Abstract
Recombinant human antibody production represents a major growing class of biopharmaceuticals based on the technological progress within the last decades especially in CHO cells. The HIV neutralizing human monoclonal antibody 2F5 was developed as hybridoma from human lymphocyte preparations. In order to estimate the potential of recombinant 2F5-expressing CHO cells, we generated different recombinant CHO cell lines by varying regulatory sequences, the codon usage, the signal peptides, and the transfection technique. These 2F5-expressing cell lines were developed by selection of the best producer, clone homogeneity, and clone stability. The gene copy number of the clones differed significantly due to methotrexate amplification. In one cell line, we identified only one copy of heavy chain and two copies of light chain. Neither the gene copy number nor the promoter was found to influence the amount of transcript exclusively emphasizing the positioning effect of the transgene. Messenger RNA levels were highest in 2F5/CO and may have resulted from a combination of the promoter and codon-optimized sequences, but unexpectedly, the amount of secreted product was not elevated in this configuration. In our example, translational and post-translational limitations are responsible for decreased antibody secretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the fact that eukaryotic and prokaryotic expression systems are available for the production of recombinant proteins, two thirds of all recombinant protein pharmaceuticals are produced in mammalian cells (Wurm 2004), with CHO cells being one of the most commonly used cell type (Andersen and Krummen 2002). Although the cultivation of prokaryotic cells is cheaper and cultures are easier to cultivate with faster growth rates, prokaryotic systems have important disadvantages. They lack, for instance, appropriate chaperones for proper protein folding and the machinery for post-translational modifications (Knappskog et al. 2007), which may be mandatory for functional and therapeutically safe proteins (Stern et al. unpublished manuscript). Among the mammalian systems, CHO cells show relative robustness to metabolic stress, can be adapted to different cultivation systems, and provide relatively high production yields, and for the optimization of recombinant protein production in mammalian cell lines, two main approaches exist, namely, genetic optimization and process optimization (Wurm 2004).
In this project, we focused on the comparison of genetic engineering approaches to enhance protein production with different promoter regions, activators, and/or enhancers (Darzacq et al. 2005; Fiering et al. 2000; Lee and Young 2000; Verrijzer et al. 1995) and modification of vectors including both the backbone and the expression cassette (Stern et al., unpublished manuscript). In this paper, we report the characterization of different CHO production cell clones, all expressing the mAb 2F5, neutralizing HIV1 (Kunert et al. 1998). Different variations of expression plasmids were used to create these stable mAb 2F5-expressing cell lines. 2F5 light (LC) and heavy chain (HC) were expressed from different plasmids under varying control elements. One system contained signal peptide derived for the naturally secreted luciferase from the copepod, Gaussia princeps, which was shown to enhance recombinant protein production (Knappskog et al. 2007). Additionally, in one of our approaches, the 2F5 complementary DNA (cDNA) was optimized with respect to AT and GC rich sequence stretches, repeat sequences, and RNA secondary structures to increase the messenger RNA (mRNA) half-life and to shorten its turnover time.
Still, the limitations for recombinant protein production in the cellular machinery and how the amplification of gene copy number (GCN) by methotrexate (MTX) affects the protein production are unclear, although several previous studies deal with these issues (Jiang et al. 2006). We tried to answer some of these questions by using real-time quantitative polymerase chain reaction (qPCR), Northern blot (NB), and flow cytometry.
Materials and methods
Expression plasmids
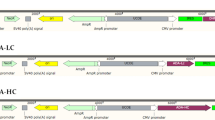
pRC/Rous sarcoma virus promoter (pRC/RSV; Invitrogen) was used as a backbone for LC and HC coding region in clones 2F5/MCB, 2F5/WCB, 2F5/NF, and 2F5/LG. The plasmid p2_dhfr, containing the mouse dhfr gene, was co-transfected to allow amplification with MTX (Alt et al. 1978) (Table 1). In 2F5/CO, the vector pCIneo (Promega) was used as expression backbone for the expression of LC behind the cytomegalovirus promoter (CMV) promoter. The genes for HC and dihydrofolate-reductase (dhfr) on pSV40/HC/dhfr were controlled by two independent SV40 promoters/terminators (Table 1). The cDNA of LC and HC was amplified from the original hybridoma, the signal peptides were from the highly germline-related antibody 3D6 (Kohl et al. 1991). The Gaussia luciferase signal peptide was used with 2F5/LG (Knappskog et al. 2007).
Recombinant cell lines
Dhfr-deficient CHO-cells DUKX-B11, ATCC CRL-9096 (Urlaub and Chasin 1980), were used to generate stable cell lines. The host cells were cultivated in Dulbecco’s modified Eagle’s medium (DMEM/Biochrom KG, Berlin, Germany) containing 4 mM l-glutamine (Life Technologies, Grand Island, NY, USA), 10% fetal calf serum (FCS; Pan, Australia), and hypoxanthine/thymidine (HT; Sigma-Aldrich Handels GmbH, Vienna, Austria). 2F5/MCB is cultivated in DMEM containing 4 mM l-glutamine, 10% dialyzed FCS, and 9.6 µM MTX (Sigma-Aldrich Handels GmbH, Vienna, Austria).
Protein-free-adapted CHO cells were cultivated in DMEM containing 4 mM l-glutamine, 0.25% soya-peptone/UF, 0.1% Pluronic-F68, in-house developed protein-free supplement (Polymun Scientific Immunbiologische Forschung GmbH, Vienna, Austria), and HT. After transfection, the cells were grown without HT but with varying levels of MTX.
Host cells were transfected either after adaptation to protein-free conditions generating 2F5/NF, 2F5/LG, and 2F5/CO, or directly while adherently growing with FCS-generating 2F5/MCB. 2F5/MCB and 2F5/WCB actually should be considered as the same cell line, just at different passages. One vial from the master cell bank (2F5/MCB) was adapted to growth in protein-free medium with a reduced MTX concentration of 0.96 µM MTX (2F5/WCB). The final level of MTX pressure applied to all clones is shown in Table 1. Transfection was performed by using either calcium phosphate precipitation (2F5/MCB; Graham and van der Eb 1973; Loyter et al. 1982), the Nucleofector Kit (2F5/NF/ AmaxaAG, Cologne, Germany), the cationic polymer polyethyleneimine (PEI) using a modified protocol of Derouazi et al (2F5/LG; Boussif et al. 1995; Derouazi et al. 2004), or by lipofection using dioleoyltrimethylammoniumpropane (DOTAP)/dioleoylphosphatidylethanolamine (DOPE) (2F5/CO; Reisinger et al. 2007). Twenty-four hours post-transfection selection with 0.5 mg ml−1 neomycin (G418) in HT-deficient medium was started for 2F5/MCB, 2F5/NF, and 2F5/LG, while 2F5/CO was selected merely with HT-deficient medium. The obtained clones were amplified with MTX starting at a concentration of 0.048 µM, and the concentration was increased by doubling with intermediate subcloning until the concentrations shown in Table 1 were reached. During the amplification with MTX, the clones were continuously tested for their antibody production, and the highest producers were chosen for further propagation. Subcloning by limited dilution combined with screening by enzyme-linked immunosorbent assay (ELISA) and FACS allowed the generation of homogenous clones. The number of subcloning steps is indicated in Table 1.
Final clones were cultivated by passaging them twice a week for at least 20 weeks to confer long-term stability. The batch experiments were carried out in 125-ml spinner flasks (Tecne). Suspension cells were inoculated at a cell density of 2 × 105 cells ml−1, and adherent cell batches were started with 6.9 × 104 cells ml−1 in roller bottles. Three different types of batches were carried out. The first batch was merely cultivated for 4 days; the second batch was harvested after the viability had declined 40%. In the third batch, l-glutamine was kept above 80 mg l−1 and glucose was kept above 1,000 mg l−1, and the cells were harvested at viability below 40%. Glucose and l-glutamine were determined using an YSI 7100 MBS (YSI Life sciences, Ohio, USA).
Determination of specific productivity
The specific productivity (qP) of mAb was calculated as picogram per cell per day (pcd). The cell concentration was determined using a Multisizer™ 3 Coulter Counter® (Beckman Coulter), and the secreted antibody was quantified using ELISA. Viability was determined via trypan blue vital stain using a Bürker–Türk chamber.
For the quantification of antibody titer in the supernatant, plates were pre-coated with goat anti-human γ-chain specific antiserum (Sigma), and detection was accomplished using horse radish peroxidase-conjugated goat anti-human κ-chain specific antiserum (Sigma) and stained with orthophenylediamine. Purified 2F5 served as a standard starting with 200 ng ml−1, and 1:2 dilution series of the samples and standard were analyzed.
Preparation of genomic DNA
Genomic DNA was prepared from all different batch experiments. 5 × 106 cells were harvested and washed with phosphate-buffered saline. The genomic DNA was isolated and purified according to the manufacturer’s instructions using the QIAamp® DNA Blood Mini kit (Qiagen) and stored at 4°C. Purity and concentration of the genomic DNA was determined with the ND-1000 Spectrophotometer (Nano-Drop®).
Preparation of RNA
Total RNA was isolated from 5 × 106 cells using Trizol reagent (Invitrogen) according to the manual and stored in RNAse-free water at −20°C. The concentration was determined using ND-1000 Spectrophotometer (Nano-Drop®).
Determination of intracellular LC and HC content
Cells were fixed and stored in 70% ethanol at 4°C. For analysis, the fixed cells were washed twice in Tris buffer (100 mM Tris–HCl, 2 mM MgCl2, pH 7.4) containing 20% FCS, resuspended in 100 μl Tris–FCS containing mouse anti-human κ-chain antibody conjugated with Quantum Red (1:60, Sigma) and goat anti-human γ-chain antiserum conjugated to fluorescein isothiocyanate (FITC; 1:60, Sigma) and incubated for 1 h at 37°C. After washing, the cells were analyzed on a FACS-Calibur flow cytometer (Becton Dickinson) equipped with a 5-W argon laser tuned to 488 and 350 nm. Cells were excited with 100 mW laser power at both wavelengths, and the fluorescence emissions measured with a 530/30 and 660/20 filter, respectively. Non-producing CHO cells served as negative controls, and compensation of spectral overlap between Fl1 and Fl3 was adjusted using cells stained only with anti γ-chain FITC antiserum.
NB analysis
A 1.5% denaturing agarose gel (Gibco, USA) and 6% formaldehyde in MOPS buffer (20 mM MOPS, 5 mM Na–acetate, 1 mM ethylenediaminetetraacetic acid, pH 7.0) were used for electrophoresis. Five hundred nanograms RNA per sample were mixed with 9 µl denaturizing buffer to an overall volume of 18 µl and incubated for 10 min at 95°C followed by 10 min on ice. Two microliters 10× gel-loading buffer was then added and the mixture loaded onto the gel and run for 2 h at 90 V.
The gel was then blotted overnight onto a positively charged nylon membrane (Roche) via capillary transfer using 20× saline-sodium citrate. Pre-incubation in hybridization buffer lasted for 70 min at 60°C, before the denatured probes (Table 2; LC and HC probes for optimized and non-optimized IgG sequences, digoxigenin labeled) were added to a concentration of 10 ng ml−1 in high-SDS hybridization buffer and incubated overnight at 60°C. Membranes were washed and blocked for 15 min in 1% blocking solution (Roche), incubated for 60 min with anti-digoxigenin-alkaline phosphatase (anti-DIG-Fab-AP; 0.75 U µl−1; Roche) 1:10,000 in 1% blocking solution and stained with 2 ml CDP-Star solution (1:100) and detected with the LumiImager™ (Boehringer Mannheim).
qPCR analysis
qPCR was performed with the Rotor-Gene 6000 (Corbett Research) utilizing the Taqman system. Four different PCR fragments served as standards, two for the HC (one non- and one codon optimized standard) and two for the LC (one non- and one codon optimized; Table 3). The standards were used in a range from 3 × 108 to 3 × 102 copies in a 1:10 dilution series. Stock solutions of the standards with a concentration of 1 × 109 copies μl−1 were stored at −20°C. Six nanograms of genomic DNA served as template per run. The standard curve was analyzed in duplicates and samples in quadruplicates. The reaction mix consisted of iQ™ Supermix (Biorad), 18 pmol of sense and antisense primers, and 3 pmol of the fluorogenic probe labeled with 6-carboxyfluorescein (FAM) and the fluorescent quencher dye 6-carboxytetramethylrhodamin (TAMRA). The primers were designed to amplify a ∼80-bp amplicon within the variable region of the antibody. Amplification was done by denaturing at 95°C for 5 min followed by 45 cycles at 95°C for 15 s and 60°C for 1 min. The optical density was measured at 495/620 nm, and results were evaluated with the Rotor-Gene 6000 software.
Validation of qPCR
For quantification and validation, eight independent analyzes of the standard curve were performed in duplicates. The results were evaluated using a statistic program based on Excel macro and evaluated by linear regression (analysis of variance), linearity (Mandel), variance homogeneity (F test), and the range. The logarithm of the copy number was calculated from the CT value (cycle when the fluorescence rises above the defined threshold) in a first order equation [CT = slope × log (copy number) + intercept].
Determination of GCN
The LC and HC GCN was quantified by analyzing 6 ng genomic DNA of each clone (representing 1,818 molecules of the genome). The calculation of the molecular weight of genomic DNA of a single cell is shown below. In this equation, the numerator describes the mass of one mole of the genome (3 × 109 bp) of a hamster cell, which consists of 6.022 × 1023 particles as described by the Loschmidt number.
The GCN was determined in four independent qPCR assays derived from different DNA preparations isolated during independent batch experiments.
Results
Determination of GCN
First, we investigated the number of transgenes per cell indicating the GCN of the LC and HC genes integrated into the chromosome. Furthermore, the GCN was correlated with the qP to gain information concerning the influence of the GCN on antibody production. Samples were collected from four independent batch experiments, while qP was analyzed from samples cultivated for 4 days growing exponentially. The recombinant cell lines 2F5/NF, 2F5/LG, and 2F5/CO displayed much lower GCN compared to the cell lines 2F5/MCB and 2F5/WCB (Fig. 1). This can be explained by the lower amplification pressure applied (0.38 µM MTX with 2F5/NF, 2F5/LG and 2F5/CO versus 9.6 µM MTX with 2F5/MCB and 0.96 µM MTX with 2F5/WCB). The fact that both 2F5/MCB and 2F5/WCB show a high GCN, although 2F5/WCB is derived from 2F5/MCB by reducing the MTX concentration and changing to protein-free cultivation medium, indicates a genetic stability of the cell lines, as ten additional passages were consumed by the adaptation and propagation of the working cell bank. This, however, is not reflected by the qP, which is considerably lower in 2F5/WCB than in 2F5/MCB. However, this genetic stability raises the question for reduced qP of 2F5/WCB in comparison to 2F5/MCB. In particular, 2F5/MCB and 2F5/WCB both contain more than 420 copies of LC cDNA and 136 or 99 copies of HC cDNA per cell. In contrast, HC and LC GCN of 2F5/NF, 2F5/LG, and 2F5/CO are both lower than 55 copies per cell.
Correlation of the qP and GCN using qPCR analysis. Samples were collected from four independent batch experiments, and qPCR was analyzed in quadruplicate. The qP was determined from samples collected during the exponential phase of two independent batches. Standard deviation was calculated from four independent experiments. Circle qP, black bar GCN LC, gray bar GCN HC
The best producing cell line is 2F5/MCB (11.7 pcd), which might be referred to adherent growth in FCS-containing medium. This qP is significantly higher than those achieved by the other clones. Surprisingly, 2F5/WCB showed only a slightly higher qP than 2F5/NF, 2F5/LG, and 2F5/CO (Table 4) despite being cultivated with the threefold amount of MTX and displaying a significantly higher number of transgenes than these cell lines. The amplification of the HC and LC genes is totally random in all cell lines except 2F5/CO, where both the HC and dhfr are on the same plasmid, but under control two different SV40 promoters. Therefore, selective pressure in that case is to get integration at a transcriptionally active site and the HC gene probably would go with the dhfr gene. In all other cases, the selective pressure to allow amplification into transcriptionally active sites is only on the dhfr gene if plasmids are not integrated at the same site. Therefore, we conclude that the GCN by itself is not sufficient for high qP as described previously (Lattenmayer et al. 2007).
NB analysis
NB analysis was used for the determination of the transcribed mRNA content of the clones. Samples were taken during exponential growth phase, and the total mRNA of five stable cell lines was isolated from 5 × 106 cells and investigated by Northern blot analysis (Fig. 2). No indication of mRNA degradation or rearrangements was observed. As expected, the LC band appeared at 710 bp, and the HC band appeared at 1,446 bp as shown in Fig. 2. Due to codon optimization in 2F5/CO and usage of different probes, it is not possible to compare hybridization intensity quantitatively because the experiments were not validated. However, 2F5/MCB, 2F5/WCB, 2F5/NF, and 2F5/LG were analyzed in parallel to enable an objective comparison. In the case of Northern blot analysis of 2F5/CO, the same experimental setup was used.
In Fig. 2, 2F5/MCB and 2F5/WCB show rather low amounts of HC and LC transcript. In contrast, 2F5/NF and 2F5/LG contained substantially more LC than HC transcript, which resembles the determined GCN ratio showing more LC than HC (Table 4), but in Northern blot, the difference seems more pronounced. The three independently transfected clones adapted to 0.38 µM MTX were all found to generate higher amounts of LC transcript despite their significantly lower LC GCN compared to 2F5/MCB and 2F5/WCB.
Quantification of qP, mRNA level, and LC to HC ratios and GCN
The next step was to look in more detail at the ratio between LC and HC at the GCN and mRNA level derived from the independent batches to verify our blot experiments (Table 4). The Northern blot ratios of LC to HC signal intensity were estimated from three independent experiments (Fig. 2 shows only one example) to calculate a LC to HC transcript ratio. Northern blots are no quantitative method, but for estimation of the ratio of LC to HC and comparison to the GCN quantification, it is a helpful indicator, especially when having the possibility to analyze the same transcript in different cell lines. In Northern blots shown in Table 4, 2F5/NF and 2F5/LG display increased signal intensity of light chain bands. Unexpectedly, 2F5/MCB and 2F5/WCB showed rather low LC mRNA amounts, and the estimation of signal intensity ratio indicated a value below one. Thus, the detected GCN ratios between LC and HC of 2F5/MCB and 2F5/WCB are not reflected in the transcript ratio (Table 4, columns 4 and 7). This observation disagrees with the postulate that higher GCN due to MTX gene amplification linearly raises the LC and HC mRNA content (Kim et al. 1998b). As already published, the high copy numbers of the expression vector after amplification may increase the specific productivity, or it may result in a detrimental effect on other cellular properties (Birch and Racher 2006). Furthermore, LC GCN of 2F5/CO is about 50-fold lower than that of 2F5/MCB and 2F5/WCB, but we detected a significantly higher level of transcript (Fig. 2), which might be referred to codon optimization or integration to a highly active transcription site. 2F5/CO coding sequences are optimized to remove cryptic splice sites or cryptic polyA-tails, unfavorable secondary structures, and to increase protein yield by using transfer RNAs that frequently occur in the expression organism (Narum et al. 2001). In the case of 2F5 expression, we realized that neither increasing the GCN like in 2F5/WCB nor increasing the amounts of transcript like in 2F5/CO lead to high secretion of mAb 2F5 (Table 4). One explanation for this inconsistency might be the adherent growth of 2F5/MCB in FCS-containing medium, enabling higher specific productivities.
Intracellular antibody content
To investigate the correlation between intracellular HC and LC content and antibody expression, we used flow cytometry analysis and ELISA during exponential growth of clones. 2F5/WCB was used as a reference by setting relative fluorescence units (rfu) of 2F5/WCB LC and HC and also the qP to 100%.
While 2F5/MCB showing the highest qP of all clones combined, the lowest levels of intracellular Ab chains were found at 70% compared to 2F5/WCB (Fig. 3).
Determination of intracellular antibody concentration using flow cytometry and comparison to clone 2F5/WCB. All data were generated in three independent experiments. 2F5/WCB was used as a reference and set 100% for LC, HC, and the qP.circle pcd, black bar intracellular content of LC, gray bar intracellular content of HC
Intracellular HC reaches the highest level in 2F5/CO at 198% compared to 2F5/WCB together with high levels of LC at 135% compared to 2F5/WCB. This observation might be ascribed to the high mRNA amount of 2F5/CO due to codon optimization, but the cells are not able to secrete the antibody efficiently.
The LC level is 30% higher than the HC level in 2F5/NF and 2F5/LG, which corresponds to the results obtained by Northern blot analysis.
Influence of cultivation conditions
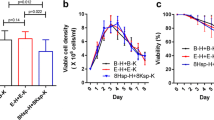
Finally, different batch experiments investigating the influence of varying cultivation times and nutrient concentrations on intracellular antibody concentration and volumetric productivity were carried out. Depending on the selection medium of the cell line, we used protein-free medium for 2F5/WCB, 2F5/NF, 2F5/LG, 2F5/CO, and 10% FCS-containing medium for 2F5 MCB. The results are given in Table 5.
The first batch was harvested after 4 days (B1), and the second batch (B2) cultivated until the viability declined significantly in protein-free medium. In batch 3 (B3), the cells were supplemented with l-glutamine and glucose and harvested after 10 days when the viability decreased significantly. Besides 2F5/MCB, all cell lines were cultivated under protein-free conditions during the entire experiments. For comparison, the rfu signal of HC and LC of B1 were defined as 100%. When cells were harvested, their intracellular content of product was highest in protein-free cultures under nutrient starvation (Table 5, B2). 2F5/CO showed a doubling of the LC and HC intracellular concentration, while 2F5/WCB, 2F5/NF, and 2F5/LG showed a doubling of HC only. Additionally, 2F5/CO completely lost viability after cell propagation. Another surprising observation was that intracellular LC and HC of 2F5/MCB increased further during B3 and that 2F5/MCB was the only clone that showed considerably increased productivity (i.e., approximately fivefold) compared to the B1 in terms of volumetric secretion of mAb 2F5.
Interestingly, in the cell lines cultivated under protein-free conditions, the titer did not increase after supplementation with glucose and l-glutamine (B3). Otherwise, under nutrition starvation, the intracellular content of chains was significantly increased but normalized to values similar to B1 in the fed-batch experiment B3.
Discussion
We investigated different recombinant cell lines each expressing the HIV-neutralizing human monoclonal antibody 2F5 (Kunert et al. 1998) that had originally been developed as hybridoma cell line (Buchacher et al. 1994). The cell lines were generated individually with different plasmids and transfection methods (Table 1), except for 2F5/WCB, which derives from 2F5/MCB by adaptation to protein-free medium. In the plasmids used for 2F5/MCB, 2F5/WCB, 2F5/NF, and 2F5/LG, both the LC and HC expression was controlled by an RSV promoter and p2-dhfr was co-transfected. The plasmids of 2F5/CO contained codon optimized LC and HC cDNAs under the control of the CMV and SV40 promoter, respectively. Additionally, the dhfr cDNA was integrated into the HC vector under control of a second SV40 promoter. In clone 2F5/LG, the signal peptide of G. princeps luciferase was used for efficient targeting of the nascent polypeptide chains of 2F5 HC and LC to the endoplasmatic reticulum. Moreover, we proofed that our established techniques employing either DOTAP/DOPE or PEI do work efficiently in comparison to commercial transfection methods during stable cell lines generation. For all cases, the MTX level had been increased stepwise and clones showing highest productivity rates and long-term stabilities (data not shown) were identified by limiting dilution subcloning and ELISA screening.
GCN in 2F5/MCB and 2F5/WCB have been found to be significantly higher than in the other clones with more than 400 copies of LC and about 100 copies of HC (Fig. 1), despite the fact that the plasmids did not carry the dhfr gene, which was co-transfected.
For the only clone that carried the dhfr gene on the HC plasmid (2F5/CO), preference for co-amplification of HC could not be detected concerning the GCN (Fig. 1). Thus, high GCN is just relevant if the gene can be transcribed efficiently.
The results for evaluation of transcription efficiency indicated that, despite the usage of the same promoter in case of 2F5/MCB, 2F5/WCB, 2F5/NF, and 2F5/LG, the determined GCN did not reflect the content of the mRNA determined by Northern blot. In particular, the clones 2F5/MCB and 2F5/WCB with significantly more transgenic LC than 2F5/NF, and 2F5/LG displayed weaker signal intensity of LC mRNA (Fig. 2). One can assume that this is caused by a positioning effect of the target gene in the chromosome (Jiang et al. 2006; Wurm 2004). Although different studies showed that the GCN is proportional to the level of MTX amplification (Pendse et al. 1992), the GCN by itself is not sufficient for high qP as described previously (Lattenmayer et al. 2007). We measured increased amounts of mRNA using codon-optimized cDNAs even under relatively low MTX pressure. In this case, the increased amount of mRNA might be driven by a prolonged mRNA half-life related to higher GC content and shorter turnover (Stern et al., unpublished manuscript). However, the beneficial effect on mRNA stability and transport into the cytoplasm has not been investigated properly (Narum et al. 2001). Additionally the 2F5/CO clone had different promoters compared to the other cell lines, which could be responsible for different transcription activity.
A novel approach is to use specific signal peptides from Gaussia luciferase mediating high level protein production (Knappskog et al. 2007). For our research, we employed the signal peptide of the G. princeps luciferase and detected similar levels of qP translated from lower amounts of mRNA (2F5/LG versus 2F5/CO) due to the optimized signal peptide, which was shown to mediate increased synthesis and more efficient secretion of a model protein (Knappskog et al. 2007).
Flow cytometry analysis confirmed that the varying protein secretion rates are often not reflected by the intracellular content of the antibody (Fig. 3) as already described by Kim et al. (1998b), and an increased mAb production during nutrient limitation in chemostat culture was observed for hybridomas (Miller et al. 2000). Others working in this field reported an inverse relationship between qP and µ (Fussenegger and Bailey 1998) and consequently suggested that cell division should be arrested to obtain a high qP because cell division drains resources required for recombinant protein production (Dinnis and James 2005). During batch cultivation under starvation conditions (B2), elevated levels of intracellular HC and LC in clones cultivated without FCS were observed (Table 5). However, whether the intracellular detected antibody chains are folded correctly or remain retarded by chaperones that might induce the intracellular protein response (Rutkowski and Kaufman 2004) is not yet known to full satisfaction.
Several in vitro studies concluded that the folding and assembly of LC and HC to obtain a functional antibody is the rate-limiting step in recombinant mAb production (Barnes and Dickson 2006; Dinnis and James 2005). Furthermore, no correlation between intracellular LC and HC mRNA content and antibody expression seems to exist (Barnes et al. 2004; Flickinger et al. 1992; Kim et al. 1998a), although the strong correlation between recombinant mAb production and LC mRNA had been postulated in several studies (Borth et al. 1999; Strutzenberger et al. 1999).
Another major bottleneck following the proper assembly of antibody chains is the ER to cis-Golgi transport, which might limit the rate of secretion (Hooker et al. 1999). It had been shown recently that active X-box binding protein (XBP1) induced synthesis of ER membrane phospholipids and could increase the surface area and volume of rough ER to direct secretion of expressed proteins (Shaffer et al. 2004). A similar effect of a better configuration of the ER and the Golgi network might be provided by the lipids of the FCS in serum-containing medium. Nowadays, great efforts focus on the replacement of fetal calf serum by synthetic compounds. However, the reason for enhanced protein secretion are complex, leaving room for different models for media optimization.
During earlier large-scale experiments, after process optimization with the same protein-free medium, low qP of mAb 2F5 had been observed and might also be referred to the fact that each antibody has a characteristic expression efficiency, affected by a combination of several parameters. One of the parameters is the primary sequence of light and heavy chain (Bentley et al. 1998), which, in combination with media composition, influences the secretion pathway of the cell.
References
Alt FW, Kellems RE, Bertino JR, Schimke RT (1978) Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem 253(5):1357–1370
Andersen DC, Krummen L (2002) Recombinant protein expression for therapeutic applications. Curr Opin Biotechnol 13(2):117–123
Barnes LM, Dickson AJ (2006) Mammalian cell factories for efficient and stable protein expression. Curr Opin Biotechnol 17(4):381–386
Barnes LM, Bentley CM, Dickson AJ (2004) Molecular definition of predictive indicators of stable protein expression in recombinant NS0 myeloma cells. Biotechnol Bioeng 85(2):115–121
Bentley KJ, Gewert R, Harris WJ (1998) Differential efficiency of expression of humanized antibodies in transient transfected mammalian cells. Hybridoma 17(6):559–567
Birch JR, Racher AJ (2006) Antibody production. Adv Drug Deliv Rev 58(5–6):671–685
Borth N, Strutzenberger K, Kunert R, Steinfellner W, Katinger H (1999) Analysis of changes during subclone development and ageing of human antibody-producing heterohybridoma cells by northern blot and flow cytometry. J Biotechnol 67(1):57–66
Boussif O, Lezoualch F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 92(16):7297–7301
Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A et al (1994) Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein–Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses 10(4):359–369
Darzacq X, Singer RH, Shav-Tal Y (2005) Dynamics of transcription and mRNA export. Curr Opin Cell Biol 17(3):332–339
Derouazi M, Girard P, Van Tilborgh F, Iglesias K, Muller N, Bertschinger M, Wurm FM (2004) Serum-free large-scale transient transfection of CHO cells. Biotechnol Bioeng 87(4):537–545
Dinnis DM, James DC (2005) Engineering mammalian cell factories for improved recombinant monoclonal antibody production: lessons from nature? Biotechnol Bioeng 91(2):180–189
Fiering S, Whitelaw E, Martin DI (2000) To be or not to be active: the stochastic nature of enhancer action. Bioessays 22(4):381–387
Flickinger MC, Goebel NK, Bibila T, Boyce-Jacino S (1992) Evidence for posttranscriptional stimulation of monoclonal antibody secretion by L-glutamine during slow hybridoma growth. J Biotechnol 22(3):201–226
Fussenegger M, Bailey JE (1998) Molecular regulation of cell-cycle progression and apoptosis in mammalian cells: implications for biotechnology. Biotechnol Prog 14(6):807–833
Graham FL, van der Eb AJ (1973) A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52(2):456–467
Hooker AD, Green NH, Baines AJ, Bull AT, Jenkins N, Strange PG, James DC (1999) Constraints on the transport and glycosylation of recombinant IFN-gamma in Chinese hamster ovary and insect cells. Biotechnol Bioeng 63(5):559–572
Jiang Z, Huang Y, Sharfstein ST (2006) Regulation of recombinant monoclonal antibody production in chinese hamster ovary cells: a comparative study of gene copy number, mRNA level, and protein expression. Biotechnol Prog 22(1):313–318
Kim NS, Kim SJ, Lee GM (1998a) Clonal variability within dihydrofolate reductase-mediated gene amplified Chinese hamster ovary cells: stability in the absence of selective pressure. Biotechnol Bioeng 60(6):679–688
Kim SJ, Kim NS, Ryu CJ, Hong HJ, Lee GM (1998b) Characterization of chimeric antibody producing CHO cells in the course of dihydrofolate reductase-mediated gene amplification and their stability in the absence of selective pressure. Biotechnol Bioeng 58(1):73–84
Knappskog S, Ravneberg H, Gjerdrum C, Trosse C, Stern B, Pryme IF (2007) The level of synthesis and secretion of Gaussia princeps luciferase in transfected CHO cells is heavily dependent on the choice of signal peptide. J Biotechnol 128(4):705–715
Kohl J, Ruker F, Himmler G, Razazzi E, Katinger H (1991) Cloning and expression of an HIV-1 specific single-chain Fv region fused to Escherichia coli alkaline phosphatase. Ann N Y Acad Sci 646:106–114
Kunert R, Ruker F, Katinger H (1998) Molecular characterization of five neutralizing anti-HIV type 1 antibodies: identification of nonconventional D segments in the human monoclonal antibodies 2G12 and 2F5. AIDS Res Hum Retroviruses 14(13):1115–1128
Lattenmayer C, Trummer E, Schriebl K, Vorauer-Uhl K, Mueller D, Katinger H, Kunert R (2007) Characterisation of recombinant CHO cell lines by investigation of protein productivities and genetic parameters. J Biotechnol 128(4):716–725
Lee TI, Young RA (2000) Transcription of eukaryotic protein-coding genes. Annu Rev Genet 34:77–137
Loyter A, Scangos GA, Ruddle FH (1982) Mechanisms of DNA uptake by mammalian cells: fate of exogenously added DNA monitored by the use of fluorescent dyes. Proc Natl Acad Sci USA 79(2):422–426
Miller WM, Blanch HW, Wilke CR (2000) A kinetic analysis of hybridoma growth and metabolism in batch and continuous suspension culture: effect of nutrient concentration, dilution rate, and pH. Biotechnol Bioeng 67(6):853–871 (Reprinted from Biotechnology and Bioengineering 32:947–965, 1988)
Narum DL, Kumar S, Rogers WO, Fuhrmann SR, Liang H, Oakley M, Taye A, Sim BK, Hoffman SL (2001) Codon optimization of gene fragments encoding Plasmodium falciparum merzoite proteins enhances DNA vaccine protein expression and immunogenicity in mice. Infect Immun 69(12):7250–7253
Pendse GJ, Karkare S, Bailey JE (1992) Effect of cloned gene dosage on cell growth and hepatitis B surface antigen synthesis and secretion in recombinant CHO cells. Biotech Bioeng 40(1):119–129
Reisinger H, Sevcsik E, Vorauer-Uhl K, Lohner K, Katinger H, Kunert R (2007) Serum-free transfection of CHO-cells with tailor-made unilamellar vesicles. Cytotechnology 54:157–168
Rutkowski DT, Kaufman RJ (2004) A trip to the ER: coping with stress. Trends Cell Biol 14(1):20–28
Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A et al (2004) XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21(1):81–93
Strutzenberger K, Borth N, Kunert R, Steinfellner W, Katinger H (1999) Changes during subclone development and ageing of human antibody-producing recombinant CHO cells. J Biotechnol 69(2–3):215–226
Urlaub G, Chasin LA (1980) Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci USA 77(7):4216–4220
Verrijzer CP, Chen JL, Yokomori K, Tjian R (1995) Binding of TAFs to core elements directs promoter selectivity by RNA polymerase II. Cell 81(7):1115–1125
Wurm FM (2004) Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22(11):1393–1398
Acknowledgments
This research was part of the Pharma-Planta Project (LSHB-CT-2003–503565), kindly funded by an EU FP6 program and partly supported by Polymun Scientific GmbH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reisinger, H., Steinfellner, W., Stern, B. et al. The absence of effect of gene copy number and mRNA level on the amount of mAb secretion from mammalian cells. Appl Microbiol Biotechnol 81, 701–710 (2008). https://doi.org/10.1007/s00253-008-1701-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1701-1