Abstract
The interaction of high-molecular dextran sulfate (DS-5000) with dimyristoylphosphatidylcholine (DMPC) monolayers and foam films (FF) at the air–water interface in the presence of Ca2+ and Na+ ions was studied. DS-5000 was added in monolayer films (MF) and in FF as monomer molecules and in liposomal form. When added in liposomal form in FF, DS-5000 decreased the stability of DMPC common black films (CBF), and no formation of Newton black films (NBF) was observed. However, when included as monomer molecules in FF, DS-5000 caused film thinning, and drastically decreased the expansion rate of the black spots and transition of thick films to NBF, thus avoiding formation of CBF. The above effects were observed in both gel and liquid-crystalline phase states of DMPC in the presence of Ca2+ ions only, and not in the presence of Na+ ions. We postulate that the interaction of DMPC with DS-5000 in the plane of FF is mediated by Ca2+ bridges and results in dehydration of the DMPC polar heads. The interaction between DMPC and DS-5000 in monolayers resulted in slower adsorption and spreading of DMPC molecules at the interface, lower monolayer surface pressure, and penetration of DS-5000 molecules to DMPC monolayers when surface lipid density was higher than 50 Å2 per DMPC molecule. The applicability of the FF model for studying the interactions of phospholipids with polysaccharides at interfaces surrounded by bulk solution, and for modeling such interactions in biological systems, e.g. LDL adhesion to the arterial walls, aggregation and fusion of liposomes, etc., is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
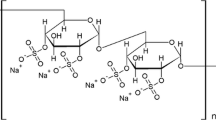
Dextran sulfate (DS) is representative of the carbohydrate moieties of glycosaminoglicane (GAG) molecules which, together with Ca2+ ions, participate in numerous interactions with films of zwitterionic phospholipids, e.g. in adhesion of low density lipoproteins (LDL) to the arterial walls (Nishida and Cogan 1970; Srinivasan et al. 1975; Kim and Nishida 1977; Huster and Arnold 1998; Huster et al. 1999) which is thought to be the key molecular process in atherogenesis (Camejo 1982; Camejo et al. 1985; Rudel et al. 1986), in aggregation and fusion of phosphatidylcholine liposomes (Arnold et al. 1990; Budker et al. 1990), etc.
In previous studies it was reported that low-molecular DS (up to DS-500) strongly interacts with films of phosphatidylcholine molecules in the presence of Ca2+ (Huster and Arnold 1998; Huster et al. 1999). It was found that the inclusion of DS-500 in liposomes resulted in liposome aggregation (Kim and Nishida 1977; Meyuhas et al 1996) and in initiation of membrane fusion (Arnold et al. 1990; Budker et al. 1990). Using monolayer films (MF) at the air–water interface, Huster et al. (1999) reported binding of DS molecules (injected as monomer molecules into the subphase of MF) to the dimyristoylphosphatidylcholine (DMPC) polar heads by formation of Ca2+ bridges without penetration of the hydrophobic region, and decreasing of surface tension by up to 3.5 mN/m at constant molecular area. It was postulated that DS/phosphatidylcholine/Ca2+ complexes with higher packing of lipids were probably formed as indicated by a higher degree of lipid-chain ordering, extended tail conformation and decreased area per lipid molecule due to drastic head-group dehydration (Steffan et al. 1994; Huster and Arnold 1998; Huster et al. 1999). These results are in agreement with the data showing that after Ca2+ bridges mediated adhesion to the carbohydrate moieties of GAGs in the phospholipid (mainly PC) monolayer of LDL, a liquid-ordered phase is formed with restricted lateral diffusion (Bihari-Varga et al. 1986; Fenske and Cushley 1990). By employing NMR measurements, it was revealed also that the repeat spacing of phosphatidylcholine multilayers was reduced to 6–7 Å due to the DS-phosphatidylcholine interaction in the presence of Ca2+ (Huster and Arnold 1998; Huster et al. 1999).
It is important that the effects of DS-induced adhesion and fusion in the presence of Ca2+ be studied by other models: (1) with DS of higher molecular weight (e.g. DS-5000) and (2) with DS-5000 involved in the phospholipid films both as monomer molecules and included in liposomes. For this purpose, in this study, we used the model system of phospholipid foam films (FF) as well as monolayer films (MF). The former, being of several types, are composed of two mutually adsorbed phospholipid monolayers, oriented “head-to-head”, (Fig. 1A–C), thus representing the contact area occurring between the cis-monolayers of two fusing bilayer membranes (Naydenova et al. 1990).
The phospholipid FF has been successfully used as a model system to study membrane–membrane interactions, fusion and adhesion, biosurfactant behavior at interfaces, lung surfactants, etc. (Naydenova et al. 1990; Lalchev et al. 1995; Lalchev 1997; Exerowa and Krugliakov 1998). The phospholipid MF (Fig. 1D) can be regarded as one half of FF or black lipid membranes (BLM) and was used for a long time as a model system for studying the interaction of membrane components with different molecules and ions injected into the liquid subphase (Lalchev et al. 1996; Lalchev and Mackie 1999; Panaiotov and Verger 2000).
The aim of the present work is to study the interactions at the air–water interface of high-molecular dextran sulfate (DS-5000) with DMPC molecules in the presence of Ca2+ and Na+ ions by using the model systems of monolayers and foam films. The goal is also to estimate the role of DS in the behavior of mixed DMPC–DS monolayer and foam films in comparison to the pure lipid films.
Materials and methods
Materials
DMPC (99% purity) and dextran sulfate with molecular mass 5,000 (DS-5000, consisting of approximately 19 monosulfated glucose monomers with mass 260.2) were purchased from Sigma, and used without further purification. CaCl2 and NaCl were purchased from Merck. Solutions were made with bi-distilled water with conductivity of less than 1 μS.
Monolayer films (MF) and foam films (FF) were formed from DMPC dispersions in water containing 0.1 M CaCl2 or 0.4 M NaCl. The dispersions were obtained by sonication of the lipid for 10 min at 50 Hz frequency in a UM-2 sonication bath containing CaCl2 or NaCl.
DS-5000 was included in MF and FF two ways: (1) as monomer molecules, by injection of a certain volume of DS-5000 solution into the subphase of DMPC monolayers and into the menisci of the biconcave drop forming the FF and (2) in liposomal form, by injection into the films of previously prepared DMPC–DS liposomes. The molar ratio DS-5000:DMPC was varied between 6.76×10−5 and 1 (corresponding to 12.8×10−4 and 19 DS monomers to 1 molecule DMPC, respectively).
Preparation of DMPC–DS liposomes was made by sonication of the lipid with DS-5000 dissolved in water containing 0.1 M CaCl2 or 0.4 M NaCl for 10 min at 50 Hz in a UM-2 sonicator. Centrifugation of the obtained liposomes for 5 min at 10,000×g at 20 °C was performed. Following the same experimental protocol, Budker et al. (1990) proved that at CaCl2 concentrations higher than 1 mM, all DS molecules were included in the liposomes.
Formation of foam films (FF)
FF were formed by the method of Scheludko and Exerowa (Exerowa and Krugliakov 1998) using the modified measuring cell as previously described (Lalchev and Mackie 1999). A biconcave drop (50 μl) of the phospholipid dispersion (pH 6.8–7.0) was incubated in the cylinder of the measuring cell at T=20 or 26 °C (corresponding to gel and liquid-crystalline phase states of DMPC) for 30 min. After sucking the solution from the drop, a thick FF with radius (r f)100 μm is formed (Fig. 1). The film spontaneously gets thinner and when critical film thickness (ca. 300 Å) is reached a black spot (local thinning) appears (Fig. 1A), and expands with a characteristic rate to fill the whole area of the film. The kinetics of this process were measured as the expansion time t 1–2 (s) detected from the moment of the first black spot appearance on the film to the moment of its expansion to the whole film area, i.e., to the formation of the black film. Under different experimental conditions two types of black FF may be formed—common black films (CBF; Fig. 1B) and Newton black films (NBF; Fig. 1C).
The probability (W) of formation of stable black FF depends strongly on the phospholipid concentration (Exerowa and Krugliakov 1990) and can be calculated by the equation W=ΔN/N, where N is the total number of trials and ΔN is the number of trials in which stable black FF are formed. Thus, W varies between 0 and 1, indicating that the films always rupture (W=0) or always form stable FF (W=1). The dependence on concentration W(C) is extremely strong which allowed a threshold concentration (C t) to be defined as the minimum phospholipid concentration at which W=1, i.e., stable FF are always formed.
It is important to underline that the W(C) relationship is strongly sensitive to the phase state of the phospholipid used, the composition of the film-forming solution, the temperature, the presence of ions, and the addition of agents in the bulk solution surrounding the film (Lalchev 1984; Exerowa and Krugliakov 1990; Naydenova et al. 1990). Changing any of the above-mentioned conditions results in shifting the W(C) dependence to the left or right along the concentration axes, thus changing C t. We used W(C) dependence of pure DMPC, C t, as a control in order to estimate the role of DS in the behavior of mixed DMPC–DS monolayer and foam films in comparison to the pure lipid films.
Formation of monolayer films (MF)
Spread monolayers of DMPC and DMPC–DS were formed in a Langmuir trough (with volumes of 7 and 25 ml and areas 709 and 1,710 mm2, respectively), and the surface tension γ (mN/m) was measured by the method of Wilhelmy as previously described, with accuracy of ±0.5 mN/m (Christova et al. 1998). For the measurements, an automatic Wilhelmy tensiometer (Biegler Electronic, Austria) with platinum float of 1×1.6 cm was used. The monolayer surface pressure π (mN/m), equilibrium π eq and maximum π max (after compression of the film to 20% from the initial surface area) were calculated by the equation π=γ 0 −γ, where γ 0 is the surface tension of pure water (72.9 mN/m) at the air/water interface and γ is surface tension of the lipid monolayer. The compression/decompression rate was 3 min per cycle. Experiments were made at T=26 °C, pH 6.8–7.0 and electrolyte concentrations C el=0.1 M CaCl2 and 0.4 M NaCl. Each experiment was performed at least three times with each sample preparation.
Adsorbed monolayers were formed in a Teflon trough with 7-ml volume of the subphase and area 706 mm2, using a Teflon-coated stirring bar. The decrease in the surface tension was measured over time by the Wilhelmy plate method using a platinum float. The trough temperature was controlled within ±0.5 °C precision, the surface tension was measured with an accuracy of 0.5 mN/m and each experiment was repeated at least three times.
The penetration of DS-5000 to DMPC monolayers was studied by adding DS with concentration C DS=2.5×10−4 M (4.7×10–3 M monomers) into the subphase of the monolayers previously formed at different surface pressures (120, 60, 50 and 40 Å2 area per DMPC molecule) containing Ca2+ ions. The values of π eq and π max were monitored during compression of the monolayers from 100 to 20% of the initial area with compression rate 1.5 min per cycle.
Results and discussion
Foam films
The dependence of the probability (W) of formation of black FF on the concentration (C) of DMPC in liquid-crystalline phase state in the presence of Ca2+ was determined (Fig. 2). It is seen that the threshold concentration (C t) of DMPC was 200 μg/ml (2.9×10–4 M), i.e., at concentrations equal to and above 200 μg/ml (2.9×10–4 M), the films of pure DMPC are stable and never rupture. The curve obtained and the value of C t for pure DMPC films were used as controls in order to investigate the interaction between DMPC and DS-5000 added into the film in the presence of Ca2+ and Na+ ions.
By addition of DS-5000 (in liposomal form) to the sample with DMPC concentration equal to C t=200 μg/ml in the presence of Ca2+ ions, we found that even at extremely low concentrations of DS-5000 up to 1×10–8 M (1.9×10–7 M monomers) the films ruptured (W=0), i.e., DS molecules had a destabilizing effect on the films. By further increasing DMPC concentration to 250 μg/ml (3.6×10–4 M) and to 300 μg/ml (4.4×10–4 M) it was possible to form stable films with increasing DS-5000 concentrations of up to 9×10–8 and 8×10–7 M, respectively (17.1×10–6 and 15.2×10–6 M monomers) (Table 1).
It is shown in Table 1 that the addition of DS-5000 concentration up to 8×10–8 M did not change the film stability (at both DMPC concentrations), and stable CBFs were formed. The DS-5000 concentration of 9×10–8 M was determined to be the minimum for rupturing the CBF of DMPC formed at 250 μg/ml (after appearance of black spot) but further increase in DMPC concentration to 300 μg/ml led to formation of stable film again. A concentration of 8×10–7 M DS-5000 was found as the minimum for rupturing the film formed at 300 μg/ml DMPC. In terms of W(C) dependence (Fig. 2), the above effect means that the W(C) curve for pure DMPC was shifted to higher DMPC concentration after addition of DS-5000, i.e., that C t increased in the presence of DS-5000. We found that at DMPC concentrations above C t the films were stable, but addition of DS-5000 in the presence of Ca2+ ions caused rupturing of the films. At C t=200 μg/ml DMPC (without DS-5000), the lipids in solution and in a film were in thermodynamic equilibrium and the film was thermodynamically stable with an indeterminate lifetime (in practice, the measured lifetime in our experiments was more than 5 h). Thus, using an expression for the free energy kT, ln(C t */C t), where C t * is the minimum DMPC concentration for stable black FF formation in the presence of DS-5000 (Table 1), we determined that the effect of dextran was to destabilize the film by a free energy of approximately 0.83 and 1.0 kJ/mol DMPC at 2.5×10–8 and 2.5×10–7 M DS-5000, respectively. Hence, the increase in the free energy of DMPC molecules in the film in the presence of DS-5000 added in liposome form is in accordance with the data for the capability of DS to induce membrane fusion.
The observed destabilizing effect of DS-5000 on the DMPC films corresponds with the conclusion of Naydenova et al. (1990) that the rupture of lipid black FF in the presence of a fusogenic agent corresponds to the monolayer merging in the process of liposome fusion, and with the report of Arnold et al. (1990) and Budker et al. (1990) that the interaction of DS in the presence of Ca2+ with PC liposomes induces liposomal fusion.
Different effects were observed when DS-5000 was added into the subphase of the DMPC FF as monomer molecules. The results from this study are shown in Table 2. It is seen that up to the DS-5000 concentration of 2.5×10–7 M, the films were formed as stable CBF with a characteristic expansion time of 15 s. Further increase in DS-5000 concentration above 8.0×10–6 M led to much faster expansion of the black spot(s), 5 s. In addition, the black spots formed in the thick film were of Newton type and their expansion led to formation of NBF (Fig. 1C), thus avoiding formation of CBF, in contrast to the case of DS-5000 in liposomal form. The transition from thick film to NBF in the presence of monomer DS-5000 molecules was not observed in the presence of Na+ ions. The NBF are known as dehydrated real bilayers and consist of two mutually adsorbed monolayers without a free water core between them. The transition to NBF provoked by DS and Ca2+ions is observed here for the first time and we interpret it as an effect of the interaction of DS-5000 with DMPC polar heads in the film plane.
We may assume that the interaction of DMPC with DS-5000 molecules in the FF plane results in dehydration of the DMPC polar heads in the presence of Ca2+ ions, leading to thinning of the liquid core between the film monolayers, and that the adhesion of DS to DMPC heads is mediated by Ca2+ bridges. This assumption is in accordance with the dehydration effect of the DS on the phosphatidylcholine polar heads observed by other authors (Steffan et al. 1994; Huster and Arnold 1998; Huster et al. 1999). Decrease in the expansion time of the black spots in phospholipid FF and provoking of transition to NBF by another fusogenic agent (PEG-4000) have been reported (Todorov 1999). Stronger adhesion between phospholipid (PC, PC plus PE) monolayer polar heads in solid-supported bilayers in the presence of PEG-8000–10000 was measured by Kuhl et al. 1996. Effects of decreasing the repeat spacing of DMPC multilayers (NMR measurements) in the presence of DS and Ca2+ were also reported by Huster and Arnold (1998) and Huster et al. (1999). These data are in agreement with the model of Akesson according to which DS adhesion to phospholipids may be via Ca2+ bridges to their polar heads rather than to the acyl chains (Akesson et al. 1989; Huster and Arnold 1998; Huster et al. 1999). Taking into account the results of the FF and the above observations, our study of the Ca2+-mediated adhesion of DS-5000 to DMPC heads in the plane of FF is presented schematically in Fig. 3.
The schematic FF structure is also confirmed by the data on NBF thickness (ca. 7–8 nm) which corresponds well to the sum of twofold thickness of DMPC molecule (ca. 5 nm) and the thickness of the film core (ca. 2–3 nm), where Ca2+ bridges between the lipid heads and the longitudinally located DS molecules are realized.
The chain-melting transition temperature of DMPC at high water content was reported to be about 24 °C (Rubenstein et al. 1980; Wilkinson and Nagle 1981; Cevc et al. 1990; Kinnunen et al. 1993). The relationships measured at 26 °C (all above) were also studied at 20 °C, thus changing the DMPC phase state from liquid-crystalline to gel. The effects caused by DS-5000 on the film behavior in the presence of Ca+ ions at 26 °C (the destabilizing effect, C t shifting, faster expansion of the spots, provoking of thinning and transition to NBF, etc.) were observed at 20 °C as well, i.e., the same effects were observed in both the gel and liquid-crystalline DMPC phase states.
Monolayer films (MF)
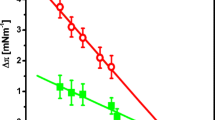
Experiments with monolayers (spread and adsorbed) were performed at molar ratio DMPC:DS=1:1.76×10–2 (1:3.34×10–1 monomer ratio) in the presence of Ca2+ions. The surface pressure–time relationship of adsorbed monolayers showed slower adsorption of DMPC and reduction in the equilibrium monolayer surface pressure by more than 8 mN/m in the presence of DS-5000 in comparison to the adsorption of pure DMPC (Fig. 4).
Dependence of surface pressure (π; mN/m) of adsorbed monolayers on time (t; min). Data were obtained in the absence (triangles) or in the presence of DS-5000 added to the subphase in monomer form (diamonds) or in liposomal form (squares). Experiments were made at molar ratio DMPC:DS-5000=1:1.76×10–2 (1:3.34×10–1 monomer ratio), T=26 °C, pH 6.8–7.0 and subphase containing 0.1 M CaCl2
The kinetics of adsorption were very slow in the presence of DS-5000 added in liposomal form, in this case the surface pressure was equal to 0 mN/m for 30 min. DMPC adsorption in the presence of DS-5000 in monomer form was much faster, in this case the surface pressure increase was detected after 10 min.
The kinetics of spreading of pure DMPC and the mixture of DMPC plus DS-5000 are shown in Fig. 5. The much slower spreading of DMPC molecules in the presence of DS-5000 and the lower equilibrium surface pressure are shown. It was also found that spreading did not depend on whether DS-5000 was used in monomer or liposomal form. A drastic increase in the equilibrium monolayer surface pressure by 22.2 mN/m was observed without DS-5000 (Fig. 5).
Kinetics of spreading of DMPC and DMPC and DS-5000 mixture at air–water interface. Data were obtained in the absence (triangles) or in presence of DS-5000 involved in monomer form (diamonds) or in liposomal form (squares). Experiments were made at a molar ratio DMPC:DS-5000=1:1.76×10–2 (1:3.34×10–1 monomer ratio), T=26 °C, pH 6.8–7.0 and subphase containing 0.1 M CaCl2
These results confirm literature data that DS binds phospholipid molecules by Ca2+ bridges in large aggregates which poorly disintegrate at the air/water interface (Akesson et al. 1989). The data also agree with the theoretical model of membrane fusion (Ohki 1988) according to which fusion agents such as DS-5000 (Arnold et al. 1990; Budker et al. 1990) decrease the lipid density (i.e., the surface pressure) in the parallel-plane head-to-head apposition between the cis-monolayers of the membranes.
We also studied the ability of DS-5000 to penetrate to DMPC monolayers that had been formed at different surface pressures (π; mN/M). DS-5000 was injected into the subphase (C DS=2.5×10–4 M, 0.1 M Ca2+) of previously formed DMPC monolayers with surface densities corresponding to 120, 60, 50 and 40 Å2 area per DMPC molecule. The values of π eq and π max (after compression to 20% from the initial monolayer area) of the monolayers are shown in Table 3.
It has been demonstrated that DS-5000 penetrates to DMPC monolayers with surface densities of 120 and 60 Å2 per DMPC molecule, thus causing an increase in π eq of ca. 5.8 mN/m. No change in π eq was observed in pure DMPC monolayers with 50 and 40 Å2 area per molecule after DS-5000 addition into the subphase. The pressure π max increased after compression of the pure DMPC monolayers with different surface densities, but from the mixed DMPC and DS-5000 monolayers only those with area 120 and 60 Å2 per molecule showed an additional increase in π max (e.g. from 42.0 to 43.7 mN/m for 60 Å2). Hence there was no penetration of DS-5000 to the monolayers with area per DMPC molecule of less than 50 Å2 (Table 3). From the data in Table 3, it would be possible to calculate some important physical quantities generally provided by the monolayer-surface balance technique (monolayer compressibility, visco-elastic modulus, free-energy change, etc.) if the experiments had been performed under equilibrium conditions. However, as was mentioned in Materials and methods, the values of π max were monitored during monolayer compression from 100 to 20% of the initial area with compression rate 1.5 min/cycle, i.e., under nonequilibrium conditions (fivefold compression of the monolayer area for 45 s).
The ability of DS to penetrate to lipid monolayers has been reported by other authors, e.g. DS-500 decreased the surface tension of spread DMPC monolayers by 3.5 mN/m by Ca2+-mediated interaction (Huster et al. 1999). Thus we can accept the ability of DS-5000 to penetrate into lipid monolayers and also to interact with phospholipids, both mediated by Ca2+ ions, as the dominant factors determining the behavior of mixed DMPC–DS monolayers and foam films studied in this work.
Conclusions
Interactions at the air/water interface of pure DMPC monolayers and foam films with high-molecular dextran sulfate (DS-5000), added in different forms in the presence of Ca2+ and Na+ ions, were studied. Thus, in addition to the widely used monolayers, we used, for the first time, foam films as a model for studying the interactions at interfaces between polysaccharides and phospholipids.
The results obtained by the two model systems clearly demonstrated that DS-5000 strongly altered the behavior of both DMPC monolayers and foam films, depending on the form of DS-5000 added (monomer or liposomal).
In comparison to the pure DMPC foam films, the interactions in mixed DMPC–DS films resulted in: (1) drastically reduced time of expansion of the black spots during the process of CBF formation; (2) destabilized CBF and shifting of the W(C) dependence to higher DMPC concentration (both effects were observed when DS-5000 was added in liposomal form), and (3) the transition between the thick FF and the NBF (bilayer lipid film), thus avoiding formation of thicker CBF-containing water core between the monolayers (the latter effect was observed when DS-5000 was added in monomer form). The effects of DS-5000 on the DMPC foam films were observed in the presence of Ca2+ ions only, but not in Na+ ions, in both gel and liquid-crystalline phase state of DMPC.
In mixed DMPC–DS monolayers, the interactions resulted in: (1) slowed adsorption and spread of DMPC molecules at the interface, (2) lowered equilibrium monolayer surface pressure, and (3) penetration of DS-5000 molecules only to DMPC monolayers with surface lipid densities greater than 50 Å2 area per DMPC molecule.
We can postulate that the ability of high-molecular DS-5000 to penetrate to lipid monolayers and thus to the monolayers of the foam films was connected with the dehydration of the phospholipid polar heads in the presence of Ca2+ ions, and mainly determined the significant alteration of the behavior of the mixed DMPC–DS foam films in comparison to the pure DMPC films. The good correlation of our results with the data obtained by other model systems demonstrates the applicability of the foam film model for studying the interactions of phospholipids with polysaccharides at interfaces surrounded by bulk solution, and for modeling such interactions in biological systems, e.g., LDL adhesion to the arterial walls, aggregation of liposomes, etc.
On the other hand, considering DS as a fusion agent (e.g., Ohki 1988; Akesson et al. 1989; Arnold et al. 1990; Budker et al. 1990) in the view of its interactions with phospholipids in the foam films, we can conclude that studying the effects caused by the fusion agent in the lipid film plane can contribute to the clarification of the mechanisms of the initial steps of the membrane fusion process. This conclusion is based on the opportunity to investigate quantitatively the effects provoked by the fusion agents on the foam film properties, such as initiation of thinning the liquid core between the film monolayers, transition of thick film to bilayer NBF, and destabilization and rupture of the film. It is important also that foam films (see their schematic structure in Figs. 1A–C, 3) appear as an adequate structural analogue of the head-to-head apposition between the cis- monolayers of the fusing membranes.
References
Akesson T, Woodward CE, Jönsson B (1989) Electric double layer forces in the presence of polyelectrolytes. J Chem Phys 91:2461–2469
Arnold K, Ohki S, Krumbiegel M (1990) Interaction of dextran sulfate with PL surfaces and liposome aggregation and fusion. Chem Phys Lipids 55(3):301–307
Bihari-Varga M, Sztatisz J, Gal S (1981) Changes in the physical behavior of low density lipoproteins in the presence of glycosaminoglycane and high density lipoproteins. Atherosclerosis 39:19–23
Budker VG, Markushin YU, Vahrusheva TE, Kiseleva EV, Mal’ceva TV, Sidorov VN (1990) Interaction of anionic polysaccharides with phosphatidylcholine vesicles in the presence of Ca2+ ions (in Russian). Biolo Membr 7(4):419–427
Camejo G (1982) The interaction of lipids and lipoproteins with the intercellular matrix of arterial tissue: its possible role in atherogenesis. Adv Lipid Res 19:1-53
Camejo G, Lopez A, Lopez F, Quinones J (1985) Interaction of low density lipoproteins with arterial proteoglycans: the role of charge and sialic acid content. Atherosclerosis 55:93–105
Cevc G, Fenzl W, Sigl L (1990) Surface induced X-ray reflection visualization of membrane orientation and fusion into multilayers. Science 249:1161–1163
Christova Y, Enchev E, Lalchev Z (1998) Effects of pulmonary surfactant proteins SP-B and SP-C and calcium ions on the surface properties of hydrophobic fractions of lung surfactant. Eur Biophys J 28:59–66
Exerowa DR, Krugliakov PM (1998) Foam and foam films: theory, experiment, application. Elsevier, Amsterdam
Fenske DB, Cushley RJ (1990) Insoluble complex formation between low density lipoprotein and heparin. A 31P- NMR study. Chem Phys Lipids 54:9-16
Huster D, Arnold K (1998) Ca2+-mediated interaction between dextran sulfate and dimiristoyl-sn-glycero-3-phosphocholine surfaces studied by 2H-nuclear magnetic resonance. Biophys J 75:909–916
Huster D, Paasche G, Dietrich U, Zachömig O, Gutberlet T, Gawrisch K, Arnold K (1999) Investigation of phospholipid area compression induced by calcium-mediated dextran sulfate interaction. Biophys J 77:879–887
Kim YC, Nishida T (1977) Nature of interaction of dextran sulfate with lecithin dispersions and lysolecithin micelles. J Biol Chem 252(4):1243–1249
Kinnunen P, Rytomaa M, Koiv A, Lehtonen J, Mustonen P, Aro A (1993) Sphingosine-mediated membrane association of DNA and its reversal by phosphatidic acid. Chem Phys Lipids 66:75–85
Kuhl T, Guo Y, Alderfer JL, Berman AD, Leckland D, Israelashvili J, Hui SW (1996) Direct measurement of polyethylene glycol induced depletion attraction between lipid bilayers. Langmuir 12:3003–3014
Lalchev Z (1984) Thin liquid films from lipids and proteins and their mixtures. PhD Thesis, Bulgarian Acadamy of Science, Sofia
Lalchev Z (1997) Surface properties of lipids and proteins at bio-interfaces. In: Birdi K (ed) Handbook of surface and colloid chemistry. CRC Press, Boca Raton
Lalchev Z, Mackie A (1999) Molecular lateral diffusion in model membrane systems. Colloids Surf B: Biointerfaces 15:147–160
Lalchev Z, Todorov R, Christova Y, Wilde P, Mackie A, Clark D (1995) Lateral mobility of phospholipid molecules in thin liquid films. Eur Biophys J 23:433–438
Lalchev Z, Todorov R, Christova Y, Wilde P, Mackie A, Clark D (1996) Molecular mobility in the monolayers of foam films stabilized by porcine lung surfactant. Biophys J 71:2591–2601
Meyuhas D, Nir S, Lichtenberg D (1996) Aggregation of phospholipid vesicles by water-soluble polymers. Biophys J 71(5):2602–2612
Naydenova S, Lalchev Z, Petrov AG, Exerowa D (1990) Pure and mixed lipid black foam films as a models of membrane fusion. Eur Biophys J 17:343–347
Nishida T, Cogan U (1970) Nature of the interaction of dextran sulfate with low density lipoproteins of the plasma. J Biol Chem 245:4689–4697
Ohki S (1988) Surface tension, hydration energy and membrane fusion. In: Ohki S, Doyle D, Flanagan TD, Hui SW, Mayhew E (eds) Molecular mechanisms of membrane fusion. Plenum Press, New York, pp 123–139
Panaiotov I, Verger R (2000) Enzymatic reactions at interfaces: interfacial and temporal organization of enzymatic lipolysis. In: Baszkin A, Norde W (eds) Physical chemistry of biological interfaces. Marcel Dekker, New York
Rubenstein J, Owicki J, McConnell H (1980) Dynamic properties of binary mixtures of phosphatidylcholines and cholesterol. Biochemistry 19:569–573
Rudel LL, Parks JS, Johnson FL, Badiak J (1986) Low density lipoproteins in atherosclerosis. J Lipid Res 27:465–474
Srinavasan R, Radhakrishnamurthy B, Berenson GS (1975) Studies of the interaction of heparin with serum lipoproteins in the presence of Ca2+, Mg2+ and Mn2+. Arch Biochim Biophys 170:334–340
Steffan G, Wulff S, Galla HJ (1994) Divalent cation-dependent interaction of sulfated polysaccharides with phosphatidylcholine/ phosphatidylglycerol liposomes. Chem Phys Lipids 74(2):141–150
Todorov R (1999) Alveolar surfactant composition and properties studied by monolayer and thin liquid films. PhD Thesis, Bulgarian Academy of Science, Sofia
Wilkinson D, Nagle J (1981) Dilatometry and calorimetry of saturated phosphatidylethanolamine dispersions. Biochemistry 20:187–192
Acknowledgements
This work was supported by grant of the fund Scientific Researches, Sofia, Bulgaria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Georgiev, G., Lalchev, Z. Model study of interactions of high-molecular dextran sulfate with lipid monolayers and foam films. Eur Biophys J 33, 742–748 (2004). https://doi.org/10.1007/s00249-004-0421-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-004-0421-4