Thermotoga maritima has been extensively studied (Bibel et al. 1998; Chhabra et al. 2003; Conners et al. 2005). Therefore, the genomic and biochemical data from T. maritima and related enzymes can be used to predict pathways for the hydrolysis of α-linked glucans and oligosaccharides. In order to confirm predicted pathways, studies of the detailed biochemical characteristics of these enzymes are necessary. Many extracellular amylolytic enzymes of T. maritima have been studied, and their physiological roles for starch metabolism have been reported (Chhabra et al. 2002; Conners et al. 2005, 2006). The broad substrate specificities of many amylolytic enzymes make them difficult to determine which enzymes are required for the breakdown of starch into glucose or maltose for subsequent use in central metabolism. Until now, the physiological role of intracellular forms of these enzymes has not been clearly understood, although two enzymes (Amy13B and Amy57C) have been found in T. maritima (Ballschmiter et al. 2006; Lim et al. 2003). The aim of this study was to characterize a putative intracellular α-amylase of the hyperthermophilic bacterium T. neapolitana and elucidate the possible physiological role of this enzyme.
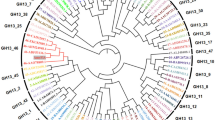
The putative intracellular α-amylase AmyB (TM1650 homolog) from T. neapolitana was successfully overexpressed in E. coli and biochemically characterized. AmyB exhibited maximum activity at 75°C and a pH of 6.5. Its thermostability was slightly enhanced by Ca2+ as observed in the Amy13B of T. maritima (Lim et al. 2003). However, a strong inhibitory effect was not observed with EDTA indicating that Ca2+ was not required for activity of T. neapolitana AmyB, unlike the dependence of Ca2+ for α-amylase (EC 3.2.1.1; Boel et al. 1990; Nielsen and Borchert 2000) and maltogenic amylase (EC 3.2.1.133; Tang et al. 2006) in the GH 13 family. Although Amy13B from T. maritima has been reported to hydrolyze starch, the activity was not extensively studied in terms of the hydrolyzed end products and hydrolyzing capacity for other α-glucans. Based on our results, AmyB displays unique features in many aspects. Although four highly conserved regions found in GH 13 family enzymes are present in AmyB, the amino acid sequence of AmyB has little similarity with α-amylases from other organisms as already described by Lim et al. (Lim et al. 2003). AmyB also lacks the N-terminal domain, which exists in other CD-/pullulan-hydrolyzing enzymes and is known to be involved in oligomerization of the enzyme (Kim et al. 2001; Yang et al. 2004). In terms of catalytic action, AmyB is quite different from CD-/pullulan-hydrolyzing enzymes such as MAase, CDase, and neopullulanase. AmyB was hydrolytically active on a variety of α-1,4-linked glucans. Apart from starch (relative activity 100%), the enzyme also cleaved amylose (58%), amylopectin (34%), and glycogen (17%) while the hydrolyses of pullulan and β-cyclodextrin, one of the preferred substrates of CD-/pullulan-hydrolyzing enzymes, were negligible (less than 10%). Acarbose is not degraded by AmyB, which is in marked contrast to its hydrolysis by CD-/pullulan-hydrolyzing enzymes and T. maritima glucosidase (TMG), which liberate glucose residues at the reducing end from maltooligosaccharides (Lee et al. 2002a, b). In phylogenetic analyses of GH 13 family enzymes, AmyB has its own separate branch with Amy13A and Amy13B from T. maritima apart from the other clusters for typical bacterial α-amylases, archaeal α-amylases, bacterial or archaeal CD-hydrolyzing enzymes, and CD-producing enzymes (CGTases; Fig. 6). The phylogenetic tree also supports the result of distinct catalytic properties of AmyB compared with many other amylolytic enzymes described thus far.
Phylogenetic relationship of AmyB with members of the GH13 family. Phylip format tree outputs from the CLUSTAL X analysis were visualized with TreeViewPPC based on the distance matrix using the neighbor-joining method. The bar at the lower left corner indicates the substitution rate (substitution/site). Bootstrap values (based on 1,000 bootstrap trials) are shown at each node. The unrooted phylogenetic tree was built from entire sequences of the following enzymes: AmyB represents intracellular α-amylase from Thermotoga neapolitana (ACF75909), Amy13B Thermotoga maritima intracellular α-amylase (AAD36717), ThMA maltogenic amylase (MAase) from Thermus sp. IM6501 (AAC15072), TpMA MAase from Thermoplasma volcanium (BAB59359), MA1 MAase from B. thermoalkalophilus (AAT94159), MA2 MAase from B. stearothermophilus (AAC46346), CD1 cyclodextrinase (CDase) from alkalophilic Bacillus sp. I-5 (AAA92925), CD2 CDase from Bacillus stearothermophilus (BAB63955), CD3 CDase from Thermococcus sp. B1001 (BAB18100), NPL neopullulanase from B. stearothermophilus (AAK15003), TVAII α-amylase II from Thermoactinomyces vulgarius (BAA02473), PFTA thermostable amylase from Pyrococcus furiosus (AAL82063), TMG Thermotoga maritima glucosidase (AAD36898), Amy13A Thermotoga maritima α-amylase (CAA72194), Bacha Bacillus halodurans α-amylase (G84015), Bacsu α-amylase from B. subtilis (AAQ83841), Bacli α-amylase from B. liqueniformis (ABF61440), Bacsp α-amylase from Bacillus sp. TS-23 (AAA63900), CGT1 cyclodextrin glucanotransferase (CGTase) from alkalophilic Bacillus sp. I-5 (AAR32682), CGT2 CGTase from B. circulans 251 (CAA55023), CGT3 CGTase from B. macerans (CAA41773), CGT4 CGTase from B. stearothermophilus ET1 (AAD00555), Thavu Thermoactinomyces vulgaris α-amylase (CAA49465), Thscu Thermomonospora curvata α-amylase (CAA41881), Essco Escherichia coli α-amylase (AAN82828), Salty Salmonella typhimurium α-amylase (AAL22523), Shifi Shigella flexineri α-amylase (AAN45063), Vibch Vibrio cholerae α-amylase (AAF96758), Yerpe Yersinia pestis α-amylase (AAM87640), Stcmu Streptococcus mutans α-amylase (AAC35010), Stmco Streptomyces coelicolor α-amylase (CAB88153), Pycfu Pyrococcus furiosus α-amylase (AAB67705), Pycsp Pyrococcus sp. KOD1 α-amylase (BAA21130), Thchy Thermococcus hydrothermalis α-amylase (AAC97877), Thcsp-Rt3 Thermococcus sp. Rt3 α-amylase (AAB87860), Thcsp-AEP Thermococcus sp. AEp11 α-amylase (AAM48113)
The kinetic data of AmyB activity with maltooligosaccharides explains that molecules with long chain length, including maltooligosaccharides and starch, are not favorable substrates for AmyB. Instead, maltotriose and maltopentaose are more favorable substrates, implying the enzyme prefers maltooligosaccharide substrates consisting of short glucose chain lengths. The catalytic pattern on maltoheptaose, maltopentaose, pNPG6, and [14C]-maltohexaose reveals that AmyB recognizes the nonreducing end of the substrate to successively liberate the maltose residue, and the type of residue at the nonreducing end seems to be important for enzymatic activity. The proposed action pattern of AmyB is summarized in Fig. 7. The results from kinetic data and the action pattern of AmyB were quite different from those of the typical extracellular α-amylases. In Amy13A from T. maritima, the enzyme activity decreased with decreasing chain length of the maltooligosaccharide substrate (Liebl et al. 1997). Furthermore, the reaction of Amy13A proceeded in an endo-type fashion, unlike that of AmyB. Overall comparisons of MAase, thermostable extracellular α-amylase, glucosidase, and AmyB are listed in Table 2. Together, these findings suggest that AmyB is a new type exo-acting α-amylase that shares some of the enzymatic characteristics of both typical α-amylases and MAases, however based on the data in this study, it can be easily distinguished from these enzymes.
On the basis of its substrate specificity and the kinetic studies for maltooligosaccharides, AmyB may be involved in either utilization of maltooligosaccharides transported through membrane following hydrolysis of extracellular α-glucan or metabolism of storage α-glucan in T. neapolitana. In the first scenario, maltooligosaccharides transported via maltose and maltotriose ABC transporters can be degraded into maltose and glucose by AmyB along with an exo-acting intracellular α-glucosidase (TM1068) and a α-glucosidase (TM1834), which are active on maltose and maltotriose but not starch, amylopectin or amylose. Sugars liberated from these enzymes can then be further metabolized through the Embden-Meyerhof or Entner-Doudoroff pathways (Bibel et al. 1998; Chhabra et al. 2002; Selig et al. 1997). However, carbohydrate-induced microarrays in T. maritima indicated that Amy13B (TM1650) and a putative α-glucosidase (Amy4C, TM1834) were not regulated by starch or maltose, whereas the extracellular α-amylase Amy13A (TM1840), an exo-acting intracellular α-glucosidase (TM1068), a 4-α-glucanotransferase (TM0364), and a pullulanase (TM1845) were upregulated in the presence of maltose or starch (Chhabra et al. 2003). Therefore, AmyB could be more likely involved in the breakdown of storage α-glucans like glycogen. In E. coli, 4-α-glucanotransferase (MalQ) uses maltose and glucose from endogenously produced maltotriose and higher maltodextrins; maltodextrin phosphorylase (MalP) then hydrolyzes the resulting maltodextrins to glucose-1-phoshate using free phosphate (Dipple and Boos 2005). Orthologs of 4-α-glucanotransferase (TM0364 and TM0767) and maltodextrin phosphorylase (TM1168) for the synthesis and degradation of α-glucan are found in T. maritima (Bibel et al. 1998). Our study showed that AmyB can hydrolyze various types of α-1,4-glucans to maltose. This activity of AmyB is also comparable to the β-amylase in plants, which plays a critical role for the degradation of glucans released from starch granules to produce maltose. The difference between AmyB and β-amylase is an ability to hydrolyze maltotriose. When maltopentaose and pNPG6 were used as substrates, β-amylase could not hydrolyze maltotriose, but AmyB further hydrolyzed maltotriose into glucose and maltose (data not shown). Therefore, AmyB could be highly applicable in producing maltose syrup by bioconversion of starch.
Since there is no further uptake of maltodextrins from the environment in this scenario, AmyB is likely to be involved in the breakdown of endogenous α-glucans-like glycogen with the cooperative action of a debranching enzyme and α-glucosidase (TM1834) to generate glucose as the energy source (Bibel et al. 1998). Future efforts should be focused on the roles of putative homologous proteins that respond to synthesis and breakdown of intracellular storage α-glucans to elucidate the physiological role of the multiple intracellular amylases found in this organism.
References
Ballschmiter M, Fütterer O, Liebl W (2006) Identification and characterization of a novel intracellular alkaline α-amylase from the hyperthermophilic bacterium Thermotoga maritima MSB8. Appl Environ Microbiol 72:2206–2211
Bibel M, Brettl C, Gosslar U, Kriegshauser G, Liebl W (1998) Isolation and analysis of genes for amylolytic enzymes of the hyperthermophilic bacterium Thermotoga maritima. FEMS Microbiol Lett 158:9–15
Boel E, Brady L, Brzozowski AM, Derewenda Z, Dodson GG, Jensen VJ, Petersen SB, Swift H, Thim L, Woldike HF (1990) Calcium binding in α-amylases: an X-ray diffraction study at 2.1-Å resolution of two enzymes from Aspergillus. Biochemistry 29:6244–6249
Bok J-D, Yernool DA, Eveleigh DE (1998) Purification, characterization, and molecular analysis of thermostable cellulases CelA and CelB from Thermotoga neapolitana. Appl Environ Microbiol 64:4774–4781
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bronnenmeier KA, Kern A, Liebl W, Staudenbauer WL (1995) Purification of Thermotoga maritima enzymes for the degradation of cellulosic materials. Appl Environ Microbiol 61:1399–1407
Cha HJ, Yoon HG, Kim YW, Lee HS, Kim JW, Kweon KS, Oh BH, Park KH (1998) Molecular and enzymatic characterization of a maltogenic amylase that hydrolyzes and transglycosylates acarbose. Eur J Biochem 253:251–262
Chhabra S, Parker KN, Lam D, Callen W, Snead MA, Mathur EJ, Short JM, Kelly RM (2001) β-mannanases from Thermotoga species. Methods Enzymol 330:224–238
Chhabra SR, Shockley KR, Ward DE, Kelly RM (2002) Regulation of endo-acting glycosyl hydrolases in the hyperthermophilic bacterium Thermotoga maritima grown on glucan- and mannan-based polysaccharides. Appl Environ Microbiol 68:545–554
Chhabra SR, Shockley KR, Conners SB, Scott KL, Wolfinger RD, Kelly RM (2003) Carbohydrate-induced differential gene expression patterns in the hyperthermophilic bacterium Thermotoga maritima. J Biol Chem 278:7540–7552
Conners SB, Montero CI, Comfort DA, Shockley KR, Johnson MR, Chhabra SP, Kelly RM (2005) An expression-driven approach to the prediction of carbohydrate transport and utilization regulons in the hyperthermophilic bacterium Thermotoga maritima. J Bacteriol 187:7267–7282
Conners SB, Mongodin EF, Johnson MR, Montero CI, Nelson KE, Kelly RM (2006) Microbial biochemistry, physiology, and biotechnology of hyperthermophilic Thermotoga species. FEMS Microbiol Rev 30:872–905
Dipple R, Boos W (2005) The maltodextrin system of Escherichia coli: metabolism and transport. J Bacteriol 187:8322–8331
Duffaud GD, McCutchen CM, Leduc P, Parker KN, Kelly RM (1997) Purification and characterization of extremely thermostable β-mannanase, β-mannosidase, and α-galactosidase from the hyperthermophilic eubacterium Thermotoga neapolitana 5068. Appl Environ Microbiol 63:169–177
Fox JD, Robyt JF (1991) Miniaturization of three carbohydrate analyses using a microsample plate reader. Anal Biochem 195:93–96
Gabelsberger J, Liebl W, Schleifer KH (1993) Cloning and characterization of β-galactoside and β-glucoside hydrolyzing enzymes of Thermotoga maritima. FEMS Microbiol Lett 109:131–137
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280:309–316
Janecek S (1994) Parallel β/α-barrels of alpha-amylase, cyclodextrin glycosyltransferase and oligo-1, 6-glucosidase versus the barrel of β-amylase: evolutionary distance is a reflection of unrelated sequences. FEBS Lett 353:119–123
Janecek S (1995) Close evolutionary relatedness among functionally distantly related members of the (α/β)8-barrel glycosyl hydrolases suggested by the similarity of their fifth conserved sequence region. FEBS Lett 377:6–8
Janecek S (2002) How many conserved sequence regions are there in the α-amylase family? Biologia 57(Suppl 11):29–41
Kim TJ, Kim MJ, Kim BC, Kim JC, Cheong TK, Kim JW, Park KH (1999) Modes of action of acarbose hydrolysis and transglycosylation catalyzed by a thermostable maltogenic amylase, the gene for which was cloned from a Thermus strain. Appl Environ Microbiol 65:1644–1651
Kim TJ, Nguyen VD, Lee HS, Kim MJ, Cho HY, Kim YW, Moon TW, Park CS, Kim JW, Oh BH, Lee SB, Svensson B, Park KH (2001) Modulation of the multisubstrate specificity of Thermus maltogenic amylase by truncation of the N-terminal domain and by a salt-induced shift of the monomer/dimmer equilibrium. Biochemistry 40:14182–14190
Lee HS, Kim MS, Cho HS, Kim JI, Kim TJ, Choi JH, Park C, Lee HS, Oh BH, Park KH (2002a) Cyclomaltodextrinase, neopullulanase, and maltogenic amylase are nearly indistinguishable from each other. J Biol Chem 277:21891–21897
Lee MH, Kim YW, Kim TJ, Park CS, Lim JW, Moon TW, Park KH (2002b) A novel amylolytic enzyme from Thermotoga maritima, resembling cyclodextrinase and α-glucosidase, that liberates glucose from the reducing end of the substrates. Biochem Biophys Res Commun 295:818–825
Liebl W (2001) Cellulolytic enzymes from Thermotoga species. Methods Enzymol 330:290–300
Liebl W, Stemplinger I, Ruile P (1997) Properties and gene structure of the Thermotoga maritima α-amylase AmyA, a putative lipoprotein of a hyperthermophilic bacterium. J Bacteriol 179:941–948
Lim WJ, Park SR, An CL, Lee JY, Hong SY, Shin EC, Kim EJ, Kim JO, Kim H, Yun HD (2003) Cloning and characterization of a thermostable intracellular α-amylase gene from the hyperthermophilic bacterium Thermotoga maritima MSB8. Res Microbiol 154:681–687
McCutchen CM, Duffaud GD, Leduc P, Petersen ARH, Tayal A, Khan SA, Kelly RM (1996) Characterization of extremely thermostable enzymatic breakers (α-1,6-galactosidase and β-1, 4-mannanase) from the hyperthermophilic bacterium Thermotoga neapolitana 5068 for hydrolysis of guar gum. Biotechnol Bioeng 52:332–339
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination reducing sugar. Anal Chem 31:426–428
Miller ES, Parker KN, Liebl W, Lam D, Callen W, Snead MA, Mathur EJ, Short JM, Kelly RM (2001) α-galactosidases from Thermotoga species. Methods Enzymol 330:246–260
Miwa I, Okudo J, Maeda K, Okuda G (1972) Mutarotase effect on colorimetric determination of blood glucose with d-glucose oxidase. Clin Chim Acta 37:538–540
Nelson KE, Clayton RA, Gill SR, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Nelson WC, Ketchum KA, McDonald L, Utterback TR, Malek JA, Linher KD, Garrett MM, Stewart AM, Cotton MD, Pratt MS, Phillips CA, Richardson D, Heidelberg J, Sutton GG, Fleischmann RD, Eisen JA, White O, Salzberg SL, Smith HO, Venter JC, Fraser CM (1999) Evidence for lateral gene transfer between archaea and bacteria from genome sequence of Thermotoga maritima. Nature 399:323–329
Nguyen TN, Borges KM, Romano AH, Noll KM (2001) Differential gene expression in Thermotoga neapolitana in response to growth substrate. FEMS Microbiol Lett 195:79–83
Nielsen JE, Borchert TV (2000) Protein engineering of bacterial α-amylases. Biochim Biophys Acta 1543:253–274
Oslancová A, Janecek S (2002) Oligo-1,6-glucosidase and neopullulanase enzyme subfamilies from the α-amylase family defined by the fifth conserved sequence region. Cell Mol Life Sci 59:1945–1959
Park KH, Kim TJ, Cheong TK, Kim JW, Oh BH, Svensson B (2000) Structure, specificity and function of cyclomaltodextrinase, a multispecific enzyme of the α-amylase family. Biochim Biophys Acta 1478:165–185
Park SH, Cha H, Kang HK, Shim JH, Woo EJ, Kim JW, Park KH (2005a) Mutagenesis of Ala290, which modulates substrate subsite affinity at the catalytic interface of dimeric ThMA. Biochim Biophys Acta 1751:170–177
Park TH, Choi KW, Park CS, Lee SB, Kang HY, Shon KJ, Park JS, Cha J (2005b) Substrate specificity and transglycosylation catalyzed by a thermostable β-glucosidase from marine hyperthermophile Thermotoga neapolitana. Appl Microbiol Biotechnol 69:411–422
Parker KN, Chhabra SR, Lam D, Callen W, Duffaud GD, Snead MA, Short JM, Mathur EJ, Kelly RM (2001a) Galactomannanases Man2 and Man5 from Thermotoga species: growth physiology on galactomannans, gene sequence analysis and biochemical properties of recombinant enzymes. Biotechnol Bioeng 75:322–333
Parker KN, Chhabra SR, Lam D, Snead MA, Mathur EJ, Kelly RM (2001b) β-Mannosidase from Thermotoga species. Methods Enzymol 330:238–246
Peist R, Schneider-Fresenius C, Boos W (1996) The MalT-dependent and malZ-encoded maltodextrin glucosidase of Escherichia coli can be converted into a dextrinyltransferase by a single mutation. J Biol Chem 271:10681–10689
Robyt JF, Mukerjea R (1994) Separation and quantitative determination of nanogram quantities of maltodextrins and isomaltodextrins by thin-layer chromatography. Carbohydr Res 251:187–202
Ruile P, Winterhalter C, Liebl W (1997) Isolation and analysis of a gene encoding α-glucuronidase, an enzyme with a novel primary structure involved in the breakdown of xylan. Mol Microbiol 23:267–279
Schumann J, Wrba A, Jaenicke R, Stetter KO (1991) Topographical and enzymatic characterization of amylases from the extremely thermophilic eubacterium Thermotoga maritima. FEBS Lett 282:122–126
Selig M, Xavier KB, Santos H, Schönheit P (1997) Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga. Arch Microbiol 167:217–232
Stam MR, Danchin EG, Rancurel C, Coutinho PM, Henrissat B (2006) Dividing the large glycoside hydrolase family 13 into subfamilies: towards improved functional annotations of α-amylase-related proteins. Protein Eng Des Sel 19:555–562
Tang SY, Le QT, Shim JH, Yang SJ, Auh JH, Park C, Park KH (2006) Enhancing thermostability of maltogenic amylase from Bacillus thermoalkalophilus ET2 by DNA shuffling. FEBS J 273:3335–3345
Thompson JD, Higgins FG, Gibson TJ (1994) CLSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4763–4680
Veith B, Zvwelov VV, Lunina NA, Berezina OV, Raasch C, Velikodvorskaya GA, Liebl W (2003) Comparative analysis of the recombinant α-d-glucosidases from the Thermotoga neapolitana and Thermotoga maritima maltodextrin utilization gene clusters. Biocatal Biotransform 21:147–158
Wassenberg D, Schurig H, Liebl W, Jaenicke R (1997) Xylanase XynA from the hyperthermophilic bacterium Thermotoga maritima: structure and stability of the recombinant enzyme and its isolated cellulose-binding domain. Protein Sci 6:1718–1726
Yang SJ, Lee HS, Park CS, Kim YR, Moon TW, Park KH (2004) Enzymatic analysis of an amylolytic enzyme from the hyperthermophilic archaeon Pyrococcus furiosus reveals its novel catalytic properties as both an α-amylase and a cyclodextrin-hydrolyzing enzyme. Appl Environ Microbiol 70:5988–5995
Yernool DA, McCarthy JK, Eveleigh DE, Bok JD (2000) Cloning and characterization of the glucooligosaccharide catabolic pathway β-glucan glucohydrolase and cellobiose phosphorylase in the marine hyperthermophile Thermotoga neapolitana. J Bacteriol 182:5172–5179
Acknowledgments
This study was supported, in part, by the Marine and Extreme Genome Research Center Program of the Ministry of Land, Transportation and Maritime Affairs and by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD; KRF-2007-521-F00056), Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kyung-Min Park and So-Young Jun contributed equally to the work.
Rights and permissions
About this article
Cite this article
Park, KM., Jun, SY., Choi, KH. et al. Characterization of an exo-acting intracellular α-amylase from the hyperthermophilic bacterium Thermotoga neapolitana . Appl Microbiol Biotechnol 86, 555–566 (2010). https://doi.org/10.1007/s00253-009-2284-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2284-1