Abstract
Gloshedobin, a thrombin-like enzyme from the venom of Gloydius shedaoensis, is usually produced as inclusion bodies in Escherichia coli cell. In this work, gloshedobin was separately fused with three fusion partners NusA, GST, and TrxA at its N terminus and then was expressed as fusion proteins in E. coli. The results showed that the NusA was the most efficient fusion partner to improve the solubility of recombinant gloshedobin. The purified NusA-fused gloshedobin with an overall yield of 64.6% was resolved as one band in the SDS-PAGE gel with molecular mass of about 90 kDa. Both fibrinogen clotting and fibrinogenolytic activities were found for the recombinant product. The purified NusA-fused gloshedobin exhibited amidolytic activity of 506 U/mg under optimal conditions of pH of 8.0 and 40°C. The inhibition study of NusA-fused gloshedobin by various inhibitors showed that serine protease inhibitors, phenylmethylsulphonyl fluoride, and N-tosyl-l-phenylalanine chloromethyl ketone, strongly inhibited its admidolytic activity, whereas ethylenediaminetetraacetic acid as well as heparin and hirudin did not, suggesting that NusA-fused gloshedobin exhibited the same characteristics as the native form of gloshedobin. The strategy of this work may contribute to improve the soluble expression level of other thrombin-like enzymes from snake venom in E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Snake venoms are very complex mixtures containing a wide variety of biologically active proteins and polypeptides (Lu et al. 2005; Markland 1998). Thrombin-like enzymes from snake venom belong to the serine protease family, exhibiting fibrinogenolytic activity that cleave the Aα-chain or the Bβ-chain of fibrinogen to release fibrinopeptide A or fibrinopeptide B, respectively (Castro et al. 2004). Since thrombin-like enzymes do not activate factor XIII, the resulting fibrin clot is not cross-linked and easily removed from circulation by the reticuloendothelial system or normal fibrinolytic system (Kini et al. 2001). Because of these physiological properties, thrombin-like enzymes have been used as antithrombotic agents for the treatment of occlusive thrombi (Koh et al. 2006; Marsh and Williams 2005).
To date, the amino acid sequences of dozens of thrombin-like enzymes have been determined partially or completely from various snake species (Castro et al. 2004; Pirkle 1998). Gloshedobin (formerly named defibrase) is a thrombin-like enzyme obtained from the venom of Gloydius shedaoensis and has been cloned and partially characterized in our previous work (Yang et al. 2002). Gloshedobin has high physiological activities of thrombin-like enzymes and, therefore, has been applied in the treatment of the thrombotic diseases. However, similar to other thrombin-like enzymes such as acutin from Agkistrodon acutus and pallabin from Agkistrodon halys pallas, gloshedobin was also expressed as biologically inactive inclusion bodies in E. coli (Pan et al. 1999; Fan et al. 1999). This may be attributed to that it is a cytotoxic protein and contains 12 cysteine residues forming six disulfide bonds (Yang et al. 2003). Many efforts have been attempted to improve the soluble expression level of recombinant thrombin-like enzymes in various microorganisms. Habutobin was expressed in a baculoviral system (Sunagawa et al. 2007). Soluble form of gloshedobin has been successfully produced in the yeast Pichia pastoris (Yang et al, 2002). However, the solubility of synthesized protein has not yet reached a satisfactory level.
Although a number of expression systems are available for production of proteins of therapeutic or commercial interest, E. coli has always been the first choice as expression host because of its low cost, fast growth, and easy operation (Baneyx 1999; Jana and Deb 2005). Many efforts such as reducing cultivation temperature (Vasina and Baneyx 1997), co-expressing of molecular chaperones (de Marco and De Marco 2004), and altering E. coli expression strain (Miroux and Walker 1996) have been attempted to overcome the difficulty in generating soluble recombinant protein in E. coli. Fusion protein expression by cloning a unique fusion tag into the N-terminal of a target protein is a preferable strategy for enhancing the solubility of recombinant proteins in E. coli (Esposito and Chatterjee 2006). Commercial fusion tags such as thioredoxin (TrxA, LaVallie et al. 1993), glutathione-S-transferase (GST, Nygren et al. 1994), N-utilization substance A (NusA, Nallamsetty and Waugh 2006), and disulfide oxidoreductase (DsbA, Zhang et al. 1998b) have been revealed to either increase the expression level or improve solubility of recombinant protein.
In this study, three soluble partners (NusA, GST, and TrxA) fused at N terminus of gloshedobin were expressed in E. coli, and the efficiency to increase the solubility of gloshedobin was compared. It was found that NusA was the most efficient fusion partner. The purified NusA-fused gloshedobin exhibited satisfactory enzymatic properties including amidolytic, fibrinogen clotting, and fibrinogenolytic activities.
Materials and methods
Host strains, plasmids, and enzymes
E. coli JM109 (Takara, Dalian, China) was used as the host for DNA manipulation. E. coli BL21-Gold (DE3; Stratagene, USA) was used as the host for expression of recombinant protein. pMD18-T Simple Vector (Takara, Dalian, China) was used as cloning vector. Plasmids pET-43.1a, pET-42a, and pET-32a (Novagen, Madison, WI, USA) were used to construct expression vectors. T4 DNA ligase, Ex Taq DNA polymerase, and all the restriction enzymes were obtained from Takara (Dalian, China).
Construction of cloning and expression vectors
The gene-encoding gloshedobin was amplified from plasmid pPIC9K-TLE (Yang et al. 2002) by polymerase chain reaction (PCR) with the N-terminal primer 5'-CGGAATTCATCATTGGAGGTGATGAA -3' (EcoRI site is underlined) and the C-terminal primer 5'-CCGCTCGAGTTAGTGGTGGTGGTGGTGG TGGTGGTGGTGGTGTGGGGGGCAGGTTGCATC-3' (XhoI site is underlined). The PCR product was digested with XhoI-EcoRI and cloned into the XhoI-EcoRI site of the pMD18-T simple vector and then transformed into E. coli JM 109. Nucleic acid sequences of the cloning DNA fragment were confirmed by DNA sequencing (BigDye™ Kit, Applied Biosystems, USA) and ABI PRISM™ 3730XL DNA Analyzer, according to the recommended protocols. The target DNA fragment was further subcloned in the same site of pET-43.1a, pET-42a, and pET-32a vectors, resulting in pQYNusA-Glo-a, pQYGST-Glo-a, and pQYTrxA-Glo-a, respectively (Fig. 1). The resulting vectors were transformed into E. coli BL21-Gold (DE3) for fusion protein expression.
Expression of gloshedobin fusion proteins
The single colony of E. coli BL21-Gold (DE3) harboring the expression vector was inoculated in 30 ml of Luria–Bertani medium containing 100 μg/ml ampicillin and then cultivated at 37°C until the optical density (OD600 ) reaches 0.6. The cells were harvested by centrifugation at 4,000×g for 10 min and resuspended in 3 l fresh Terrific Broth medium containing 100 μg/ml ampicillin and 1% (w/v) glucose. Expression of the fusion protein was induced with isopropyl-β-d-thiogalactoside (IPTG) at a final concentration of 1.0 mM, and the culture was incubated for an additional 8 h at 25°C. The cells were harvested by centrifugation at 8,000×g for 15 min and washed with TE buffer (20 mM Tris-HCl, 10 mM ethylenediaminetetraacetic acid (EDTA), pH 8.0). Approximated 30 g (wet weight) cells were obtained from 3 l culture. After centrifugation, the cell pellets was resuspended in 40 ml (for 1 l culture) ice-cold extraction buffer (20 mM Tris-HCl, 100 mM NaCl, 1 mM EDTA, pH 8.0, 0.5 % (w/v) glycerol) and lysed by ultrasonication at ice-cold temperature using UP400S instrument (Dr. Hielscher GmbH, Stuttgart, Germany). The cell lysis was centrifuged at 12,000 rpm for 15 min to separate soluble (supernatant) and precipitated (pellet) fractions.
Purification of gloshedobin fusion protein
Solid ammonium sulfate was added into the supernatant to reach 1 M. After centrifugation at 12,000 rpm for 20 min at 4°C, the supernatant was loaded onto a Phenyl Sepharose FF column (GE Healthcare-Amersham Biosciences) followed by elution with a gradient of 1–0 M ammonium sulfate. Active fractions were collected and loaded onto a Q-Sepharose HP column (GE Healthcare-Amersham Biosciences) followed by a Mono Q column (GE Healthcare-Amersham Biosciences), eluting by a gradient of 0.2–0.4 and 0.15–0.4 M of NaCl, respectively. The active fractions were concentrated by ultracentrifuge (Amicon Ultra-15, Millipore, USA) and loaded onto a Superdex 200 column (GE Health-Amersham Biosciences) by eluting with elution buffer (50 mM Tris-HCl, 150 mM NaCl, pH 7.5). Aliquots of the resulting purified protein were frozen at –20°C for subsequent analysis. Enzymatic activity assay by using substrate Nα-benzoyl-dl-arginine-p-nitroanilide (BApNA) was applied to monitor the elution of active fractions during every purification step.
SDS-PAGE and western blot analysis
SDS-PAGE analysis was performed using 10% resolving gel and 5% stacking gels. The protein bands were visualized by Coomassie brilliant blue R-250 and then analyzed by image-density analysis software (Gel-Pro, USA). Protein samples were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membrane (Millipore, USA). The membranes were blocked with 5% defatted milk at room temperature for 60 min and then incubated with anti-snake venom horse serum (1:500, Shanghai Institute of Biologic Products, Shanghai, China) at room temperature for 3 h. The membranes were washed five times with TBS-T buffer (20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.15% (v/v) Tween-20) and then incubated with horseradish peroxidase-conjugated rabbit anti-horse IgG (1:5,000, Shanghai Institute of Biologic Products, Shanghai, China) at room temperature for 60 min. Finally, the membranes were washed five times as above, and a 3'-diaminobenzidine kit was used for color development detected (Sigma, St. Louis, Mo, USA).
Amidolytic activity assay
Amidolytic activity was determined by incubating 195 μl of the substrate, 2 mM BApNA (20 mM Tris-HCl, pH 7.5) with 5 µl of gloshedobin (1.0 µg )in a 96-well plate. The amount of -p-nitroaniline released was determined by measuring the change in absorbance at 405 nm using a Sinrise Spectrophotometer (Tecan, Austria). One unit of amidolytic activity was defined as the quantity of enzyme required for hydrolyzing 1.0 µM/min BApNA at 25°C.
Fibrinogen clotting and fibrinogenolytic activity assay
Fibrinogen clotting activity was determined by incubating 100 µl of purified NusA-fused gloshedobin with 200 µl of fibrinogen (4 mg/ml) in 20 mM Tris-HCl (pH 7.5) containing 150 mM NaCl at 37°C for 6 h in glass tube, and then the occurrence of the fibrinogen clotting phenomenon was verified by photos. The fibrinogen from bovine, purchased from Sigma, is plasminogen-free and of electrophoretical-grade purity.
Fibrinogenolytic activity was determined by incubating 25 µl of purified NusA-fused gloshedobin (1.0 µg) with 100 µl of fibrinogen (0.2 mg/ml) in 20 mM Tris-HCl (pH 7.8) containing 150 mM NaCl at 37°C for 0.5, 1, 1.5, 2, 2.5, 4, 6, 8, 10, 12, 19, and 24 h, respectively. Then, the reaction mixture was separated by 10% SDS-PAGE to examine the cleavage pattern of fibrinogen chains digested by gloshedobin.
Effects of pH and temperature on amidolytic activity
The effect of pH on amidolytic activity was determined by preincubating 1.0 µg of enzyme in citrate buffer (20 mM, pH 4–6) or Tris-HCl buffer (20 mM, pH 7–10) at 25°C for 20 min. The reaction was initiated by addition of 2 mM substrate solution (BApNA, 20 mM Tris-HCl, pH 7.5). The effect of temperature on amidolytic activity was determined by preincubating 1.0 μg of enzyme in 20 mM Tris-HCl (pH 7.5) at different temperatures (20°C, 30°C, 40°C, 50°C, and 60°C) for 20 min. The reaction was initiated by addition of 2 mM substrate solution (BApNA, 20 mM Tris-HCl, pH 7.5).
Effect of enzyme inhibitors on the amidolytic activity
The effect of enzyme inhibitors on the amidolytic activity was determined by preincubating 1.0 μg of enzyme in 20 mM Tris-HCl (pH 7.5) with inhibitors including 0.1 or 1 mM phenylmethylsulphonyl fluoride (PMSF), 0.1 or 1 mM N-tosyl-l-phenylalanine chloromethyl ketone (TPCK), 1 mg/ml heparin, 0.4 mg/ml hirudin, 3 mM dithiothretol (DTT), 3 mM β-mercaptoethanol (β-ME), 10 mM EDTA at 25°C for 20 min. The reaction was initiated by addition of 2 mM substrate solution (BApNA, 20 mM Tris-HCl, pH 7.5).
Results
Construction of the expression vectors
Gloshedobin gene was successfully amplified from the plasmid pPIC9K-TLE and ligated into plasmid pET-43.1a, pET-42a, and pET-32a vectors, resulting in pQYNusA-Glo-a, pQYGST-Glo-a, and pQYTrxA-Glo-a, respectively. In these recombinant plasmids, gloshedobin was fused with fusion partners NusA, GST, and TrxA, respectively, under the control of T7 promoter (Fig. 1). All constructs were confirmed by DNA sequencing.
Expression of NusA-, GST-, and TrxA-fused gloshedobin
The fusion expression vectors (pQYTrxA-Glo-a, pQYGST-Glo-a, and pQYNusA-Glo-a) encoding the target protein combined with the soluble tags were transformed into E. coli BL21-Gold (DE3) to express proteins of interest, respectively. When the culture was propagated at 37°C until the optical density (OD600) reaches 0.6, the expression of the fusion protein was induced with 1.0 mM of IPTG, and then the culture was grown for an additional 8 h at 25°C. Consequently, approximated 10 g (wet weight) cells were obtained from 1 l culture. After lysed by ultrasonication, the supernatant and pellet fractions of fusion proteins were analyzed by western blotting using horse anti-snake venom antibody. As shown in Fig. 2, although TrxA-, GST-, and NusA-fused gloshedobins appeared in soluble fraction of the cell lysate after induced with IPTG-, TrxA-, and GST-fused gloshedobin were mainly in the form of inclusion bodies in E. coli. Moreover, TrxA- and GST-fused gloshedobin were also expressed even without IPTG induction, which was also seen in Fig. 2. Unlike these two, NusA-fused gloshedobin was only produced after the induction of IPTG and mainly in the soluble form in the cytosol of E. coli. Therefore, NusA-fused gloshedobin was selected as example in the subsequent experiments including.
Western blot analysis of the recombinant gloshedobin fused with soluble fusion tags. Lane S (+) represents soluble faction of cell lysates after IPTG induction; Lane S (–) represents soluble faction of cell lysates before IPTG induction; Lane P (+) represents precipitates after IPTG induction; Lane P (–) represents precipitates before IPTG induction; Lane C host strain alone; Lane M1, M2 protein molecular weight marker
Purification of NusA-fused gloshedobin
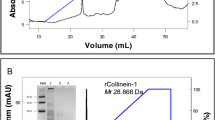
NusA-fused gloshedobin was purified in this study by four chromatographic steps: hydrophobic interaction chromatography, two anion exchanges chromatographies, and gel filtration chromatography. Specific activity of NusA-fused gloshedobin was determined by its amidolytic activity by using the substrate, BApNA. As shown in Fig. 3, SDS-PAGE showed that the purified NusA-fused gloshedobin was observed as a single band in the gel with molecular masses of about 90 kDa. After these four-step chromatographies, the specific activity and overall recovery of purified NusA-fused gloshedobin was 506 U/mg and 64.6%, respectively (Table 1). The recovery and purification factors of each chromatography step are summarized in Table 1.
SDS-PAGE analysis of purified NusA-fused gloshedobin by four-step chromatographies. Lane M protein molecular weight marker; lane 1 cell lysates; lane 2 the eluent from hydrophobic interaction chromatography using Phenyl Sepharose FF column; lane 3 the eluent from anion exchanges chromatography using Q-Sepharose HP column; lane 4 the eluent from anion exchange chromatography using Mono Q column; lane 5 the eluent from gel filtration chromatography using Superdex 200 column
Fibrinogen clotting and fibrinogenlytic activities of purified NusA-fused gloshedobin
In vitro, thrombin-like enzymes are serine protease, which resemble thrombin, play a key role in coagulation because they cleave fibrinogen to cross-linked fibrin by releasing either fibrinopeptide A or fibrinopeptide B. Therefore, thrombin-like enzymes exhibit fibrinogen clotting and fibrinogenlytic activities in vitro. However, in vivo thrombin-like enzyme different from thrombin do not activate factor XIII. Consequently, fibrin cleaved by these proteases cannot be cross-linked, and then easily removed from circulation by the reticuloendothelial system or normal fibrinolytic system.
In the case of fibrinogen clotting activity assay, the fibrinogen was incubated with purified NusA-fused gloshedobin at 37°C for 6 h in glass tube, and then the occurrence of the fibrinogen clotting phenomenon was verified by photos. As shown in Fig. 4, the solution containing fibrinogen and purified NusA-fused gloshedobin was turbid; however, other glass tube was clear. These results indicated that the addition of purified NusA-fused gloshedobin only led to the formation of a clotting phenomenon. In the case of fibrinogenolytic activity assay, the degradation products of fibrinogen digested with NusA-fused gloshedobin were analyzed by SDS-PAGE. As shown in Fig. 5, the band of α chain of fibrinogen became invisible on the gel after 2 h of the reaction. Moreover, the β chain of fibrinogen was fully digested after 24 h of the reaction. However, the remaining γ chain of fibrinogen seems to be stable, since the band of γ chain was intact even after 24 h. These results indicated that NusA-fused gloshedobin exhibited the thrombin-like activities.
Fibrinogen clotting activity of purified NusA-fused gloshedobin; 1 0.4 mg/ml of fibrinogen only; 2 0.4 mg/ml of fibrinogen with purified NusA-fused gloshedobin; 3 0.4 mg/ml of fibrinogen with concentrated culture medium after IPTG induction; 4 0.4 mg/ml of fibrinogen with cytosol of E. coli carring empty plasmid after IPTG induction
Effects of pH, temperature, and inhibitors on amidolytic activity
The amidolytic activity of the purified NusA-fused gloshedobin was determined using BApNA as substrate. As shown in Fig. 6, the optimum conditions for the enzyme’s amidolytic activity were determined to be 40°C and pH 8.0 (Fig. 6a, b).
The effect of various inhibitors on the amidolytic activity of NusA-fused gloshedobin was evaluated. As shown in Table 2, the concentration of 1 mM of PMSF completely inhibited this activity. The amidolytic activity of NusA-fused gloshedobin was partially affected by TPCK, DTT, and β-ME. However, heparin and EDTA had no effect on this activity.
Discussion
The expression of recombinant thrombin-like enzymes in microorganisms is still of research interest because the amount of thrombin-like enzymes extracted from snake venom is very limited. Some thrombin-like enzymes have been successfully synthesized in various microorganisms by recombinant DNA technology. Salmobin, a thrombin-like enzyme obtained from Agkistrodon halys, was expressed by cell surface display on E. coli using ice nucleation protein (Jeong et al. 2001). In addition, soluble form of batroxobin and ancrod has successfully been obtained in the yeast stain P. pastoris (You et al. 2004; Yu et al. 2007). However, the expression level of proteins synthesized by these systems could not satisfy requirements of biochemical analysis, therapeutics, or structural studies.
E. coli is the preferred host cell for the expression of recombinant proteins because of its well-characterized genetics, rapid growth, and the availability of numerous vectors (Baneyx 1999; Jana and Deb 2005). However, thrombin-like enzymes are difficult to be expressed in the soluble form in E. coli because they are cytotoxic and single-chain cystein-rich proteins. Correct folding of recombinant thrombin-like enzymes in term of enzymatic activities is still a big challenge.
Calobin, a thrombin-like enzyme obtained from Agkistrodon caliginous, was co-expressed with disulfide isomerase (DsbC) and TrxA (Yuan et al. 2004). The free form of TrxA and DsbC were efficient for the soluble expression of calobin. However, the recombinant calobin-TrxA-DsbC only retained the fibrinogenolytic activity but lost most of amidolytic activity and even all the fibrinogen clotting activity. Therefore, in this work, NusA, GST, and TrxA genes were expressed as fusion partners, not in the free form. As shown in Fig. 2, although NusA, GST, and TrxA were all efficient for the soluble expression of gloshedobin, the solubility of NusA-fused gloshedobin was the most satisfactory one. In addition, the expression vectors, pQYTrxA-Glo-a and pQYGST-Glo-a, failed to be strictly controlled under T7 promoter because recombinant proteins were observed to be produced even without IPTG induction (Fig. 2). These results indicated that NusA was the most efficient fusion partner for improving the soluble expression level of gloshedobin.
During molecular cloning, his-tag at C terminus of recombinant gloshedobin was designed in an attempt to simplify the purification procedure. Although his-tag purification strategy is widely applied in the simplification of purification of recombinant proteins, it is unfortunate that most gloshedobin existed in unbound fractions, which was observed by enzymatic activity assay. Similarly, it is noted for its ineffective binding to Ni-chelating ligands (Yuan et al. 2004; Debeljak et al. 2006). The reason may not be clear until detailed structural information is revealed. Therefore, in this work, four chromatographic steps were used to purify the NusA-fused gloshedobin, which contained hydrophobic interaction chromatography, two anion exchanges chromatographies, and gel filtration chromatography. According to SDS-PAGE analysis shown in Fig. 3, the nonspecific bands gradually disappeared, and the NusA-fused gloshedobin finally became a single band. These results indicated that although four chromatography steps were cumbersome, they were very effective.
The fibrinogenolytic activity is a main physiological property of thrombin-like enzymes in terms of their potential clinical application in thrombosis and anticoagulation. In this work, the purified NusA-fused gloshedobin preferentially digested the Aα chain then slowly cleaved the Bβ chain, however, did not affect the γ chain (Fig. 5). This is very similar to most of thrombin-like enzymes (Nishida et al. 1994; Zhang et al. 1998a), although other individual thrombin-like enzymes which preferentially degraded the Aα or Bβ chains (Sant’Ana et al. 2008) or degraded both Aα and Bβ chains with similar efficiency, were reported (Castro et al. 2004). In addition, the purified NusA-fused gloshedobin in this work also exhibited fibrinogen clotting activity and amidolytic activity, suggesting that NusA fusion tag did not affect the thrombin-like enzyme characteristics of gloshedobin.
The optimum conditions for the enzyme’s amidolytic activity were determined to be 40°C and pH 8.0, similar to that of other thrombin-like enzymes. That serine protease inhibitors PMSF (1 mM) and TPCK (1 mM) inhibited the amidolytic activity of NusA-fused gloshedobin by 100% and 77%, respectively (Table 2), is consistent with the fact that it belongs to the serine protease family. The chelator EDTA did not affect its amidolytic activity, also confirming that the recombinant gloshedobin was a serine protease.
High concentration of DTT (10 mM) and β-ME (10 mM) had little effect on the amidolytic activity of the recombinant gloshedobin in this study, suggesting that recombinant gloshedobin was a relatively stable protein in comparison with other thrombin-like enzymes. Bothrombin (Nishida et al. 1994) and stejnobin (Zhang et al. 1998a) were reported to be significantly inhibited by DTT and β-ME. However, like gloshedobin, harobin, a fibrinogenolytic serine protease from the sea snake Lapemis hardwickii (He et al. 2007), was reported to be resistant to 10 mM DTT. Heparin, a natural thrombin inhibitor, did not affect the amidolytic activity, suggesting that the recombinant gloshedobin differed from thrombin in structure. From a clinical point of view, it is important that heparin as well as hirudin does not inhibit its enzymatic activity, which indicates that gloshedobin could combine with heparin or hirudin in the treatment of thromboembolic and cardiopulmonary diseases.
In conclusion, we investigated the soluble expression of gloshesdobin fused with three soluble tags and found that the solubility of NusA-fused gloshedobin was the best. The purified NusA-fused gloshedobin exhibited satisfactory thrombin-like enzyme activities including amidolytic activity, fibrinogen clotting activity, and fibrinogenolytic activity. This study provided a method useful for the preparation of other recombinant thrombin-like enzymes of clinical interest.
References
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10:411–421
Castro HC, Zingali RB, Albuquerque MG, Pujol-Luz M, Rodriques CR (2004) Snake venom thrombin-like enzymes: from reptilase to now. Cell Mol Life Sci 61:843–856
Debeljak N, Feldman L, Davis KL, Komel R, Sytkowski AJ (2006) Variability in the immunodetection of His-tagged recombinant proteins. Anal Biochem 359:216–223
de Marco A, De Marco V (2004) Bacteria co-transformed with recombinant proteins and chaperones cloned in independent plasmids are suitable for expression tuning. J Biotechnol 109:45–52
Esposito D, Chatterjee DK (2006) Enhancement of soluble protein expression through the use of fusion tags. Curr Opin Biotechnol 17:353–358
Fan CY, Qian YC, Yang SL, Gong Y (1999) Cloning, sequence analysis and expression in E. coli of the cDNA of the thrombin-like enzyme (pallabin) from the venom of Agkistrodon halys pallas. Biochem Mol Biol Int 47:217–225
Jana S, Deb JK (2005) Strategies for efficient production of heterologous proteins in Escherichia coli. Appl Microbiol Biotechnol 67:289–298
Jeong H, Yoo S, Kim E (2001) Cell surface display of salmobin, a thrombin-like enzyme from Agkistrodon halys venom on Escherichia coli using ice nucleation protein. Enzyme Microb Technol 28:155–160
He J, Chen S, Gu J (2007) Identification and characterization of Harobin, a novel fibrino(geno)lytic serine protease from a sea snake (Lapemis hardwickii). FEBS Lett 581:2965–2973
Kini RM, Rao VS, Joseph JS (2001) Procoagulant proteins from snake venoms. Haemostasis 31:218–224
Koh DC, Armugam A, Jeyaseelan K (2006) Snake venom components and their applications in biomedicine. Cell Mol Life Sci 63:3030–3041
Lavallie ER, Diblasio EA, Kovacic S, Grant KL, Schendel PF, McCoy JM (1993) A thioredoxin gene fusion expression system that circumvents inclusion body formation in the Escherichia-coli cytoplasm. Biotechnology 11:187–193
Lu Q, Clemetson JM, Clemetson KJ (2005) Snake venoms and hemostasis. J Thromb Haemost 3:1791–1799
Markland FS (1998) Snake venoms and the hemostatic system. Toxicon 36:1794–1800
Marsh N, Williams V (2005) Practical applications of snake venom toxins in haemostasis. Toxicon 45:1171–1181
Miroux B, Walker JE (1996) Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol 260:289–298
Nallamsetty S, Waugh DS (2006) Solubility-enhancing proteins MBP and NusA play a passive role in the folding of their fusion partners. Protein Expr Purif 45:175–182
Nishida S, Fujimura Y, Miura S, Ozaki Y, Usami Y, Suzuki M, Titani K, Yoshida E, Suqimoto M, Yoshioka A, Fukui H (1994) Purification and characterization of bothrombin, a fibrinogen-clotting serine protease from the venom of Bothrops jararaca. Biochemistry 33:1843–1849
Nygren PA, Stahl S, Uhlen M (1994) Engineering proteins to facilitate bioprocessing. Trends Biotechnol 12:184–188
Pan H, Du X, Yang G, Zhou Y, Wu X (1999) cDNA cloning and expression of acutin. Biochem Biophys Res Commun 255:412–415
Pirkle H (1998) Thrombin-like enzymes from snake venoms: an updated inventory. Thromb Haemost 79:675–683
Sant’Ana CD, Ticli FK, Oliveira LL, Giqlio JR, Rechia CG, Fuly AL, de Araújo S, Franco JJ, Stabeli RG, Soares AM, Sampaio SV (2008) BjussuSp-I: a new thrombin-like enzyme isolated from Bothrops jararacussu snake venom. Comp Biochem Physiol A Mol Inteqr Physiol 151:443–454
Sunagawa M, Nakamura M, Kosugi T (2007) Cloning of Habutobin cDNA and antithrombotic activity of recombinant protein. Biochem Biophys Res Commun 362:899–904
Vasina JA, Baneyx F (1997) Expression of aggregation-prone recombinant proteins at low temperatures: a comparative study of the Escherichia coli cspA and tac promoter systems. Protein Expr Purif 9:211–218
Yang Q, Li M, Xu J, Bao Y, Lei X, An L (2003) Expression of gloshedobinm a thrombin-like enzyme from the venom of Gloydius shedoensis, in Escherichia coli. Biotechnol Lett 25:101–104
Yang Q, Xu XM, Li M, Yuan XD, Su ZG, Jason JC (2002) Cloning and expression of defibrase cDNA from the venom of Gloydius shedoensis. Biotechnol Lett 24:135–138
You WK, Choi WS, Koh YS, Shin HC, Jang Y, Chung KH (2004) Functional characterization of recombinant batroxobin, a snake venom thrombin-like enzyme, expressed from Pichia pastoris. FEBS Lett 571(1–3):67–73
Yuan S, Duan H, Liu C, Liu X, Liu T, Tao H, Zhang Z (2004) The role of thioredoxin and disulfide isomerase in the expression of the snake venom thrombin-like enzyme calobin in Escherichia coli BL21 (DE3). Protein Expr Purif 38:51–60
Yu X, Li Z, Xia X, Fang H, Zhou C, Chen H (2007) Expression and purification of ancrod, an anticoagulant drug, in Pichia pastoris. Protein Expr Purif 55(2):257–261
Zhang Y, Gao R, Lee WH, Zhu SW, Xiong YL, Wang WY (1998a) Characterization of a fibrinogen-clotting enzyme from Trimeresurus stejnegeri venom, and comparative study with other venom proteases. Toxicon 36:131–142
Zhang Y, Olsen DR, Nguyen KB, Olson PS, Rhodes ET, Mascarenhas D (1998b) Expression of eukaryotic proteins in soluble form in Escherichia coli. Protein Expres Purif 12:159–165
Acknowledgements
The authors thank the National Natural Science Foundation of China (20536010), the State Key Laboratory of Bio-Organic & Natural Products Chemistry (Shanghai, China), the National Special Fund for State Key Laboratory of Bioreactor Engineering (ECUST; Shanghai, China), the Key Laboratory of Natural Pesticide & Chemical Biology of Ministry of Education (Wuhan, China), and Shanghai Key Lab of Chemical Biology (China).
Author information
Authors and Affiliations
Corresponding author
Additional information
X. Jiang and J. Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jiang, X., Xu, J. & Yang, Q. Soluble expression, purification, and characterization of Gloydius shedaoensis venom gloshedobin in Escherichia coli by using fusion partners. Appl Microbiol Biotechnol 85, 635–642 (2010). https://doi.org/10.1007/s00253-009-2141-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2141-2