Abstract
The development of spores represents one of the main morphogenetic processes in spore-forming bacterial groups, involving more than 150 genes and a variety of sigma factors. These factors control not only the transcription of sporulation-specific operons, involved in spore formation and maturation, but also the manufacture of a variety of products, including important antibiotics, traditionally linked to secondary metabolism. The present chapter encompasses a detailed description of the genes involved in the bacterial sporulation process, their location in the chromosome of Bacillus subtilis, and a description of the main antibiotics produced by different Bacillus species. It also includes a review of alternative bacterial secondary metabolites, such as lanthipeptides, parasporal crystals, and toxins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Endospore-forming bacilli constitute a prominent group of bacteria, not only for the pathogenic species it includes (i.e., Clostridium botulinum, Clostridium difficile, Bacillus anthracis), but also for its saprophytic (i.e., Bacillus subtilis) and industrially important microorganisms (i.e., C. acetobutylicum). Additional significant spore-forming species include the genera Desulfotomaculum, Paenibacillus, and Alicyclobacillus. Other recently described Gram-positive bacteria such as Caldalkalibacillus thermarum TA2.A1 (Peddie et al. 1999; Xue et al. 2006), which is a member of alkaliphilic bacteria but otherwise related to the Bacillales order, has been recently shown to contain at least three annotated operons involved in spore germination (de Jong et al. 2020), including the genes gerABC and yndE. As this alkalophilic bacterium is old in terms of evolution, it has to be assumed that the ability of endospore-forming emerged soon in the evolution of Gram-positive bacteria.
The bacteria exhibiting this exclusive ability, when encounter unappropriate physicochemical conditions initiate the formation of important small molecules, that are collectively known as “alarmones” which are part of the heat shock response in B. subtilis (Schäfer et al. 2020); two of them classic and well known such as pppGpp, ppGpp, and the newest one pGpp, with at last recognized effect as alarmone (Yang et al. 2020), all of them involved in a classical bacterial response known as “stringent response” and recently also found in metazoan (Ito et al. 2020). These elements (highly conserved in Nature and known for more than five decades; Cashel and Gallant 1969) show a variety of pleiotropic effects and are involved in a number of metabolic pathways in bacteria, including the development of endospores. Therefore the alarmones represent a new way for bacterial survival (Fernández-Coll and Cashel 2020). In addition, it has been shown recently that the ComX quorum sensing peptide of B. subtilis positively affects the sporulation process (Špacapan et al. 2020). Differentiation processes in B. subtilis, such as endospore formation, involve multiple paralog Rap-Phr systems that are highly redundant, and that according to Gastélum and colleagues in 2020, interconnect this first-order morphogenetic event with others such as the development of competence.
B. subtilis is, therefore, and without a doubt, the best-known Gram-positive bacterial rod, and contains three subspecies [i.e., subtilis (Nakamura et al. 1999), spizizenii (Nakamura et al. 1999), and inaquosorum (Rooney et al. 2009)]. These three subspecies are so similar (they share ca 3300 ORFs) that they can only be differentiated by phylogenetic analysis of multiple proteins, as their 16S rRNAs exhibit an extremely high sequence identity (for a genomic insight into the taxonomic status of the three B. subtilis subspecies, see Yi et al. 2014).
Although endospore-forming bacteria can exhibit different metabolic and genetic abilities, they all belong to the phylum Firmicutes and share the capacity to survive harsh environmental conditions via the production of highly resistant endospores; this is a superior biological development, normally subjected to catabolic regulation (Schaeffer et al. 1965). These highly resistant structures have been recently reviewed from the point of view of the different technologies usable today that cause endospore death (Cho and Chung 2020). Espores from B. subtilis have been used recently in chickens with positive results as adjuvants in vaccines against the avian influenza H9N2 orthomyxovirus (Lee et al. 2020)
Endospore formation follows the same genetic program in all bacteria, with little variation from species to species; this fact led Hutchison and coworkers to suggest in 2014 that “a robust and sophisticated developmental framework was already in place in the last common ancestor of all extant Firmicutes.” Nearly 90 different bacterial genera can form endospores and, although Gram-positive microorganisms are predominant among them, this endospore-forming group also includes many Gram-negative species.
This survival structure was originally described by Ferdinand Julius Cohn, in the nineteenth century (1875). The author, although a botanist, became one of the founding fathers of modern bacteriology and microbiology by demonstrating the ability of Bacillus to form endospores and describing the basic steps in spore formation (Drews 2000). Cohn, due to his background in algal taxonomy, also made a significant contribution to bacterial taxonomy, although his bacterial classification was not accepted by many of his colleagues, who still believed that bacteria could spontaneously arise from decaying biological matter (Cohn 1875; Drews 2000).
The number of endospores produced by bacteria can vary from one (monosporic species), two (bisporic), or many (polysporic), and they always are genetically identical copies of the vegetative cells. The morphogenetic process resulting in endospore production is usually initiated by a lack of nutrients essential for vegetative growth (mainly nitrogen source depletion). This process is tightly regulated by SPO genes and different σ factors, that define the sporulation stages, and terminates with the formation of a multilayer, refractive, highly resistant structure that can withstand the challenges posed by factors such as extreme temperatures and DNA-damaging agents (Errington 2003). This survival structure is what microbiologists call “endospore,” characterized by its metabolically inactive “dormant state.” In some bacterial groups, however, the sporulation process gives rise to multiple intracellular offsprings, some of which do not undergo a dormancy period; many of these spore-forming bacteria, although hard to grow axenically, were identified as Clostridia, one of the endospore-forming bacterial group (Hutchison et al. 2014).
Unraveling the mechanism of endospore formation, triggered by starvation, resulted not only in the understanding of this basic bacterial morphogenetic process and in obtaining a variety of mutants with different metabolic and genetic abilities, but also in the discovery of novel non-Firmicutes and remote bacterial strains displaying certain characteristics of the Firmicutes. One of these traits is the resistance to soil-dwelling predatory microorganisms, such as the delta proteobacterium Myxococcus xanthus. It is well known that nondomesticated strains of B. subtilis, capable of producing bacillaene (a polyene antibiotic), can resist the attack of the predatory bacteria, eventually forming spores and hence becoming fully resistant to the predator. On the other hand, laboratory strains of B. subtilis, usually unable to produce the antibiotic, are easily predated by M. xanthus (Müller et al. 2014).
Starvation-induced sporulation is the last survival resort for some bacteria. The sporulation process involves a cellular decision-making stage (commitment point), that can last several hours depending on the bacterium, accompanied by the development of actinomycin resistance (Sterlini and Mandelstam 1969). During this time, the bacterium explores other possibilities of survival, such as the secretion of enzymes to use alternative food sources, production of antibiotics to eliminate competing microorganisms, and the induction of cell competence to uptake exogenous DNA. Sporulation is suppressed until all other possibilities are shown inviable and, once the commitment point is reached, sporulation is irreversible. The sporulation process is spatially and temporally orchestrated and represents one of the most thoroughly investigated cellular processes. Some of the genes involved were mapped on the B. subtilis chromosome (Piggot and Coote 1976; Piggot and Hoch 1985) by means of either transformation or transduction. Sporulation studies in Bacillus and Clostridium determined that, although the process is continuous, it can be structured into several stages. Sporulation starts with Stage 0: the decision to sporulate and ends with Stage VI/VII: spore release (Fig. 1), as proposed by Ryter in 1965. Already in 1974, Hranueli et al. proposed that spore formation in Bacillus involves at least 37 operons. For a recent review, see Setlow and Johnson (2019).
Sporulation stages in the Gram-positive bacteria Paenibacillus favisporus. (a, b) Fore-spore formation; (c) spore maturation, displaying the typical surface of the spores from this species; d, e, and f) lysis of sporangium and spore release (modified from Velázquez et al. 2004). Scale bar is 0.7 mm (a, b, c, and d) or 0.2 mm (e and f)
The years 1996 and 1997 saw the publication, in Microbiology and Nature respectively, of first the computerized genetic map of B. subtilis (Biaudet et al. 1996) and then the complete sequence of B. subtilis genome (Kunst et al. 1997). B. subtilis genome spans 4,214,810 base pairs, encompassing 4100 protein-coding genes, as well as at least ten prophages or their remnants and a large number of genes for using a variety of nutrients, many of plant origin. More recently, the publication of complete genome sequences, such as that of Clostridium perfringens (Shimizu et al. 2002), has permitted to carry out comparative genomics with other important Gram-positive anaerobic sporulating rods.
Unraveling this complex genetic and biochemical pathway not only contributes to a better knowledge of the biology of sporulating Gram-positive bacteria, but could also result in the discovery of novel antibiotics, or even contribute to the knowledge of associated flavors in certain beverages, such as the Chinese Maotai Liquor (Wang et al. 2018).
Endospore formation is a major morphological feature used in bacterial taxonomy and the characteristics of the spore, such as location within the sporangium (mother cell), sporangium distension and number of spore per sporangium, are also important for the classification of both aerobic and anaerobic spore-forming Gram-positive bacilli.
Starvation is not the only trigger for sporulation, in fact, siderophore production is another factor affecting endospore formation. Grandchamp and coworkers demonstrated in 2017 that the production of bacillibactin facilitates sporulation, and even enterobactin (a siderophore from E. coli) induces B. subtilis sporulation. However, while the uptake of either siderophore involves binding to just one protein (FeuA), the onset of sporulation in the presence of the siderophores requires a different protein for each siderophore, such as the esterase BesA for bacillibactin and the esterase YbbA for enterobactin (Grandchamp et al. 2017).
B. subtilis spores have recently found quite unusual applications (Sun et al. 2020). The authors used spore coat proteins CotB and CotC as anchors for the heterogenous antigen in a system grass carp reovirus combined with the genes cwlJ and sleB able to control the pore germination. Heterologous antigens using this method were able to elicit a strong humoral as well as cellular response in Ctenopharyngodon idella.
One tends to consider the SPO proteins (all those so far related to the sporulation process) as exclusive of those bacteria able to carry on with the formation of endospores, but the truth of the matter is that there is a large variety of bacterial species (including Escherichia coli) that contain sporulation-related repeated domains, known to bind peptidoglycan and also to enhance the activity of the penicillin-binding proteins and hence of the transpeptidase activity (Pazos et al. 2020).
The study of endospore formation in B. subtilis has been an important increase of our knowledge in terms of genetics, biochemistry, and developmental biology, but indeed it has resulted in practical applications. One of these has been the development of a new strain of B. subtilis that harboring the β-lactam-induced regulatory system BlaR1/BlaI from Staphylococcus aureus, which can be used as an efficient biosensor to evaluate the presence of β-lactams in solution (Lautenschläger et al. 2020). Another interesting application involving the spores of Bacillus subtilis is related to the use of these spores to prepare vaccines against B. anthracis. So, Oh, and colleagues reported in 2020 the obtention of a new B. subtilis strain that originates spores with the anthrax protective antigen on the surface. All in all, and as Errington and van der Aart have recently proposed B. subtilis has been and still is a workhorse as a model for studying cellular development, including the generation of asymmetry, cell fate, and prokaryotic morphogenesis in general (Errington and van der Aart 2020).
2 Genes and Factors Affecting Endospore Formation
The initiation of sporulation is a prime example of developmental biology in Gram-positive bacteria that strongly involves biochemical and genetic factors. It occurs in Nature constantly in this group of bacteria, when encountering inappropriate physicochemical conditions, and the picture of the whole process may be altogether blurred by the continuous growing of B. subtilis under laboratory conditions in what has been denoted as “loss of social traits during domestication process of Bacillus subtilis” (Barreto et al. 2020).
Endospore formation depends on a major signal transduction system known as “the sporulation phosphorelay” that controls phosphorylation of the key Spo0A transcription factor (Burbulys et al. 1991; Ohlsen et al. 1994; Wang et al. 2001), as well as the synthesis of sporulation-specific sigma factors (Fimlaid et al. 2015) involved in the subsequent sporulation stages. Most of the biochemical changes during sporulation appear to occur during the first two “sporulation stages” mentioned above (0 and II); during this period, a new cell differentiates within the mother cell and isolates itself, although it maintains a specialized connection system to the mother cell, to receive from her a variety of nurturing compounds, such as activators and sigma factors.
Initiation of sporulation in B. subtilis stops normal growth (stage 0; Fig. 2), this is followed by the synthesis of a septum (stage II, see below). The Spo0A protein is activated through phosphorylation (Sonenshein 2000) in stage 0 and is responsible for the regulation, either directly or indirectly, of more than 500 genes (Fawcett et al. 2000). When studying the σ factors involved in the sporulation process, it soon became clear that a single vegetative σ factor could not be responsible for the RNA transcription carried out from a variety of promoters which, in addition, are different from those responsible for vegetative growth and primary metabolism. Further proof of this was provided by Linn and coworkers that, already in 1973, demonstrated that the activity of the vegetative sigma subunit of B. subtilis RNA polymerase dramatically decreases once sporulation starts, and its levels remained low throughout the sporulation process. These findings were confirmed by Brevet the following year (Brevet 1974).
Key stages of the sporulation cycle in Bacillus subtilis. Sporulation phases 0 to VI are indicated in the diagram, and the main genes involved in the process are summarized. Stage VI represents the final events leading to spore maturation inside the mother cell, while stage VII requires mother cell lysis for the spore to be released
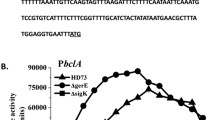
An example of activation of sporulation-specific genes/regulons is the cascade reaction initiated by the arbB gene, which encodes a protein (ArbB) that acts as a repressor of spo0H expression. The gene spo0H encodes the σH protein (Weir et al. 1991), which regulates the expression of σF protein, responsible for entering sporulation stage II (Wu et al. 1992; Sonoda et al. 2015). Figure 3 summarizes the different σ factors involved in the main sporulation stages.
Summary of sigma factors involved in Bacillus subtilis sporulation. SpoIIT is involved in the activation of σE in the mother cell, whereas SpoIIIL is required for σG activity in the forespore (Meeske et al. 2016)
Briefly, the spore formation pathway mainly depends on two pivotal kinases integrated into the phosphorelay process of sporulation. The main activators and repressors systems required for sporulation initiation are depicted in Tables 1, 2, 3, 4, and 5.
Bacteria rely on histidine kinases to react to a variety of external signals, and this also applies to sporulation. KinA is perhaps the main histidine kinase involved in the initiation of endospore formation in the family Bacillaceae. Winnen and collaborators described in 2013 that this kinase had an N-terminal region (residues 1–382) spanning three tandem Per-ARNT-Sim (PAS) domains, believed to constitute the sensor sporulation module. Upon nutrient starvation in endospore-forming bacteria, KinA inhibits the antikinase activity of KipI (gene homologues of kipI are found almost throughout all bacterial kingdom; Jacques et al. 2011a), hence allowing sporulation. KipI and KipA are the fourth and fifth genes, respectively, of a seven-cistron operon that is upregulated by high glucose concentrations and down-regulated in the presence of nitrogen. The combined actions of KinA and Kipl trigger the regulatory pathway known as the sporulation phosphorelay, which in turn activates Spo0A (the main component of the sporulation cascade). The protein Sda (Fig. 4) is also involved in KinA phosphorylation, as well as in replication and sporulation coordination (Veening et al. 2009). The gene products involved in stage 0 are depicted in Tables 1 and 2.
Sporulating stages II and III involve a differentiation program that lasts 5 hours, and, according to Eichenberger and coworkers in 2004, it involves 383 genes epistatically controlled by transcription factors σE, σF, σG (they activate 81 genes), and σK. This stage is characterized by an asymmetric division that gives rise to a sporangium, formed by the mother cell and separated from the future forespore by a closing Z-ring that leaves a narrow tunnel, also known as the “feeding tube” (Mastny et al. 2013) that links both compartments. The tunnel also contains a DNA filament that extends from the mother cell. As it is, and before the beginning of the asymmetric division to form the prospore, an axial DNA filament is formed containing two chromosomes along the longest axis of the cell, and firmly attached to each pole thanks to proteins such as RacA, Soj, Spo0J, and MinD (Wu and Errington 2003; Willis et al. 2020). In this way, when the prespore is finally formed, it tapes ca. 30% of one chromosome and the remaining 70% of the chromosome relays on the feeding tube, and particularly on the translocase SpoIIIE (Bath et al. 2000; Willis et al. 2020), which is a hexameric protein that embraces the double-stranded DNA, and translocates each arm into the prespore, presumably through the formation of small pores. It is known that the terminus chromosomal region in B. subtilis is comprised between 152 and 187°, and that this region is the last one to be translocated into the prospore (Willis et al. 2020). The feeding tube, therefore, is crucial for spore formation and maturation, as this process requires many gene products expressed by the mother cell genes that are transferred to the forespore through this tunnel. The genes involved in this stage and their function are summarized in Table 3.
Sporulating stage III is characterized by the engulfment of the forespore by the mother cell; this results in the forespore being covered by a double-membrane, inner and outer membranes (McKenney et al. 2013), within the mother cell cytosol. This phase is accompanied by a simultaneous synthesis of modified peptidoglycan, which contains the modified sugar muramic-δ-lactam and a low level of peptide cross-links between the glycan strands (Popham 2002), located between the inner and outer membranes. Deposition of a proteinaceous layer takes place mainly externally, thus completing the formation of the spore “cortex,” that constitutes the characteristic structure of Stage IV (see Tables 4 and 5 for its main components and functions).
A peculiarity of B. subtilis sporulation is that there is a temporal dissociation between the occurrence of late events and the expression of genes that determine them (Jenkinson et al. 1980), as the proteins responsible for the changes during stages V and VI are already synthesized by the end of stage IV. Stage V is characterized by the formation of the spore coat, which contains approximately 70 proteins, originated from the mother cell, many of which started migrating to the spore surface at the time of engulfment (Popham 2002). Stage VI (maturation and sporangium lysis; summarized in Table 6) starts with the synthesis of dicarboxylic dipicolinic acid (derived from l-aspartate, see Fig. 5), that chelates high amounts of Ca++ and transforms the spore into a refractile structure containing the coat proteins (Fig. 6), selectively stainable by malachite green at high temperature, and thus forming a spore crust (which is the outer most layer of spores lacking sporangium). The crust structure is composed of several coat proteins such as CotV, Cot W, CotX, CotY, CotZ, and CgeA (Bartels et al. 2019), being CotY the most important in the crust structure in terms of scaffolding and morphogenetic functions (Shuster et al. 2019; Dubois et al. 2020) along with CoX and CotZ. In addition to these coat proteins, the crust contains a variety of glycans with functions largely unknown, although at least two different glycans have been proposed: one linked to the outer coat proteins and another strictly linked to the crust (Shuster et al. 2019; Dubois et al. 2020). In this sense, the genes spsM, spsABCDEFGIJKL, yfnHGFED, ytdA-ytcABC, and cgeAB-cgeCDE have been involved in the synthesis of the surface proteins (Cangiano et al. 2014). Lately, it has been demonstrated (Dubois et al. 2020) that these sps genes encode the legionaminic acid pathway that is required for crust assembly. The legionaminic acid is a 9 carbon, beta-neuraminic acid derivative (5,7-diamino-3,5,7,9-tetradeoxy-d-glycero-beta-d-galacto-non-2-ulopyranosonic acid), also found on the flagellin of Helicobacter pylori and Campylobacter jejuni.
Finally, the mature spore is normally released, as a dormant resistant cell, by lysis of the sporangium wall (old mother cell’s). The spore can remain dormant for a long period of time, many years until reactivation (germination) takes place when environmental conditions, such as food and temperature, permit it. During the first phases of germination, efflux of ions occurs and step by step also disassembly of the coat proteins and the cortex; most of the previously captured calcium is released. During all the time the spore was a dormant structure, well within the spore core there were a variety of unaltered mRNAs. The question is, are these mRNAs functional during germination?. This question and several others have been recently proposed by Setlow and Christie in their recent review of 2020. The existence of fully functional mRNAs in spores would indeed speed up the germination processes, since the germinating spores would pass directly to translation as the ribosomes became, in turn, functional.
Futuristically, it would be interesting if B. subtilis had receptors for 4,5-dihydroxy-2,3-pentanedione derivatives, collectively known as “autoinducers AI-2” and involved in quorum sensing responses. This, without a doubt, would facilitate the coordination of sporulation in an otherwise asynchronous culture (a recent communication on the role of autoinducer AI-2 may be found in Zhang and colleagues in 2020).
3 Secondary Metabolites Produced During Endospore Formation
3.1 Antibiotics
Spore-forming bacteria are excellent secondary metabolite producers, including antibiotics. Bu’Lock already described in 1961 the relationship between intermediary metabolism and antibiotic synthesis (Bu’Lock 1961), while Weinberg summarized the main characteristics of secondary metabolites (Weinberg 1964). According to this author, a secondary metabolite has a restricted distribution (best if species-specific), does not play an obvious role in general metabolism, and is rapidly synthesized even when bacterial growth is minimal or non-existent. Sermonti concluded in 1980 that secondary metabolism is a primitive type of metabolism. Kalenova et al. (2017) recently reported that secondary metabolites produced by Bacillus sp., isolated from late Neogene permafrost, have a very potent effect on cytokine production by human peripheral blood mononuclear cells. These metabolites induced the production of both proinflammatory (TNF-α, IL-1β, IL-8, IL-2, and IFNγ) and anti-inflammatory (IL-4 and IL-10) cytokines, and the secretion levels of cytokines were far higher than those induced by B. cereus, medicinal strain IP5832, metabolites. These results propound a putative role for these secondary metabolites in the development of immunomodulating drugs.
Manganese and copper are two transition metals that appear to be important both in endospore formation (Weinberg 1964; Krueger and Kolodziej 1976) and in secondary metabolite synthesis (i.e., iron for mycobacillin or cobalt for d-glutamyl peptide; Jansen and Hirschmann 1944; Foster and Woodruff 1946). Manganese, in particular, appears to be essential as, according to Weinberg, no other biologically active element can substitute it. Apart from transition metals, starvation (depletion of a usable nitrogen source) triggers both sporulation and secondary metabolism (including synthesis of antibiotics), originating a metabolic state known as the “stringent response” that involves GTP and active ribosomes (Lukin et al. 1983; Ochi and Ohsawa 1984).
We envisage that research into novel sources of antibiotics and secondary metabolites (as well as other pharmaceutically relevant compounds) in the near future will involve the study of yet unknown microorganisms isolated from insects, plants, or animals. Indeed, insects represent the most diverse group of animals and should constitute an excellent source of microorganisms capable of producing bioactive molecules as secondary metabolites. In his review, Bode provides prime examples of entomopathogenic bacteria as sources of secondary metabolites, these include Bacillus thuringiensis, Pseudomonas entomophila, Xenorhabdus, and Photorhabdus (Bode 2009).
The genus Bacillus is an eminent antibiotic producer (mostly polypeptidic), with already 167 peptides described by Berdy in 1974 and a number of new ones characterized since (see review by Katz and Demain 1977). The classical antibiotics produced by B. subtilis include mycobacillin, subtilin, bacilysin, bacillomycin, fungistatin, bulbiformin, bacillin, bacillaene, subsporin, bacillocin, mycosubtilin, fungocin, iturin, neocidin, and eumycin. B. brevis secretes gramicidin S, tyrocidine, linear gramicidin, brevin, edeine, eseine, bresseine, and brevistin. B. pumilus synthesizes micrococcin P, pumilin, and tetain, while B. mesentericus produces esperin, and B. licheniformis generates bacitracin, licheniformin, and proticin. Antibiotic production in B. polymyxa includes polymyxin, colistin, gatavalin, and jolipeptin, while B. circulans secretes butirosin, circulin, polypeptin, EM-49, and xylostatin. B. cereus makes biocerin, cerexin and thiocillin, and B. laterosporus synthesizes laterosporamine and laterosporin.
Those described above are all peptide antibiotics, listed by Katz and Demain in 1977, and all share the following basic properties: (1) their size is much smaller than “normal” antibiotics; (2) they are usually produced as a close family of peptides; (3) they can be constituted by either amino acids only, or be complexed with other compounds, such as polymyxins, that contain either 6-methyloctanoic acid or 6-ethylheptanoic acid as a fatty acid residue; (4) frequently contain d-amino acids not found in proteins, and (5) they are mainly resistant to hydrolysis by peptidases and proteases.
The peptide antibiotic synthesis requirements are the same for all of them; they all require amino acids, ATP, the appropriate synthase (that can be purified from cell-free extracts), Mg2+ ion, and a reducing agent. The antibiotic extends from the N-terminal to the C-terminal end, as is the case in protein synthesis, and only enzyme-bound intermediates are involved (Katz and Demain 1977). Lipmann and collaborators proposed a mechanism for the synthesis of cyclic peptide antibiotics, such as gramicidin S, which involves peptidyl transfers from enzyme-bound thioester intermediates (Gevers et al. 1969; Lipmann 1973). When the peptide antibiotic is linear (i.e., gramicidins) the pentadecapeptide remains thioester-linked, and formylation occurs after completion of the polypeptide synthesis (Bauer et al. 1972). Despite all the advances in our knowledge of the genetics, biochemistry, and synthesis of sporulation-related antibiotics, little is known about the role (or roles) that these compounds play in the producing organism. The suggested function as a biochemical sink has its merit, although, as indicated by Katz and Demain (1977), these antibiotics are produced specifically when the cell detects harsh conditions and could either be packaged in the Bacillus spore to provide a favorable environment (by eliminating competitors) during germination or inhibit spore germination until environmental conditions are favorable.
Antifungal antibiotics produced by Bacillus are somehow linked to sporulation, as they are secondary metabolites. They are not common in these bacteria, although there are some lipopeptides (Hamley 2015) with antifungal action, such as fengycin, surfactin, and iturin family compounds (Dunlap et al. 2013); and more recently, Knight and coworkers described one secreted by B. subtilis subsp inaquosorum (Knight et al. 2018). All these antibiotics are synthesized by synthetases not linked to ribosomes, they exhibit different types of cyclization and varied length of the fatty acid chain. Fengycin was the first antifungal identified (Vanittanakom et al. 1986), although surfactin is perhaps the most powerful biosurfactant and the iturin family displays a broad-spectrum antifungal activity (Knight et al. 2018).
Iturins are a group of lipopeptide antifungal amphiphilic antibiotics that act on the cytoplasmic membrane altering K+ permeability. Iturins increase membrane permeability by forming ion-conducting pores, due to their interaction with sterols and phospholipids present in the membrane. The antifungal activity of these compounds increases with the number of aggregates formed and depends on the type of amino acids contained by the lipopeptide, as well as the type of sterols present in the cytoplasmic membrane.
Iturin A (Fig. 7) is the archetype for B. subtilis lipopeptide (Besson et al. 1976). It is encoded by the iturin A operon, which spans over 38 kb and contains four open reading frames, ituD, ituA, ituB, and ituC (Tsuge et al. 2001). Recently (Zhou et al. 2020) have reported on the isolation from deep sea, of a new bacterial strain, tentatively classified within the Bacillus genus, that synthesizes two new iturin-like lipopeptides, designated as C14 iturin W, and C15 iturin W, with fungicidal activity by introducing damage into the fungal plasmalemma. Mycosubtilin, also produced by some B. subtilis strains, is similar to iturin, although there are minor differences between the two antibiotics, both in the conformation of serine and asparagine and in the order the two amino acids are found on the lipopeptides.
Iturins may have additional roles as biocontrol agents. It has been reported lately (Wang et al. 2020), that iturin A directly extracted from B. subtilis strain WL-2 readily exerts a controlling role on the fungus Phytophthora infestans (potato late blight disease that shapes a threat worldwide for Solanum tuberosum culture) through disruption of the cellular membrane and oxidative stress.
Plipastatin (A and B) are potent Bacillus antimicrobial lipopeptides (inhibitors of phospholipase A2; Volpon et al. 2000), thought to replace shortly conventional treatments in plant–fungal infections. B. subtilis synthesizes this antibiotic directed by the operon ppsABCDE operon (Vahidinasab et al. 2020); the authors accomplished the construction of a new strain able to produce in a constitutive manner, increased amounts of plipastatin.
Interestingly, recently it has been reported that some fungal–bacterial interactions are able to select mutants able to synthesize increased levels of compounds with antifungal activity (Albarracín-Orio et al. 2020). Surprisingly, the authors found that interactions of B. subtilis with the fungus Setophoma terrestris, originated bacterial variants which had lost the ability to form lipopeptides and instead had gained the capability to synthesize compounds with antifungal activity.
Genome mining applied to B. subtilis NCD-2 is giving positive results as far as unraveling the potential to find antimicrobial compounds in this strain, and also to determine the specificity of respective gene clusters (Su et al. 2020). The strain is a good one to fight soil-borne plant pathogenic fungi, since it has been described as producer of broad-spectrum antifungal compounds. Additional species of the Bacillus genus, such as B. velezensis have been described as bein g good sources of L ipopeptides and polyketides (Ruiz-García et al. 2005; Rabbee and Baek 2020), that allows the bacterium to exert quite positive antagonistic effects against plant pathogens, such as Verticillium dahlia that causes wilt in olive trees (Castro et al. 2020), or to promote the growth of Malus hupehensis Rehd (Wang et al. 2019) while related to B. subtilis, is different in that it contains nine gene clusters (namely, srf, bmy, fen, dhb, bac, mln, bae, dfn, and nrs) by which the bacterium produces a large variety of antimicrobial compounds (Rabbee and Baek 2020).
Table 7 summarizes the most relevant antibiotics produced by Bacillus.
3.2 Alkanes
Alkanes, with a general molecular formula of CnH2n+2, represent the simplest organic molecules that are widely distributed in nature; they are stable due to their backbone carbon atoms, having attained their octet of electrons through the formation of four covalent bonds.
Alkanes can be used as an advanced biofuel because of their high-energy content, which is 30% higher than ethanol. Although it has been reported that recombinant E. coli strains can produce a different range of alkanes, such as pentadecane and heptadecane (Choi and Lee 2013), the use of these compounds is far from being industrially exploited, and this includes the alkanes produced as secondary metabolites in Gram-positive sporulating bacteria. However, most sporulating bacteria appear to be good alkane degraders. Efficient microbial biosynthesis of alkanes with long carbon chains is difficult to achieve in a single organism (Lehtinen et al. 2018), as this process requires a two-step pathway. Hence, the first step of CO2 reduction to acetate should be carried out by a homoacetogenic bacterium following the Wood–Ljungdahl pathway. Transformation into long-chain hydrocarbons, on the other hand, would be best achieved by a second engineered microorganism expressing the enzymes acyl-ACP reductase (AAR) and aldehyde deformylating oxygenase (ADO); ADO is regarded as the bottleneck for the alkane biosynthesis, due to the low activity of the enzyme.
The available data indicate that aerobic Gram-positive sporulating bacteria do not naturally exhibit the ability to generate alkanes, at least not in enough quantities to be industrially relevant. In fact, some results suggest that these microorganisms are totally unable to do so unless they are genetically engineered. However, this appears not to be the case for anaerobic clostridia; Bagaeva and Zinurova reported in 2004 that Clostridium pasteurianum could in fact synthesize alkanes (C25-C35 intracellular and C11-C24 extracellular) at the end of its logarithmic growth phase, in an atmosphere formed by a mixture of CO2+H2/ argon. A particularity of this bacterial species, not present in Gram-negative bacteria, is its ability to produce branched alkanes. Despite the aforementioned, there have been recent papers describing the ability of certain strains of B. subtilis to form a “volatilome” formed by secondary metabolites that include hydrocarbons, ketones, alcohols, aldehydes, ester, acids, among many others (up to 231), and some having the property to control the fungal population in the rhizosphere (Kai 2020).
3.3 Parasporal Crystals
Parasporal crystals constitute one of the few examples in Biology in which a cell contains a crystallized structure with biological activity. The archetypes for these structures are the bipyramidal parasporal crystals of B. thuringiensis, a Gram-positive, endospore-forming bacterium closely related to both B. cereus and B. anthracis, the causative agent of anthrax. The crystals are synthesized during endospore formation and are hence associated with the secondary metabolism of Bacillaceae. This microorganism was initially described by Ishiwatari Shigetane (1901) in the silkworm and named Bacillus sotto. It was later renamed as B. thuringiensis after Berliner (1915) isolated it from the gut of the flour moth caterpillar in Thuringia, Germany (Milner 1994). There are currently several known B. thuringiensis subspecies (all producing parasporal crystals) that display different toxicity towards insects, such as Lepidoptera, Coleoptera, Diptera, Hymenoptera, and Nematoda (Schnepf et al. 1998; Wei et al. 2003; Soberón et al. 2013). The proteinaceous nature (δ-endotoxin or cry proteins) of the parasporal crystal was described by Hannay and Fitz-James in 1955, while the crystal-specific toxicity towards caterpillars of the lepidopteran species Pieris brassicae was known since 1965 (Lecadet and Martouret 1965). This research defined the type subspecies, Berliner, while further subspecies, such as kurstaki, israelensis, and aizawa, were later described. In 1968 de Barjac and Bonnefoi carried out the first attempt to rationalize the taxonomy of B. thuringiensis subspecies and varieties. Cry proteins are encoded by cry genes, which are located on a plasmid in most B. thuringiensis strains. In 1979 both Robert A. Zakharian and coworkers and Miteva independently reported the plasmid location of the cry genes, suggesting a role for the plasmid in both endospore and crystal formation (Zakharian et al. 1979; Miteva 1979)
There are multiple studies on the mode of action of B. thuringiensis toxins (i.e., Koch et al. 2015) which, unlike chemical pesticides, are effective only after being ingested by the insect. The parasporal Cry proteins are approximately 70–140 kDa and, once within the gastrointestinal tract of insects, they become activated by proteases and specifically bind to epithelial cells receptors (mostly cadherin-like glycoproteins); they create pores, formed by oligomers of six Cry molecules (this is essential for lethality), that cause a dramatic cellular osmotic imbalance which eventually leads to the death of the insect.
Since the cry genes were cloned in 1981 (Schnepf and Whiteley 1981) there have been many successful attempts to express them in transgenic crop plants, such as corn, some of which involved biotechnological companies such as Monsanto. The initial concerns about the possible negative effects of the thuringiensis toxins, either released into the environment through the roots of the transgenic plants, or present in the foodstuffs, resulted in the experiments being concealed from the public, such as the work by Saxena and Stotzky in 2000. In fact, there was no need for such concern, as indicated by Koch and coworkers in 2015: “Cry proteins are very limited in their duration of effectiveness because they can be washed off the plant (e.g., by rain) or inactivated by sunlight within days after application, and they require considerable water, heat, and feedstock to produce, and must be manually applied, either by hand sprayer on small plots or by machine if applied to large tracts.” Because of their safety of use, a variety of Cry proteins have been approved for use in at least one country to protect against lepidopteran pests, and these include: Cry1Ab inserted into maize by Monsanto; Cry1Ac expressed in cotton, corn, brinjal, and soy by Monsanto; Cry1A.105 + Cry2Ab2 and Cry1Ac + Cry2Ab2 were introduced in maize varieties by Monsanto; Cry1Ac + Cry1F in cotton and soy by Dow; Cry1Fa2 in maize by Dow; Cry1Ac + Cry1F in cotton and soy by Dow; Cry1Ab + Cry2Ae in cotton by Bayer. In addition, Cry34Ab1 + Cry35Ab1 were expressed in maize by Dow and DuPont to protect from Coleoptera (Koch et al. 2015). The economic importance of Cry proteins in crop protection was reviewed by Marques and coworkers in 2019. As for the price for the production of these proteins, it passes through the obtention of Cry protein-overproducing strains. An easy way of doing this was recently reported by Quan and coworkers in 2020. The authors, by simply deleting the leu B gene (encodes for the 3-isopropylmalate dehydrogenase in the leucine synthesis pathway) in a conditionally asporogenous B. thuringiensis, were able to overexpress such a protein.
The isolation of new and natural strains of B. thuringiensis must proceed at whatever pace, since Nature has always provided new useful mutations for human industrial applications. In this sense, Liu and colleagues reported in 2020 the isolation of a new strain B. thuringiensis, X023, which exhibits enhanced insecticidal (against Plutella xylostella) activity by copper ions. This ion promoted the expression of cry1Ac and vip3Aa, the synthesis of aminoacids, the glyoxalate pathway, as well as the poly-β-hydroxybutyrate accumulation; all these compounds are necessary for the synthesis of parasporal crystals (Liu et al. 2020).
Concerning the safety of use of these biocides, they are generally considered as safe, as they are quite specific in their mode of action against lepidopteran or Diptera insects; however, their use may disturb the general metabolism of other insects initially thought not to be susceptible to the cry toxins. In this sense, Nawrot-Esposito and colleagues reported in 2020 that these bioinsecticides cause defects in the larval development of Drosophila melanogaster, by reducing the protein digestion. Differential side-effects of thuringiensis biocides have also been reported on this fly by Babin and coworkers in 2020 non-target Drosophila flies.
Late reports (Ursino et al. 2020) have shown that B. subtilis may be directly used to produce mosquitocidal toxins against species of Aedes, known to transmit some arbovirus-caused diseases. Some of these diseases include Dengue fever and Yellow fever (transmitted by Aedes aegypti), Japanese Encephalitis and Rift Valley fever (transmitted by Culex tritaeniorhynchus), among others. It is clear that the genetic background of B. subtilis is by far better known than that of B. thuringiensis; so any genetic manipulation with projection in the industry (i.e., increase production of lepidopteran or dipteral toxins, or obtention of altogether different toxins) should have a better outcome if developed in B. subtilis. The deepest study on this topic follows in the next chapter.
3.4 Lanthipeptides
Lanthipeptides constitute “natural products,” ribosomally synthesized by bacilli as secondary metabolites, and are posttranslationally modified peptides (RiPPs) (Nolan and Walsh 2009; Dias et al. 2015). These modifications include the formation of meso-lanthionine and 3-methyllanthionine, as well as dehydrated amino acids. Xin and coworkers classified lanthipeptides into four groups in 2015, depending on the enzymes involved in post-translational processing. In group I, amino acid dehydration is carried out by a dedicated lanthipeptide dehydratase, and cyclization is catalyzed by a lanthipeptide cyclase; in group II, the lanthipeptide is modified by specific proteins; whereas in groups III and IV, lanthipeptide dehydration and cyclization reactions are carried out by multifunctional enzymes. B. thuringiensis and B. cereus are able to produce more than 20 bacteriocins, many with potential usage both in the Food Science industry and in the clinical control of pathogenic bacteria (Rea et al. 2010). Cerecidins merit a special citation among the lanthipeptides produced by the cereus group, for their prospective usefulness in controlling pathogenic bacteria (Wang et al. 2014). In fact, cerecidins A1 and A7 are known to be active against Gram-positive bacteria, displaying remarkable efficacy against both multidrug-resistant S. aureus (MRSA strains) and vancomycin-resistant Enterococcus faecalis.
As a general rule, lanthipeptides are encoded by structural genes (lanA), normally synthesized as non-active precursors that are later hydrolyzed into an N-terminal peptide and a C-terminal peptide; the N-terminal leader peptide is important for post-translational modifications (Yang and van der Donk 2013; Dias et al. 2015). The structural genes for these peptides (lanA) frequently cluster with genomic islands, this is the case for lanthipeptides synthesized by Bacillus methylotrophicus (Dias et al. 2015), and this supports the notion that their production might be the result of evolutionary adaptation to best achieve their in vivo function, either as controllers of other microorganisms (Wang et al. 2014) or as plant growth promoters (Hao et al. 2012). It appears that Gram-positive spore-forming bacteria require antimicrobial lanthipeptides to conquer harsh environments, as the strains and bacterial species isolated from harder habitats seem to produce novel lanthipeptides with new characteristics (Othoum et al. 2018). The structural lanthipeptide genes have been cloned (Ongey et al. 2018) and are in the process of being genetically modified in order to both increase production of these compounds, that are normally produced in low amounts by their “natural” bacterial species, and broaden their application. Lanthipeptides are very promising bioactive compounds with a great potential use not only in human and veterinary medicine but also in the control of bacteria that cause food spoilage.
4 Secondary Metabolites in the Environment
Microbiologists are still blatantly ignorant concerning the number of bacterial species on earth and can only hypothesize to estimate the enormous number (perhaps up to 80%) of bacteria that cannot yet be grown in axenic conditions in the laboratory. This is either due to the lack of appropriate culture media or because microorganisms are rarely found in nature in pure culture (only pathogenic microorganisms constitute a monoculture when causing a disease), and to flourish, they need to be in contact with other microorganisms, often through “quorum sensing” mechanisms, or may require secondary metabolites such as antibiotics or lanthipeptides. Zengler and coworkers researched this topic in their interesting publication entitled “Cultivating the uncultured” (2002), putting forward a proposal for a universal method to detect, or at least estimate, the numerous unculturable microorganisms present in the environment. According to Nai and Meyer (2018) “Only a paradigm shift in cultivation techniques—from axenic to mixed cultures—can allow a full comprehension of the (chemical) communication of microorganisms, with profound consequences for natural product discovery, microbial ecology, symbiosis, and pathogenesis.” This means that it is essential to develop the microbial co-culture technology, as well as understand the effects of secondary metabolites produced by a given microbial specimen on the biological development of neighboring organisms. Despite our lack of knowledge in these basic research areas, some advances are slowly taking place, among them are the early reports by Johnson and colleagues and Patel and Roth, both in 1978. More recently, Shank (2013) studied bacterial co-cultures to examine the influence of secondary metabolites on microbial interspecies interactions in the natural environment. In addition, Nai and Meyer (2018) reported that the three technical approaches currently used (3D-bioprinting, single-cell metabolomics, and microfluidics) can allow systematic co-culture of three or more microorganisms. Hopefully, the next few decades would bring a much better understanding of the complex microbial relationships that occur in “natural” environments.
This knowledge and understanding could revitalize the search for novel natural compounds with antimicrobial activity, such as antibiotics, a task currently practically abandoned by pharmaceutical companies throughout the world. Some authors estimate that there are still up to 1000 novel antimicrobials awaiting discovery, as well as a great number of yet unknown enzybiotics (Veiga-Crespo et al. 2007). Production of novel drugs could be attained by microbial co-cultures in which the secondary metabolites secreted by one species induce expression of antibiotics or antimicrobials in another species (Bertrand et al. 2014). Gram-positive organisms and spore-forming bacteria, together with members of the Pseudomonadaceae family, are prime candidates to use in co-culture experiments, as they are among the best secondary metabolite producers. Although the number of combinations for laboratory co-culture experiments is high, the family Bacillaceae (B. subtilis, B. cereus, B. licheniformis, B. thuringiensis, or B. brevis) can be anticipated as good candidates for co-culture with antibiotic-producing fungi, such as Penicillium, Aspergillus, or Acremonium. These co-cultures could result in the production of novel, improved β-lactams. Other good contenders for co-culture experiments are members of the Streptococcaceae and Myxococcaceae families, as they constitute well known antibiotic producers. This opens up the exciting possibility of obtaining new and improved antibacterials in the near future, as long as both governments and private companies are willing to invest in this new venture. This research is essential for the future of antibiotic development and must be done now to find new antimicrobials to counteract the threat of poly-resistant bacterial strains. Antibiotic resistance was described by the World Health Organization in 2018 as “one of the biggest threats to global health, food security, and development” facing humanity today.
5 Toxins
The ability of spore-forming Gram-positive bacilli (such as Bacillus or Clostridium) to produce toxins is very high and, in most bacteria, it is linked to secondary metabolism. These compounds include some of the most potent neurotoxins known in nature (i.e., C. botulinum, C. perfringens, C. sordellii, or Cl. tetani). Although the toxigenic phenotype has mainly been assigned to the strict anaerobic Clostridium genus, this ability is also displayed by some species of the mostly aerobic Bacillus genus, such as B. cereus and B. anthracis. Clostridium botulinum was named Bacillus botulinus by Emile van Ermengem, who originally isolated it from spoiled ham (1897). The American bacteriologist Ida Albertina Bengtson (1881–1952), the first woman hired to work at the National Institutes of Health (Lindenmann 2005), renamed it as Clostridium in 1924, as it is an anaerobic organism, hence restricting the genus Bacillus to aerobic spore-forming rods. Despite this, the bacterium was still referred to as Bacillus in publications well into the 1950s, such as in the article by Bulatova and Matveev (1957) concerning clostridial species. Finally, Collins et al. (1994) reorganized and redefined the species included in the genus Clostridium.
These neurotoxins produced by these bacteria are proteinaceous in nature and composed of two subunits (α and β). Botulism toxin was originally purified and crystallized by Lamanna et al. (1946), and is classified into eight types, referred to as A to H (Dover et al. 2014); A and B are the most important to humans. This toxin prevents the release of the neurotransmitter acetylcholine from axon endings at the neuromuscular junction and causes flaccid paralysis. The botulinic toxin is currently used in a number of medical applications, ranging from wrinkle reduction to the treatment of limb spasticity after a stroke (Sun et al. 2019); it is also applied in esthetic plastic surgery to treat facial sagging (Zhou et al. 2019), as well as in the treatment of Parkinson’s disease (Cardoso 2018), bruxism (Tinastepe et al. 2015) and strabism (Scott 1981).
Eklund et al. demonstrated in 1971 that, when C. botulinum type C is cured of its prophage, the bacteriophage Ceβ, it ceases to produce toxin and becomes nontoxigenic C. novyi type A. This discovery could open the possibility of toxin gene movilization among different clostridial species (Eklund et al. 1974). In the late twentieth century, a neurotoxigenic Clostridium butyricum strain, isolated from food, was found to be involved in an outbreak of food-borne type E botulism (Aureli et al. 1986; Meng et al. 1997). In addition, Cassir and coworkers recently demonstrated (2016) that Clostridium butyricum, normally used as a probiotic, could become a new emerging pathogen. Enterococcus faecium has also been reported as a potential producer of botulinum toxin, presumably due to horizontal transmission of the toxic gene from a clostridial strain (Zhang et al. 2018)
References
Adams DW, Errington J (2009) Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol 7:642–653
Akita E, Ito T, Tsuruoka T, Niida T (1970) Synthesis of an aminocyclitol antibiotic, SF-733 (ribostamycin). Antimicrob Agents Chemother (Bethesda) 10:33–37
Albarracín-Orio AG, Petras D, Tobares RA, Aksenov AA, Wang M, Juncosa F, Sayago P, Moyano AJ, Dorrestein PC, Smania AM (2020) Fungal–bacterial interaction selects for quorum sensing mutants with increased production of natural antifungal compounds. Commun Biol 3:670. https://doi.org/10.1038/s42003-020-01342-0
Alvarez CE (2008) On the origins of arrestin and rhodopsin. BMC Evol Biol 8:222
Amon JD, Yadav AK, Ramirez-Guadiana FH, Meeske AJ, Cava F, Rudner DZ (2020) SwsB and SafA are required for CwlJ-dependent spore germination in Bacillus subtilis. J Bacteriol 202:e00668-19
Antoniewski C, Savelli B, Stragier P (1990) The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J Bacteriol 172:86–93
Arabolaza AL, Nakamura A, Pedrido ME, Martelotto L, Orsaria L, Grau RR (2003) Characterization of a novel inhibitory feedback of the anti-anti-sigma SpoIIAA on Spo0A activation during development in Bacillus subtilis. Mol Microbiol 47:1251–1263
Asen I, Djuranovic S, Lupas AN, Zeth K (2009) Crystal structure of SpoVT, the final modulator of gene expression during spore development in Bacillus subtilis. J Mol Biol 386:962–975
Atsumi K, Oiwa R, Omura S (1975) Production of bacillin by Bacillus sp. strain no. KM-208 and its identity with tetaine (bacilysin). J Antibiot (Tokyo) 28:77–78
Aureli P, Fenicia L, Pasolini B, Gianfranceschi M, McCroskey LM, Hatheway CL (1986) Two cases of type E infant botulism caused by neurotoxigenic Clostridium butyricum in Italy. J Infect Dis 154:207–211
Azumi M, Ogawa K, Fujita T, Takeshita M, Furumai T, Igarashi Y, Yoshida R (2008) Bacilosarcins A and B, novel bioactive isocoumarins with unusual heterocyclic cores from the marine-derived bacterium Bacillus subtilis. Tetrahedron 64:6420–6425
Babin A, Nawrot-Esposito M-P, Gallet A, Gatti J-L, Poirié M (2020) Differential side-effects of Bacillus thuringiensis bioinsecticide on non-target Drosophila flies. Sci Rep 10:16241
Bagaeva TV, Zinurova EE (2004) Comparative characterization of extracellular and intracellular hydrocarbons of Clostridium pasteurianum. Biochemistry (Mosc) 69:427–428
Bagyan I, Setlow P (2002) Localization of the cortex lytic enzyme CwlJ in spores of Bacillus subtilis. J Bacteriol 184:1219–1224
Bai U, Mandic-Mulec I, Smith I (1993) SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-protein interaction. Genes Dev 7:139–148
Baldus JM, Buckner CM, Moran CP Jr (1995) Evidence that the transcriptional activator Spo0A interacts with two sigma factors in Bacillus subtilis. Mol Microbiol 17:281–290
Barák I, Behari J, Olmedo G, Guzmán P, Brown DP, Castro E, Walker D, Westpheling J, Youngman P (1996) Structure and function of the Bacillus SpoIIE protein and its localization to sites of sporulation septum assembly. Mol Microbiol 19:1047–1060
Barnes EM (1949) Laterosporin A and laterosporin B, antibiotics produced by B. laterosporus. Br J Exp Pathol 30:100–104
Barnes EM, Newton GG (1953) Brevin: an antibiotic produced by Bacillus brevis. Antibiot Chemother (Northfield) 3:866–872
Barreto HC, Cordeiro TN, Henriques AO, Gordo I (2020) Rampant loss of social traits during domestication of a Bacillus subtilis natural isolate. Sci Rep 10:18886. https://doi.org/10.1038/s41598-020-76017-1
Barsby T, Kelly MT, Gagne SM, Andersen RJ (2001) Bogorol A produced in culture by a marine Bacillus sp. reveals a novel template for cationic peptide antibiotics. Org Lett 3:437–440
Barsby T, Kelly MT, Andersen RJ (2002) Tupuseleiamides and basiliskamides, new acyldipeptides and antifungal polyketides produced in culture by a Bacillus laterosporus isolate obtained from a tropical marine habitat. J Nat Prod 65:1447–1451
Bartels J, Blüher A, López Castellanos S, Richter M, Günther M, Mascher T (2019) The Bacillus subtilis endospore crust: protein interaction network, architecture and glycosylation state of a potential glycoprotein layer. Mol Microbiol 112:1576–1592
Bath J, Wu LJ, Errington J, Wang JC (2000) Role of Bacillus subtilis SpoIIIE in DNA transport across the mother cell-prespore division septum. Science 290:995–997
Bauer K, Roskoski R Jr, Kleinkauf H, Lipmann F (1972) Synthesis of a linear gramicidin by a combination of biosynthetic and organic methods. Biochemistry 11:3266–3271
Beall B, Moran CP (1994) Cloning and characterization of spoVR, a gene from Bacillus subtilis involved in spore cortex formation. J Bacteriol 176:2003–2012
Belitsky BR, Sonenshein AL (2008) Genetic and biochemical analysis of CodY-binding sites in Bacillus subtilis. J Bacteriol 190:1224–1236
Berdy J (1974) Recent developments of antibiotic research and classification of antibiotics according to chemical structure. Adv Appl Microbiol 18:309–406
Bertrand S, Bohni N, Schnee S, Schumpp O, Gindro K, Wolfender JL (2014) Metabolite induction via microorganism co-culture: a potential way to enhance chemical diversity for drug discovery. Biotechnol Adv 32:1180–1204
Besson F, Peypoux F, Michel G, Delcambe L (1976) Characterization of iturin A in antibiotics from various strains of Bacillus subtilis. J Antibiot (Tokyo) 29:1043–1049
Bhate DS (1955) Pumilin, a new antibiotic from Bacillus pumilus. Nature 175:816–817
Biaudet V, Samson F, Anagnostopoulos C, Ehrlich SD, Bessières P (1996) Computerized genetic map of Bacillus subtilis. Microbiology 142:2669–2729
Bidnenko V, Nicolas P, Grylak-Mielnicka A, Delumeau O, Auger S, Aucouturier A, Guerin C, Repoila F, Bardowski J, Aymerich S, Bidnenko E (2017) Termination factor Rho: from the control of pervasive transcription to cell fate determination in Bacillus subtilis. PLoS Genet 13:e1006909
Bode HB (2009) Entomopathogenic bacteria as a source of secondary metabolites. Curr Opin Chem Biol 13:224–230
Borowski E (1953) Isolation of tetaine, an antibiotic from the strain of Bacillus pumilus. Biul Panstw Inst Med Morsk Trop J W Gdansku 5:294–309
Bouvier J, Stragier P, Bonamy C, Szulmajster J (1984) Nucleotide sequence of the spo0B gene of Bacillus subtilis and regulation of its expression. Proc Natl Acad Sci U S A 81:7012–7016
Brevet J (1974) Direct assay for sigma factor activity and demonstration of the loss of this activity during sporulation in Bacillus subtilis. Mol Gen Genet. 128:223–231
Brinsmade SR, Alexander EL, Livny J, Stettner AI, Segrè D, Rhee KY, Sonenshein AL (2014) Hierarchical expression of genes controlled by the Bacillus subtilis global regulatory protein CodY. Proc Natl Acad Sci U S A 111:8227–8232
Bugaichuk UD, Piggot PJ (1986) Nucleotide sequence of the Bacillus subtilis developmental gene spoVE. J Gen Microbiol 132:1883–1890
Bukowska-Faniband E, Hederstedt L (2013) Cortex synthesis during Bacillus subtilis sporulation depends on the transpeptidase activity of SpoVD. FEMS Microbiol Lett 346:65–72
Bulatova TI, Matveev KI (1957) Relation between central nervous system injury by Bacillus perfringens and Bacillus oedematiens toxins and the blood antitoxin titre. Biull Eksp Biol Med 43:71–75
Bu’Lock JD (1961) Intermediary metabolism and antibiotic synthesis. Advan Appl Microbiol 3:293–342
Burbulys D, Trach KA, Hoch JA (1991) The initiation of sporulation in Bacillus subtilis is controlled by a multicomponent phosphorelay. Cell 64:545–552
Butler PD, Mandelstam J (1987) Nucleotide sequence of the sporulation operon, spoIIIE, of Bacillus subtilis. J Gen Microbiol 133:2359–2370
Cai D, Zhang B, Zhu J, Xu H, Liu P, Wang Z, Li J, Yang Z, Ma X, Chen S (2020) Enhanced bacitracin production by systematically engineering S-adenosylmethionine supply modules in Bacillus licheniformis. Front Bioeng Biotechnol 8:305
Callow RK, Glover RE, Hart PD (1947) Licheniformin, the antibiotic material from Bacillus licheniformis; concentration and some chemical and biological properties. Biochem J 41:xxvii
Camp AH, Losick R (2009) A feeding tube model for activation of a cell-specific transcription factor during sporulation in Bacillus subtilis. Genes Dev 23:1014–1024
Camp AH, Wang AF, Losick R (2011) A small protein required for the switch from σF to σG during sporulation in Bacillus subtilis. J Bacteriol 193:116–124
Cangiano G, Sirec T, Panarella C, Isticato R, Baccigalupi L, De Felice M, Ricca E (2014) The sps gene products affect the germination, hydrophobicity, and protein adsorption of Bacillus subtilis spores. Appl Environ Microbiol 80:7293–7302
Cardoso F (2018) Botulinum toxin in parkinsonism: the when, how, and which for botulinum toxin injections. Toxicon 147:107–110
Cashel M, Gallant J (1969) Two compounds implicated in the function of the RC gene of Escherichia coli. Nature 221:838–841
Cassir N, Benamar S, La Scola B (2016) Clostridium butyricum: from beneficial to a new emerging pathogen. Clin Microbiol Infect 22:37–45
Castro D, Torres M, Sampedro I, Martínez-Checa F, Torres B, Béjar V (2020) Biological control of Verticillium Wilt on olive trees by the salt-tolerant strain Bacillus velezensis XT1. Microorganisms 8:1080
Cattoni DI, Thakur S, Godefroy C, Le Gall A, Lai-Kee-Him J, Milhiet PE, Bron P, Nöllmann M (2014) Structure and DNA-binding properties of the Bacillus subtilis SpoIIIE DNA translocase revealed by single-molecule and electron microscopies. Nucleic Acids Res 42:2624–2636
Chakraborty M, Mahmud NU, Gupta DR, Tareq FS, Shin HJ, Islam T (2020) Inhibitory effects of linear lipopeptides from a marine Bacillus subtilis on the wheat blast fungus Magnaporthe oryzae Triticum. Front Microbiol 11:665
Chan WC, Bycroft BW, Leyland ML, Lian LY, Yang JC, Roberts GC (1992) Sequence-specific resonance assignment and conformational analysis of subtilin by 2D NMR. FEBS Lett 300:56–62
Chen NY, Jiang SQ, Klein DA, Paulus H (1993) Organization and nucleotide sequence of the Bacillus subtilis diaminopimelate operon, a cluster of genes encoding the first three enzymes of diaminopimelate synthesis and dipicolinate synthase. J Biol Chem 268:9448–9465
Chen B, Himes P, Liu Y, Zhang Y, Lu Z, Liu A, Yan H, Kroos L (2014) Structure of bacterial transcription factor SpoIIID and evidence for a novel mode of DNA binding. J Bacteriol 196:2131–2142
Chen M, Lyu Y, Feng E, Zhu L, Pan C, Wang D, Liu X, Wang H (2020) SpoVG is necessary for sporulation in Bacillus anthracis. Microorganisms 8:548
Cho W, Chung M-S (2020) Bacillus spores: a review of their properties and inactivation processing technologies. Food Sci Biotechnol 29:1447–1461
Choi YJ, Lee SY (2013) Microbial production of short-chain alkanes. Nature 502:571–574
Clarkson J, Campbell ID, Yudkin MD (2004) Efficient regulation of sigmaF, the first sporulation-specific sigma factor in B. subtilis. J Mol Biol 342:1187–1195
Cohn F (1875) Untersuchungen über Bakterien II. Beitr Biol Pflanz 1:141–207
Collins MD, Lawson PA, Willems A, Cordoba JJ, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow JA (1994) The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol 44:812–826
Cosby WM, Zuber P (1997) Regulation of Bacillus subtilis sigmaH (spo0H) and AbrB in response to changes in external pH. J Bacteriol 179:6778–6787
Crater DL, Moran CP Jr (2002) Two regions of GerE required for promoter activation in Bacillus subtilis. J Bacteriol 184:241–249
Cutting S, Oke V, Driks A, Losick R, Lu S, Kroos L (1990) A forespore checkpoint for mother cell gene expression during development in B. subtilis. Cell 62:239–250
Daniel RA, Drake S, Buchanan CE, Scholle R, Errington J (1994) The Bacillus subtilis spoVD gene encodes a mother-cell-specific penicillin-binding protein required for spore morphogenesis. J Mol Biol 235:209–220
Dartois V, Djavakhishvili T, Hoch JA (1996) Identification of a membrane protein involved in activation of the KinB pathway to sporulation in Bacillus subtilis. J Bacteriol 178:1178–1186
de Barjac H, Bonnefoi A (1968) A classification of strains of Bacillus thuringiensis Berliner with a key to their differentiation. J Invertebr Pathol 11:335–347
de Jong SI, van den Broek MA, Merkel AY, Cortes PT, Kalamorz F, Cook GM, van Loosdrecht MCM, McMillan DGG (2020) Genomic analysis of Caldalkalibacillus thermarum TA2.A1 reveals aerobic alkaliphilic metabolism and evolutionary hallmarks linking alkaliphilic bacteria and plant life. Extremophiles 24:923–935
Delcambe L (1952) Some properties of iturin. Arch Int Physiol 60:554–555
den Blaauwen T, Hamoen LW, Levin PA (2017) The divisome at 25: the road ahead. Curr Opin Microbiol 36:85–94
Devi SN, Kiehler B, Haggett L, Fujita M (2015) Evidence that autophosphorylation of the major sporulation kinase in Bacillus subtilis is able to occur in trans. J Bacteriol 197:2675–2684
Dias L, Caetano T, Pinheiro M, Mendo S (2015) The lanthipeptides of Bacillus methylotrophicus and their association with genomic islands. Syst Appl Microbiol 38:525–533
Dover N, Barash JR, Hill KK, Xie G, Arnon SS (2014) Molecular characterization of a novel botulinum neurotoxin type H gene. J Infect Dis 209:192–202
Drews G (2000) The roots of microbiology and the influence of Ferdinand Cohn on microbiology of the 19th century. FEMS Microbiol Rev 24:225–249
Driks A, Roels S, Beall B, Moran CP Jr, Losick R (1994) Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev 8:234–244
Dubnau EJ, Cabane K, Smith I (1987) Regulation of spo0H, an early sporulation gene in bacilli. J Bacteriol 169:1182–1191
Dubnau E, Weir J, Nair G, Carter L 3rd, Moran C Jr, Smith I (1988) Bacillus sporulation gene spo0H codes for sigma 30 (sigma H). J Bacteriol 170:1054–1062
Dubois T, Krzewinski F, Yamakawa N, Lemy C, Hamiot A, Brunet L, Lacoste A-S, Knirel Y, Guerardel Y, Faille C (2020) The sps genes encode an original legionaminic acid pathway required for crust assembly in Bacillus subtilis. mBio 11:e01153-20
Dubos RJ, Hotchkiss RD (1941) The production of bactericidal substances by aerobic sporulating bacilli. J Exp Med 73:629–640
Duncan L, Losick R (1993) SpoIIAB is an anti-sigma factor that binds to and inhibits transcription by regulatory protein sigma F from Bacillus subtilis. Proc Natl Acad Sci U S A 90:2325–2329
Duncan L, Alper S, Losick R (1996) SpoIIAA governs the release of the cell-type specific transcription factor sigma F from its anti-sigma factor SpoIIAB. J Mol Biol 260:147–164
Dunlap CA, Bowman MJ, Schisler DA (2013) Genomic analysis and secondary metabolite production in Bacillus amyloliquefaciens AS 43.3: a biocontrol antagonist of Fusarium Head Blight. Biol Control 64:166–175
Ebata M, Miyazaki K, Takahashi Y (1969) Studies on subsporin. I. Isolation and characterization of subsporins A, B and C. J Antibiot (Tokyo) 22:467–472
Eichenberger P, Jensen ST, Conlon EM, van Ooij C, Silvaggi J, González-Pastor JE, Fujita M, Ben-Yehuda S, Stragier P, Liu JS, Losick R (2003) The sigmaE regulon and the identification of additional sporulation genes in Bacillus subtilis. J Mol Biol 327:945–972
Eichenberger P, Fujita M, Jensen ST, Conlon EM, Rudner DZ, Wang ST, Ferguson C, Haga K, Sato T, Liu JS, Losick R (2004) The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis. PLoS Biol 2:e328
Eijlander RT, Holsappel S, de Jong A, Ghosh A, Christie G, Kuipers OP (2016) SpoVT: from fine-tuning regulator in Bacillus subtilis to essential sporulation protein in Bacillus cereus. Front Microbiol 7:1607
Eklund MW, Poysky FT, Reed SM, Smith CA (1971) Bacteriophage and the toxigenicity of Clostridium botulinum type C. Science 172:480–482
Eklund MW, Poysky FT, Meyers JA, Pelroy GA (1974) Interspecies conversion of Clostridium botulinum type C to Clostridium novyi type A by bacteriophage. Science 186:456–458
Errington J (2003) Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol 1:117–126
Errington J, van der Aart LT (2020) Microbe profile: Bacillus subtilis: model organism for cellular development, and industrial workhorse. Microbiology (Reading) 166:425–427
Errington J, Rong S, Rosenkrantz MS, Sonenshein AL (1988) Transcriptional regulation and structure of the Bacillus subtilis sporulation locus spoIIIC. J Bacteriol 170:1162–1167
Errington J, Appleby L, Daniel RA, Goodfellow H, Partridge SR, Yudkin MD (1992) Structure and function of the spoIIIJ gene of Bacillus subtilis: a vegetatively expressed gene that is essential for sigma G activity at an intermediate stage of sporulation. J Gen Microbiol 138:2609–2618
Fan N, Cutting S, Losick R (1992) Characterization of the Bacillus subtilis sporulation gene spoVK. J Bacteriol 174:1053–1054
Fawcett P, Eichenberger P, Losick R, Youngman P (2000) The transcriptional profile of early to middle sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A 97:8063–8068
Fernandes CG, Moran CP Jr, Henriques AO (2018) Autoregulation of SafA assembly through recruitment of a protein cross-linking enzyme. J Bacteriol 200(14):pii:e00066-18. https://doi.org/10.1128/JB.00066-18
Fernández-Coll L, Cashel M (2020) Possible roles for basal levels of (p)ppGpp: growth efficiency vs. surviving stress. Front Microbiol 11:592718
Ferrari FA, Lang D, Ferrari E, Hoch JA (1982) Molecular cloning of the spo0B sporulation locus in bacteriophage lambda. J Bacteriol 152:809–814
Ferrari FA, Trach K, LeCoq D, Spence J, Ferrari E, Hoch JA (1985) Characterization of the spo0A locus and its deduced product. Proc Natl Acad Sci U S A 82:2647–2651
Fimlaid KA, Jensen O, Donnelly ML, Siegrist MS, Shen A (2015) Regulation of Clostridium difficile spore formation by the SpoIIQ and SpoIIIA proteins. PLoS Genet 11:e1005562
Fort P, Errington J (1985) Nucleotide sequence and complementation analysis of a polycistronic sporulation operon, spoVA, in Bacillus subtilis. J Gen Microbiol 131:1091–1105
Foster JW, Woodruff HB (1946) Bacillin, a new antibiotic substance from a soil isolate of Bacillus subtilis. J Bacteriol 51:363–369
Fujikawa K, Suketa Y, Hayashi K, Suzuki T (1965) Chemical structure of circulin A. Experientia 21:307–308
Fujita M, Kobayashi Y (1985) Cloning of sporulation gene spoIVC in Bacillus subtilis. Mol Gen Genet 199:471–475
Fujita M, Sadaie Y (1998) Feedback loops involving Spo0A and AbrB in in vitro transcription of the genes involved in the initiation of sporulation in Bacillus subtilis. J Biochem 124:98–104
Fukuoka T, Moriya S, Yoshikawa H, Ogasawara N (1990) Purification and characterization of an initiation protein for chromosomal replication, DnaA, in Bacillus subtilis. J Biochem 107:732–739
Gao CH, Tian XP, Qi SH, Luo XM, Wang P, Zhang S (2010) Antibacterial and antilarval compounds from marine gorgonian-associated bacterium Bacillus amyloliquefaciens SCSIO 00856. J Antibiot 63:191–193
Garson W, McLeod C, Tetrault PA, Koffler H, Peterson DH, Colingsworth DR (1949) On the naming of two antibiotics from members of the Bacillus circulans group: circulin and polypeptin. J Bacteriol 58:115–116
Gastélum G, de la Torre M, Rocha J (2020) Rap Protein Paralogs of Bacillus thuringiensis: a multifunctional and redundant regulatory repertoire for the control of collective functions. J Bacteriol 202:e00747–e00719
Gerard J, Haden P, Kelly MT, Andersen RJ (1996) Loloatin B, cyclic decapeptide antibiotic, produced in culture by a tropical marine bacterium. Tetrahedron Lett 37:7201–7294
Gevers W, Kleinkauf H, Lipmann F (1969) Peptidyl transfers in gramicidin S bisoynthesis from enzyme-bound thioester intermediates. Proc Natl Acad Sci U S A 63:1335–1342
Gómez M, Cutting SM (1996) Expression of the Bacillus subtilis spoIVB gene is under dual sigma F/sigma G control. Microbiology 142:3453–3457
Grandchamp GM, Caro L, Shank EA (2017) Pirated siderophores promote sporulation in Bacillus subtilis. Appl Environ Microbiol 83:e03293–e03216
Guillen N, Weinrauch Y, Dubnau DA (1989) Cloning and characterization of the regulatory Bacillus subtilis competence genes comA and comB. J Bacteriol 171:5354–5361
Guillot C, Moran CP (2007) Essential internal promoter in the spoIIIA locus of Bacillus subtilis. J Bacteriol 189:7181–7189
Gutierrez J, Smith R, Pogliano K (2010) SpoIID-mediated peptidoglycan degradation is required throughout engulfment during Bacillus subtilis sporulation. J Bacteriol 192:3174–3186
Guzmán P, Westpheling J, Youngman P (1988) Characterization of the promoter region of the Bacillus subtilis spoIIE operon. J Bacteriol 170:1598–1609
Halder S, Parrell D, Whitten D, Feig M, Kroos L (2017) Interaction of intramembrane metalloprotease SpoIVFB with substrate Pro-σK. Proc Natl Acad Sci U S A 114:E10677–E10686
Hamley IW (2015) Lipopeptides: from self-assembly to bioactivity. Chem Commun 41:8574–8583
Hannay CL, Fitz-James P (1955) The protein crystals of Bacillus thuringiensis Berliner. Can J Microbiol 1:694–710
Hao K, He P, Blom J, Rueckert C, Mao Z, Wu Y, He Y, Borriss R (2012) The genome of plant growth-promoting Bacillus amyloliquefaciens subsp. plantarum strain YAU B9601-Y2 contains a gene cluster for mersacidin synthesis. J Bacteriol 194:3264–3265
Hayashi K, Kensuke T, Kobayashi K, Ogasawara N, Ogura M (2000) Bacillus subtilis RghR (YvaN) represses rapG and rapH, which encode inhibitors of expression of the srfA operon. Mol Microbiol 59:1714–1729
Henriques AO, Beall BW, Roland K, Moran CP Jr (1995) Characterization of cotJ, a sigma E-controlled operon affecting the polypeptide composition of the coat of Bacillus subtilis spores. J Bacteriol 177:3394–3406
Howell SF (1950) Polypeptin, an antibiotic from a member of the Bacillus circulans group. II. Purification, crystallization, and properties of polypeptin. J Biol Chem 186:863–877
Howell SF, Tauber H (1948) Subtenolin; an antibiotic from Bacillus subtilis; isolation and chemical properties. Proc Soc Exp Biol Med 67:432–435
Howells JD, Anderson LE, Coffey GL, Senos GD, Underhill MA, Vogler DL, Ehrlich J (1972) Butirosin, a new aminoglycosidic antibiotic complex: bacterial origin and some microbiological studies. Antimicrob Agents Chemother 2:79–83
Hranueli D, Piggot PJ, Mandelstam J (1974) Statistical estimate of the total number of operons specific for Bacillus subtilis sporulation. J Bacteriol 119:684–690
Hutchison EA, Miller DA, Angert ER (2014) Sporulation in bacteria: beyond the standard model. Microbiol Spectr 2(5). https://doi.org/10.1128/microbiolspec.TBS-0013-2012
Illing N, Errington J (1990) The spoIIIA locus is not a major determinant of prespore-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol 172:6930–6936
Illing N, Errington J (1991) The spoIIIA operon of Bacillus subtilis defines a new temporal class of mother-cell-specific sporulation genes under the control of the sigma E form of RNA polymerase. Mol Microbiol 5:1927–1940
Imamura D, Zhou R, Feig M, Kroos L (2008) Evidence that the Bacillus subtilis SpoIIGA protein is a novel type of signal-transducing aspartic protease. J Biol Chem 283:15287–15299
Imamura D, Kuwana R, Takamatsu H, Watabe K (2010) Localization of proteins to different layers and regions of Bacillus subtilis spore coats. J Bacteriol 192:518–524
Ionesco H, Michel J, Cami B, Schaeffer P (1970) Symposium on bacterial spores: II. Genetics of sporulation in Bacillus subtilis Marburg. J Appl Bacteriol 33:13–24
Ireton K, Gunther NW 4th, Grossman AD (1994) spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J Bacteriol 176:5320–5329
Ishikawa S, Yamane K, Sekiguchi J (1998) Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J Bacteriol 180:1375–1380
Ito M, Koyama Y (1972) Localization of jolipeptin and colistin in their producing strain, Bacillus polymyxa var. colistinus Koyama. J Antibiot (Tokyo) 25:147–148
Ito D, Kawamura H, Oikawa A, Ihara Y, Shibata T, Nakamura N, Asano T, Kawabata S-I, Suzuki T, Masuda S (2020) ppGpp functions as an alarmone in metazoan. Commun Biol 3:671
Jacques DA, Langley DB, Hynson RM, Whitten AE, Kwan A, Guss JM, Trewhella J (2011a) A novel structure of an antikinase and its inhibitor. J Mol Biol 405:214–226
Jacques DA, Langley DB, Kuramitsu S, Yokoyama S, Trewhella J, Guss JM (2011b) The structure of TTHA0988 from Thermus thermophilus, a KipI-KipA homologue incorrectly annotated as an allophanate hydrolase. Acta Crystallogr D Biol Crystallogr 67:105–111
James MN, Watson KJ (1966) Chemistry of micrococcin P. IX. The crystal and molecular structure of micrococcinic acid bis-4-bromoanilide. J Chem Soc Perkin 1 16:1361–1371
Jansen EF, Hirschmann DJ (1944) Subtilin-an antibacterial product of Bacillus subtilis. Culturing conditions and properties. Arch Biochem 4:297–309
Jenkinson HF, Kay D, Mandelstam J (1980) Temporal dissociation of late events in Bacillus subtilis sporulation from expression of genes that determine them. J Bacteriol 141:793–805
Jeong SY, Ishida K, Ito Y, Okada S, Murakami M (2003) Bacillamide, a novel algicide from the marine bacterium, Bacillus sp. SY-1, against the harmful dinoflagellate, Cochlodinium polykrikoides. Tetrahedron Lett 44:8005–8007
Jiang M, Grau R, Perego M (2000a) Differential processing of propeptide inhibitors of Rap phosphatases in Bacillus subtilis. J Bacteriol 182:303–310
Jiang M, Shao W, Perego M, Hoch JA (2000b) Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol Microbiol 38:535–542
Johnson EA, Burdon KL (1946) Eumycin, a new antibiotic active against pathogenic fungi and higher bacteria, including bacilli of tuberculosis and diphtheria. J Bacteriol 51:591
Johnson BA, Anker H, Meleney FL (1945) Bacitracin: a new antibiotic produced by a member of the B. subtilis group. Science 102:376–377
Johnson CW, West HD, Jones HL, Long CJ (1949) Biocerin: an antibiotic produced by Bacillus cereus. J Bacteriol 57:63–65
Johnson EA, Villa TG, Lewis MJ, Phaff HJ (1978) Simple method for the isolation of astaxanthin from the basidiomycetous yeast Phaffia rhodozyma. Appl Environ Microbiol 35:1155–1159
Jonas RM, Weaver EA, Kenney TJ, Moran CP Jr, Haldenwang WG (1988) The Bacillus subtilis spoIIG operon encodes both sigma E and a gene necessary for sigma E activation. J Bacteriol 170:507–511
Kai M (2020) Diversity and distribution of volatile secondary metabolites throughout Bacillus subtilis isolates. Front Microbiol 11:559
Kalenova LF, Kolyvanova SS, Bazhin AS, Besedin IM, Mel’nikov VP (2017) Effects of secondary metabolites of permafrost Bacillus sp. on cytokine synthesis by human peripheral blood mononuclear cells. Bull Exp Biol Med 163:235–238
Katz E, Demain AL (1977) The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriol Rev 41:449–474
Kevany BM, Rasko DA, Thomas MG (2009) Characterization of the complete zwittermicin A biosynthesis gene cluster from Bacillus cereus. Appl Environ Microbiol 75:1144–1155
Kim EY, Tyndall ER, Huang KC, Tian F, Ramamurthi KS (2017) Dash-and-recruit mechanism drives membrane curvature recognition by the small bacterial protein SpoVM. Cell Syst 5:518–526.e3
Knight C, Bowman MJ, Frederick L, Day A, Lee C, Dunlap CA (2018) The first report of antifungal lipopeptide production by a Bacillus subtilis subsp. inaquosorum strain. Microbiol Res 216:40–46
Kobayashi K, Shoji K, Shimizu T, Nakano K, Sato T, Kobayashi Y (1995) Analysis of a suppressor mutation ssb (kinC) of sur0B20 (spo0A) mutation in Bacillus subtilis reveals that kinC encodes a histidine protein kinase. J Bacteriol 177:176–182
Koch MS, Ward JM, Levine SL, Baum JA, Vicini JL, Hammond BG (2015) The food and environmental safety of Bt crops. Front Plant Sci 6:283
Kodama T, Takamatsu H, Asai K, Kobayashi K, Ogasawara N, Watabe K (1999) The Bacillus subtilis yaaH gene is transcribed by SigE RNA polymerase during sporulation, and its product is involved in germination of spores. J Bacteriol 181:4584–4591
Koyama Y, Kurosasa A, Tsuchiya A, Takakuta K (1950) A new antibiotic ‘colistin’ produced by spore-forming soil bacteria. J Antibiot (Tokyo) 3:457–458
Krueger WB, Kolodziej BJ (1976) Measurement of cellular copper levels in Bacillus megaterium during exponential growth and sporulation. Microbios 17:141–147
Kudoh J, Ikeuchi T, Kurahashi K (1984) Identification of the sporulation gene spoOA product of Bacillus subtilis. Biochem Biophys Res Commun 122:1104–1109
Kudoh J, Ikeuchi T, Kurahashi K (1985) Nucleotide sequences of the sporulation gene spo0A and its mutant genes of Bacillus subtilis. Proc Natl Acad Sci U S A 82:2665–2668
Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessières P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Caldwell B, Capuano V, Carter NM, Choi SK, Cordani JJ, Connerton IF, Cummings NJ, Daniel RA, Denziot F, Devine KM, Düsterhöft A, Ehrlich SD, Emmerson PT, Entian KD, Errington J, Fabret C, Ferrari E, Foulger D, Fritz C, Fujita M, Fujita Y, Fuma S, Galizzi A, Galleron N, Ghim SY, Glaser P, Goffeau A, Golightly EJ, Grandi G, Guiseppi G, Guy BJ, Haga K, Haiech J, Harwood CR, Hènaut A, Hilbert H, Holsappel S, Hosono S, Hullo MF, Itaya M, Jones L, Joris B, Karamata D, Kasahara Y, Klaerr-Blanchard M, Klein C, Kobayashi Y, Koetter P, Koningstein G, Krogh S, Kumano M, Kurita K, Lapidus A, Lardinois S, Lauber J, Lazarevic V, Lee SM, Levine A, Liu H, Masuda S, Mauël C, Médigue C, Medina N, Mellado RP, Mizuno M, Moestl D, Nakai S, Noback M, Noone D, O’Reilly M, Ogawa K, Ogiwara A, Oudega B, Park SH, Parro V, Pohl TM, Portelle D, Porwollik S, Prescott AM, Presecan E, Pujic P, Purnelle B, Rapoport G, Rey M, Reynolds S, Rieger M, Rivolta C, Rocha E, Roche B, Rose M, Sadaie Y, Sato T, Scanlan E, Schleich S, Schroeter R, Scoffone F, Sekiguchi J, Sekowska A, Seror SJ, Serror P, Shin BS, Soldo B, Sorokin A, Tacconi E, Takagi T, Takahashi H, Takemaru K, Takeuchi M, Tamakoshi A, Tanaka T, Terpstra P, Togoni A, Tosato V, Uchiyama S, Vandebol M, Vannier F, Vassarotti A, Viari A, Wambutt R, Wedler H, Weitzenegger T, Winters P, Wipat A, Yamamoto H, Yamane K, Yasumoto K, Yata K, Yoshida K, Yoshikawa HF, Zumstein E, Yoshikawa H, Danchin A (1997) The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249–256
Kurylo-Borowska Z (1959) Isolation and properties of pure edeine, an antibiotic of the strain Bacillus brevis Vm4. Biul Inst Med Morsk Gdansk 10:151–163
Kuwana R, Yamamura S, Ikejiri H, Kobayashi K, Ogasawara N, Asai K, Sadaie Y, Takamatsu H, Watabe K (2003) Bacillus subtilis spoVIF (yjcC) gene, involved in coat assembly and spore resistance. Microbiology 149:3011–3021
Kuwana R, Takamatsu H, Watabe K (2007) Expression, localization and modification of YxeE spore coat protein in Bacillus subtilis. J Biochem 142:681–689
Lamanna C, McElroy OE, Eklund HW (1946) The purification and crystallization of Clostridium botulinum type A toxin. Science 103:613–614
Lambert EA, Popham DL (2008) The Bacillus anthracis SleL (YaaH) protein is an N-acetylglucosaminidase involved in spore cortex depolymerization. J Bacteriol 190:7601–7607
Lautenschläger N, Popp PF, Mascher T (2020) Development of a novel heterologous β-lactam-specific whole-cell biosensor in Bacillus subtilis. J Biol Eng 14:21
Lecadet M, Martouret D (1965) The enzymic hydrolysis of Bacillus thuringiensis Berliner crystals, and the liberation of toxic fractions of bacterial origin by the chyle of Pieris brassicae (Linnaeus). J Invertebr Pathol 20:105–108
LeDeaux J, Grossman AD (1995) Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to the sporulation sensor kinases KinA and KinB in Bacillus subtilis. J Bacteriol 177:166–175
Lee JE, Kye Y-C, Park S-M, Shim B-S, Yoo S, Hwang E, Kim H, Kim S-J, Han SH, Park TS, Park BC, Yun C-H (2020) Bacillus subtilis spores as adjuvants against avian influenza H9N2 induce antigen-specific antibody and T cell responses in White Leghorn chickens. Vet Res 51:68
Lehtinen T, Virtanen H, Santala S, Santala V (2018) Production of alkanes from CO2 by engineered bacteria. Biotechnol Biofuels 11:228
Levin PA, Fan N, Ricca E, Driks A, Losick R, Cutting S (1993) An unusually small gene required for sporulation by Bacillus subtilis. Mol Microbiol 9:761–771
Lewis GM, Hopper ME, Shultz S (1946) In vitro fungistasis by a Bacterium (Bacillus subtilis var. XG and XY). Arch Derm Syphilol 54:300–307
Lewis RJ, Brannigan JA, Smith I, Wilkinson AJ (1996) Crystallisation of the Bacillus subtilis sporulation inhibitor SinR, complexed with its antagonist, SinI. FEBS Lett 378:98–100
Li Y, Davis A, Korza G, Zhang P, Li YQ, Setlow B, Setlow P, Hao B (2012) Role of a SpoVA protein in dipicolinic acid uptake into developing spores of Bacillus subtilis. J Bacteriol 194:1875–1884
Lin GH, Chen CL, Tschen JS, Tsay SS, Chang YS, Liu ST (1998) Molecular cloning and characterization of fengycin synthetase gene fenB from Bacillus subtilis. J Bacteriol 180:1338–1341
Lindenmann J (2005) Women scientists in typhus research during the first half of the twentieth century. Gesnerus 62:257–272
Linn TG, Greenleaf AL, Shorenstein RG, Losick R (1973) Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci U S A 70:1865–1869
Lipmann F (1973) Nonribosomal polypeptide synthesis on polyenzyme templates. Acc Chem Res 6:361–367
Liu Z, Xie J, Deng Z, , Wang M, Dang D, Luo S, Wang Y, Sun Y, Xia L, Ding X (2020) Enhancing the insecticidal activity of new Bacillus thuringiensis X023 by copper ions. Microb Cell Fact 19:195
Louie P, Lee A, Stansmore K, Grant R, Ginther C, Leighton T (1992) Roles of rpoD, spoIIF, spoIIJ, spoIIN, and sin in regulation of Bacillus subtilis stage II sporulation-specific transcription. J Bacteriol 174:3570–3576
Lu S, Cutting S, Kroos L (1995) Sporulation protein SpoIVFB from Bacillus subtilis enhances processing of the sigma factor precursor Pro-sigma K in the absence of other sporulation gene products. J Bacteriol 177:1082–1085
Lukin AA, Planutene MV, Rozov AN (1983) Role of the ribosomes in controlling cell differentiation and secondary metabolism in sporulating bacteria. II. The suppression of the phenotypic expression of ribosomal mutations (strA) as affected by RNA-polymerase mutations (rfm) in Bacillus subtilis. Genetika 19:737–743
Ma Y, Kong Q, Qin C, Chen Y, Chen Y, Lv R, Zhou G (2016) Identification of lipopeptides in Bacillus megaterium by two-step ultrafiltration and LC-ESI-MS/MS. AMB Express 6:79
Majumdar SK, Bose SK (1958) Mycobacillin, a new antifungal antibiotic produced by B. subtilis. Nature 181:134–135
Mandic-Mulec I, Doukhan L, Smith I (1995) The Bacillus subtilis SinR protein is a repressor of the key sporulation gene spo0A. J Bacteriol 177:4619–4627
Manwaring WH (1940) Dubos’ “Gramicidin”. Cal West Med 53:256–257
Marques LH, Santos AC, Castro BA, Moscardini VF, Rosseto J, Silva OABN, Babcock JM (2019) Assessing the efficacy of Bacillus thuringiensis (Bt) pyramided proteins Cry1F, Cry1A.105, Cry2Ab2, and Vip3Aa20 expressed in Bt maize against lepidopteran pests in Brazil. J Econ Entomol 112:803–811
Martin NI, Hu H, Moake MM, Churey JJ, Whittal R, Worobo RW, Vederas JC (2003) Isolation, structural characterization, and properties of mattacin (polymyxin M), a cyclic peptide antibiotic produced by Paenibacillus kobensis M. J Biol Chem 278:13124–13132
Mastny M, Heuck A, Kurzbauer R, Heiduk A, Boisguerin P, Volkmer R, Ehrmann M, Rodrigues CD, Rudner DZ, Clausen T (2013) CtpB assembles a gated protease tunnel regulating cell-cell signaling during spore formation in Bacillus subtilis. Cell 155:647–658
Matsuno K, Sonenshein AL (1999) Role of SpoVG in asymmetric septation in Bacillus subtilis. J Bacteriol 181:3392–3401
McKenney PT, Driks A, Eichenberger P (2013) The Bacillus subtilis endospore: assembly and functions of the multilayered coat. Nat Rev Microbiol 11:33–44
McQuade RS, Comella N, Grossman AD (2001) Control of a family of phosphatase regulatory genes (phr) by the alternate sigma factor sigma-H of Bacillus subtilis. J Bacteriol 183:4905–4909
Meeske AJ, Sham LT, Kimsey H, Koo BM, Gross CA, Bernhardt TG, Rudner DZ (2015) MurJ and a novel lipid II flippase are required for cell wall biogenesis in Bacillus subtilis. Proc Natl Acad Sci U S A 112:6437–6442
Meeske AJ, Rodrigues CD, Brady J, Lim HC, Bernhardt TG, Rudner DZ (2016) High-throughput genetic screens identify a large and diverse collection of new sporulation genes in Bacillus subtilis. PLoS Biol 14:e1002341
Menez J, Buckingham RH, de Zamaroczy M, Campelli CK (2002) Peptidyl-tRNA hydrolase in Bacillus subtilis, encoded by spoVC, is essential to vegetative growth, whereas the homologous enzyme in Saccharomyces cerevisiae is dispensable. Mol Microbiol 45:123–129
Meng X, Karasawa T, Zou K, Kuang X, Wang X, Lu C, Wang C, Yamakawa K, Nakamura S (1997) Characterization of a neurotoxigenic Clostridium butyricum strain isolated from the food implicated in an outbreak of food-borne type E botulism. J Clin Microbiol 35:2160–2162
Milner RJ (1994) History of Bacillus thuringiensis. Agric Ecosyst Environ 49:9–13
Mirouze N, Parashar V, Baker MD, Dubnau DA, Neiditch MB (2011) An atypical Phr peptide regulates the developmental switch protein RapH. J Bacteriol 193:6197–6206
Miteva V (1979) Plasmids and crystal formation in Bacillus thuringiensis. Acta Microbiol Bulg 5:36–41
Molle V, Fujita M, Jensen ST, Eichenberger P, González-Pastor JE, Liu JS, Losick R (2003) The Spo0A regulon of Bacillus subtilis. Mol Microbiol 50:1683–1701
Mondol MA, Shin HJ, Islam MT (2013) Diversity of secondary metabolites from marine Bacillus species: chemistry and biological activity. Mar Drugs 11:2846–2872
Mountford SJ, Mohanty B, Roberts KD, Yu HH, Scanlon MJ, Nation RL, Velkov T, Li J, Thompson PE (2017) The first total synthesis and solution structure of a polypeptin, PE2, a cyclic lipopeptide with broad spectrum antibiotic activity. Org Biomol Chem 15:7173–7180
Muchová K, Chromiková Z, Bradshaw N, Wilkinson AJ, Barák I (2016) Morphogenic protein RodZ interacts with sporulation specific SpoIIE in Bacillus subtilis. PLoS One 11:e0159076
Muchová K, Chromiková Z, Barák I (2020) Linking the Peptidoglycan Synthesis Protein Complex with Asymmetric Cell Division during Bacillus subtilis Sporulation. Int J Mol Sci 21:4513
Müller S, Strack SN, Hoefler BC, Straight PD, Kearns DB, Kirby JR (2014) Bacillaene and sporulation protect Bacillus subtilis from predation by Myxococcus xanthus. Appl Environ Microbiol 80:5603–5610
Mysliwiec TH, Errington J, Vaidya AB, Bramucci MG (1991) The Bacillus subtilis spo0J gene: evidence for involvement in catabolite repression of sporulation. J Bacteriol 173:1911–1919
Nai C, Meyer V (2018) From axenic to mixed cultures: technological advances accelerating a paradigm shift in microbiology. Trends Microbiol 26:538–554
Najafi SM, Willis AC, Yudkin MD (1995) Site of phosphorylation of SpoIIAA, the anti-anti-sigma factor for sporulation-specific sigma F of Bacillus subtilis. J Bacteriol 177:2912–2913
Nakajima N, Chihara S, Koyama Y (1972) A new antibiotic, gatavalin. I. Isolation and characterization. J Antibiot (Tokyo) 25:24324–24327
Nakamura LK, Roberts MS, Cohan FM (1999) Relationship of Bacillus subtilis clades associated with strains 168 and W23: a proposal for Bacillus subtilis subsp. subtilis subsp. nov. and Bacillus subtilis subsp. spizizenii subsp. nov. Int J Syst Bacteriol 49:1211–1215
Nawrot-Esposito MP, Babin A, Pasco M, Poirié M, Gatti J-L, Gallet A (2020) Bacillus thuringiensis bioinsecticides induce developmental defects in non-target Drosophila melanogaster larvae. Insects 11:697
Newton GG (1949) Antibiotics from a strain of B. subtilis; bacilipin A and B and bacilysin. Br J Exp Pathol 30:306–319
Nolan EM, Walsh CT (2009) How nature morphs peptide scaffolds into antibiotics. ChemBioChem 10:34–53
Nunes F, Fernandes C, Freitas C, Marini E, Serrano M, Moran CP Jr, Eichenberger P, Henriques AO (2018) SpoVID functions as a non-competitive hub that connects the modules for assembly of the inner and outer spore coat layers in Bacillus subtilis. Mol Microbiol 110:576–595
Ochi K, Ohsawa S (1984) Initiation of antibiotic production by the stringent response of Bacillus subtilis Marburg. J Gen Microbiol 130:2473–2482
Oh Y, Kim JA, Kim C-H, Cho S-K, Pan J-G (2020) Bacillus subtilis spore vaccines displaying protective antigen induce functional antibodies and protective potency. BMC Vet Res 16:259
Ohlsen KL, Grimsley JK, Hoch JA (1994) Deactivation of the sporulation transcription factor Spo0A by the Spo0E protein phosphatase. Proc Natl Acad Sci USA 91:1756–1760
Ongey EL, Giessmann RT, Fons M, Rappsilber J, Adrian L, Neubauer P (2018) Heterologous biosynthesis, modifications and structural characterization of Ruminococcin-A, a lanthipeptide from the gut bacterium Ruminococcus gnavus E1, in Escherichia coli. Front Microbiol 9:1688
Othoum G, Bougouffa S, Razali R, Bokhari A, Alamoudi S, Antunes A, Gao X, Hoehndorf R, Arold ST, Gojobori T, Hirt H, Mijakovic I, Bajic VB, Lafi FF, Essack M (2018) In silico exploration of Red Sea Bacillus genomes for natural product biosynthetic gene clusters. BMC Genomics 19(1):382
Özcengiz G, Öğülür İ (2015) Biochemistry, genetics and regulation of bacilysin biosynthesis and its significance more than an antibiotic. N Biotechnol 32:612–619
Ozin AJ, Henriques AO, Yi H, Moran CP (2000) Morphogenetic proteins SpoVID and SafA form a complex during assembly of the Bacillus subtilis spore coat. J Bacteriol 182:1828–1833
Ozin AJ, Samford CS, Henriques AO, Moran CP (2001) SpoVID guides SafA to the spore coat in Bacillus subtilis. J Bacteriol 183:3041–3049
Parashar V, Mirouze N, Dubnau DA, Neiditch MB (2011) Structural basis of response regulator dephosphorylation by Rap phosphatases. PLoS Biol 9:e1000589
Patel GB, Roth LA (1978) Acetic acid and hydrogen metabolism during coculture of an acetic acid producing bacterium with methanogenic bacteria. Can J Microbiol 24:1007–1010
Patel PS, Huang S, Fisher S, Pirnik D, Aklonis C, Dean L, Meyers E, Fernandes P, Mayerl F (1995) Bacillaene, a novel inhibitor of procaryotic protein synthesis produced by Bacillus subtilis: production, taxonomy, isolation, physico-chemical characterization and biological activity. J Antibiot (Tokyo) 48:997–1003
Pazos M, Peters K, Boes A, Safaei Y, Kenward C, Caveney NA, Laguri C, Breukink E, Strynadka NCJ, Simorre J-P, Terrak M, Vollme W (2020) SPOR Proteins are required for functionality of class A penicillin-binding proteins in Escherichia coli. mBio 11:e02796-20. https://doi.org/10.1128/mBio.02796-20
Peddie CJ, Cook GM, Morgan HW (1999) Sodium-dependent glutamate uptake by an alkaliphilic, thermophilic Bacillus strain, TA2.A1. J Bacteriol 181:3172–3177
Perego M (2001) A new family of aspartyl phosphate phosphatases targeting the sporulation transcription factor Spo0A of Bacillus subtilis. Mol Microbiol 42:133–143
Perego M, Hoch JA (1987) Isolation and sequence of the spo0E gene: its role in initiation of sporulation in Bacillus subtilis. Mol Microbiol 1:125–132
Perego M, Hoch JA (1988) Sequence analysis and regulation of the hpr locus, a regulatory gene for protease production and sporulation in Bacillus subtilis. J Bacteriol 170:2560–2567
Perego M, Spiegelman GB, Hoch JA (1988) Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol Microbiol 2:689–699
Perego M, Cole SP, Burbulys D, Trach K, Hoch JA (1989) Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. J Bacteriol 171:6187–6196
Perego M, Glaser P, Hoch JA (1996) Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis. Mol Microbiol 19:1151–1157
Peters HK 3rd, Haldenwang WG (1994) Isolation of a Bacillus subtilis spoIIGA allele that suppresses processing-negative mutations in the Pro-sigma E gene (sigE). J Bacteriol 176:7763–7766
Pettit GR, Knight JC, Herald DL, Pettit RK, Hogan F, Mukku VJRV, Hamblin JS, Dodson M, Chapuis JC (2009) Antineoplastic agents. 570. Isolation and structure elucidation of bacillistatins 1 and 2 from a marine Bacillus silvestris. J Nat Prod 72:366–371
Peypoux F, Besson F, Michel G, Delcambe L (1981) Structure of bacillomycin D, a new antibiotic of the iturin group. Eur J Biochem 118:323–327
Piggot PJ (1973) Mapping of asporogenous mutations of Bacillus subtilis: a minimum estimate of the number of sporulation operons. J Bacteriol 114:1241–1253
Piggot PJ, Coote JG (1976) Genetic aspects of bacterial endospore formation. Bacteriol Rev 40:908–962
Piggot PJ, Hoch JA (1985) Revised genetic linkage map of Bacillus subtilis. Microbiol Rev 49:158–179
Popham DL (2002) Specialized peptidoglycan of the bacterial endospore: the inner wall of the lockbox. Cell Mol Life Sci 59:426–433
Popham DL, Stragier P (1991) Cloning, characterization, and expression of the spoVB gene of Bacillus subtilis. J Bacteriol 173:7942–7949
Präve P, Sukatsch D, Vértesy L (1972) Proticin, a new phosphorus-containing antibiotic. I. Taxonomy, fermentation, isolation, and biological properties. J Antibiot (Tokyo) 25:1–3
Quan M, Peng J, Zhu Z, Zhou P, Luo S, Xie J, Xia L, Sun Y, Ding X (2020) Construction of a conditionally asporogenous Bacillus thuringiensis recombinant strain overproducing cry protein by deletion of the leuB gene. Front Microbiol 11:1769
Rabbee MF, Baek K-H (2020) Antimicrobial activities of lipopeptides and polyketides of Bacillus velezensis for agricultural applications. Molecules 25:4973
Ragkousi K, Eichenberger P, van Ooij C, Setlow P (2003) Identification of a new gene essential for germination of Bacillus subtilis spores with Ca2+-dipicolinate. J Bacteriol 185:2315–2329
Ramamurthi KS, Clapham KR, Losick R (2006) Peptide anchoring spore coat assembly to the outer forespore membrane in Bacillus subtilis. Mol Microbiol 62:1547–1557
Ramírez-Guadiana FH, Meeske AJ, Rodrigues CDA, Barajas-Ornelas RDC, Kruse AC, Rudner DZ (2017) A two-step transport pathway allows the mother cell to nurture the developing spore in Bacillus subtilis. PLoS Genet 13:e1007015
Ramirez-Peralta A, Stewart KA, Thomas SK, Setlow B, Chen Z, Li YQ, Setlow P (2012) Effects of the SpoVT regulatory protein on the germination and germination protein levels of spores of Bacillus subtilis. J Bacteriol 194:3417–3425
Rea MC, Sit CS, Clayton E, O’Connor PM, Whittal RM, Zheng J, Vederas JC, Ross RP, Hill C (2010) Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci U S A 107:9352–9357
Resnekov O, Driks A, Losick R (1995) Identification and characterization of sporulation gene spoVS from Bacillus subtilis. J Bacteriol 177:5628–5635
Ricca E, Cutting S, Losick R (1992) Characterization of bofA, a gene involved in intercompartmental regulation of pro-sigma K processing during sporulation in Bacillus subtilis. J Bacteriol 174:3177–3184
Rigden DJ, Galperin MY (2008) Sequence analysis of GerM and SpoVS, uncharacterized bacterial ‘sporulation’ proteins with widespread phylogenetic distribution. Bioinformatics 24:1793–1797
Roels S, Driks A, Losick R (1992) Characterization of spoIVA, a sporulation gene involved in coat morphogenesis in Bacillus subtilis. J Bacteriol 174:575–585
Rogers MJ, Cundliffe E, McCutchan TF (1998) The antibiotic micrococcin is a potent inhibitor of growth and protein synthesis in the malaria parasite. Antimicrob Agents Chemother 42:715–716
Rooney AP, Price NP, Ehrhardt C, Swezey JL, Bannan JD (2009) Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. inaquosorum subsp. nov. Int. J Syst Evol Microbiol 59:2429–2436
Rosenthal KS, Ferguson RA, Storm DR (1977) Mechanism of action of EM 49, membrane-active peptide antibiotic. Antimicrob Agents Chemother 12:665–672
Rowland SL, Burkholder WF, Cunningham KA, Maciejewski MW, Grossman AD, King GF (2004) Structure and mechanism of action of Sda, an inhibitor of the histidine kinases that regulate initiation of sporulation in Bacillus subtilis. Mol Cell 13:689–701
Rudner DZ, LeDeaux JR, Ireton K, Grossman AD (1991) The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J Bacteriol 173:1388–1398
Ruiz-García C, Béjar V, Martínez-Checa F, Llamas I, Quesada E (2005) Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Vélez in Málaga, southern Spain. Int J Syst Evol Microbiol 55:191–195
Ryter A (1965) Etude morphologique de la sporulation de Bacillus subtilis. Ann Inst Pasteur (Paris) 108:40–60
Sadaie Y, Kada T (1983) Formation of competent Bacillus subtilis cells. J Bacteriol 153:813–821
Sato T, Samori Y, Kobayashi Y (1990) The cisA cistron of Bacillus subtilis sporulation gene spoIVC encodes a protein homologous to a site-specific recombinase. J Bacteriol 172:1092–1098
Saxena D, Stotzky G (2000) Bacillus thuringiensis (Bt) toxin released from root exudates and biomass of Bt corn has no apparent effect on earthworms, nematodes, protozoa, bacteria, and fungi in soil. Soil Biol Biochem 33:1225–1230
Schaeffer P, Millet J, Aubert JP (1965) Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A 54:704–711
Schäfer H, Beckert B, Frese CK, Steinchen W, Nuss AM, Beckstette M, Hantke I, Driller K, Sudzinová P, Krásný L, Kaever V, Dersch P, Bange G, Wilson DN, Turgay K (2020) The alarmones (p)ppGpp are part of the heat shock response of Bacillus subtilis. PLoS Genet 16:e1008275
Schmidt R, Margolis P, Duncan L, Coppolecchia R, Moran CP Jr, Losick R (1990) Control of developmental transcription factor sigma F by sporulation regulatory proteins SpoIIAA and SpoIIAB in Bacillus subtilis. Proc Natl Acad Sci U S A 87:9221–9225
Schnepf HE, Whiteley HR (1981) Cloning and expression of the Bacillus thuringiensis crystal protein gene in Escherichia coli. Proc Natl Acad Sci U S A 78:2893–2897
Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62:775–806
Schyns G, Buckner CM, Moran CP Jr (1997) Activation of the Bacillus subtilis spoIIG promoter requires interaction of Spo0A and the sigma subunit of RNA polymerase. J Bacteriol 179:5605–5608
Scott AB (1981) Botulinum toxin injection of eye muscles to correct strabismus. Trans Am Ophthalmol Soc 79:734–770
Sermonti G (1980) Primitive nature of secondary metabolism. Riv Biol 73:353–371
Serrano M, Côrte L, Opdyke J, Moran CP Jr, Henriques AO (2003) Expression of spoIIIJ in the prespore is sufficient for activation of sigma G and for sporulation in Bacillus subtilis. J Bacteriol 185:3905–3917
Setlow P (2012) Dynamics of the assembly of a complex macromolecular structure—the coat of spores of the bacterium Bacillus subtilis. Mol Microbiol 83:241–244
Setlow P, Christie G (2020) Bacterial spore mRNA—what’s up with that? Front Microbiol 11:596092
Setlow P, Johnson E (2019) Spores and their significance. In: Doyle M, Diez-Gonzalez F, Hill C (eds) Food microbiology: fundamentals and frontiers, 5th edn. ASM Press, Washington, DC, pp 23–63
Seyler RW Jr, Henriques AO, Ozin AJ, Moran CP Jr (1997) Assembly and interactions of cotJ-encoded proteins, constituents of the inner layers of the Bacillus subtilis spore coat. Mol Microbiol 25:955–566
Shank EA (2013) Using coculture to detect chemically mediated interspecies interactions. J Vis Exp 80:e50863
Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Ogasawara N, Hattori M, Kuhara S, Hayashi H (2002) Complete genome sequence of Clostridium perfringens, an anaerobic flesheater. Proc Natl Acad Sci U S A 99:996–1001
Shimotsu H, Kawamura F, Kobayashi Y, Saito H (1983) Early sporulation gene spo0F: nucleotide sequence and analysis of gene product. Proc Natl Acad Sci U S A 80:658–662
Shoji J, Hinoo H, Wakisaka Y, Koizumi K, Mayama M (1975) Isolation of two new related peptide antibiotics, cerexins A and B (studies on antibiotics from the genus Bacillus. I). J Antibiot (Tokyo) 28:56–59
Shoji J, Sakazaki R, Wakisaka Y, Koizumi K, Mayama M (1976a) Isolation of brevistin, a new peptide antibiotic. Studies on antibiotics from the genus Bacillus. IX. J Antibiot (Tokyo) 29:375–379
Shoji J, Sakazaki R, Wakisaka Y, Koizumi K, Mayama M (1976b) Isolation of a new antibiotic, laterosporamine. Studies on antibiotics from the genus Bacillus. XIII. J Antibiot (Tokyo) 29:390–393
Shoji J, Kato T, Yoshimura Y, Tori K (1981) Structural studies on thiocillins I, II and III (studies on antibiotics from the genus Bacillus XXIX). J Antibiot (Tokyo) 34:1126–1136
Shuster B, Khemmani M, Abe K, Huang X, Nakaya Y, Maryn N, Buttar S, Gonzalez AN, Driks A, Sato T, Eichenberger P (2019) Contributions of crust proteins to spore surface properties in Bacillus subtilis. Mol Microbiol 111:825–843
Siranosian KJ, Ireton K, Grossman AD (1993) Alanine dehydrogenase (ald) is required for normal sporulation in Bacillus subtilis. J Bacteriol 175:6789–6796
Soberón M, López-Díaz JA, Bravo A (2013) Cyt toxins produced by Bacillus thuringiensis: a protein fold conserved in several pathogenic microorganisms. Peptides 41:87–93
Sonenshein AL (2000) Control of sporulation initiation in Bacillus subtilis. Curr Opin Microbiol 3:561–566
Sonenshein AL (2005) CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr Opin Microbiol 8:203–207
Sonoda Y, Mizutani K, Mikami B (2015) Structure of Spo0M, a sporulation-control protein from Bacillus subtilis. Acta Crystallogr F Struct Biol Commun 71:1488–1497
Špacapan M, Danevčič T, Štefanic P, Porter M, Stanley-Wall NR, Mandic-Mulec I (2020) The ComX quorum sensing peptide of Bacillus subtilis affects biofilm formation negatively and sporulation positively. Microorganisms 8:1131
Stansly PG, Schlosser ME (1947) Studies on polymyxin: isolation and identification of Bacillus polymyxa and differentiation of polymyxin from certain known antibiotics. J Bacteriol 54:549–556
Stephenson S, Mueller C, Jiang M, Perego M (2003) Molecular analysis of Phr peptide processing in Bacillus subtilis. J Bacteriol 185:4861–4871
Sterlini JM, Mandelstam J (1969) Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J 113:29–37
Stevens CM, Daniel R, Illing N, Errington J (1992) Characterization of a sporulation gene, spoIVA, involved in spore coat morphogenesis in Bacillus subtilis. J Bacteriol 174:586–594
Stragier P, Losick R (1996) Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet 30:297–341
Strauch MA, Aronson AI, Brown W, Schreier H, Sonenshein AL (1988) Sequence of the Bacillus subtilis glutamine synthetase gene region. Gene 71:257–265
Strauch M, Webb V, Spiegelman G, Hoch JA (1990) The SpoOA protein of Bacillus subtilis is a repressor of the abrB gene. Proc Natl Acad Sci U S A 87:1801–1805
Su Z, Chen X, Liu X, Guo Q, Li S, Lu X, Zhang X, Wang P, Dong L, Zhao W, Ma P (2020) Genome mining and UHPLC–QTOF–MS/MS to identify the potential antimicrobial compounds and determine the specificity of biosynthetic gene clusters in Bacillus subtilis NCD-2. BMC Genomics 21:767. https://doi.org/10.1186/s12864-020-07160-2
Sun LC, Chen R, Fu C, Chen Y, Wu Q, Chen R, Lin X, Luo S (2019) Efficacy and safety of botulinum toxin type A for limb spasticity after stroke: a meta-analysis of randomized controlled trials. Biomed Res Int. https://doi.org/10.1155/2019/8329306
Sun R, Zhang M, Chen H, Wei Y, Ning D (2020) Germination-arrest Bacillus subtilis spores as an oral delivery vehicle of grass carp reovirus (GCRV) Vp7 antigen augment protective immunity in grass carp (Ctenopharyngodon idella). Genes (Basel) 11:1351. https://doi.org/10.3390/genes11111351
Takahashi F, Sumitomo N, Hagihara H, Ozaki K (2015) Increased dipicolinic acid production with an enhanced spoVF operon in Bacillus subtilis and medium optimization. Biosci Biotechnol Biochem 79:505–511
Takamatsu H, Chikahiro Y, Kodama T, Koide H, Kozuka S, Tochikubo K, Watabe K (1998) A spore coat protein, CotS, of Bacillus subtilis is synthesized under the regulation of sigmaK and GerE during development and is located in the inner coat layer of spores. J Bacteriol 180:2968–2974
Takamatsu H, Kodama T, Imamura A, Asai K, Kobayashi K, Nakayama T, Ogasawara N, Watabe K (2000) The Bacillus subtilis yabG gene is transcribed by SigK RNA polymerase during sporulation, and yabG mutant spores have altered coat protein composition. J Bacteriol 182:1883–1888
Takamatsu H, Imamura D, Kuwana R, Watabe K (2009) Expression of yeeK during Bacillus subtilis sporulation and localization of YeeK to the inner spore coat using fluorescence microscopy. J Bacteriol 191:1220–1229
Tareq FS, Shin HJ (2017) Bacilotetrins A and B, anti-staphylococcal cyclic-lipotetrapeptides from a marine-derived Bacillus subtilis. J Nat Prod 80:2889–2892
Theeragool G, Miyao A, Yamada K, Sato T, Kobayashi Y (1993) In vivo expression of the Bacillus subtilis spoVE gene. J Bacteriol 175:4071–4080
Tinastepe N, Küçük BB, Oral K (2015) Botulinum toxin for the treatment of bruxism. Cranio 33:291–298
Tojo S, Hirooka K, Fujita Y (2013) Expression of kinA and kinB of Bacillus subtilis, necessary for sporulation initiation, is under positive stringent transcription control. J Bacteriol 195:1656–1665
Tovar-Rojo F, Chander M, Setlow B, Setlow P (2002) The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J Bacteriol 184:584–587
Trach KA, Chapman JW, Piggot PJ, Hoch JA (1985) Deduced product of the stage 0 sporulation gene spo0F shares homology with the Spo0A, OmpR, and SfrA proteins. Proc Natl Acad Sci U S A 82:7260–7264
Trempy JE, Bonamy C, Szulmajster J, Haldenwang WG (1985) Bacillus subtilis sigma factor sigma 29 is the product of the sporulation-essential gene spoIIG. Proc Natl Acad Sci U S A 82:4189–4192
Trischman JA, Jensen PR, Fenical W (1994) Halobacillin: a cytotoxic cyclic acylpeptide of the iturin class produced by a marine Bacillus. Tetrahedron Lett 35:5571–5574
Tsuge K, Akiyama T, Shoda M (2001) Cloning, sequencing, and characterization of the iturin A operon. J Bacteriol 183:6265–6273
Tzeng YL, Feher VA, Cavanagh J, Perego M, Hoch JA (1998) Characterization of interactions between a two-component response regulator, Spo0F, and its phosphatase, RapB. Biochemistry 37:16538–16545
Ursino E, Albertini AM, Fiorentino G, Gabrieli P, Scoffone VC, Pellegrini A, Gasperi G, Di Cosimo A, Barbieri G (2020) Bacillus subtilis as a host for mosquitocidal toxins production. Microb Biotechnol 13:1972–1982
Üstok FI, Chirgadze DY, Christie G (2015) Structural and functional analysis of SleL, a peptidoglycan lysin involved in germination of Bacillus spores. Proteins 83:1787–1799
Vahidinasab M, Lilge L, Reinfurt A, Pfannstiel J, Henkel M, Heravi KM, Hausmann R (2020) Construction and description of a constitutive plipastatin mono-producing Bacillus subtilis. Microb Cell Fact 19:205. https://doi.org/10.1186/s12934-020-01468-0
van Ermengem EP (1897) Ueber einen neuen anaëroben Bacillus und seine Beziehungen zum Botulismus. Zeitschrift für Hygiene und Infektionskrankheiten 26:1–56
Van Hoy BE, Hoch JA (1990) Characterization of the spoIVB and recN loci of Bacillus subtilis. J Bacteriol 172:1306–1311
Vanittanakom N, Loeffler W, Koch U, Jung G (1986) Fengycin-a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J Antibiot 39:888–901
Vasudeva RS, Subbaiah TV, Sastry MLN, Rangaswamy G, Iyengar MRS (1958) ‘Bulbiformin’, an antibiotic produced by Bacillus subtilis. Ann Appl Biol 46:336–345
Veening JW, Murray H, Errington J (2009) A mechanism for cell cycle regulation of sporulation initiation in Bacillus subtilis. Genes Dev 23:1959–1970
Vega-Cabrera LA, Wood CD, Pardo-López L (2018) Spo0M: structure and function beyond regulation of sporulation. Curr Genet 64:17–23
Veiga-Crespo P, Ageitos JM, Poza M, Villa TG (2007) Enzybiotics: a look to the future, recalling the past. J Pharm Sci 96:1917–1924
Velázquez E, de Miguel T, Poza M, Rivas R, Rosselló-Mora R, Villa TG (2004) Paenibacillus favisporus sp. nov., a xylanolytic bacterium isolated from cow faeces. Int J Syst Evol Microbiol 54:59–64
Vepachedu VR, Setlow P (2007) Role of SpoVA proteins in release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J Bacteriol 189:1565–1572
Vértesy L (1972) Proticin, a new phosphorus-containing antibiotic. II. Characterization and chemical studies. J Antibiot (Tokyo) 25:4–10
Volpon L, Besson F, Lancelin J-M (2000) NMR structure of antibiotics plipastatins A and B from Bacillus subtilis inhibitors of phospholipase A2. FEBS Lett 485:76–80
Wakeley PR, Dorazi R, Hoa NT, Bowyer JR, Cutting SM (2000) Proteolysis of SpolVB is a critical determinant in signalling of Pro-sigmaK processing in Bacillus subtilis. Mol Microbiol 36:1336–1348
Walker JE, Abraham EP (1970) The structure of bacilysin and other products of Bacillus subtilis. Biochem J 118:563–570
Walton RB, Woodruff HB (1949) A crystalline antifungal agent, mycosubtilin, isolated from subtilin broth. J Clin Invest 28:924–926
Wang L, Grau R, Perego M, Hoch JA (1997) A novel histidine kinase inhibitor regulating development in Bacillus subtilis. Genes Dev 11:2569–2579
Wang L, Fabret C, Kanamaru K, Stephenson K, Dartois V, Perego M, Hoch JA (2001) Dissection of the functional and structural domains of phosphorelay histidine kinase A of Bacillus subtilis. J Bacteriol 183:2795–2802
Wang J, Zhang L, Teng K, Sun S, Sun Z, Zhong J (2014) Cerecidins, novel lantibiotics from Bacillus cereus with potent antimicrobial activity. Appl Environ Microbiol 80:2633–2643
Wang W, Liu R, Shen Y, Lian B (2018) The potential correlation between bacterial sporulation and the characteristic flavor of Chinese Maotai liquor. Front Microbiol 9:1435
Wang C, Zhao D, Qi G, Mao Z, Hu X, Du B, Liu K, Ding Y (2019) Effects of Bacillus velezensis FKM10 for Promoting the Growth of Malus hupehensis Rehd and Inhibiting Fusarium verticillioides. Front Microbiol 10:2889
Wang Y, Zhang C, Liang J, Wu L, Gao W, Jiang J (2020) Iturin A extracted from Bacillus subtilis WL-2 affects phytophthora infestans via cell structure disruption, oxidative stress, and energy supply dysfunction. Front Microbiol 11:536083
Washington TA, Smith JL, Grossman AD (2017) Genetic networks controlled by the bacterial replication initiator and transcription factor DnaA in Bacillus subtilis. Mol Microbiol 106:109–128
Waterman R, Lewis J, Waterman KC (2017) Accelerated stability modeling for peptides: a case study with bacitracin. AAPS PharmSciTech 18:1692–1698
Wei JZ, Hale K, Carta L, Platzer E, Wong C, Fang SC, Aroian RV (2003) Bacillus thuringiensis crystal proteins that target nematodes. Proc Natl Acad Sci USA 100:2760–2765
Weinberg ED (1964) Manganese requirement for sporulation and other secondary biosynthetic processes of Bacillus. Appl Microbiol 12:436–441
Weir J, Dubnau E, Ramakrishna N, Smith I (1984) Bacillus subtilis spo0H gene. J Bacteriol 157:405–412
Weir J, Predich M, Dubnau E, Nair G, Smith I (1991) Regulation of spo0H, a gene coding for the Bacillus subtilis sigma H factor. J Bacteriol 173:521–529
Willis C, Errington J, Wu LJ (2020) Cohesion of sister chromosome termini during the early stages of sporulation in Bacillus subtilis. J Bacteriol 202:e00296–e00220
Winkelman JT, Blair KM, Kearns DB (2009) RemA (YlzA) and RemB (YaaB) regulate extracellular matrix operon expression and biofilm formation in Bacillus subtilis. J Bacteriol 191:3981–3991
Winnen B, Anderson E, Cole JL, King GF, Rowland SL (2013) Role of the PAS sensor domains in the Bacillus subtilis sporulation kinase KinA. J Bacteriol 195:2349–2358
Wojciechowska H, Zgoda W, Borowski E, Dziegielewski K, Ulikowski S (1983) The antibiotic edeine. XII. Isolation and structure of edeine F. J Antibiot (Tokyo) 36:793–798
Wolf D, Rippa V, Mobarec JC, Sauer P, Adlung L, Kolb P, Bischofs IB (2016) The quorum-sensing regulator ComA from Bacillus subtilis activates transcription using topologically distinct DNA motifs. Nucleic Acids Res 44:2160–2172
Wu LJ, Errington J (1994) Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science 264:572–575
Wu LJ, Errington J (2003) RacA and the Soj-Spo0J system combine to effect polar chromosome segregation in sporulating Bacillus subtilis. Mol Microbiol 49:1463–1475
Wu JJ, Schuch R, Piggot PJ (1992) Characterization of a Bacillus subtilis sporulation operon that includes genes for an RNA polymerase sigma factor and for a putative DD-carboxypeptidase. J Bacteriol 174:4885–4892
Xin B, Zheng J, Xu Z, Song X, Ruan L, Peng D, Sun M (2015) The Bacillus cereus group is an excellent reservoir of novel lanthipeptides. Appl Environ Microbiol 81:1765–1774
Xue Y, Zhang X, Zhou C, Zhao Y, Cowan DA, Heaphy S, Grant WD, Jones BE, Ventosa A, Ma Y (2006) Caldalkalibacillus thermarum gen. nov., sp. nov., a novel alkalithermophilic bacterium from a hot spring in China. Int J Syst Evol Microbiol 56:1217–1221
Yang X, van der Donk WA (2013) Ribosomally synthesized and post-translationally modified peptide natural products: new insights into the role of leader and core peptides during biosynthesis. Chemistry 19:7662–7677
Yang J, Anderson BW, Turdiev A, Turdiev H, Stevenson DM, Amador-Noguez D, Lee VT, Wang JD (2020) The nucleotide pGpp acts as a third alarmone in Bacillus, with functions distinct from those of (p) ppGpp. Nat Commun 11:5388. https://doi.org/10.1038/s41467-020-19166-1
Yi H, Chun J, Cha CJ (2014) Genomic insights into the taxonomic status of the three subspecies of Bacillus subtilis. Syst Appl Microbiol 37:95–99
Yu YT, Kroos L (2000) Evidence that SpoIVFB is a novel type of membrane metalloprotease governing intercompartmental communication during Bacillus subtilis sporulation. J Bacteriol 182:3305–3309
Zakharian RA, Israelian IA, Agabalian AS, Tatevosian PE, Akopian SM (1979) Bacillus thuringiensis plasmid DNA. Mikrobiologiia 48:226–229
Zengler K, Toledo G, Rappe M, Elkins J, Mathur EJ, Short JM, Keller M (2002) Cultivating the uncultured. Proc Natl Acad Sci U S A 99:15681–15686
Zeytuni N, Flanagan KA, Worrall LJ, Massoni SC, Camp AH, Strynadka NCJ (2018) Structural and biochemical characterization of SpoIIIAF, a component of a sporulation-essential channel in Bacillus subtilis. J Struct Biol 204:1–8
Zhang HL, Hua HM, Pei YH, Yao S (2004) Three new cytotoxic cyclic acylpeptides from marine Bacillus sp. Chem Pharm Bull 52:1029–1030
Zhang S, Lebreton F, Mansfield MJ, Miyashita SI, Zhang J, Schwartzman JA, Tao L, Masuyer G, Martínez-Carranza M, Stenmark P, Gilmore MS, Doxey AC, Dong M (2018) Identification of a botulinum neurotoxin-like toxin in a commensal strain of Enterococcus faecium. Cell Host Microbe 23:169–176
Zhang L, Li S, Liu X, Wang Z, Jiang M, Wang R, Xie L, Liu Q, Xie X, Shang D, Li M, Wei Z, Wang Y, Fan C, Luo Z-Q, Shen X (2020) Sensing of autoinducer-2 by functionally distinct receptors in prokaryotes. Nat Commun 11:5371
Zharikova GG, Kherat DM, Maksimov VN, Silaev AB (1972) Application of the method of mathematical design of experiments to the biosynthesis of the antibiotics esein and bresein. Dokl Akad Nauk SSSR 204:465–467
Zheng G, Slavik MF (1999) Isolation, partial purification and characterization of a bacteriocin produced by a newly isolated Bacillus subtilis strain. Lett Appl Microbiol 28:363–367
Zhou Y, Choi YL, Sun M, Yu ZN (2008) Novel roles of Bacillus thuringiensis to control plant diseases. Appl Microbiol Biotechnol 80:563–572
Zhou R, Fei Y, Sun L, Guo J, Zhou X, Zhang X (2019) BTX-A rejuvenation: regional botulinum toxin-A injection of the platysma in patients with facial sagging. Aesthetic Plast Surg doi. https://doi.org/10.1007/s00266-019-01396-4
Zhou S, Liu G, Zheng R, Sun C, Wu S (2020) Structural and functional insights into iturin W, a novel lipopeptide produced by the deep-sea bacterium Bacillus sp. strain wsm-1. Appl Environ Microbiol 86:e01597–e01520
Zuber P, Losick R (1987) Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol 169:2223–2230
Acknowledgment
T. G. V. owes a debt of gratitude to his co-authors for their continual help and support, both in research and teaching, during his many years in the Department of Microbiology at the Universities of Salamanca and Santiago de Compostela, Spain.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Villa, T.G. et al. (2021). Genetics and Biochemistry of Sporulation in Endospore-Forming Bacteria (Bacillus): A Prime Example of Developmental Biology. In: Villa, T.G., de Miguel Bouzas, T. (eds) Developmental Biology in Prokaryotes and Lower Eukaryotes. Springer, Cham. https://doi.org/10.1007/978-3-030-77595-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-77595-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-77594-0
Online ISBN: 978-3-030-77595-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)