Abstract
The effect of medium supplementation on heterologous production of human serum transferrin (hTF) in the fission yeast Schizosaccharomyces pombe has been investigated. The productivity of recombinant hTF was low in wild-type S. pombe cells. To overcome this impediment, culture media supplements were screened for their ability to improve secretion of hTF. Casamino acids (CAA), which have been reported to increase heterologous protein productivity in Pichia pastoris, improved the secretion hTF by more than fourfold. An anion surfactant deoxycholate or polyethylene glycol also improved the secretion hTF. Interestingly, dextran sodium sulfate (DSS), a poly-anion surfactant, was found to enhance production of secreted hTF better than any other supplement tested. Addition of DSS in the presence of 2% CAA exhibited a synergistic effect on increasing hTF secretion, resulting in an increase of about sevenfold relative to conventional conditions. Cell growth was not found to be affected by the addition of DSS or CAA. DSS may act as a surfactant and may also facilitate the anchoring of liposomes, and these properties may contribute to efficient secretion or exocytosis through the plasma membrane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transferrins are a homologous family of Fe3+-binding proteins that include serum transferrin, ovotransferrin, lactoferrin, and melanotransferrin (Aisen and Listowsky 1980; Bullen et al. 2000). They are monomeric glycoproteins of approximately 80 kDa containing two domains (N-lobe and C-lobe) that play an important role in iron metabolism due to their ability to bind iron (Baker et al. 2003). Serum transferrins play a role in iron transport. Lactoferrins are found in milk, tears, and other bodily secretions in numerous mammals. Ovotransferrins are found in avian egg white, and melanotransferrins are found on the surface of melanocytes (Bullen et al. 2000). Within the transferrin family, human serum transferrin (hTF), which is secreted as a 679-residue protein with 20 disulfide bonds and two N-glycan chains on the C-lobe, functions to transport iron from sites of absorption and storage to sites of utilization, such as developing red blood cells, but also protects against free radical damage caused by catalytic activity of iron (von Bonsdorff et al. 2001). Although the protein was identified more than half a century ago (Schade and Caroline 1946), the complete mechanism of iron loading and release remains to be elucidated.
In order to study factors affecting this mechanism, the expression of recombinant full-length transferrin has proved to be an important tool. The most common system for producing transferrin is baby hamster kidney cells (Mason et al. 2001), although this system is not particularly efficient. The relatively low yields may be due to the multiple disulfide bonds in transferrin. Several transferrin expression systems have been attempted in a variety of host organisms. While a number of laboratories have reported using these expression systems (Steinlein et al. 1995; Mason et al. 1996), the final protein products were not glycosylated. Pichia pastoris is a widely used host for heterologous gene expression, although full-length transferrin production has been difficult. Only secretion of either N-lobe transferrin or C-lobe transferrin has been reported (Mason et al. 1996).
Schizosaccharomyces pombe is the most intensely studied and well-characterized yeast species in terms of molecular genetics and cell biology. S. pombe is taxonomically and evolutionarily distant from the budding yeast (Takegawa et al. 2009). Unlike budding yeast, S. pombe has not been broadly used to make fermented foods such as wine, beer, and bread, but it shares greater similarity to multicellular organisms than budding yeast in terms of mRNA splicing, cell division control, and post-translational modification (Zhao and Lieberman 1995). S. pombe is now considered an attractive host for the expression of molecules with complicated structures, such as glycosylated proteins derived from higher animals. Recently, many types of human recombinant proteins have been expressed successfully in fission yeast, including human antithrombin III (Broker et al. 1987), human papillomavirus E7 protein (Tommasino et al. 1992), human D2S dopamine receptor (Sander et al. 1994), and human growth hormone (hGH; Idiris et al. 2006a, b), as well as recombinant proteins from other organisms (Giga-Hama and Kumagai 1999). Our group has developed S. pombe protein-production systems that have been useful for producing many types of heterologous proteins (Okada et al. 1998a, b; Giga-Hama and Kumagai 1999; Isoai et al. 2002; Ikeda et al. 2004). Moreover, Idiris et al. (2006a, b) recently demonstrated the possibility of improving the efficiency of heterologous production of protease-sensitive proteins by using a host deleted for multiple proteases. This host yielded 20-fold higher levels of hGH relative to other hosts; however, expression and secretion of hTF in this same host was unexpectedly low (Idiris, personal communication). hGH is a relatively small protein of 22 kDa and has two disulfide bonds; whereas, hTF possess 20 disulfide bonds and two N-glycan chains. Unlike Saccharomyces cerevisiae, N-linked oligosaccharides of S. pombe contain core structure and a large outer chain with 100–200 residues of galactomannan (Bush et al. 1974; Ballou et al. 1994). This complexity of the hTF protein may influence the low level of hTF secretion. In order to increase levels of secreted hTF, we have therefore attempted to optimize culture conditions for S. pombe. In this paper, we report the effects of medium supplements on heterologous production of hTF and demonstrate that levels of secreted hTF can be increased up to sevenfold relative to conventional conditions.
Materials and methods
Strains, media, and growth conditions
The S. pombe strains used in this study were ARC039 (h −leu1-32 ura4-C190T) and a strain deficient in eight different proteases named A8 (h −leu1-32 ura4-C190T Δpsp3, Δisp6, Δppp53, Δppp16, Δppp22, Δsxa2, Δppp80, and Δppp20). A8, which has an additional disruption at ppp20+, was derived from A7-3 (h −leu1-32 ura4-C190T, Δpsp3, Δisp6, Δppp53, Δppp16, Δppp22, Δsxa2, and Δppp80), which was previously constructed by gene replacement of the target open reading frames (ORFs) using a ura4 cassette (Idiris et al. 2006a, b). These strains were grown in the following media: yeast extract medium (YES) with supplements [YES: 0.5% bacto-yeast extract (Becton, Dickinson and Company, NJ, USA), 3% glucose, and SP supplements (Qbiogene, Montreal, Canada)]; yeast extract-polypeptone-dextrose (YPD) [1% bacto-yeast extract (BD), 2% bactopeptone (BD), and 2% glucose]; or minimum media (MM) which of 1,000 ml contain 3 g KH2PO4, 2.2 g Na2HPO4, 5 g NH4Cl, 20 g glucose, 1.05 g MgCl2 6H20, 11 mg CaCl2, 1 g KCl, 40 mg Na2SO4, 1 mg pantothenic acid, 10 mg nicotinic acid, 10 mg inositol, 10 μg biotin, 0.5 mg boric acid, 0.4 mg MnSO4, 0.4 mg ZnSO4 7H2O, 0.2 mg FeCl2 6H2O, 0.04 mg H2MoO4 H2O, 0.1 mg KI, 0.04 mg CuSO4 5H2O, 1 mg citric acid, 75 mg adenine, histidine, and lysine. A total of 37.5 mg of uracil or 75 mg of leucine was added per 1,000 ml to cover auxotrophies. Transformants were plated onto minimum media with agar (MMA), MMA Ura− (MMA without uracil), or MMA Leu− (MMA without leucine) and grown at 30°C. Escherichia coli strain DH10B (Invitrogen) was used for plasmid preparation.
Construction of an hTF and m-hTF expression vector and transformation of fission yeast cells
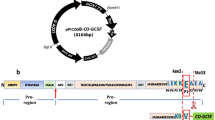
A chromosome-integrative multicassette vector for secretory expression of hTF was constructed essentially based on the D-amino acid oxidase expression vector as described (Isoai et al. 2002). Briefly, 2,097 bp of the ORF of hTF was artificially synthesized by GeneArt (Regensburg, Germany) according to a highly biased codon table obtained from three high-expression genes, adh1, tpi1, and gdp1, in S. pombe and containing a tagged sequence encoding a FLAG epitope. The synthetic hTF gene fragment was inserted between the human cytomegalovirus promoter (hCMV-p) and human lipocortin I (LPI) terminator to construct the hTF expression cassette. This construct was then inserted into the multiple cloning sites of the integration vector pXL1 (Isoai et al. 2002) to construct pTL2OSTFN-CF (Fig. 1a). Next, pTL2OSTFN-CF-2XL was constructed by ligation of PvuI-NheI and PvuI-SpeI fragments containing the hTF expression cassette from pTL2OSTFN-CF. A multicassette expression vector, pTL2OSTFN-CF-4XL, consisting of four tandem copies of the hTF expression cassette (hCMV-p/hTF-ORF/LPI terminator) was constructed by ligation of PvuI-NheI and PvuI-SpeI fragments containing two hTF expression cassettes from pTL2OSTFN-CF-2XL. For construction of the unglycosylated hTF (m-hTF) expression vector, pTL2P3hTF(S449A/T647A)-SL6, a 2,040-bp S. pombe high-bias codon-type synthetic m-hTF gene lacking 57 bp from the 5′-terminus of hTF was subcloned downstream of the P3-secretion signal sequence of pSL6P3. The constructed vector was linearized by digestion with BsiWI and was inserted into the leu1 locus of host S. pombe strains by transformation according to the lithium acetate-based transformation method (Okazaki et al. 1990). Transformed cells were then grown for 1 h at 30°C in YES medium before being plated on MMA Leu plates. Transformants secreting hTF were screened by western blotting.
Structure of the chromosome-integrative hTF and unglycosylated hTF (m-hTF) expression vectors. AmpR ampicillin-resistance-encoding gene, hCMV cytomegarovirus, LPI human lipocortin I, leu1+ β-isopropylmalate dehydrogenase gene (two separate parts in the full gene cassette are indicated by the 5′-half and the 3′-half, respectively), hTF human transferrin gene, ORF open reading frame, ori, replication origin, ter 3-terminator, P3 P-factor secretion signal. a hTF expression vector pTL2OSTFN-CF-4XL. Four hTF expression cassettes were inserted into the vector. b m-hTF expression vector pTL2P3hTF(S449A/T647A)-SL6. A 2,040-bp Schizosaccharomyces pombe high-bias codon-type synthetic m-hTF gene lacking 57 bp from the 5′-end of m-hTF, which contains the secretion signal sequence was subcloned downstream of the P3-secretion signal sequence. After linearizing the resultant vectors by digestion with BsiWI, whose sites are located upstream and downstream of the two leu1+ gene components, the cassette regions were integrated into the leu1-locus of the S. pombe strains by transformation
Western blot analysis
Cell culture supernatants of hTF- or m-hTF-producing recombinant strains were added into equal volumes of acetone and incubated overnight, centrifuged at 10,000 × g for 10 min, and dried after removal of the supernatant. These extracts were then dissolved in 500 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and incubated at 80°C for 5 min. A total of 20 μl of sample was applied to an SDS-PAGE gel containing a 5–20% gradient and electro-blotted onto a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc., Malvern, PA, USA). Immunodetection analysis was performed on the blotted membrane with SNAP i.d. (Millipore) using mouse polyclonal anti-hTF antibody (Cederlane Lab. Ltd.) as the first antibody at a dilution of 1:500 and horseradish peroxidase-conjugated rabbit anti-mouse IgG antiserum (GE healthcare, Piscataway, NJ, USA) as the second antibody at a dilution of 1:3,000. Human hTF (Apo) purified from blood (Wako Chemical Co. Ltd.) was used as a standard. hTF-specific signals were visualized by enhanced chemiluminescence (ECL-plus, GE healthcare, Piscataway, NJ, USA) and were detected with an LAS4000 imaging system (Fuji Film Co. Ltd., Japan). Detected hTF bands were quantified by using a multi-gauge image analyzer (Fuji Film Co. Ltd).
RNA preparation and northern blotting
RNA was prepared by using a Qiagen RNeasy kit according to the manufacturer’s instructions for isolating total RNA minipreps from yeast. RNA was run on a formaldehyde gel, followed by blotting to a Pall Biodyne Transfer Membrane (Pall Corp., Washington, NY, USA). Pre-hybridization and hybridization were performed as described (Cooper et al. 1997) with an AlkPhos Direct kit module (GE healthcare, Piscataway, NJ, USA). Transcripts were visualized by CDP-Star detection reagent (GE healthcare, Piscataway, NJ, USA) and detected with an LAS4000 Imaging System (Fuji Film Co. Ltd., Japan).
Results
Construction of the hTF and m-hTF vector and protein expression in S. pombe
For expression and secretion of hTF in fission yeast, we used the chromosome-integrative multicassette vector pTL2OSTFN-CF-4XL (Fig. 1a). In this vector, four tandemly positioned hTF genes driven by a strong promoter, hCMV-p, were ligated between two parts of a leu1 + gene, which were then used to direct integration of the multiple hTF expression cassettes into the leu1 locus of the S. pombe genome. These approaches were previously found to be effective in achieving high and stable expression of heterologous proteins in fission yeast (Isoai et al. 2002; Ikeda et al. 2004; Idiris et al. 2006a, b). We also constructed an expression vector for unglycosylated hTF (m-hTF), a recombinant transferrin mutant lacking the two N-linked glycosylation sites (N-K-S449A and N-V-T647A). Thirty-four amino-acid residues of the S. pombe P-factor secretion signal P3 were used to direct secretion of the recombinant proteins. The P3-conjugated m-hTF protein was subcloned under the CMV promoter in the chromosome-integrative expression vector pSL6P3 to generate pTL2P3hTF(S449A/T647A)-SL6 (Fig. 1b). These expression vectors were linearized with BsiWI and were introduced into appropriate strains by transformation. Transformants were selected on medium lacking leucine. Transformants were cultured in liquid medium for 2–3 days at 30°C with shaking. Secretion of hTF and m-hTF was confirmed by western blot analysis of culture supernatants concentrated by acetone precipitation as described in “Materials and methods”.
Medium supplements enhance secretion of hTF in S. pombe
For expression and secretion of recombinant hTF, we introduced the expression vector pTL2OSTFN-CF-4XL into a strain deficient in eight different proteases named A8 (Δpsp3, Δisp6, Δppp53, Δppp16, Δppp22, Δsxa2, Δppp80, and Δppp20), which was derived from a parent strain A7-3, which was isolated as a strain showing minimum of proteolytic degradation of hGH (Idiris et al. 2006b). A transformant producing the greatest amount of secreted hTF was confirmed by western blot analysis and was analyzed for hTF productivity. From the western blot analysis, the secretion level of hTF was elevated in the A8 strain compared to that in WT (data not shown), possibly due to low endogenous proteolytic activity.
The transformant was then grown in MM or YES medium, both of which are widely used for growing fission yeast with or without casamino acids (CAA) addition. In P. pastoris, it has been reported that supplementing fermentation medium with CAA reduces extracellular proteolysis and stabilizes secreted proteins (Coppella and Dhurjati 1989; Clare et al. 1991; Chen et al. 2000; Cha et al. 2005; Wang et al. 2005; Rahbarizadeh et al. 2006; Toonkool et al. 2006). Cells were cultured for 60 h, and the supernatant was concentrated and analyzed by western blotting. A smaller amount of secreted hTF was observed in YES medium than in MM (Fig. 2a, lane 3). When 2% CAA was added to MM or YPD medium, the amount of secreted hTF increased markedly (Fig. 2a, lanes 5 and 7). The increase in secretion resulting from the addition of CAA may have been caused by a reduction in proteolysis because the A8 strain still possesses more than 50 putative protease genes (Idiris et al. 2006a), and CAA may reduce the activity of these remaining proteases. Regardless of CAA supplementation, the amount of secreted hTF was similar in cells grown in YPD and those grown in MM even though YPD has a greater nitrogen content (Fig. 2a, lanes 4 to 7). This result suggests that the productivity of hTF was not dependent on the amount of nitrogen. One of the purposes of our study was to produce low-cost recombinant proteins in S. pombe cells. Therefore, MM was chosen over YPD on the basis of these observations.
Western blot analysis of secreted recombinant transferrin. Culture supernatants from Schizosaccharomyces pombe A8 strain expressing transferrin were concentrated by acetone precipitation and dissolved in sample buffer, and then applied to a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel using 500 μl of culture supernatant. Western blotting was performed as described in “Materials and methods”. a Amount of secreted transferrin in different culture media. Control human serum transferrin (hTF) 100 ng (lane 1), MagicMark™ XP Western Protein Standard (Invitrogen; lane 2), cultured in yeast extract medium (lane 3), cultured in mineral medium (MM; lane 4), cultured in MM with 2% casamino acid (CAA; lane 5), cultured in yeast extract-polypeptone-dextrose (YPD) medium (lane 6), and cultured in YPD medium with 2% CAA (lane 7). b Effect of medium supplements on secretion of transferrin. Cells were cultured in MM with appropriate supplements for 60 h at 30°C and medium supernatants were prepared; 100 ng control hTF (lanes 1 and 11), cultured without supplements (lane 2), cultured with dextran sodium sulfate (DSS; lanes 3 and 4, 0.01% and 0.002%, respectively), dextran (lane 5), Tween20 (lane 6, 0.001%), 0.002% TritonX-100 (lane 7), deoxycholic acid (lanes 8 and 9, 0.01% and 0.002%, respectively), and polyethylene glycol 8,000 (lane 10, 0.1%). c Effect of DSS and CAA on secretion of unglycosylated hTF (m-hTF). A 60-h culture supernatant from cells expressing m-hTF was concentrated by acetone precipitation, subjected to SDS-PAGE, and detected by western blot analysis. Cultured in MM without supplements (lane 1), cultured in MM with 0.01% DSS (lane 3), 2% CAA (lane 4), and 0.01% DSS and 2% CAA (lane 5), 100 ng control hTF (lanes 2 and 6). d Quantitative determination of transferrin. Cells were cultured in MM with or without supplements for 60 h at 30°C with shaking, and culture supernatants were then analyzed by western blot analysis. hTF-specific signals were visualized by enhanced chemiluminescence and detected with an LAS4000 imaging system. Bands were quantified using a multi-gauge image analyzer. The Y-axis represents relative values, where the amount of hTF for lane 1 was defined as 1.0. Quantity of hTF from culture supernatant without supplements (column 1), with 0.01% DSS (column 2), with 2% CAA (column 3), and with 0.01% DSS and 2% CAA (column 4). e Growth curve of cells cultured with medium supplements. Incubation of hTF-expressing cells was started at OD610 = 0.1 and observed for 126 h. Closed circles, cells cultured in MM Leu− without supplements; open circles, cells cultured in MM Leu− medium containing 0.01% DSS; X, cells cultured in medium containing 2% CAA; squares, cells cultured in medium containing 0.01% DSS and 2% CAA
Next, we examined various media supplements to increase the levels of secreted hTF. Idiris et al. (2006b) reported that production levels of hTF were not significantly changed between strains harboring four and eight integrated hTF expression cassettes. We speculated that newly synthesized hTF might accumulate in the secretory pathway, specifically, in the ER, and might be degraded via ER quality control (ERAD), although existence of the ERAD pathway has not been clearly demonstrated in S. pombe. In the secretory pathway, the majorities of secreted protein are transported through the ER and Golgi and are then secreted extracellular by exocytosis. In addition, regions of the plasma membrane around the cell wall or periplasm of unicellular organisms functions as an effective permeability barrier with respect to protein secretion. Previously, the presence of polyethylene glycol, M. W. 8000 (PEG8000), anionic (SDS, deoxycholate) or non-ionic (Tweens) surfactants caused a substantial increase in the yield of secreted α-amylase in Geobacillus thermoleovorans (Uma Maheswar Rao and Satyanarayana 2003). We therefore analyzed whether various surfactants influenced hTF secretion (Fig. 2b). Surfactants were added to MM at a concentration ranging from 0.001% to 0.1%, and cells were incubated with shaking for 60 h at 30°C. Culture supernatants were concentrated, and hTF was detected by western blot analysis. The fission yeast cells did not grow in the presence of more than 0.001% of the anionic surfactant SDS. Deoxycholate is also an anionic surfactant; however, addition of deoxycholate less than 0.01% was observed to cause the most significant increase in levels of secreted hTF (Fig. 2b, lane 8 and 9). The effects of non-ionic surfactants were also investigated. Addition of Tween 20 caused a growth defect at concentrations greater than 0.001% and reduced levels of secreted hTF (Fig. 2b, lane 6). Addition of 0.002% non-ionic TritonX-100 had no apparent effect on growth, but a significant increase in levels of secreted hTF was not observed (Fig. 2b, lane 7).
PEG is a synthetic polymer that is commonly used for transformation of fission yeast (Hood and Stachow 1992; Morita and Takegawa 2004), because it is thought to increase permeability of the cell membrane (Arnold et al. 1990). Our investigation revealed that addition of PEG8000 at 0.1% also increased levels of secreted hTF (Fig. 2b, lane 10). A growth defect was observed at 1% PEG8000. Interestingly, addition of dextran sodium sulfate (DSS) was observed to generate a marked increase in levels of secreted hTF (Fig. 2b, lanes 3 and 4). DSS is a weak poly-anionic surfactant that has branched chains of anhydroglucose units containing approximately two to three sulfate groups per glucosyl residue. Our investigation revealed that at least 0.002% DSS, whose average molecular weight is 5,000, drastically increased levels of secreted hTF. Next, we investigated whether DSS could increase secretion in the presence of CAA. hTF-expressing cells were cultured in MM with 0.01% DSS or 2% CAA or both 0.01% DSS and 2% CAA for 60 h, and supernatants were concentrated and prepared for western blotting. The amount of secreted hTF increased about threefold after addition of DSS as compared to the no-supplement control (Fig. 2c, lanes 1 and 3, Fig. 2d, column 1 and 2). Moreover, addition of DSS in the presence of 2% CAA exhibited a synergistic effect, increasing levels of secreted hTF about sevenfold as compared to a culture without supplements (Fig. 2c, lanes 1 and 5, Fig. 2d, columns 1 and 4); whereas, CAA alone increased level of hTF secretion about fivefold (Fig. 2c, lanes 1 and 4 and Fig. 2d, column 1 and 3).
Next, we observed the growth rate for 126 h to determine whether cell growth was influenced by addition of DSS or CAA (Fig. 2e). The final cell yield was not changed by either additive, which suggested that these additives were not used as nutrients for growth. Addition of DSS alone had no effect on growth rate at an early stage (24 to 60 h); whereas, addition of CAA slightly decreased the growth rate at an early stage. This decrease in growth rate may be caused by the load of excessive heterologous protein expression, because addition of CAA or CAA + DSS increased the hTF secretion level by five- to sevenfold as compared to a culture with no additives. We also confirmed that a larger molecular weight (average M.W. 500,000) form of DSS exhibited a similar effect on secretion of hTF (data not shown). Moreover, dialyzed DSS was not found to lose activity.
We also analyzed the effect of the neutral polysaccharide dextran (average M.W., 5,000) as a supplement; this polymer has the same backbone as DSS but no sulfate groups. The hTF-expressing A8 strain was grown in MM medium containing dextran at 0.001%, 0.01%, 0.1%, and 1%; however, no effect on levels of secreted hTF was observed (Fig. 2b, lane 5). These data suggest that the polysaccharide backbone of DSS is not critical, but rather that multiple anionic residues may cause the observed increase in hTF secretion.
Northern blot analysis of hTF gene expression
Densitometry of the hTF protein bands obtained by western blot analysis revealed that the culture media containing CAA and DSS contained more than sevenfold greater levels of secreted hTF protein as compared with media lacking supplements. We therefore examined whether the mRNA transcription level of hTF was influenced by the supplements. Cells were incubated with appropriate supplements in MM for either 16 or 48 h at 30°C, followed by overnight pre-incubation in MM. The cells were then harvested by centrifugation and processed for Northern blot analysis as described in “Materials and methods”. An approximately 500-bp DNA fragment of the hTF ORF was used as a probe for Northern blotting.
The cells cultured with supplements strongly expressed hTF mRNA (Fig. 3, lanes 2–4), because hTF mRNA was transcribed under the CMV promoter, which expressed constitutively in S. pombe; however, these expression levels of hTF mRNA were the same as those in control cells without supplements at either 16 or 48 h (Fig. 3, lane 1). These data suggest that the medium supplements did not influence transcription of hTF.
Northern blot analysis of transferrin mRNA. Cells were cultured in mineral medium (MM) containing appropriate supplements for 16 and 48 h at 30°C. Cells were then harvested, and total RNA was extracted as described in “Materials and methods”. RNA from cells cultured in MM alone (lane 1), cultured in MM with 0.01% dextran sodium sulfate (DSS; lane 2), with 2% casamino acid (CAA; lane 3), and with 0.01% DSS and 2% CAA (lane 4). Upper panel indicates transferrin mRNA, and lower panel indicates rRNA for representation for quantity control
Influence of medium supplements on secretion of unglycosylated hTF (m-hTF)
Transferrin possesses two N-linked glycosylation sites at residues 432 and 629, corresponding to the N-glycosylation motif sequence Asn-Xaa-Ser/Thr. Heterologous expression of unglycosylated recombinant hTF has been reported in S. cerevisiae (Sargent et al. 2006) and in P. pastoris (Mason et al. 1996), for yield of hTF might be affected by glycosylation on hTF. Our study suggested DSS or CAA greatly increased the secretion level of glycosylated hTF. However, the influence of medium supplements on expression of hTF has not yet been reported. Moreover, there are no reports concerning how DSS might be able to increase protein secretion. In S. pombe, N-linked oligosaccharide is composed of core structure and a large outer chain of galactomannan (Bush et al. 1974; Ballou et al. 1994). This huge carbohydrate structures could be a possible site of action of DSS for increase of hTF secretion level. We therefore observed whether the secretion level of unglycosylated hTF is also influenced by additives. Recombinant hTF with a mutation (N-K-S449A and N-V-T647A) corresponding to unglycosylated hTF (m-hTF) was constructed (Yang et al. 1993). S. pombe A8 was transformed with this construct and grown by the same method as the hTF transformant on MM containing the appropriate supplement, CAA or DSS. Cells were cultured for 60 h, and supernatants were then concentrated and analyzed by western blotting (Fig. 4a). Densitometry of the m-hTF bands obtained by western blot analysis revealed that levels of secreted m-hTF were also increased by the addition of CAA or DSS (Fig. 4b). DSS increased secretion about threefold relative to unsupplemented MM. CAA and CAA + DSS supplementation increased levels 4.2- and 6.3-fold, respectively, although both ratios were slightly lower than levels of secreted hTF. We speculate that the unglycosylated protein is less stable than glycosylated hTF, possibly due to proteolytic activity in the culture supernatant. These data suggest that the observed increase in levels of secreted hTF caused by DSS supplementation was independent of the glycosylation state of hTF.
Effect of dextran sodium sulfate (DSS) and casamino acid (CAA) on secretion of unglycosylated human serum transferrin (m-hTF). a 60-h culture supernatant from cells expressing m-hTF was concentrated by acetone precipitation, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and detected by western blot analysis; 50 ng control hTF (lanes 1 and 6), cultured in mineral medium (MM) without supplements (lane 2), with 0.01% DSS (lane 3), with 2% CAA (lane 4) with 0.01% DSS and 2% CAA (lane 5). b Quantitative determination of m-hTF. Cells were cultured in MM with or without supplements for 60 h at 30°C with shaking. Culture supernatants were then analyzed by western blot analysis. m-hTF-specific signals were visualized by enhanced chemiluminescence and detected with an LAS4000 imaging system. The m-hTF band was quantified using a multi-gauge image analyzer. The Y-axis represents relative values, where the quantity of transferrin for lane 1 was defined as 1.0. Quantity of transferrin from culture supernatant without supplements (column 1), with 0.01% DSS (column 2), with 2% CAA (column 3), and with 0.01% DSS and 2% CAA (column 4)
Discussion
The S. pombe system is being used extensively for high-level heterologous protein production, primarily because it shares many genetic and biochemical features with higher eukaryotes (Russel 1989; Giga-Hama 1997). However, optimization is required for high levels of production, especially in the case of unstable proteins. Several protease activities have been detected in culture supernatants of S. pombe (Idiris et al. 2006a). Recently, Idiris et al. (2006a, b) constructed a strain harboring deletions of multiple proteases and demonstrated significantly enhanced hGH productivity. Although improved stability of secreted hTF was observed in this strain, the productivity of the hTF secreted was unacceptably low, although almost all hTF was secreted extracellular medium, for whole cell extract contained only a faint hTF (data not shown). In the present paper, we analyzed the effects of media supplements on increasing levels of secreted recombinant hTF in S. pombe. As compared to conventional conditions, supplementation with at least 0.002% DSS increased levels of secreted hTF up to threefold. Moreover, a synergistic effect was found between DSS and CAA, resulting in an increase in secreted hTF of about sevenfold. CAA is known to be a protease inhibitor. For example, addition of CAA and adjustment of pH resulted in a 3.5-fold increase in a heterogenous recombinant merozoite surface protein in P. pastoris (Wang et al. 2005). To our knowledge, however, there have been no reports on whether CAA also increases protein production in S. pombe.
It is also interesting that addition of DSS increased the amount of secreted hTF by S. pombe cells. DSS is a biodegradable and biocompatible polymer that has been widely used in pharmaceutical applications. For example, DSS has been found to be an important anti-atherosclerotic drug (Radhakrishnamurthy et al. 1978) and recently has been applied to gene delivery systems (Tiyaboonchai et al. 2003; Nimesh et al. 2006). DSS is a negatively charged macromolecule, and Zschornig et al. (1992) reported that DSS enhances the fusion of liposomes containing cationic stearylamine and phosphatidylcholine or phosphatidylethanolamine. These data suggest that DSS might increase the efficiency of secretion by mechanisms including exocytosis of transport vesicles. We also demonstrated that the anionic surfactant deoxycholate increased levels of secreted hTF. Small amounts of surfactants could lead to destabilization of membranes, including those found in transport vesicles or the plasma membrane itself, leading to enhanced membrane fusion.
DSS also possesses a weak anionic surfactant character (Tomasic et al. 2005) that might enhance vesicle fusion. Georgiev and Lalchev (2004) suggested that DSS worked as a fusion agent by interacting with cationic polar heads of lipids in the presence of Ca2+ ions. This anchoring phenomenon resembles the interaction of PEG and membranes, where strong adhesion between phospholipid (phosphatidylcholine or phosphatidylcholine plus phosphatidylethanolamine) monolayer polar heads in solid-supported bilayers was observed in the presence of PEG8000/10000 and measured by Kuhl et al. (1996). Our investigation showed that PEG8000 also increased levels of secreted hTF. In Bacillus subtilis, low-molecular weight PEG600 in the medium was observed to increase enzyme release by altering the phospholipid layers of the cell membrane (Andersson et al. 1987, 2000). These data suggest that DSS may possess both surfactant properties and the anchoring ability of a hydrophilic polymer such as PEG. These properties may contribute to improved secretion or exocytosis through the plasma membrane. Additional studies will be necessary to clarify the precise function of DSS in increasing levels of secreted hTF. However, because DSS was able to effect an improvement at low concentration, addition of DSS could be applied economically at an industrial scale. In future work, we aim to elucidate the mechanism underlying the increase in secreted protein levels and applying it to other recombinant proteins produced in S. pombe.
References
Aisen P, Listowsky I (1980) Iron transport and storage proteins. Annu Rev Biochem 49:357–393
Andersson E, Ramgren M, Hahn-Hagerdal B (1987) The influence of PEG on alpha-amylase production with Bacillus species. Ann N Y Acad Sci 506:613–616
Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS, Champlin RE, Vaughan WP (2000) Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 6(5A):548–554
Arnold K, Ohki S, Krumbiegel M (1990) Interaction of dextran sulfate with phospholipid surfaces and liposome aggregation and fusion. Chem Phys Lipids 55(3):301–307
Baker HM, Anderson BF, Baker EN (2003) Dealing with iron: common structural principles in proteins that transport iron and heme. Proc Natl Acad Sci U S A 100(7):3579–3583
Ballou CE, Ballou L, Ball G (1994) Schizosaccharomyces pombe glycosylation mutant with altered cell surface properties. Proc Natl Acad Sci U S A 91(20):9327–9331
Broker M, Ragg H, Karges HE (1987) Expression of human antithrombin III in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Biochim Biophys Acta 908(3):203–213
Bullen J, Griffiths E, Rogers H, Ward G (2000) Sepsis: the critical role of iron. Microbes Infect 2(4):409–415
Bush DA, Horisberger M, Horman I, Wursch P (1974) The wall structure of Schizosaccharomyces pombe. J Gen Microbiol 81(1):199–206
Cha HJ, Dalal NN, Bentley WE (2005) Secretion of human interleukin-2 fused with green fluorescent protein in recombinant Pichia pastoris. Appl Biochem Biotechnol 126(1):1–11
Chen DC, Wang BD, Chou PY, Kuo TT (2000) Asparagine as a nitrogen source for improving the secretion of mouse alpha-amylase in Saccharomyces cerevisiae protease A-deficient strains. Yeast 16(3):207–217
Clare JJ, Romanos MA, Rayment FB, Rowedder JE, Smith MA, Payne MM, Sreekrishna K, Henwood CA (1991) Production of mouse epidermal growth factor in yeast: high-level secretion using Pichia pastoris strains containing multiple gene copies. Gene 105(2):205–212
Cooper JP, Nimmo ER, Allshire RC, Cech TR (1997) Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature 385(6618):744–747
Coppella SJ, Dhurjati P (1989) alpha-Factor directed expression of the human epidermal growth factor in Saccharomyces cerevisiae. Biotechnol Bioeng 33(8):976–983
Georgiev G, Lalchev Z (2004) Model study of interactions of high-molecular dextran sulfate with lipid monolayers and foam films. Eur Biophys J 33(8):742–748
Giga-Hama Y (1997) Fission yeast Schizosaccharomyces pombe: an attractive host for heterologous protein production. Springer-Verlag, Berlin
Giga-Hama Y, Kumagai H (1999) Expression system for foreign genes using the fission yeast Schizosaccharomyces pombe. Biotechnol Appl Biochem 30(Pt 3):235–244
Hood MT, Stachow C (1992) Influence of polyethylene glycol on the size of Schizosaccharomyces pombe Electropores. Appl Environ Microbiol 58(4):1201–1206
Idiris A, Bi K, Tohda H, Kumagai H, Giga-Hama Y (2006a) Construction of a protease-deficient strain set for the fission yeast Schizosaccharomyces pombe, useful for effective production of protease-sensitive heterologous proteins. Yeast 23(2):83–99
Idiris A, Tohda H, Bi KW, Isoai A, Kumagai H, Giga-Hama Y (2006b) Enhanced productivity of protease-sensitive heterologous proteins by disruption of multiple protease genes in the fission yeast Schizosaccharomyces pombe. Appl Microbiol Biotechnol 73(2):404–420
Ikeda S, Nikaido K, Araki K, Yoshitake A, Kumagai H, Isoai A (2004) Production of recombinant human lysosomal acid lipase in Schizosaccharomyces pombe: development of a fed-batch fermentation and purification process. J Biosci Bioeng 98(5):366–373
Isoai A, Kimura H, Reichert A, Schorgendorfer K, Nikaido K, Tohda H, Giga-Hama Y, Mutoh N, Kumagai H (2002) Production of D-amino acid oxidase (DAO) of Trigonopsis variabilis in Schizosaccharomyces pombe and the characterization of biocatalysts prepared with recombinant cells. Biotechnol Bioeng 80(1):22–32
Kuhl T, Guo Y, Alderfer JL, Berman AD, Leckland D, Israelashvili J, Hui SW (1996) Direct measurement of polyethylene glycol induced depletion attraction between lipid bilayers. Langmuir 12:3003–3014
Mason AB, Woodworth RC, Oliver RW, Green BN, Lin LN, Brandts JF, Tam BM, Maxwell A, MacGillivray RT (1996) Production and isolation of the recombinant N-lobe of human serum transferrin from the methylotrophic yeast Pichia pastoris. Protein Expr Purif 8(1):119–125
Mason AB, He QY, Adams TE, Gumerov DR, Kaltashov IA, Nguyen V, MacGillivray RT (2001) Expression, purification, and characterization of recombinant nonglycosylated human serum transferrin containing a C-terminal hexahistidine tag. Protein Expr Purif 23(1):142–150
Morita T, Takegawa K (2004) A simple and efficient procedure for transformation of Schizosaccharomyces pombe. Yeast 21(8):613–617
Nimesh S, Kumar R, Chandra R (2006) Novel polyallylamine-dextran sulfate-DNA nanoplexes: highly efficient non-viral vector for gene delivery. Int J Pharm 320(1–2):143–149
Okada H, Sekiya T, Yokoyama K, Tohda H, Kumagai H, Morikawa Y (1998a) Efficient secretion of Trichoderma reesei cellobiohydrolase II in Schizosaccharomyces pombe and characterization of its products. Appl Microbiol Biotechnol 49(3):301–308
Okada H, Tada K, Sekiya T, Yokoyama K, Takahashi A, Tohda H, Kumagai H, Morikawa Y (1998b) Molecular characterization and heterologous expression of the gene encoding a low-molecular-mass endoglucanase from Trichoderma reesei QM9414. Appl Environ Microbiol 64(2):555–563
Okazaki K, Okazaki N, Kume K, Jinno S, Tanaka K, Okayama H (1990) High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res 18(22):6485–6489
Radhakrishnamurthy B, Ruiz HA, Srinivasan SR, Preau W, Dalferes ER Jr, Berenson GS (1978) Studies of glycosaminoglycan composition and biologic activity of Vessel, a hypolipidemic agent. Atherosclerosis 31(2):217–229
Rahbarizadeh F, Rasaee MJ, Forouzandeh M, Allameh AA (2006) Over expression of anti-MUC1 single-domain antibody fragments in the yeast Pichia pastoris. Mol Immunol 43(5):426–435
Russel P (1989) Gene cloning and expression in fission yeast. Academic, San Diego
Sander P, Grunewald S, Reilander H, Michel H (1994) Expression of the human D2S dopamine receptor in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe: a comparative study. FEBS Lett 344(1):41–46
Sargent PJ, Farnaud S, Cammack R, Zoller HM, Evans RW (2006) Characterisation of recombinant unglycosylated human serum transferrin purified from Saccharomyces cerevisiae. Biometals 19(5):513–519
Schade AL, Caroline L (1946) An iron-binding component in human blood plasma. Science 104(2702):340–341
Steinlein LM, Graf TN, Ikeda RA (1995) Production and purification of N-terminal half-transferrin in Pichia pastoris. Protein Expr Purif 6(5):619–624
Takegawa K, Tohda H, Sasaki M, Idiris A, Ohashi T, Mukaiyama H, Giga-Hama Y, Kumagai H (2009) Production of heterologous proteins using the fission-yeast (Schizosaccharomyces pombe) expression system. Biotechnol Appl Biochem 53(Pt 4):227–235
Tiyaboonchai W, Woiszwillo J, Middaugh CR (2003) Formulation and characterization of DNA-polyethylenimine-dextran sulfate nanoparticles. Eur J Pharm Sci 19(4):191–202
Tomasic V, Tomasic A, Smit I, Filipovic-Vincekovic N (2005) Interactions in mixed cationic surfactants and dextran sulfate aqueous solutions. J Colloid Interface Sci 285(1):342–350
Tommasino M, Contorni M, Cavalieri F (1992) HPV16 E7 phosphorylation in fission yeast: characterization and biological effects. Gene 111(1):93–98
Toonkool P, Metheenukul P, Sujiwattanarat P, Paiboon P, Tongtubtim N, Ketudat-Cairns M, Ketudat-Cairns J, Svasti J (2006) Expression and purification of dalcochinase, a beta-glucosidase from Dalbergia cochinchinensis Pierre, in yeast and bacterial hosts. Protein Expr Purif 48(2):195–204
Uma Maheswar Rao JL, Satyanarayana T (2003) Enhanced secretion and low temperature stabilization of a hyperthermostable and Ca2+-independent alpha-amylase of Geobacillus thermoleovorans by surfactants. Lett Appl Microbiol 36(4):191–196
von Bonsdorff L, Tolo H, Lindeberg E, Nyman T, Harju A, Parkkinen J (2001) Development of a pharmaceutical apotransferrin product for iron binding therapy. Biologicals 29(1):27–37
Wang J, Nguyen V, Glen J, Henderson B, Saul A, Miller LH (2005) Improved yield of recombinant merozoite Surface protein 3 (MSP3) from Pichia pastoris using chemically defined media. Biotechnol Bioeng 90(7):838–847
Yang B, Hoe MH, Black P, Hunt RC (1993) Role of oligosaccharides in the processing and function of human transferrin receptors. Effect of the loss of the three N-glycosyl oligosaccharides individually or together. J Biol Chem 268(10):7435–7441
Zhao Y, Lieberman HB (1995) Schizosaccharomyces pombe: a model for molecular studies of eukaryotic genes. DNA Cell Biol 14(5):359–371
Zschornig O, Arnold K, Richter W, Ohki S (1992) Dextran sulfate-dependent fusion of liposomes containing cationic stearylamine. Chem Phys Lipids 63(1)–2:15–22
Acknowledgments
This work was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and the Project for Development of a Technological Infrastructure for Industrial Bioprocesses on R&D of New Industrial Science and Technology Frontiers by the Ministry of Economy, Trade and Industry (METI) of Japan, as supported by the New Energy and Industrial Technology Development Organization (NEDO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukaiyama, H., Giga-Hama, Y., Tohda, H. et al. Dextran sodium sulfate enhances secretion of recombinant human transferrin in Schizosaccharomyces pombe . Appl Microbiol Biotechnol 85, 155–164 (2009). https://doi.org/10.1007/s00253-009-2130-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2130-5