Abstract
Online foam separation was proposed to recover nisin during fermentation of Lactococcus lactis subsp. lactis ATCC 11454. Firstly, the optimal pH profile of nisin fermentation was investigated including different realkalization set values and pH drop gradients. Then the selected pH profiles of 5.75 ± 0.05 and 6.25–5.75 (±0.02) were used to perform nisin fermentation coupling with foam separation. The results showed that pH profile of 5.75 ± 0.05 was better than that of 6.25–5.75 (±0.02) for online foam separation. With the optimal pH profile, an aeration of 20 ml min−1 that started at 8 h of incubation and lasted for 2 h resulted in 6.6 times higher specific productivity than that of the fermentation without aeration. Nisin synthesis was therefore prolonged with low sucrose concentration in the culture broth, which indicated that the feedback inhibition of nisin is more influential than the substrate limitation of sucrose in the late phase of nisin fermentation. Total nisin production (4,870 ± 180 IU ml−1) was increased by 30.3% with online foam separation. This effective online recovery method for nisin production could be easily scaled up due to the facile operation of foaming process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nisin is an antimicrobial peptide produced by some strains of Lactococcus lactis. It is effective against a wide range of Gram-positive bacteria and most heat-resistant spores in foods and beverages (Goulhen et al. 1999). Nisin has been studied for decades due to its effective application as a food additive (Rayman et al. 1981; Ruiz et al. 2009).
Commercial nisin is produced mainly by liquid fermentation, and surely it is of great significance to improve productivity. Besides some of the traditional factors such as producer strain, composition of medium, temperature, and aeration that can affect the production of nisin, there are also several special factors that must be considered because of the unique characteristic of nisin production such as feedback inhibition of nisin, adsorption of nisin onto the producer cells, and enzymatic degradation (Parente and Ricciardi 1999). A dramatic decrease in nisin level after reaching the peak value was suspected to be a result of proteolytic degradation and/or adsorption of nisin by producer cells. The best method to decrease the loss of nisin is to recover the product during fermentation and protect it at low pH as soon as possible. Several attempts have been made to solve the problem. Online removal of nisin based on adsorption mechanism by using Amberlite XAD-4 had been investigated, and the results indicated that nisin production also occurred in the stationary phase of nisin fermentation (Tolonen et al. 2004). A similar experiment had also been reported by Pongtharangkul and Demirci, in which study silicic acid was used as the adsorbent to perform the online removal of nisin, and the production of nisin was significantly enhanced by 3.9 times (Pongtharangkul and Demirci 2007). In Kim’s study, a solvent (phenyl–methyl silicone oil) was introduced to the aqueous phase to form a two-phase batch culture system for nisin production and a 24% increase of nisin was obtained (Kim 1997). High-specific cellular nisin production was maintained by cycle changing the medium and an increase of 19-fold more nisin was achieved (Şimşek and Saris 2009).
Though the methods mentioned above have led to more or less increases of nisin production, it is hard for these techniques to be scaled up because of the high cost or operational difficulties. Thus, it is necessary to find a new method to decrease the feedback inhibition of nisin as well as nisin lost during fermentation. Foam separation is a simple and low-cost method and belongs to the adsorptive bubble separation techniques. It has already been successfully applied in productions of many biosurfactants such as surfactin and cellulase (Davis et al. 2001; Chen et al. 2006; Zhang et al. 2007). Due to the marked hydrophobic nature of nisin as an antimicrobial peptide, foam separation could be a feasible method to perform the online recovery of nisin. However, the adsorption of nisin onto various surfaces, such as cytoplasmic membrane, glass container, and polypropylene tubes, is seriously depending on the pH of the culture broth, which also influences the solubility as well as stability of nisin. Hurst and Dring (Hurst and Dring 1968) found that at pH 6.80 (controlled fermentation) more than 80% of the nisin synthesized was bound to the cells, whereas at pH below 6.0, more than 80% of the nisin was in the culture fluid. Yang et al. (Yang et al. 1992) found that the optimal pH for bacteriocin adsorption to cells ranges from pH 5.5 to 6.5. On the other hand, it had been reported that at pH 6.4, about 1% and 23% of nisin activity were lost after the nisin preparation had been stored at 4 °C and 20 °C, respectively, for 3 h, and the higher the pH value, the more the nisin activity would lose (Huot et al. 1996). It is clear that with the increase of pH from 5.5 to 6.8, the adsorption of nisin enhanced, but the solubility and stability decreased sharply, and this pH range is the usual condition that nisin fermentation was performed. As a result, it is necessary to select a proper pH profile of nisin fermentation for online foam separation to be carried out.
In this study, effects of pH profiles on nisin production were studied, firstly, to determine a proper pH control strategy. Then, nisin fermentation coupling with foam separation was investigated under the predetermined pH profile to increase the nisin production.
Materials and methods
Bacterial strains, growth media, and analytical methods
The fermentation medium of the nisin-producing strain (Lactococcus lactis subsp. lactis ATCC 11454) consisted of 40 g sucrose, 10 g KH2PO4, 15 g peptone, 15 g yeast extract, 2 g NaCl and 0.2 g MgSO4·7H2O per liter of distilled water with initial pH of 7.6. Micrococcus flavus NCIB 8166, purchased from China General Microbiological Collection Center, was used as the indicator strain in the nisin bioactivity assay. It was grown in the SI medium (initial pH 7.2), which consisted of 5 g glucose, 2 g Na2HPO4, 8 g tryptone, 3 g yeast extract powder, 5 g NaCl, 5 ml Tween 20, and 10 g agar (if necessary) per liter of distilled water. All media were autoclaved at 121 °C for 20 min and stored at 4 °C.
Nisin activity was determined by a modified dual-dosage agar diffusion assay (Wu et al. 2009). Biomass was estimated by optical density at 600 nm (OD600). The value was converted to cell dry weight (CDW) from a standard curve (CDW = 6.7547 × OD600 – 0.0247). The concentration of sucrose was measured by Roe colorimetric method. Briefly, the sample was centrifuged at 10,000 × g, 4 °C for 10 min, and the supernatant was collected and diluted to 40 times with distilled water. Then 1.8 ml diluent was mixed with 0.2 ml 2 M NaOH and kept in boiling water bath for 10 min. After being cooled in ice water for 1 min, the sample was mixed with 2 ml resorcinol solution (prepared by dissolving 0.1 g resorcinol in 100 ml 6 M HCl) and 6 ml 10 M HCl and then kept in water bath (60 °C) for 40 min. After being cooled in room temperature for 15 min, the sample was estimated by optical density at 500 nm (OD500) and the value was converted to residual sucrose (RS) from a standard curve (RS = 69.0 × OD500). The pH value of the culture broth was continuously recorded by a pH probe.
Experimental design
Nisin fermentation under different pH profiles
In order to find the optimal pH profile for online foam separation during nisin fermentation, realkalization set value (the constant pH value at which nisin fermentation was controlled) and pH drop gradient were studied as follows.
(1) pH was allowed to drop freely from the initial pH 7.6 till the realkalization set value and then controlled at different levels from 6.50 to 5.25 (±0.05) with 6 M NaOH.
(2) Various combinations of realkalization set values were employed to carry out the fermentation. When the pH decreased from the upper limit to the lower limit of each autoacidification cycle, 6 M NaOH was added to the culture broth so that the pH could reach the upper limit of such gradient again.
Nisin fermentation coupling with foam separation
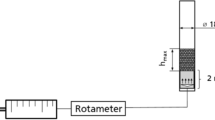
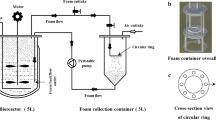
Nisin fermentation was conducted in a 500 ml jar as the fermentor loaded with 400 ml culture broth (6% v/v inoculation) at 30 °C. A glass sinter of pore size 150–250 microns was inserted into the fermentor by linking to a glass tube that allowed the sterilized air to be blown into the culture broth at the bottom of the fermentor. A cylindrical foaming column with an internal diameter of 17 mm and a height of 18 cm was used for foam collection. After incubation for 8 h, half of the culture broth was withdrawn and maintained in another fermentor without aeration as the control. In the original fermentor remaining 200 ml culture broth, foam was generated by 20 ml min−1 aeration and collected with 0.02 M HCl, in which nisin could be dissolved quickly without antifoam. The aeration lasted 2 h and the fermentation was performed until 14 h.
Measurement of each sample was performed in triplicate. All experiments described above were carried out in duplicate.
Results
Effects of pH profiles on nisin fermentation
Figure 1a shows that the cell growth of L. lactis subsp. lactis with different realkalization set values could be divided into three types. (1) High pH profile of 6.50 ± 0.05 led to a well growth at the beginning of fermentation and then a significant decrease of the CDW after reaching the set value for 3.42 h. The CDW was 28% lower than that of the fermentation controlled at pH 5.75 ± 0.05 at the end of incubation. (2) Moderate pH profiles of 6.25, 6.00, and 5.75 (±0.05) caused similar cell growths as pH 6.50 ± 0.05 at early phase of fermentation. But the growths separated from each other after incubating for 9 h and the higher the pH value, the lower the CDW. (3) Low pH profiles of 5.50 and 5.25 (±0.05) carried out a slow growth rate at the beginning but a constant increase of CDW in the whole range of fermentation. Cell growth of fermentation controlled at pH 5.50 ± 0.05 markedly excelled that of fermentation controlled at pH 5.25 ± 0.05 (14% higher) and even reached the same value of fermentation controlled at pH 5.75 ± 0.05 (4.41 g l−1) at the end of fermentation. Figure 1b shows that nisin production was maximized (3,790 ± 130 IU ml−1) when the batch fermentation was controlled at pH 5.75 ± 0.05. Whereas other pH profiles gave more or less negative effects on nisin production, which effectively corresponded to the growth of L. lactis subsp. lactis under different realkalization set values as shown in Fig. 1a.
As in each cycle of autoacidification, the pH drop accords with a linear decrease till the late phase of nisin fermentation, it is reasonable to illustrate the condition of pH drop gradient by average velocity of [H+] variation (AVHV). Figure 2a shows the AVHV of nisin fermentation fulfilled various pH drop gradients and the corresponding data of CDW and nisin activity are shown in Fig. 2b and c, respectively. It is clear that with the pH drop gradients of 6.50–5.50, 6.50–5.75, and 6.50–6.00 (±0.02), nisin productions decreased after incubating for 10 h, and the corresponding CDW had similar performances. Nisin production was maximized (3,720 ± 190 IU ml−1) with the pH drop gradient of 6.25–5.75 (±0.02). Consequently, pH profiles of 5.75 ± 0.05 and 6.25–5.75 (±0.02) were selected to perform the nisin fermentation coupling with foam separation.
Effects of pH drop gradients on nisin fermentation. Calculated average velocity of [H+] variation (a), cell growth (b), and nisin production (c). [H+] was converted from pH value, average velocity (M h−1) = increment of [H+] of an autoacidification cycle (M)/time needed (h). The standard deviation is indicated by error bars
Effect of online foam separation on nisin fermentation
Figure 3 shows the production of nisin with online foam separation controlled with the two pH profiles described previously, and the parameters of the foamate generated by the foaming process are shown in Table 1. It is obvious that online foam separation was successfully applied to decrease nisin concentration in the culture broth by online recovery of nisin. The synthesis of nisin was therefore prolonged with low nisin concentration in the culture broth. At the end of fermentation, total nisin production (sum of nisin activity of the culture broth with online foam separation and the foamate) was 4,870 ± 180 and 4,640 ± 170 IU ml−1 with the pH profiles of 5.75 ± 0.05 and 6.25–5.75 (±0.02), respectively, which were 30.3% and 25.4% more than that of the fermentation without online foam separation. Accordingly, because of the higher recovery rate (37.3% compared with 35.1%), lower total nisin lost (5.9% compared with 9.7%) and higher nisin production (4,870 ± 180 IU ml−1 compared with 4,640 ± 170 IU ml−1), the pH profile of 5.75 ± 0.05 was better than that of 6.25–5.75 (±0.02) for online foam separation.
Nisin production of Lactococcus lactis subsp. lactis ATCC 11454 with pH profiles of 5.75 ± 0.05 (a) and 6.25–5.75 (±0.02) (b). With online foam separation (filled circles), without online foam separation (filled squares), and the total nisin (filled triangle). Total nisin (IU ml−1) = (nisin quantity of the culture broth with online foam separation (IU) + nisin quantity of foamate (IU))/200 ml. The standard deviation is indicated by error bars
In order to illustrate the effect of online foam separation on specific nisin productivity, CDW (data not shown) was converted to colony forming unit (CFU) by using \( CDW = 2.02\, \times {10^{ - 9}} CFU + 0.012 \) equation (Shimizu et al. 1999) and then specific productivity of nisin fermentation with the pH profile of 5.75 ± 0.05 was calculated (Fig. 4). It is clear that as nisin concentration in the culture broth increased, the specific productivity decreased. When online foam separation was finished at 10 h, specific productivity of nisin with online foam separation reached the value of 338 × 10−9 IU h−1 per cell, while such value of fermentation without online foam separation was 51 × 10−9 IU h−1 per cell. At the end of fermentation, the specific productivity (with online foam separation) decreased to−7 × 10−9 IU h−1 per cell when the nisin concentration reached 3,690 ± 180 IU ml−1.
Specific productivity with (black column) and without (gray column) online foam separation when pH was controlled at 5.75 ± 0.05 and the corresponding nisin activity in the culture broth. With online foam separation (circle), without online foam separation (square). Specific productivity (IU h−1 per cell) = nisin increment (IU ml−1)/time needed (h)/CFU (cell ml−1)
Discussion
A definite realkalization set value used to perform the fermentation of L. lactis subsp. lactis could take dual effects on cell growth in different phases of nisin fermentation. In the early phase of fermentation, high set values (ranging from 6.50 to 5.75) were beneficial to cell growth whereas low set values like 5.50 and 5.25 (±0.05) would slow down the growth rate. When the fermentation was conducted in the stationary phase, high set values like 6.50 and 6.25 (±0.05) would take considerable detrimental effects on nisin fermentation, which was mainly caused by the adsorption of nisin on the producer cells (Hurst and Dring 1968; Yang et al. 1992). On the contrary, low set values such as 5.50 and 5.25 (±0.05) would prolong the growth of L. lactis subsp. lactis with low growth rate by inhibiting the adsorption of nisin. As a result, a moderate realkalization set value (5.75 ± 0.05) could be suitable for cell growth in the whole range of nisin fermentation.
When the fermentation was subjected to different pH drop gradients, the adsorbed nisin could be released into the culture broth at low pH of each gradient, and nisin synthesis could be continued at high pH of the next autoacidification cycle. As the extracellular pH (pHex) decreased owing to the production of lactic acid, the intracellular pH (pHin) would also drop to a new level to maintain a constant transmembrane pH gradient (Siegumfeldt et al. 2000). It implied that the pHex would affect not only the adsorption and stability of nisin but also the central metabolic pathways of L. lactis (Even et al. 2002). Consequently, such a pH drop gradient should not be too far away from the optimal pH of fermentation (5.75 ± 0.05 in this study). Among the gradients investigated in this study, the pH drop gradient of 6.25–5.75 (±0.02) provided a moderate AVHV and obtained the highest nisin production (Fig. 2). Whereas other gradients that have pH 6.50 ± 0.02 as its upper limit or 5.50 ± 0.02 as its lower limit are detrimental to nisin fermentation because of the adsorption of nisin onto the producer cells at high pH or the changes of the central metabolic pathways at low pH, respectively.
It has been reported that different nisin producers produced nisin to different ceiling concentrations and once the host-specific ceiling concentration of nisin was reached, the nisin production would be stopped even if the producing strain continued to grow well (Kim et al. 1998). Such ceiling concentration of nisin in this study was 3,860 ± 190 IU ml−1 (with regard to pH profile of 5.75 ± 0.05) when fermentation had been conducted for 11 h without online foam separation, because the specific productivity of nisin decreased below zero from then on. However, with the online foam separation that made the nisin concentration in culture broth 38.9% lower than that of the control, the specific productivity of nisin was maintained at relative high level of 338 × 10−9 IU h−1 per cell compared with 51 × 10−9 IU h−1 per cell of the control when the aeration was finished. Nisin synthesis was therefore prolonged with increments of 287, 280, and 71 × 10−9 IU h−1 per cell compared with the specific productivity of the control at 10.5, 11.5, and 12.5 h, respectively. As the prolonged nisin synthesis was obtained when the nisin concentration had been decreased by online foam separation and the sucrose was almost depleted (data not shown), it is reasonable to conclude that the feedback inhibition of nisin is more influential than the substrate limitation of sucrose in the late phase of nisin fermentation.
In this study, total nisin was decreased by 5.9% and 9.7%, when the aeration of 20 ml min−1 was finished at 10 h, with pH profiles of 5.75 ± 0.05 and 6.25–5.75 (±0.02), respectively (Table 1). These decreases indicated that the 2-h aeration had led to a considerable loss of total nisin during the foaming procedure. However, it had been reported that aeration up to an air flow rate of 3.5 v/v/m (volume of air/volume of fermentor/min) had positive effect on nisin production and the specific production at 60% or 90% initial air saturation was eightfold higher than that at 0% (Amiali et al. 1998). Cabo et al. (Cabo et al. 2001) also found that nisin production at the maximum biomass point quadrupled when the oxygen saturation percentage increased from 50% to 100%. Consequently, it is reasonable to conclude that the decrease of total nisin is caused by other characteristics of nisin during online foam separation such as nonspecific adsorption of nisin on the bioreactor’s surface and the foaming column. If so, the decrease of total nisin may possibly be solved by addition of Tween 80 in the culture broth (Joosten and Nunez 1995). In addition, as had been described in the introduction section, pH of the culture broth could affect not only the adsorption but also the solubility and stability of nisin. Compared with the pH profile of 6.25–5.75 (±0.02), pH controlled at 5.75 ± 0.05 could make nisin more soluble and stable in fermentation system. As a result, when foam was generated by online foam separation more nisin could be enriched on the bubbles whereas less nisin would be lost because of the high solubility and stability of nisin with low pH.
In conclusion, online foam separation proved to be an effective online recovery method to decrease the feedback inhibition of nisin during nisin fermentation by L. lactis subsp. lactis. Synthesized nisin could therefore be recovered and protected immediately at low pH. Nisin production of 4,870 ± 180 IU ml−1 (30.3% more than that of the control) could be achieved by fermentation coupling with foam separation which could be easily scaled up due to the facile operation of foaming process.
References
Amiali MN, Lacroix C, Simard RE (1998) High nisin Z production by Lactococcus lactis UL719 in whey permeate with aeration. World J Microbiol Biotechnol 14:887–894
Cabo ML, Murado MA, Gonzalez MP et al (2001) Effects of aeration and pH gradient on nisin production. A mathematical model. Enzym Microb Technol 29:264–273
Chen C-Y, Baker SC, Darton RC (2006) Batch production of biosurfactant with foam fractionation. J Chem Technol Biotechnol 81:1923–1931
Davis DA, Lynch HC, Varley J (2001) The application of foaming for the recovery of surfactin from B. subtilis ATCC 21332 cultures. Enzym Microb Technol 28:346–354
Even S, Lindley ND, Loubiere P et al (2002) Dynamic response of catabolic pathways to autoacidification in Lactococcus lactis: transcript profiling and stability in relation to metabolic and energetic constraints. Mol Microbiol 45:1143–1152
Goulhen F, Meghrous J, Lacroix C (1999) Production of a nisin Z/pediocin mixture by pH-controlled mixed-strain batch cultures in supplemented whey permeate. J Appl Microbiol 86:399–406
Huot E, Barrena-Gonzalez C, Petitdemange H (1996) Comparative effectiveness of nisin and bacteriocin J46 at different pH values. Lett Appl Microbiol 22:76–79
Hurst A, Dring GJ (1968) The relation of the length of lag phase of growth to the synthesis of nisin and other basic proteins by Streptococcus lactis grown under different cultural conditions. J Gen Microbiol 50:383–390
Joosten HMLJ, Nunez M (1995) Adsorption of nisin and enterocin 4 to polypropylene and glass surfaces and its prevention by Tween 80. Lett Appl Microbiol 21:389–392
Kim WS (1997) Nisin production by Lactococcus lactis using two-phase batch culture. Lett Appl Microbiol 25:169–171
Kim WS, Hall RJ, Dunn NW (1998) Improving nisin production by increasing nisin immunity/resistance genes in the producer organism Lactococcus lactis. Appl Microbiol Biotechnol 50:429–433
Parente E, Ricciardi A (1999) Production, recovery and purification of bacteriocins from lactic acid bacteria. Appl Microbiol Biotechnol 52:628–638
Pongtharangkul T, Demirci A (2007) Online recovery of nisin during fermentation and its effect on nisin production in biofilm reactor. Appl Microbiol Biotechnol 74:555–562
Rayman MK, Aris B, Hurst A (1981) Nisin: a possible alternative or adjunct to nitrite in the preservation of meats. Appl Environ Microbiol 41:375–380
Ruiz A, Williams SK, Djeri N et al (2009) Nisin, rosemary, and ethylenediaminetetraacetic acid affect the growth of Listeria monocytogenes on ready-to-eat turkey ham stored at four degrees Celsius for sixty-three days. Poult Sci 88:1765–1772
Shimizu H, Mizuguchi T, Tanaka E et al (1999) Nisin production by a mixed-culture system consisting of Lactococcus lactis and Kluyveromyces marxianus. Appl Environ Microbiol 65:3134–3141
Siegumfeldt H, Rechinger KB, Jakobsen M (2000) Dynamic changes of intracellular pH in individual lactic acid bacterium cells in response to a rapid drop in extracellular pH. Appl Environ Microbiol 66:2330–2335
Şimşek Ö, Saris PEJ (2009) Cycle changing the medium results in increased nisin productivity per cell in Lactococcus lactis. Biotechnol Lett 31:415–421
Tolonen M, Saris PEJ, Siika-aho M (2004) Production of nisin with continuous adsorption to Amberlite XAD-4 resin using Lactococcus lactis N8 and L. lactis LAC48. Appl Microbiol Biotechnol 63:659–665
Wu Z, Wang L, Jing Y et al (2009) Variable volume fed-batch fermentation for nisin production by Lactococcus lactis subsp. lactis W28. Appl Biochem Biotechnol 152:372–382
Yang R, Johnson MC, Ray B (1992) Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl Environ Microbiol 58:3355–3359
Zhang Q, Lo C-M, Ju L-K (2007) Factors affecting foaming behavior in cellulase fermentation by Trichoderma reesei Rut C-30. Bioresour Technol 98:753–760
Acknowledgment
This study was supported by Nature Science Foundation of Tianjin (08JCZDJC25200) and Tianjin Kangyi Bioengineering Co., Ltd. of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
W. Liu and H. Zheng contributed equally.
Rights and permissions
About this article
Cite this article
Liu, W., Zheng, H., Wu, Z. et al. Effects of pH profiles on nisin fermentation coupling with foam separation. Appl Microbiol Biotechnol 85, 1401–1407 (2010). https://doi.org/10.1007/s00253-009-2217-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2217-z