Abstract
The effects of cortex-lysis related genes with the pdaA, sleB, and cwlD mutations of Bacillus subtilis (natto) NAFM5 on sporulation and germination were investigated. Single or double mutations did not prevent normal sporulation, but did affect germination. Germination was severely inhibited by the double mutation of sleB and cwlD. The quality of natto made with the sleB cwlD double mutant was tested, and the amounts of glutamic acid and ammonia were very similar to those in the wild type. The possibility of industrial development of natto containing a reduced number of viable spores is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natto is a traditional and popular food in Japan (Murooka and Yamashita 2008). It has unique features regarding stickiness and taste, which are related to the digestion of soy proteins. It is made from steamed soybeans using Bacillus subtilis (natto) as a starter. B. subtilis (natto) forms spores that are found in the natto. Bacillus spores are extremely stable as to chemical and/or physical treatments. Therefore, the killing of spores in natto is not easy without loss of food quality, i.e. taste, flavor, color, and/or stickiness. For these reasons, the utilization of natto for processed foods has not increased and natto containing a decreased amount of spores has been desired. For the industrial development of natto, sporulation and/or germination mutants are needed as starters. Since enzymes produced during the sporulation phase usually affect the quality of natto, germination mutants are expected to be the strongest candidates. In B. subtilis, various germination-deficient mutants have been investigated. The major group of germination-deficient mutants is related to the germination receptors for germinants [l-alanine, AGFK (a mixture of l-asparagine, d-glucose, d-fructose and KCl), and Ca2+-dipicolinic acid]. This group contains gerA, gerB, gerK, and gerD (Paidhungat and Setlow 2002; Ragkousi et al. 2003). The mutants of this group are inadequate for our purpose, because germinants comprise not only l-alanine, AGFK, and Ca2+-dipicolinic acid but also possible unknown nutrients (inosine is known as a good germinant for other bacilli; Moir 1992). The second group is related to spore coat deficiency. The gerT and gerQ mutants belong to this group but their spores germinate at high frequency on rich media (Ragkousi et al. 2003; Ferguson et al. 2007). The third group affects vegetative growth and/or sporulation. The gerC, gerF, gerG, and gerE mutants belong to this group (Paidhungat and Setlow 2002; Piggot and Losick 2002; Moir 1981). These mutants are also inadequate because the mutants are not clear germination-defective mutants and may affect the quality of natto. The fourth group is related to cortex-lysis or modification and it contains cwlD, sleB, cwlJ, and pdaA (Sekiguchi et al. 1995; Moriyama et al. 1996; Ishikawa et al. 1998). CwlD, SleB, and CwlJ were considered to be cortex-lytic enzymes. SleB recognizes muramic-delta-lactam, which is a unique peptidoglycan component in the cortex (Warth and Strominger 1969). Muramic-delta-lactam is produced by PdaA (polysaccharide deacetylase) and CwlD (N-acetylmuramoyl-L-alanine amidase homologue) (Sekiguchi et al. 1995; Fukushima et al. 2002; Gilmore et al. 2004; Fukushima et al. 2005). The mutations in the fourth group affect neither vegetative growth nor sporulation, but severely affect germination. Therefore, in this study we investigated the effect of disruption of pdaA, cwlD, and/or sleB on the germination of B. subtilis (natto) and applied the mutants to the production of natto.
Materials and methods
Bacterial strains and plasmids
The strains of B. subtilis 168, B. subtilis (natto) NAFM5 (Kimura et al. 2004), and Escherichia coli and the plasmids used in this study are listed in Table 1. NAFM5 was a derivative of the major natto starter, Miyagino strain, and NAFM5 had the competence ability. B. subtilis was grown on LB medium (Sambrook et al. 1989) at 37°C overnight, inoculated into DSM (Schaeffer sporulation medium) (Schaeffer et al. 1965), and then shaken at 37°C. If necessary, kanamycin and tetracycline were added to the medium to final concentrations of 20 and 10 μg ml−1, respectively. E. coli was grown in LB medium at 37°C. If necessary, kanamycin, tetracycline, and ampicillin were added to the medium to final concentrations of 20, 20, and 50 μg ml−1, respectively.
Plasmids construction
To construct pdaA mutants, the upstream region of pdaA was amplified by PCR using two primers, pdaAUP1 and pdaAUP2 (Table 2), with B. subtilis (natto) NAFM5 DNA as a template. The PCR fragment was digested with KpnI and BamHI and ligated to pUC118 at the corresponding sites, followed by transformation of E. coli JM109, plasmid pDA1 being generated. pDG780 was digested with BamHI and SalI, and the 1.5-kb fragment (kanamycin cassette) was inserted into pDA1 at the corresponding sites to generate plasmid pDA2. Similarly, the downstream region of pdaA was cloned into pUC118 with two primers, pdaADW1 and pdaADW2, to generate plasmid pDA3. pDA2 was digested with KpnI and SalI and the 2.5-kbp fragment was inserted into pDA3 at the corresponding sites. The resulting plasmid, pDA4, was used to construct a pdaA-deficient mutant. pDA4-Tc containing the tetracycline cassette from pDG1515 instead of the kanamycin cassette of pDA4 was used to construct a pdaA sleB-double mutant.
Likewise, for sleB mutants, pSB4 was constructed using the upstream and downstream regions of sleB and the kanamycin cassette from pDG780. The fragment was amplified by PCR with B. subtilis (natto) NAFM5 DNA as a template and primers sleBUP1 and sleBUP2 or primers sleBDW1 and sleBDW2. For cwlD mutants, pCD4 was constructed using the upstream and downstream regions of cwlD and the tetracycline cassette from pDG1515. The fragment was amplified by PCR with B. subtilis (natto) NAFM5 DNA as a template and primers cwlDUP1 and cwlDUP2 or primers cwlDDW1 and cwlDDW2.
Mutant construction
pdaA-deficient mutants, 16A and NAA, were constructed by transformation of B. subtilis 168 and B. subtilis (natto) NAFM5, respectively, with pDA4. Disruption of the pdaA gene was confirmed by PCR using primers pdaAUP1 and pdaADW2. sleB-deficient mutants, 16B and NAB, were constructed by transformation of 168 and NAFM5, respectively, with pSB4. cwlD-deficient mutants, 16D and NAD, were constructed by transformation of 168 and NAFM5, respectively, with pCD4. pdaA and sleB double-deficient mutants, 16AB and NAAB, were constructed by transformation of 16B and NAB, respectively, with pDA4-Tc. pdaA cwlD-double mutants, 16AD and NAAD, were constructed by transformation of 16A and NAA, respectively, with pCD4. sleB cwlD-double mutants, 16BD and NABD, were constructed by transformation of 16B and NAB, respectively, with pCD4.
Transformation of E. coli and B. subtilis strains
E. coli transformation was performed as described by Sambrook et al. (1989) and B. subtilis and B. subtilis (natto) transformation was performed by the procedure of Anagnostopoulos and Spizizen (1961).
Viable spore counts
B. subtilis 168, 16A, 16B, and 16D and B. subtilis (natto) NAFM5, NAA, NAB, and NAD were shake-cultured in LB medium overnight at 37°C. These precultures were inoculated into DSM followed by shake-culturing for 3 days at 37°C. The numbers of spores were determined with a bacterial counting chamber on a microscope. Heat resistance spores (80°C, 10 min) were counted by a standard agar method (Nissui Pharm) as viable colonies after 3 days at 37°C.
Spore germination
B. subtilis 168, 16AB, 16AD, and 16BD and B. subtilis (natto) NAFM5, NAAB, NAAD, and NABD were shake-cultured in DSM for 3 days at 37°C. The spores were purified by lysozyme treatment followed by salt and detergent washes as described by Nicholson and Setlow (1990). The purified spores were adjusted to A 600 = 1 and their viability was assayed as described above. To measure spore germination as the loss of optical density and release of dipicolinic acid, spores were diluted with a 10 mM Tris–HCl buffer (pH 8.4). Germination was initiated by the addition of l-Ala (10 mM). At appropriate times, A 600 of the mixture was measured and a 3-ml sample was taken and centrifuged with a microcentrifuge. The supernatant was used for the measurement of released dipicolinic acid as described by Nicholson and Setlow (1990).
Analysis of natto
B. subtilis (natto) NAFM5 and NABD were shake-cultured in LB medium at 37°C overnight and then the cultures were diluted to 105 cells ml−1 with saline. To make natto, 1 ml of a diluent was inoculated into 50 g of autoclaved soybeans (121°C, 50 min), followed by fermentation at 40°C for 17 h. One hundred milliliters of saline were added to the natto, followed by agitating for 1 h at 4°C. Then the supernatant was used to count the viable spores and to measure the glutamate and ammonia contents for evaluation of the natto. The amounts of glutamate and ammonia were determined with an F-kit l-glutamic acid (Roche) and an F-kit ammonia, respectively, according to the manufacturer’s instructions. The organoleptic evaluation of stickiness and flavor were performed by lifting the natto after mixing with a spatula.
Results
Effects of single mutants as to cortex-lysis-related genes
SleB is a cortex-lytic enzyme (Moriyama et al. 1996) and is assumed to digest the cortex-containing muramic delta-lactam, a unique peptidoglycan component (Warth and Strominger 1969). The delta-lactam structure is produced by CwlD and PdaA (Sekiguchi et al. 1995; Fukushima et al. 2002; Gilmore et al. 2004; Fukushima et al. 2005). Since the genome organizations of B. subtilis 168 and B. subtilis (natto) are very similar (Qiu et al. 2004), we attempted amplification of the upstream and downstream regions of sleB, cwlD, and pdaA of B. subtilis (natto) with B. subtilis (natto) DNA as a template and primers based on the sequences of B. subtilis 168. We were able to amplify these three gene fragments and then three disruption plasmids, pDA4 (for pdaA), pSB4 (sleB), and pCD4 (cwlD), were constructed. The corresponding genes in B. subtilis 168 and B. subtilis (natto) were disrupted by conventional transformation with these plasmids. Then we examined the spore germination frequencies of the sleB, cwlD, and pdaA mutants of B. subtilis 168 and B. subtilis (natto) NAFM5 (Fig. 1). Microscopic observation revealed that all the mutants formed spores and the numbers of spores were similar to those in the wild type (108 to 109 spores ml−1). In the case of B. subtilis 168, the viable numbers of mutant spores measured as heat resistant ones were different from those in the wild type. In particular, the pdaA and cwlD mutants showed greatly decreased numbers of viable spores (2.4 × 105 and 5.1 × 104 spores ml−1, respectively), but the sleB mutation did not affect viability significantly (1.7 × 108 spores ml−1). In the case of B. subtilis (natto) NAFM5, the pdaA and cwlD mutants showed decreased numbers of viable spores (1.5 × 106 and 1.6 × 106 spores ml−1, respectively), but the effects were much less than those in B. subtilis 168. The sleB mutation in B. subtilis (natto) decreased the number of viable spores (1.1 × 107 spores ml−1). The degrees of the effects of these mutations in B. subtilis 168 were ΔcwlD, ΔpdaA, and ΔsleB in decreasing order and those in B. subtilis (natto) were (ΔpdaA, ΔcwlD) and ΔsleB, in decreasing order.
The spore numbers of the single mutants and the wild types. The single mutants and the wild types of B. subtilis 168 and B. subtilis (natto) NAFM5 were cultured in DSM for 3 days at 37°C. The numbers of spores were determined under a microscope (a). Heat-resistant spores (80°C, 10 min), as viable spores, were plated, followed by counting after 3 days at 37°C (b). Open and slashed boxes indicate B. subtilis 168 and B. subtilis (natto) NAFM5, respectively. c.f.u. colony forming units
Effects of double mutants as to cortex-lysis-related genes
Although the single mutants as to the cortex-lysis-related genes showed decreased numbers of viable spores, the effects were not complete. Therefore, we examined the effect of double mutants as to cortex-lysis-related genes, i.e. the pdaA and sleB, pdaA and cwlD, and sleB and cwlD mutations. All the double mutants formed spores at frequencies similar to in the single mutants. To analyze the germination of mutants, the spores were purified and then they (A 600 = 1) were heated for 10 min at 80°C, followed by plating on agar medium to determine viable spore numbers (Fig. 2). Colonies were counted after 3 days, because the colonies of mutants were very small at 24 h.
The spore numbers of the double mutants and the wild types. The double mutants and the wild types of B. subtilis 168 and B. subtilis (natto) NAFM5 were cultured in DSM for 3 days at 37°C. The numbers of spores were determined under a microscope (a). Heat-resistant spores (80°C, 10 min) were counted after cultivation for 3 days at 37°C (b). Open and slashed boxes indicate 168 and NAFM5, respectively
The most dramatic effect was found for the double mutant as to sleB and cwlD. B. subtilis 168 spores with these mutations gave 8.6 × 102 viable cells ml−1 and B. subtilis (natto) spores gave 1.0 × 102 viable cells ml−1 (Fig. 2b). Since the sleB gene product was considered to only degrade the cortex with muramic-delta-lactam (Masayama et al. 2006), the effects of the mutations were different from each other (sleB is not associated with muramic-delta lactam formation). Therefore, the synergetic effect of the sleB and cwlD mutations was found for both B. subtilis 168 and B. subtilis (natto). From a practical aspect, the sleB cwlD-double mutant is a good candidate for producing natto with spores deficient in significant germination.
Analysis of spore germination
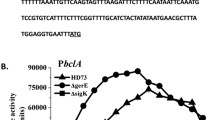
Since the sleB cwlD-double mutants exhibited the lowest germination, germination was compared among 168, 16BD (ΔsleB ΔcwlD), NAFM5, and NABD (ΔsleB ΔcwlD) spores (Fig. 3). During germination in l-Ala buffer, the 16BD and NABD spores showed a slower decrease in A 600 than the wild-type spores of 168 and NAFM5, respectively. Therefore, the germination of both strains was severely affected by the double mutations. Dipicolinic acid release from spores is also a marker of the early germination event. But the levels of the release of dipicolinic acid from these spores were almost the same as that in the wild type. These results suggest that an early germination event (i.e., dipicolinic acid release) was not affected by the double mutations in either strain. These data were supported by the results for each single mutation in B. subtilis (Sekiguchi et al. 1995; Moriyama et al. 1996). Phase-contrast microscopy of the wild type and mutants is shown in Fig. 4. The refractility of spores of the wild types, 168 and NAFM5, changed from bright to dark after 6 h, whereas 16BD and NABD spores became phase gray (or blight) under the same conditions. After 24 h, the 16BD and NABD spores were still phase gray (Fig. 4f, h). These results correspond to the spore viability and the loss of optical density. On the other hand, it is interesting that the dipicolinic acid content of B. subtilis (natto) spores was 1.5 times larger that that of B. subtilis ones (Fig. 3b).
The germination properties of the sleB cwlD-double mutants and the wild types. The germination of spores of the sleB cwlD-double mutants and the wild types was monitored at A 600 at the indicated times after addition to a germination buffer (10 mM l-alanine, 10 mM Tris–HCl (pH 8.4)). Relative absorbance is shown in (a). The dipicolinic acid (DPA) released into the supernatants of spore suspensions during germination is shown in (b). Open and closed circles indicate 168 and its sleB cwlD-double mutant (16BD), respectively. Open and closed triangles indicate NAFM5 and its sleB cwlD-double mutant (NABD), respectively
Phase-contrast microscopy of spores of the sleB cwlD-double mutants derived from 168 and NAFM5. Spores were germinated at 37°C for 6 h (a to d) or 24 h (e to h) in the germination buffer. a and e Show 168 and b and f show the sleB cwlD-double mutant of 168 (16BD). c and g Show NAFM5 and d and h show the sleB cwlD-double mutant of NAFM5 (NABD). Bar = 5 μm
Analysis of natto
Since the sleB cwlD-double mutants formed spores but viable spores were very few, we made natto with NAFM5 and NABD, and then determined the numbers of spores and quality of natto (Fig. 5). Microscopy of extracts of natto indicated that the spore formation of NABD was normal, but the viable spore number decreased similarly in a liquid culture (Fig. 5). The organoleptic test of stickiness and flavor and both the glutamic acid and ammonia contents of NABD were similar to those of the wild type (Fig. 5 and data not shown). These results indicated that the natto made with NABD exhibited normal qualities.
The spore numbers and the amounts of glutamic acid and ammonia in natto. Natto made with the sleB cwlD-double mutant and the wild type was added to saline, followed by agitation for 1 h at 4°C. a The numbers of spores in the supernatants were determined under a microscope or by plating after heat treatment (80°C, 10 min) followed by cultivation for 3 days at 37°C. b The amounts of l-glutamic acid and ammonia in the supernatants were measured with F-kits to evaluate the quality of the natto. Open and slashed boxes indicate NAFM5 and its sleB cwlD-double mutant (NABD), respectively
Discussion
Spores of B. subtilis were extremely stable as to chemical or physical attack. Natto is a soybean-fermented food and B. subtilis (natto) is used as a starter, therefore natto contains many spores. On the other hand, low bacterial numbers in processed foods are desirable from the viewpoint of shelf life. For this reason, natto is not used as a processed food. Spores from natto contaminate machinery used for manufacturing foods and thus the foods have high initial viable cell counts. Therefore, natto with no or few spores is required.
There have been many reports about germination-defective mutants of B. subtilis. Fukushima et al. (2002) reported that the germination rate of pdaA mutants was 0.0008%. In this study, the germination rates of the pdaA mutants were 0.075% and 0.17% for 168 and NAFM5, respectively. The germination rate of the NAFM5 mutant was apparently higher than that of the 168 mutant. The germination rate of the 168 mutant shown in this study was higher than that previously reported. Ishikawa et al. (1998) reported the germination rate of the sleB mutant in B. subtilis was 43% and our results were 61% and 1.2% for 168 and NAFM5, respectively. The mutation of sleB was more effective in NAFM5 than in 168. Sekiguchi et al. (1995) reported that the germination rate of the cwlD mutant in B. subtilis was less than 3.7 × 10–8, but our results were 1.9 × 10–4 and 1.7 × 10–3 for 168 and NAFM5, respectively. Popham et al. (1996) showed that the germination rate of the cwlD mutant in B. subtilis was 5 × 10–4 (with the respect to the wild type).
We investigated the effects of double mutations of these three genes. The cwlD and sleB double mutation strongly affected the germination frequency. The loss of optical density of the spores of the cwlD sleB-double mutant was low compared with in the wild type and the refractility of the spores stopped at the phase gray stage. But the DPA release level was almost the same as that in the wild type. These results indicate that the germination of the cwlD sleB-double mutant was blocked at a late stage of germination. This phenomenon resembled the case of the sleB cwlJ-double mutant (Ishikawa et al. 1998). The release of dipicolinic acid of NAFM5 was higher than that of 168. The reason was not clear but the spore size was larger than that of 168 (Fig. 4). The difference of DPA release may be attributed to the different spore size.
Stickiness and the amounts of glutamic acids and ammonia are important for natto qualities. The natto made with the cwlD sleB-double mutant has the same qualities as the wild type. This indicates that the cwlD sleB-double mutant has no effect on natto qualities. But natto contains vegetative cells in addition to spores. The vegetative cells in natto also affect the shelf life of processed foods. Therefore, process to decrease the number of vegetative cells is required. Such processes are heating, freezing, and addition of preservatives such as glycine or lysozyme and they are milder than killing spores. Consequently, the effect on food quality seems to be very little. Similarly, decolonization of vegetative cells contaminated on machine is easier than that of spores. The introduction of a cold-sensitive mutation to the germination mutant may become an improvement process. Moreover, natto contains abundant vitamin K that is expected to prevent osteoporosis. However, because vitamin K weakens the effect of an anti-blood coagulant pharmaceutical agent, it is not preferable that patients take agent with natto (Homma et al. 2006). It is known that B. subtilis spores germinate in intestines (Casula and Cutting 2002). It means B. subtilis produces vitamin K in intestines (Kaneki et al. 2001). In contrast to spores, vegetative cells do not have the tolerance to peptic juice (Duc et al. 2003). Therefore, the control of the germination of spores in intestines is preferable and thus the germination mutant may be able to be used for its application. In this manuscript, we present the possibility of the improved natto production with a sleB cwlD germination-defective mutant.
References
Anagnostopoulos C, Spizizen J (1961) Requirements for transformation in Bacillus subtilis. J Bacteriol 81:741–746
Casula G, Cutting SM (2002) Bacillus probiotics: spore germination in the gastrointestinal tract. Appl Environ Microbiol 68:2344–2352
Duc le H, Hong HA, Cutting SM (2003) Germination of the spore in the gastrointestinal tract provides a novel route for heterologous antigen delivery. Vaccine 21:4215–4224
Ferguson CC, Camp AH, Losick R (2007) gerT, a newly discovered germination gene under the control of the sporulation transcription factor σK in Bacillus subtilis. J Bacteriol 189:7681–7689
Fukushima T, Yamamoto H, Atrih A, Foster SJ, Sekiguchi J (2002) A polysaccharide deacetylase gene (pdaA) is required for germination and for production of muramic delta-lactam residues in the spore cortex of Bacillus subtilis. J Bacteriol 184:6007–6015
Fukushima T, Kitajima T, Sekiguchi J (2005) A polysaccharide deacetylase homologue, PdaA, in Bacillus subtilis acts as N-acetylmuramic acid deacetylase in vitro. J Bacteriol 187:1287–1292
Gilmore ME, Bandyopadhyay D, Dean AM, Linnstaedt SD, Popham DL (2004) Production of muramic delta-lactam in Bacillus subtilis spore peptidoglycan. J Bacteriol 186:80–89
Homma K, Wakana N, Suzuki Y, Nukui M, Daimatsu T, Tanaka E, Tanaka K, Koga Y, Nakajima Y, Nakazawa H (2006) Treatment of natto, a fermented soybean preparation, to prevent excessive plasma vitamin K concentrations in patients taking warfarin. J Nutr Sci Vitaminol (Tokyo) 52:297–301
Ishikawa S, Yamane K, Sekiguchi J (1998) Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J Bacteriol 180:1375–1380
Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, Ishida N, Nakagawa M, Takechi M, Sano Y, Mizuno Y, Hoshino S, Miyao M, Inoue S, Horiki K, Shiraki M, Ouchi Y, Orimo H (2001) Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition 17:315–321
Kimura K, Tran LS, Uchida I, Itoh Y (2004) Characterization of Bacillus subtilis gamma-glutamyltransferase and its involvement in the degradation of capsule poly-gamma-glutamate. Microbiology 150:4115–4123
Masayama A, Fukuoka H, Kato S, Yoshimura T, Moriyama M, Moriyama R (2006) Subcellular localization of a germination-specific cortex-lytic enzyme, SleB, of bacilli during sporulation. Genes Genetic Syst 81:163–169
Moir A (1981) Germination properties of a spore coat-defective mutant of Bacillus subtilis. J Bacteriol 146:1106–1116
Moir A (1992) Spore germination. In: Doi RH, McGloughlin M (eds) Biology of bacilli: applications to industry. Butterworth-Heinemann, Boston, pp 23–38
Moriyama R, Hattori A, Miyata S, Kudoh S, Makino S (1996) A gene (sleB) encoding a spore cortex-lytic enzyme from Bacillus subtilis and response of the enzyme to l-alanine-mediated germination. J Bacteriol 178:6059–6063
Murooka Y, Yamashita M (2008) Traditional healthful fermented products of Japan. J Ind Microbiol Biotechnol 35:791–798
Nicholson WL, Setlow P (1990) Sporulation, germination and outgrowth. In: Harwood CR, Cutting SM (eds) Molecular biological methods for Bacillus. Wiley, Chichester, pp 391–450
Paidhungat M, Setlow P (2002) Spore germination and outgrowth. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and its closest relatives: from genes to cells. ASM, Washington DC, pp 537–548
Piggot PJ, Losick R (2002) Sporulation genes and intercompartmental regulation. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and its closest relatives: from genes to cells. ASM, Washington DC, pp 483–517
Popham DL, Helin J, Costello CE, Setlow P (1996) Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci USA 93:15405–15410
Qiu D, Fujita K, Sakuma Y, Tanaka T, Ohashi Y, Oshima H, Tomita M, Itaya M (2004) Comparative analysis of physical maps of four Bacillus subtilis (natto) genomes. Appl Environ Microbiol 70:6247–6256
Ragkousi K, Eichenberger P, van Ooij C, Setlow P (2003) Identification of a new gene essential for germination of Bacillus subtilis spores with Ca2+-dipicolinate. J Bacteriol 185:2315–2329
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Schaeffer P, Millet J, Aubert JP (1965) Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA 54:704–711
Sekiguchi J, Akeo K, Yamamoto H, Khasanov FK, Alonso JC, Kuroda A (1995) Nucleotide sequence and regulation of a new putative cell wall hydrolase gene, cwlD, which affects germination in Bacillus subtilis. J Bacteriol 177:5582–5589
Warth AD, Strominger JL (1969) Structure of the peptidoglycan of bacterial spores: occurrence of the lactam of muramic acid. Proc Natl Acad Sci USA 64:528–535
Acknowledgements
The authors would like to thank Dr. Keitarou Kimura (National Food Research Institute) for the supply of B. subtilis (natto) NAFM5. This work was supported by Grants-in-Aid for Scientific Research (B) (19380047) and for the Global COE program of the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MECSSTJ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitsui, N., Murasawa, H. & Sekiguchi, J. Development of natto with germination-defective mutants of Bacillus subtilis (natto). Appl Microbiol Biotechnol 82, 741–748 (2009). https://doi.org/10.1007/s00253-009-1894-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1894-y