Abstract
The targeting of recombinant proteins for secretion to the culture medium of Escherichia coli presents significant advantages over cytoplasmic or periplasmic expression. However, a major barrier is inadequate secretion across two cell membranes. In the present study, we attempted to circumvent this secretion problem of the recombinant α-cyclodextrin glycosyltransferase (α-CGTase) from Paenibacillus macerans strain JFB05-01. It was found that glycine could promote extracellular secretion of the recombinant α-CGTase for which one potential mechanism might be the increase in membrane permeability. However, further analysis indicated that glycine supplementation resulted in impaired cell growth, which adversely affected overall recombinant protein production. Significantly, delayed supplementation of glycine could control cell growth impairment exerted by glycine. As a result, if the supplementation of 1% glycine was optimally carried out at the middle of the exponential growth phase, the α-CGTase activity in the culture medium reached 28.5 U/ml at 44 h of culture, which was 11-fold higher than that of the culture in regular terrific broth medium and 1.2-fold higher than that of the culture supplemented with 1% glycine at the beginning of culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the production of recombinant proteins, Escherichia coli is the most commonly used host because of its simplicity, safety, and known genetic properties (Baneyx 1999; Shokri et al. 2003). However, overexpression of recombinant proteins often results in the formation of inactive protein aggregates (inclusion bodies) in the cytoplasm, which is a significant barrier of gene expression in E. coli (Choi and Lee 2004; Makrides 1996). To avoid the formation of inclusion bodies, considerable efforts have been made to target recombinant proteins outside the cytoplasm, mostly to the periplasm (Jeang et al. 2005; Kim et al. 2005). Although secretion of these proteins into the periplasm can lead to the rapid isolation of recombinant proteins for biological evaluation, its application on an industrial scale was also limited by the unavailability of efficient large-scale methods for the selective release of periplasmic proteins from the cell (Yang et al. 1998), while protein secretion into the culture medium facilitates downstream purification efforts substantially by eliminating the need to disrupt cells for protein recovery. Moreover, extracellular secretion can allow higher recombinant protein expression titers because product accumulation is not restricted by the limited volume of the periplasmic compartment (Makrides 1996). Nevertheless, despite these advantages, extracellular secretion of recombinant proteins has been limited, mainly due to the difficulties in protein translocation across the two membranes of E. coli cells (Choi and Lee 2004; Koebnik et al. 2000).
Various attempts have been made to facilitate extracellular secretion of recombinant proteins in E. coli. These included either molecular approaches, such as coexpression of molecular chaperones, manipulation of the various transport pathways, and fusing of the product to a carrier protein that is normally secreted extracellularly (Makrides 1996; Blight et al. 1994; Sorensen and Mortensen 2005; Jana and Deb 2005), or cultivation strategies, such as the changes of culture medium composition, temperature, pH, or aeration, medium supplements, and taking advantage of the growth-coupled effects on membrane components (Rinas and Hoffmann 2004; Mergulhao et al. 2005; Shokri et al. 2003). Thereinto, the strategy using medium supplements, such as lysozyme, magnesium, calcium, ethylenediaminetetraacetic acid (EDTA), glycine, and Triton X-100, was frequently employed (Choi and Lee 2004; Mergulhao et al. 2005; Yang et al. 1998; Jang et al. 1999).
Cyclodextrin glycosyltransferase (EC 2.4.1.19, CGTase) is an extracellular enzyme capable of converting starch or starch derivates into cyclodextrins through an intramolecular transglycosylation reaction. Cyclodextrins are cyclic, nonreducing oligoglucopyranose molecules linked via α(1,4)-glycosidic bonds (Chang et al. 1998). They can form inclusion complexes with various hydrophobic guest molecules (Saenger 1980). As a consequence of complex formation, the properties of the included molecules are altered (Lucas-Abellan et al. 2008; Mourtzinos et al. 2007), which leads to many potential applications of cyclodextrins in the industries related to food, pharmaceuticals, agriculture, etc. (Szente and Szejtli 2004; Li et al. 2007). To overcome the low CGTase productivity of wild strains, the overexpression of the recombinant CGTase in E. coli has been expected. However, the extracellular secretion of the recombinant CGTase in E. coli remained a challenging task since this enzyme was usually accumulated in the periplasmic space and/or formed biologically inactive inclusion bodies (Kim et al. 2005; Jeang et al. 1999, 2005). This greatly limited the industrial applications of the recombinant CGTase.
Previously, we had constructed an E. coli expression system of the cgt gene encoding α-CGTase from Paenibacillus macerans strain JFB05-01 (Chen et al. 2008). However, the extracellular production efficiency of the recombinant α-CGTase needed to be improved. In the present study, we showed that extracellular secretion of the recombinant α-CGTase was markedly enhanced by delayed supplementation of glycine. Possible mechanisms were also discussed.
Materials and methods
Bacterial strain, plasmid, and chemicals
The recombinant plasmid cgt/pET-20b(+) in which the cgt gene coding the mature wild-type CGTase from P. macerans strain JFB05-01 (CCTCC M203062) was placed downstream of a DNA sequence coding pelB signal peptide, and E. coli BL21(DE3) harboring plasmid cgt/pET-20b(+) were constructed previously (Chen et al. 2008; Li et al. 2009). Peptone and yeast extract powder were obtained from Oxoid (Basingstoke, Hampshire, United Kingdom). Glycine and methyl orange were purchased from ShangHai Chemical Reagent Ltd. attached to China Medicine Group (ShangHai, China). Isopropyl β-d-1-thiogalactopyranoside (IPTG), O-nitrophenyl-β-d-galactopyranoside (ONPG), and N-phenyl-α-naphthylamine (NPN) were purchased from Beyotime Institute of Biotechnology (Nantong, China). Glucose-6-phosphate, Triton X-100, NADP, and NADPH were purchased from Sigma-Aldrich (Steinheim, Germany). All inorganic compounds were of reagent grade or higher quality.
Expression of α-CGTase
A single colony of E. coli BL21(DE3) cells harboring plasmid cgt/pET-20b(+) was inoculated into 10 ml Luria–Bertani medium containing 100 µg/ml ampicillin and grown at 37°C overnight. Overnight culture (1 ml) was then diluted into 100 ml of terrific broth (TB) containing 100 µg/ml ampicillin in a 500-ml flask and incubated on a rotary shaker (200 rpm) at 30°C until optical density at 600 nm (OD600) of the culture reached 0.6. IPTG was added to a final concentration of 0.01 mM to induce the expression of the target protein. Protein induction was performed at 25°C and continued for 90 h. To investigate the effects of glycine on cell growth or extracellular secretion of the recombinant α-CGTase, TB medium was supplemented with 0.25–1.25% glycine at a certain time point of culture. Samples were withdrawn hourly and analyzed for OD600 and enzyme activities.
Cell fractionation
Cell fractionation was performed as described by Kato et al. (1983) with some modifications. The extracellular fraction was obtained by centrifugation of the culture broth at 10,000×g for 20 min at 4°C. To separate the periplasmic fraction, 1-ml culture was harvested and resuspended in 1 ml of 30 mM Tris–HCl solution (pH 7.0) containing 25% (w/v) sucrose and 1 mM EDTA. The cell suspension was incubated on ice for 2 h and pelleted by centrifugation at 10,000×g for 20 min at 4°C. The supernatant was collected as a periplasmic fraction. The pellet was then resuspended in l ml of 50 mM phosphate buffer (pH 6.0) and disrupted by ultrasonication with a SONIFER 450 (Branson, Danbury, CT, USA). After centrifugation at 10,000×g for 20 min at 4°C, the supernatant and cell debris were collected as a soluble cytoplasmic and insoluble inclusion body fraction, respectively. The cell debris was resuspended in 0.l ml of sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris–HCl, pH 7.6; 100 mM 2-mercaptoethanol; 2% SDS; 5% glycerol; 0.15 mM bromophenol blue) and boiled for 10 min to solubilize the inclusion bodies.
Assay of α-CGTase
The α-cyclodextrin-forming activity was determined by the methyl orange method as described previously (Lejeune et al. 1989) with some modifications; 0.1 ml of the culture, periplasmic, or cytoplasmic supernatant (appropriately diluted in 50 mM phosphate buffer, pH 6.0) was incubated with 0.9 ml of 3% (w/v) soluble starch in 50 mM phosphate buffer (pH 6.0) at 40°C for 10 min. The reaction was terminated by the addition of 1.0 M HCl (1.0 ml) and then 1.0 ml of 0.1 mM methyl orange in 50 mM phosphate buffer (pH 6.0) was added. After the reaction mixture was incubated at 16°C for 20 min, the amount of α-cyclodextrin in the mixture was spectrophotometrically determined by measuring the absorbance at 505 nm. One unit of α-CGTase activity was defined as the amount of enzyme that was able to produce 1 µmol of α-cyclodextrin per minute.
N-terminal amino acid sequencing
To check whether the pelB signal peptide was cleaved from the insoluble inclusion body, isolated inclusion bodies were subjected to automated Edman degradation sequencing by using a 492cLC protein sequencer (Applied Biosystems, Foster City, CA, USA).
Protein renaturation
The inclusion body fraction was washed twice with 1% Triton X-100 and any residual Triton X-100 was removed by washing with 50 mM phosphate buffer (pH 6.0). Renaturation of inclusion body proteins was performed as described previously (Kim et al. 2000).
Assay of glucose-6-phosphate dehydrogenase
Glucose-6-phosphate dehydrogenase (G6PD) activity in the extracellular or cytoplasmic fraction was determined as described previously (Sanwal 1970) with some modifications. The incubation mixture contained 1 mM glucose-6-phosphate, 0.075 mM NADP, 10 mM MgCl2, 50 mM Tris–HCl (pH 8.0), and the culture or cytoplasmic supernatant in a volume of 1.0 ml. The increase in the absorbance at 340 nm was measured at 25°C in a spectrophotometer (UV-2450; Shimadzu Co., Kyoto, Japan). One unit of G6PD activity was defined as the amount of enzyme that was able to produce 1 µmol of NADPH per minute.
Assay of outer membrane permeability
Outer membrane permeability was measured using NPN access assay as described previously (Loh et al. 1984). Samples of E. coli BL21(DE3) cells were withdrawn at different growth stages, rinsed once using centrifugation at 3,000×g, and resuspended in 10 mM phosphate buffer (pH 7.4) to an OD600 of 0.5. NPN was added to a final concentration of 10 mM into quartz cuvette containing 2 ml of cell suspension. The sample was mixed by inversion of the cuvette immediately prior to fluorescence monitoring. Fluorescence was measured using a Shimadzu RF-1501 spectrofluorometer (Shimadzu Co., Kyoto, Japan) with slit widths set to 1 mm and excitation and emission wavelengths set to 350 and 420 nm, respectively.
Assay of inner membrane permeability
Permeability of the inner membrane was assessed by measuring the access of ONPG to the cytoplasm essentially as described previously (Lehrer et al. 1988). Briefly, ONPG was added to a final concentration of 100 µg/ml into the quartz cuvette containing 2 ml of cell suspension prepared as described above, and substrate cleavage by β-galactosidase was monitored by light absorption measurements at 420 nm in a spectrophotometer (UV-2450; Shimadzu Co., Kyoto, Japan).
Miscellaneous methods
Cell density was determined by measuring the OD600 of the culture using a spectrophotometer (UV-2450; Shimadzu Co., Kyoto, Japan) after an appropriate dilution. Colony-forming unit (CFU) per milliliter of culture per OD600 was estimated by plating a suitably diluted culture sample and counting colonies which appeared after 12 h of incubation at 37°C. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE, 10% gel) was performed under denaturing conditions with a Mini-PROTEAN II electrophoresis unit (Bio-Rad Laboratories, Richmond, CA, USA). Protein bands were visualized by staining with 0.25% Coomassie brilliant blue R-250.
Results
Effect of glycine supplement time on extracellular secretion of α-CGTase
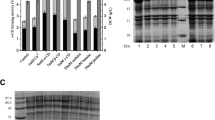
When E. coli cells were cultured in regular TB medium, very few recombinant α-CGTase was secreted into the culture medium within the first 40 h (Figs. 1 and 2). SDS-PAGE analysis showed that, at 40 h of culture, many recombinant enzymes were accumulated in the periplasmic space and a few insoluble inclusion bodies were formed (Fig. 2). In the periplasmic fraction, an α-CGTase activity of 12.8 U/ml could be detected at 40 h of culture. Furthermore, the amino acid sequence at the N terminus of inclusion body was determined to mainly be Met-Lys-Tyr-Leu-Leu, which was identical to the N-terminal sequence of the pelB signal peptide (Choi and Lee 2004), indicating that most inclusion bodies had the pelB signal peptide and thus were formed in the cytoplasm. In addition, after the renaturation of inclusion body proteins, almost no α-CGTase activity could be detected, suggesting that the α-CGTases fused to the pelB signal peptide might not be folded correctly. After the lag phase of extracellular secretion, the activity of α-CGTase in the culture medium increased gradually and reached 22.5 U/ml at 90 h of culture (Fig. 1).
Effect of glycine supplement time on extracellular activity of α-CGTase. TB medium was supplemented with 1.0% glycine at 0 h (open triangles), 8 h (asterisks), 14 h (open circles), 20 h (open diamonds), or 26 h (open squares) of culture. E. coli cells were grown in regular TB medium as control (filled circles). Each value represents the mean of three independent measurements, and the deviation from the mean is below 5%
SDS-PAGE analysis of α-CGTase expressed in E. coli. α-CGTase distribution in the different fractions was analyzed after 40 h of culture. E. coli cells were grown in regular TB medium (lanes 3, 6, and 9), or TB medium supplemented with 1% glycine at the beginning of culture (lanes 2, 5, and 8) or 14 h of culture (lanes 1, 4, and 7), respectively. Lane M protein size marker, lanes 1, 2, and 3 extracellular fraction (10 µl), lanes 4, 5, and 6 periplasmic fraction (10 µl), lanes 7, 8, and 9 insoluble fraction (5 µl)
When E. coli cells were cultured in TB medium supplemented with 1% glycine, the enhancement in the extracellular secretion of the recombinant α-CGTase was obviously observed (Figs. 1 and 2). However, the extracellular secretion of the recombinant enzyme was significantly affected by glycine supplement time (Fig. 1). Glycine supplementation at the middle of the exponential growth phase (approximately 14 h of culture) resulted in higher extracellular activity of the recombinant α-CGTase when compared to that at other time points of culture. The α-CGTase activity in the culture medium reached 28.5 U/ml at 44 h of culture, which was 11-fold higher than that of the culture in regular TB medium at the same time point of culture (Fig. 1). If TB medium was supplemented with 1% glycine at the beginning of culture, although more recombinant enzymes were secreted into the culture medium within the first 30 h, the α-CGTase activity in the culture medium at 44 h of culture was approximately 15% lower than that in the culture supplemented with 1% glycine at the middle of the exponential growth phase (Fig. 1). If TB medium was supplemented with 1% glycine at the late stage of the exponential growth phase (such as 20 or 26 h of culture) or later, the α-CGTase activity in the culture medium increased relatively slowly. Thus, at 44 h of culture, it was much lower than that in the culture supplemented with 1% glycine at the middle of the exponential growth phase.
SDS-PAGE analysis showed that, when E. coli cells were cultured in TB medium supplemented with 1% glycine at the beginning of culture, no appreciable recombinant enzyme was accumulated in the periplasmic space and almost no insoluble inclusion body was formed at 40 h of culture (Fig. 2). By comparison, delayed supplementation of glycine resulted in the increases in periplasmic accumulation and inclusion body formation of the recombinant enzyme. Nevertheless, glycine supplementation at the middle of exponential growth phase caused only slight increases, as demonstrated by few recombinant enzymes accumulated in the periplasmic space and few insoluble inclusion bodies formed in the cytoplasm at 40 h of culture (Fig. 2). Further analysis confirmed that the α-CGTase activity in the periplasmic fraction was only 1.8 U/ml. In addition, in all cultures, no α-CGTase activity was detected in the soluble cytoplasmic fraction.
Effect of glycine concentration on extracellular secretion of α-CGTase
The extracellular secretion of the recombinant α-CGTase was also significantly affected by glycine concentration. As shown in Fig. 3, when the TB medium was supplemented with 0.25–1.25% glycine at the middle of the exponential growth phase, the optimal glycine concentration for extracellular recombinant protein production was 1%. In the culture with a lower concentration of glycine, the α-CGTase activity in the culture medium increased more slowly and thus extracellular production efficiency of the recombinant α-CGTase was lower than that in the culture with 1% glycine. It was worth noting that, with glycine concentration further increasing to 1.25% or higher, the enhancing effect of glycine on the extracellular activity of the recombinant enzyme was significantly reduced (Fig. 3).
Effect of glycine concentration on extracellular activity of α-CGTase. E. coli cells were grown in regular TB medium (filled circles) or TB medium supplemented with 0.25% (open squares), 0.5% (open triangles), 0.75% (×), 1.0% (open circles), or 1.25% (open diamonds) glycine at 14 h of culture, respectively. Each value represents the mean of three independent measurements, and the deviation from the mean is below 5%
When E. coli cells were cultured in TB medium supplemented with 0.25–1.25% glycine at the beginning of culture, the optimal concentration of glycine was also approximately 1% (data not shown).
Effect of glycine supplementation on cell membrane permeability
The hydrophobic fluorescent probe NPN was used as an indicator of outer membrane integrity. NPN has a low fluorescence quantum yield in aqueous solution but fluoresces strongly in the hydrophobic environment of a biological membrane. Normally, NPN is excluded from E. coli by the lipopolysaccharide layer of the outer membrane but can enter at points where membrane integrity is compromised (Eriksson et al. 2002). Thus, fluorescence value and its increased rate indicate outer membrane permeability. Permeability of the inner membrane was evaluated using the β-galactosidase substrate ONPG as a probe. When ONPG passes the inner membrane, it can be cleaved by β-galactosidase localized within the cytoplasm, resulting in the appearance of a yellow color. Thus, absorbance at 420 nm and its increased rate indicate inner membrane permeability.
To explore the mechanism for the promotive role of glycine in extracellular secretion of the recombinant enzyme, we analyzed the effect of glycine supplementation on cell membrane permeability. As shown in Fig. 4, when E. coli cells were cultured in regular TB medium, both outer and inner cell membranes had very low permeability during the first 36 h of culture. Upon further culture, cell aging resulted in the increases in cell membrane permeability (data not shown), which might be the main reason for the increase in extracellular secretion of the recombinant α-CGTase at the later stage of culture. When E. coli cells were cultured in TB medium supplemented with 1% glycine at the beginning of culture, the outer and inner membranes had high permeability soon after the culture started (Fig. 4). By comparison, delayed supplementation of glycine resulted in lower membrane permeabilities during the first 36 h of culture. Nevertheless, in the culture supplemented with 1% glycine at the middle of the exponential growth phase, although, at 24 h of culture, the permeabilities of the outer and inner membranes were lower than those of the culture supplemented with 1% glycine at the beginning of culture, they were still much higher than those of the culture in regular TB medium (Fig. 4). Furthermore, membrane permeabilities kept on increasing and, at 36 h of culture, reached almost the same levels as those of the culture supplemented with 1% glycine at the beginning of culture (Fig. 4). In addition, the enhancing effects of glycine on membrane permeability were dose-dependent (data not shown).
Effects of glycine supplement time on the permeabilities of the outer (a) and inner (b) membranes. After E. coli cells had been grown in regular TB medium for 12 h (filled squares), 24 h (filled diamonds), and 36 h (filled triangles), respectively, or in TB medium supplemented with 1.0% glycine at the beginning of culture (solid line) or 14 h of culture (dashed line) for 12 h (open squares), 24 h (open diamonds), and 36 h (open triangles), respectively, they were harvested, rinsed, and resuspended in 10 mM phosphate buffer (pH 7.4) to an OD600 of 0.5. NPN or ONPG was added into cell suspension. NPN fluorescence and the absorbance at 420 nm were monitored for 60 min, respectively. Each value represents the mean of three independent measurements, and the deviation from the mean is below 5%
Effect of glycine supplementation on cell growth
In fact, the culture with higher extracellular activity of the recombinant α-CGTase did not completely correspond to higher cell membrane permeabilities. To reveal the underlying reason for this, the effect of glycine supplementation on cell growth was also investigated. The results showed that glycine supplementation had a negative effect on cell growth. Cell density (OD600) of the culture is an indicator of total cell number, while CFU denotes viable cell number, suggesting cell viability. As shown in Figs. 5 and 6, after TB medium was supplemented with 1% glycine, the OD600 and CFU values at each culture time point were lower than those of the culture in regular TB medium, indicating the reductions in total cell number and cell viability. G6PD is a cytoplasmic protein and the level of this enzyme in the culture medium is expected to be very low unless substantial cell lysis has occurred (Sanwal 1970). Thus, G6PD activity in the culture medium can be used as an indicator of cell lysis. It was found that, when E. coli cells were cultured in regular TB medium, G6PD activity in the soluble cytoplasmic fraction reached 2.30 U/ml at 40 h of culture, while G6PD activity in the culture medium was very low during the first 40 h of culture (Fig. 6), indicating almost no cell lysis. In the culture with 1% glycine, G6PD activity in the culture medium at 40 h of culture was higher than that of the culture in regular TB medium (Fig. 6), indicating the increase in cell lysis.
Effect of glycine supplement time on cell density (OD600). TB medium was supplemented with 1.0% glycine at 0 h (open triangles), 8 h (asterisks), 14 h (open circles), 20 h (open diamonds), or 26 h (open squares) of culture. E. coli cells were grown in regular TB medium as control (filled circles). Each value represents the mean of three independent measurements, and the deviation from the mean is below 5%
Effects of glycine supplement time on cell viability and cell lysis. E. coli cells were grown in regular TB medium (filled circles) or TB medium supplemented with 1% glycine at the beginning of culture (open triangles) or 14 h of culture (open circles), respectively. Time courses of CFU per milliliter of culture per OD600 (solid line) and G6PD activity in the culture medium (dashed line) were analyzed. Each value represents the mean of three independent measurements, and the deviation from the mean is below 5%
Cell growth impairment exerted by glycine was affected by glycine supplement time. Obviously, glycine supplementation at the beginning of culture resulted in the most significant reductions in total cell number and viability. When E. coli cells were cultured in TB medium supplemented with 1% glycine at the beginning of culture, the OD600 and CFU values at 40 h of culture were approximately 48% and 52% lower than those of the culture in regular TB medium, respectively (Figs. 5 and 6). Moreover, G6PD activity in the culture medium increased gradually and reached 0.35 U/ml at 40 h of culture (Fig. 6), which was approximately 32% of G6PD activity in the soluble cytoplasmic fraction (1.10 U/ml), indicating obvious lysis of E. coli cells. By comparison, delayed supplementation could reduce cell growth impairment exerted by glycine. In the culture supplemented with 1% glycine at the middle of the exponential growth phase, OD600 and CFU values at 40 h of culture were 45% and 68% higher than those in the culture supplemented with 1% glycine at the beginning of culture, respectively (Figs. 5 and 6), while G6PD activity in the culture medium at 40 h of culture had an approximately 66% reduction (Fig. 6). Moreover, G6PD activity in the culture medium was only 7% of that in the soluble cytoplasmic fraction (1.73 U/ml), indicating no obvious cell lysis.
In addition, the negative effect of glycine on cell growth was dose-dependent. If TB medium was supplemented with 0.5%, 1%, or 1.25% glycine at the middle of the exponential growth phase, the OD600 value at 40 h of culture decreased by approximately 15%, 25%, or 40 %, respectively, when compared to that of the culture in regular TB medium (Fig. 7). Furthermore, the supplementation of a higher concentration of glycine resulted in lower CFU value of culture and higher G6PD activity in the culture medium (data not shown).
Effect of glycine concentration on cell density (OD600). E. coli cells were grown in regular TB medium (filled circles) or TB medium supplemented with 0.25% (open squares), 0.5% (open triangles), 0.75% (×), 1.0% (open circles), or 1.25% (open diamonds) glycine at 14 h of culture, respectively. Each value represents the mean of three independent measurements, and the deviation from the mean is below 5%
Discussion
The targeting of recombinant proteins for secretion to the culture medium of E. coli presents significant advantages over cytoplasmic or periplasmic expression (Mergulhao et al. 2005). However, when E. coli cells were cultured in regular TB medium, although the pelB signal peptide enabled the translocation of α-CGTase to the periplasmic space through sec-dependent secretion pathway, it was very difficult to overcome the outer membrane barrier for the translocation of the recombinant α-CGTase directed to the periplasmic space, as demonstrated by very few recombinant enzymes in the culture medium and a high-level periplasmic accumulation of the recombinant enzyme at 40 h of culture. Even across the inner membrane, the translocation of α-CGTase was inadequate as demonstrated by the formation of many insoluble inclusion bodies in the cytoplasm. Just due to the inadequate protein secretion across two cell membranes, the extracellular recombinant α-CGTase production was limited. Thus, in the present study, we attempted to circumvent this secretion problem of this protein in E. coli by glycine supplementation.
Glycine was a commonly used medium supplement for promoting the secretion of the recombinant enzyme into the culture medium of E. coli. In the present study, it was found that glycine efficiently enhanced the extracellular activity of the recombinant α-CGTase. Such enhancement of heterologous protein secretion into the culture medium by glycine has also been reported by some investigators (Yang et al. 1998; Tang et al. 2008; Kaderbhai et al. 1997; Aristidou et al. 1993; Jang et al. 1999; Yu et al. 1991). One potential mechanism for this enhancement was generally considered to be the increases in cell membrane permeability (Yang et al. 1998), which might be induced by the modification of peptidoglycan that resulted from the incorporation of glycine into the nucleotide-activated peptidoglycan precursors (Hammes et al. 1973). Here, our quantitative analysis confirmed the role of glycine in increasing cell membrane permeability. Specifically, the increased outer membrane permeability allowed release of the recombinant α-CGTases directed to the periplasmic space as demonstrated by the decrease in periplasmic accumulation, while the increase in inner membrane permeability and the relief of the stress exerted by periplasmic accumulation might promote translocation of this protein across the inner membrane, as demonstrated by the loss or decrease in insoluble inclusion bodies formed in the cytoplasm (Manting and Driessen 2000; Shin and Chen 2008). Additionally, cell lysis might also contribute to the extracellular release of the recombinant enzyme. However, it was generally undesirable for extracellular recombinant protein production, mainly due to more contaminants from the intracellular compartment (Shokri et al. 2003; Shin and Chen 2008).
In previous reports, glycine supplementation was carried out, generally at the beginning of culture. However, while glycine supplementation at the beginning of culture resulted in high membrane permeability soon after the culture started, it was found to have a markedly negative effect on cell growth as demonstrated by the significant reductions in total cell number and viability and the obvious increase in cell lysis, which in turn could affect overall recombinant protein production (Sugamata and Shiba 2005). Thus, in the culture supplemented with 1% glycine at the beginning of culture, although the translocations of the recombinant proteins across the outer and inner membranes were significantly improved, cell growth impairment prevented the increase in the extracellular activity of the recombinant α-CGTase.
To further enhance the extracellular enzyme activity, it was desirable to promote healthy cell growth under the premise of adequate protein secretion. A previous study had shown that the combined use of glycine at a relative low concentration (about 0.5%) and bacteriocin release protein at a relative low induction level resulted in higher total and extracellular activities of the recombinant enzyme without significantly affecting cell growth, when compared to those in the culture supplemented with a relatively high concentration of glycine (about 1.0%) alone (Yu et al. 1991). In search for a simple solution, here, we found that delayed supplementation of glycine could control cell growth impairment exerted by glycine due to the accumulations of many healthy cells in the culture medium before glycine supplementation. In the culture supplemented with 1% glycine at the middle of the exponential growth phase, cell number and viability were much higher than those in the culture supplemented with 1% glycine at the beginning of culture, while cell lysis was notably decreased. In the meantime, before glycine supplementation, although outer membrane permeability was not sufficient to allow the release of the recombinant α-CGTases directed to the periplasmic space, most recombinant proteins could be successfully translocated across the inner membrane and firstly accumulated in the periplasmic space at this stage, as demonstrated by only few inclusion bodies formed in the cytoplasm. Subsequently, glycine could induce the increases in the membrane permeability, which rendered the cell membranes permeable enough to allow extracellular secretion of the recombinant α-CGTase. As a result, glycine supplementation at the middle of exponential growth phase facilitated healthy cell growth under the premise of almost sufficient membrane permeabilities, thereby leading to higher extracellular activity of the recombinant α-CGTase than that in the culture supplemented with 1% glycine at the beginning of culture. Importantly, in this culture, the relative low level of G6PD in the culture medium indicated that cell lysis was much less responsible for extracellular recombinant protein production than that in the culture supplemented with 1% glycine at the beginning of culture.
Noteworthily, if TB medium was supplemented with 1% glycine at the early stage of exponential growth phase, it was limited to control cell growth impairment exerted by glycine. On the other hand, if glycine supplementation was carried out at the late stage of the exponential growth phase or later, a few insoluble inclusion bodies might have been formed before glycine supplementation, which was similar to the culture in regular TB medium. Furthermore, too late supplementation of glycine could not result in the rapid increases in membrane permeability of some mature cells. Thus, for extracellular recombinant α-CGTase production, the optimal glycine supplement time should be the middle of the exponential growth phase at which glycine supplementation could obtain the better balance between the amount of enzyme producer and the increased membrane permeability.
In summary, our study demonstrated that delayed glycine supplementation markedly enhanced the extracellular secretion of the recombinant α-CGTase. To the best of our knowledge, this is the first report about the enhancing effect of delayed glycine supplementation on the extracellular secretion of a recombinant protein in E. coli. This approach could be used for extracellular secretion of other heterogeneous proteins in E. coli.
References
Aristidou AA, Yu P, San KY (1993) Effects of glycine supplement on protein production and release in recombinant Escherichia coli. Biotechnol Lett 15:331–336
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10:411–421
Blight MA, Chervaux C, Holland IB (1994) Protein secretion pathway in Escherichia coli. Curr Opin Biotechnol 5:468–474
Chang HY, Irwin PM, Nikolov ZL (1998) Effects of mutations in the starch-binding domain of Bacillus macerans cyclodextrin glycosyltransferase. J Biotechnol 65:191–202
Chen J, Wu J, Li ZF, Li B, Cheng CC (2008) Cloning and expression of the gene encoding α-cyclodextrin glycosyltransferase. CN Patent 200810024162.3
Choi JH, Lee SY (2004) Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol 64:625–635
Eriksson M, Nielsen PE, Good L (2002) Cell permeabilization and uptake of antisense peptide-peptide nucleic acid (PNA) into Escherichia coli. J Biol Chem 277:7144–7147
Hammes W, Schleifer KH, Kandler O (1973) Mode of action of glycine on the biosynthesis of peptidoglycan. J Bacteriol 116:1029–1053
Jana S, Deb JK (2005) Strategies for efficient production of heterologous proteins in Escherichia coli. Appl Microbiol Biotechnol 67:289–298
Jang KH, Seo JW, Song KB, Kim CH, Rhee SK (1999) Extracellular secretion of levansucrase from Zymomonas mobilis in Escherichia coli. Bioprocess Biosyst Eng 21:453–458
Jeang CL, Wung CH, Chang BY, Yeh SS, Lour DW (1999) Characterization of the Bacillus macerans cyclodextrin glucanotransferase overexpressed in Escherichia coli. Proc Natl Sci Counc Repub China B 23:62–68
Jeang CL, Lin DG, Hsieh SH (2005) Characterization of cyclodextrin glycosyltransferase of the same gene expressed from Bacillus macerans, Bacillus subtilis, and Escherichia coli. J Agric Food Chem 53:6301–6304
Kaderbhai N, Karim A, Hankey W, Jenkins G, Venning J, Kaderbhai MA (1997) Glycine-induced extracellular secretion of a recombinant cytochrome expressed in Escherichia coli. Biotechnol Appl Biochem 25(Pt1):53–61
Kato C, Kudo T, Watanabe K, Horikoshi K (1983) Extracellular production of Bacillus penicillinase by Escherichia coli carrying pEAP2. Appl Microbiol Biotechnol 18:339–343
Kim CI, Kim MD, Park YC, Han NS, Seo JH (2000) Refolding of Bacillus macerans cyclodextrin glucanotransferase expressed as inclusion bodies in recombinant Escherichia coli. J Microbiol Biotechnol 10:632–637
Kim SG, Kweon DH, Lee DH, Park YC, Seo JH (2005) Coexpression of folding accessory proteins for production of active cyclodextrin glycosyltransferase of Bacillus macerans in recombinant Escherichia coli. Protein Expr Purif 41:426–432
Koebnik R, Locher KP, Van Gelder P (2000) Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol Microbiol 37:239–253
Lehrer R, Barton A, Ganz T (1988) Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J Immunol Methods 108:153–158
Lejeune A, Sakaguchi K, Imanaka T (1989) A spectrophotometric assay for the cyclization activity of cyclomaltohexaose (α-cyclodextrin) glucanotransferase. Anal Biochem 181:6–11
Li ZF, Wang M, Wang F, Gu ZB, Du GC, Wu J, Chen J (2007) γ-Cyclodextrin: a review on enzymatic production and applications. Appl Microbiol Biotechnol 77:245–255
Li ZF, Zhang JY, Wang M, Gu ZB, Du GC, Li JK, Wu J, Chen J (2009) Mutations at subsite −3 in cyclodextrin glycosyltransferase from Paenibacillus macerans enhancing alpha-cyclodextrin specificity. Appl Microbiol Biotechnol 83:483–490
Loh B, Grant C, Hancock REW (1984) Use of the fluorescent probe 1-n-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother 26:546–551
Lucas-Abellan C, Gabaldon-Hernandez JA, Penalva J, Fortea MI, Nunez-Delicado E (2008) Preparation and characterization of the inclusion complex of chlorpyrifos in cyclodextrins to improve insecticide formulations. J Agric Food Chem 56:8081–8085
Makrides SC (1996) Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev 60:512–538
Manting EH, Driessen AJ (2000) Escherichia coli translocase: the unravelling of a molecular machine. Mol Microbiol 37:226–238
Mergulhao FJ, Summers DK, Monteiro GA (2005) Recombinant protein secretion in Escherichia coli. Biotechnol Adv 23:177–202
Mourtzinos I, Salta F, Yannakopoulou K, Chiou A, Karathanos VT (2007) Encapsulation of olive leaf extract in β-cyclodextrin. J Agric Food Chem 55:8088–8094
Rinas U, Hoffmann F (2004) Selective leakage of host-cell proteins during high-cell-density cultivation of recombinant and non-recombinant Escherichia coli. Biotechnol Prog 20:679–687
Saenger W (1980) Cyclodextrin inclusion compounds in research and industry. Angew Chem 19:344–362
Sanwal BD (1970) Regulatory mechanisms involving nicotinamide adenine nucleotides as allosteric effectors III. Control of glucose 6-phosphate dehydrogenase. J Biol Chem 245:1626–1631
Shin HD, Chen RR (2008) Extracellular recombinant protein production from an Escherichia coli lpp deletion mutant. Biotechnol Bioeng 101:1288–1296
Shokri A, Sanden AM, Larsson G (2003) Cell and process design for targeting of recombinant protein into the culture medium of Escherichia coli. Appl Microbiol Biotechnol 60:654–664
Sorensen HP, Mortensen KK (2005) Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol 115:113–128
Sugamata Y, Shiba T (2005) Improved secretory production of recombinant proteins by random mutagenesis of hlyB, an α-hemolysin transporter from Escherichia coli. Appl Environ Microbiol 71:656–662
Szente L, Szejtli J (2004) Cyclodextrins as food ingredients. Trends Food Sci Technol 15:137–142
Tang JB, Yang HM, Song SL, Zhu P, Ji AG (2008) Effect of glycine and Triton X-100 on secretion and expression of ZZ-EGFP fusion protein. Food Chem 108:657–662
Yang J, Moyana T, MacKenzie S, Xia Q, Xiang J (1998) One hundred seventy-fold increase in excretion of an FV fragment-tumor necrosis factor alpha fusion protein (sFV/TNF-α) from Escherichia coli caused by the synergistic effects of glycine and Triton X-100. Appl Environ Microbiol 64:2869–2874
Yu P, Aristidou AA, San KY (1991) Synergistic effect of glycine and bacteriocin release protein in the release of periplasmic protein in recombinant E. coli. Biotechnol Lett 13:311–316
Acknowledgments
This work was supported financially by the Natural Science Foundation of Jiangsu Province (BK2007019), the National Outstanding Youth Foundation of China (20625619), the National High-tech Research and Development Program of China (863 Program; 2006AA10Z335), Research Program of State Key Laboratory of Food Science and Technology (SKLF-MB-200802), Program of Innovation Team of Jiangnan University (2008CXTD01), and the Graduate Student Creative Research Program of Jiangsu Province in 2008 (CX08B_127Z).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Z., Gu, Z., Wang, M. et al. Delayed supplementation of glycine enhances extracellular secretion of the recombinant α-cyclodextrin glycosyltransferase in Escherichia coli . Appl Microbiol Biotechnol 85, 553–561 (2010). https://doi.org/10.1007/s00253-009-2157-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2157-7