Abstract
In this study, we assessed the potential of PMR1-disrupted Pichia pastoris (Pppmr1) expressing human serum albumin and interferon alpha2b fusion protein (HSA-IFN-alpha2b) in large-scale fermentation. The high osmotic pressure of standard basal salts medium (BSM) was detrimental to the growth and viability of Pppmr1. HSA-IFN-alpha2b was secreted into a supernatant with a concentration of up to 112 mg/L after 20 h of induction and then began to decline. In vitro stability tests indicated that the disappearance of HSA-IFN-alpha2b was ascribed to proteolytic degradation. Decreasing the salt concentration of BSM medium to one quarter of the original formula improved the growth and viability of Pppmr1. As a result of reduced cell lysis and protease release, HSA-IFN-alpha2b was stable in the supernatant, which enabled a longer production phase (30 h) and a higher expression level (215 mg/L). Lowering the culture temperature to 20°C increased the cell viability during carbon source transition and alleviated the oxygen and methanol limitation, which extended the production phase to 40 h and increased the expression level to 680 mg/L. The addition of 2% Soytone prolonged the production phase to 60 h and increased the expression level to 1,260 mg/L, which was more than tenfold higher than that of Pppmr1 cultured under the conditions recommended by Invitrogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pichia pastoris has evolved as a successful recombinant protein expression host in recent years (Cereghino and Cregg 2000). The most attractive attribute of P. pastoris is its high efficiency: for some proteins, expression levels of more than 10 g/L can be achieved (Werten et al. 1999). But such success cannot be achieved for all proteins, and, for some difficult-to-express proteins, the expression levels were extremely low (<1 mg/L) or undetectable (Murphy et al. 1998; Sinclair and Choy 2002).
Great efforts have been made to increase the productivity of the P. pastoris expression system. These efforts mainly include gene optimization (Chang et al. 2006; Woo et al. 2002), overexpression of chaperones (Damasceno et al. 2007; Zhang et al. 2006), and culture condition optimization (Zhang et al. 2007). Besides the abovementioned methods, PMR1 gene disruption is an effective alternative strategy to increase the secretion capacity of P. pastoris.
Yeast PMR1 encodes a P-type Ca2+-ATPase localized in the Golgi apparatus (Antebi and Fink 1992). Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic-reticulum-associated protein degradation (Dürr et al. 1998). It had been demonstrated that disruption of PMR1 gene increased the secretion capacity of Saccharomyces cerevisiae, Kluyveromyces lactis, and Hansenula polymorpha (Melnick et al. 1990; Uccelletti et al. 2004; Agaphonov et al. 2007). Recently, P. pastoris PMR1 has been cloned (Dux and Inan 2006). Previous studies in our laboratory showed that disruption of P. pastoris PMR1 gene increased the secretion of human serum albumin (HSA) and interferon alpha2b (IFN-alpha2b) fusion protein (HSA-IFN-alpha2b), a promising protein pharmaceutical for the treatment of hepatitis C (Subramanian et al. 2007; Zhao et al. 2007). However, this effect was counteracted by the decreased viability of PMR1-deficient mutant (Pppmr1) during prolonged cultivation (Zhao et al. 2008).
Despite the extensive research that has been conducted to study the effects of PMR1 disruption on the secretion of heterologous proteins secretion from yeast, no large-scale fermentation studies have been published, probably due to concern that the Ca2+-dependent growth defect of PMR1 disrupted mutants may be an obstacle to large-scale application (Ko et al. 2002). However, it has been demonstrated that in high-cell-density P. pastoris cultures, to achieve optimized expression, the growth rate must be kept low, either by feeding the carbon source at a limiting level or lowering the culture temperature (Schenk et al. 2008; Jahic et al. 2003). Thus, it was hypothesized that, for hosts that could grow rapidly and grow to high cell density, decreased growth rate would not be a major limitation for large-scale fermentation.
In this study, the effects of the salt concentration of medium, the culture temperature, and the addition of complex medium on the growth and heterologous protein expression of Pppmr1 cultured in a 30-L fermentor were studied in detail.
Materials and methods
Strains
P. pastoris GS115 and Pppmr1-expressing HSA-IFN-alpha2b were described elsewhere (Zhao et al. 2008). The gene encoding HSA-IFN-alpha2b is an artificial gene synthesized according to the P. pastoris codon usage preference. HSA-IFN-alpha2b expression cassette is under the direction of prepro secretion signal of HSA and under the control of AOX1 promoter. Both strains contain one copy of expression cassette and are of Mut+ phenotype.
Media
Buffered minimal glycerol medium (BMG medium) containing 13.4 g/L yeast nitrogen base without amino acids, 10 g/L glycerol, 0.4 mg/L biotin, and 10 mM CaCl2 in 100-mM potassium phosphate buffer, pH 6.0, was used for cell growth in shake flask culture.
Standard basal salts medium (BSM) or low-salts BSM (LS-BSM) with a trace element solution PTM1 were used for fermentation runs. BSM contained (per liter): H3PO4 (85%), 26.7 ml; CaSO4, 0.93 g; K2SO4, 18.2 g; MgSO4·7H2O, 14.9 g; KOH, 4.13 g; glycerol, 40 g. LS-BSM contained (per liter): (NaPO3)6, 6.5 g; CaSO4, 0.23 g; K2SO4, 4.55 g; MgSO4·7H2O, 3.73 g; KOH, 1.03 g; glycerol, 40 g. PTM1 contained (per liter): CuSO4·5H2O, 6.0 g; NaI, 0.08 g; MnSO4·H2O, 3.0 g; Na2MoO4·2H2O, 0.2 g; H3BO3, 0.02 g; CoCl2, 0.5 g; ZnCl2, 20.0 g; FeSO4·7 H2O, 65.0 g; biotin, 0.3 g; and concentrated H2SO4, 5 ml (Surribas et al. 2007).
Shake flask cultivation
Inoculum for the fed-batch fermentation was grown in 1 L of BMG in a 3-L shake flask at 200 rpm and 30°C until OD600 reached 10. Two 1-L cultures were combined and used for the bioreactor inoculum.
Fed-batch fermentation process
Fed-batch fermentation was performed in a 30-L fermentor (BioStat UD30, B Braun Biotech International). The fermentation process was divided into three different stages:
Glycerol batch phase
The fermentor containing 20 L BSM or LS-BSM medium was sterilized at 120°C for 30 min. After cooling, the temperature was set to 30°C; the pH was adjusted to 6.0 with 28% ammonium hydroxide, and 90 mL PTM1 trace salts was added aseptically. The fermentor was inoculated with 2-L seed culture, and agitation and aeration were set to keep the dissolved oxygen (DO) above 20%.
Glycerol fed-batch phase
Once the glycerol in the BSM or LS-BSM medium was completely consumed, which was indicated by an increase in the DO (DO spike), a glycerol feed was initiated to increase the cell biomass under limiting conditions. Glycerol was added as 50% solution containing 12 mL/L PTM1, and the feed rate was set to 240 mL/h. The glycerol fed-batch phase lasted for 4 h.
Methanol induction phase
The methanol induction phase was further divided into two different phases:
Methanol-nonlimited fed-batch phase
At the end of abovementioned glycerol fed-batch phase, the induction of expression was initiated by a bolus injection of 50 mL methanol to give a methanol concentration of 0.2%. For the subsequent cultivation, methanol concentration was monitored by a methanol sensor (East China University of Science and Technology, Shanghai, China) and maintained at this value by continuous methanol feeding through a peristaltic pump. Methanol was added as 100% solution containing 12 mL/L PTM1. DO was kept above 20% by the adjustment of agitation and aeration.
Methanol-limited fed-batch phase
Once agitation and aeration had reached maximum (1,000 rpm and 3 vvm), the DO was maintained above 20% by the adjustment of the methanol feeding rate.
The fermentation was terminated when HSA-IFN-alpha2b ceased accumulation.
For batches induced at low temperature, the temperature was decreased to 20°C at the end of glycerol fed-batch phase.
For batches supplemented with complex medium, Soytone (BD Company) was added to a final concentration of 2% at the end of glycerol fed-batch phase.
Stability of HSA-IFN-alpha2b in the fermentation supernatant
Fermentation supernatant was spiked with purified HSA-IFN-alpha2b to a final concentration of 1.2 mg/mL, which matched the expression level of the optimized fermentation runs. The sample was incubated at 30°C for different periods of time (0, 1, 2, 4, 8, 12, 24 h). The remaining HSA-IFN-alpha2b was detected by nonreducing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Analytical methods
Biomass analysis
Cell concentration was monitored by measuring the optical density at 600 nm (OD600).
HSA-IFN-alpha2b expression level
The expression level of HSA-IFN-alpha2b was determined by gel densitometry (Xue et al. 2005).
Cell viability assay
The cell viability was assessed by propidium iodide (PI) staining. FACSCalibur flow cytometry (BD Company) equipped with a 488-nm argon laser was used for this analysis. Samples taken from the fermentor were diluted with phosphate-buffered saline (160 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, and 1 mM KH2PO4, pH 7.3). For staining, 25 μL of a stock solution containing 200 μg/mL of PI dissolved in water was added to 975 μL of diluted sample. After gently mixing on a vortex, cells were incubated for 10 min at room temperature. Samples were then analyzed at a cell rate of about 1,500 counts per second. A count of 50,000 was collected in each measurement. PI-positive cells were considered as dead and the PI-negative cells were considered as viable (Xiao et al. 2006).
Results
Optimizing the Ca2+ concentration in the BSM medium for the growth of Pppmr1
Pppmr1 displayed Ca2+-dependent growth defect (Zhao et al. 2008). To determine the optimal Ca2+ concentration for the growth of Pppmr1, we evaluated the growth characteristics of Pppmr1 in BSM medium supplemented with different concentrations of CaCl2 (0, 10, 20, 30 mM). As can be seen from Fig. 1, 10 mM Ca2+ was optimal for the growth of Pppmr1 in BSM medium, while excess Ca2+ resulted in the precipitation of salts, and was inhibitory to the growth of Pppmr1. Considering that the standard BSM medium already contained about 6 mM Ca2+, for low-salt BSM medium that contained about 1 mM Ca2+, 15 mM CaCl2 was added to give a final calcium concentration of about 16 mM.
Cultivation of Pppmr1 in standard BSM medium
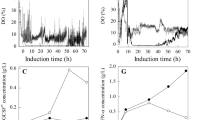
As a first step, Pppmr1 was cultured in a 30-L fermentor following Pichia fermentation process guidelines provided by Invitrogen. As can be seen from Table 1 and Fig. 2a, when cultured in BSM medium supplemented with 10 mM Ca2+ at 30°C, Pppmr1 had a decreased growth rate compared to its parent strain GS115 grown under the same condition. The glycerol batch phase of Pppmr1 was about 4.4 h longer than that of GS115. The biomass of Pppmr1 at the end of fermentation was only 68% of GS115. HSA-IFN-alpha2b secreted from Pppmr1 reached its maximal concentration (about 112 mg/L) after 20 h of induction and rapidly disappeared from the supernatant in the subsequent cultivation (Fig. 2b). Cell viability assay revealed that the cell death rate before induction was 12%. Upon methanol induction, cell death grew exponentially to 35%, and at the end of induction it increased to 49% (Fig. 3). For comparison, the cell viability of GS115 was almost 100% before induction, and it never fell below 80% during the whole fermentation process. In vitro stability tests using purified HSA-IFN-alpha2b as substrate revealed that this fusion protein was subjected to severe proteolytic degradation in the fermentation supernatant. After being incubated at 30°C for 24 h, HSA-IFN-alpha2b disappeared from the supernatant (Fig. 4).
Stability of HSA-IFN-alpha2b in the fermentation supernatant of Pppmr1 from different fermentation runs. Fermentation supernatant was spiked with purified HSA-IFN-alpha2b to a final concentration of 1.2 mg/ml. The sample was incubated at 30°C for indicated periods of time (0, 1, 2, 4, 8, 12, 24 h). The remaining HSA-IFN-alpha2b was detected by nonreducing SDS-PAGE. BSM basal salts medium; LS-BSM low-salts basal salts medium
Effects of decreasing the salt concentration of BSM medium on the growth and heterologous protein expression of Pppmr1
A recent study demonstrated that decreasing the salts concentration of BSM medium improved the cell viability of GS115 and reduced cell lysis (Surribas et al. 2007). Consistent with these observations, it was demonstrated in this study that decreasing the salt concentration of BSM medium to one quarter of the original formula prompted the growth of Pppmr1, which was manifested by a 3.4-h decrease of glycerol batch phase and a 25% increase of the biomass at the end of fermentation (Table 1, Fig. 2a). The expression level of HSA-IFN-alpha2b reached 215 mg/L after 30 h of induction and was kept almost constant for subsequent cultivation (Fig. 2b). Cell viability assay showed that the dead cell rate before induction decreased from 12% to 2%. The carbon source switch from glycerol to methanol increased the cell death rate to 18%. Thereafter, cell death percentage remained nearly constant at a value of about 20% (Fig. 3). Stability tests revealed that HSA-IFN-alpha2b was stable in the fermentation supernatant of Pppmr1 cultured in low-salts BSM medium. The amount of HSA-IFN-alpha2b remained nearly unchanged after incubated at 30°C for 24 h (Fig. 4).
Effects of lowering the culture temperature on the growth and heterologous protein expression of Pppmr1
Temperature is a critical parameter in P. pastoris fermentation. Several studies have demonstrated that decreasing the culture temperature improved cell viability and heterologous protein expression (Jahic et al. 2003; Lin et al. 2007). P. pastoris is a quite psychrotolerant organism that can grow at a temperature as low as 12°C (Jahic et al. 2003). As shown in Table 1, when Pppmr1 was induced at 20°C, it had a longer methanol-nonlimited fed-batch phase (MNLFB) phase. The effect of lowering the culture temperature on the growth of Pppmr1 was not straightforward: at the early phase of induction, it had a negative effect on the biomass, while, at the late phase of induction, it prompted the accumulation of biomass (Fig. 2a). Decreasing the induction temperature to 20°C prolonged the production phase to 48 h and increased the expression level to 680 mg/L (Fig. 2b). Consistent with early observation, when Pppmr1 was shifted to grow on methanol at low temperature, the cell death rate increased less significantly. During the whole methanol induction phase, the dead cell percentage never exceeded 10% (Fig. 3). In vitro stability tests showed that HSA-IFN-alpha2b was not subjected to degradation. After a 24-h incubation at 30°C, no obvious decrease of the band density of HSA-IFN-alpha2b could be observed (Fig. 4).
Effects of the addition of complex medium on the growth and heterologous protein expression of Pppmr1
The addition of complex media such as yeast extract, casamino acids or Soytone have been shown to increase protein expression level, reduce protein degradation, or prevent protein aggregation in P. pastoris fermentation (Woo et al. 2004, 2008; Wang et al. 2005). In this study, 2% animal-free Soytone was added to the fermentation medium before induction. The addition of Soytone prompted the growth of Pppmr1, prolonged the production phase to 60 h, and increased the expression level to 1,260 mg/L (Table 1, Fig. 2). The addition of complex medium had little effect on cell viability (Fig. 3). Stability tests showed that only slight degradation had occurred to HSA-IFN-alpha2b, and the band density remained largely unchanged after being incubated at 30°C for 24 h (Fig. 4).
Application of this fermentation strategy to the cultivation of GS115
It was observed that GS115 could also benefit from the revised fermentation strategy (low-salts BSM + 2% Soytone, 20°C), as GS115 grown under these conditions had higher biomass, prolonged production phase, and, most important of all, a 66% increase of the expression level (from 480 to 750 mg/L; Table 1, and Fig. 2).
Discussion
Great efforts have been made to increase the productivity of P. pastoris in recent years. Most of the techniques aimed to increase the secretion capacity of this important recombinant protein expression system were transplanted from a more well-established yeast expression host S. cerevisiae. PMR1 gene was first identified by the observation that its disruption caused a “supersecretion” phenotype in S. cerevisiae (Smith et al. 1985). This effect has been validated in K. lactis, H. polymorpha, and recently P. pastoris (Uccelletti et al. 2004; Agaphonov et al. 2007; Zhao et al. 2008). However, most studies were restricted to small-scale shake flask culture, probably due to a concern that the Ca2+-dependent growth defect of PMR1-disrupted mutant will extend the duration of large-scale fermentation (Ko et al. 2002).
As shown in this study, when Pppmr1 was cultured in standard BSM medium, its glycerol batch phase lasted for 23.8 h, which was 4.4 h longer that of its parent strain GS115 cultured under the same condition. By optimizing the composition of BSM medium, the glycerol batch phase of Pppmr1 was shortened to 20.4 h, which was only 1 h longer than that of GS115 cultured under the conditions recommended by Invitrogen. Since a typical P. pastoris fermentation process lasts for tens of hours, such minor a increase of the duration will not be a major limitation for large-scale application. In fact, our experiments revealed that the major obstacle of Pppmr1 for large-scale fermentation was its decreased viability during prolonged culture, which led to cell lysis, protease release, and target protein degradation. The decreased viability of PMR1-disrupted mutant has been documented for other yeasts such as H. polymorpha and Candida albicans (Agaphonov et al. 2007; Bates et al. 2005).
Since disruption of PMR1 gene has been shown to affect the cell wall integrity of Schizosaccharomyces pombe (Cortés et al. 2004), it is plausible that Pppmr1 is more sensitive to osmotic pressure as compared to GS115. Decreasing the salt concentration of BSM medium to one quarter of the standard formula improved cell viability and, more important for heterologous protein expression, decreased cell lysis and protease release. This was supported by the results of in vitro stability tests with purified HSA-IFN-alpha2b as substrate.
Temperature is an important parameter for P. pastoris fermentation, and its effects can be interpreted from several different perspectives. From the point view of cellular physiology, decreasing the temperature reduces folding stress imposed on the cell and leads to a higher rate of correctly folded product (Gasser et al. 2007). Lower temperature enables P. pastoris to adapt to methanol induction more smoothly and results in lower death rate (Jahic et al. 2003). From the point of view of fermentation parameter control, lower temperature alleviates the oxygen and methanol limitation which is inevitably encountered for high-cell-density fermentation of P. pastoris. As shown in this study, Pppmr1 cultured at 20°C had a longer MNLFB phase, thus lowering the temperature created a more favorable external environment for heterologous protein expression.
As shown in this study, for all the fermentation runs except in standard BSM medium at 30°C, the biomass of Pppmr1 accumulated steadily for the whole cultivation process, while the accumulation of HSA-IFN-alpha2b did not. Such phenomenon has been termed as a “decoupling” of cell growth and heterologous protein expression (Plantz et al. 2006). It has been suggested that methanol alone cannot support the carbon and energy demands to sustain both growth and recombinant protein production (Plantz et al. 2006). The addition of a complex medium helped to alleviate such carbon and energy starvation and extended the production phase.
Our results showed that the high heterologous protein expression level of Pppmr1 under optimized culture conditions enabled it to be a promising host for large-scale fermentation for the commercial production of HSA-IFN-alpha2b fusion protein.
References
Agaphonov MO, Plotnikova TA, Fokina AV, Romanova NV, Packeiser AN, Kang HA, Ter-Avanesyan MD (2007) Inactivation of the Hansenula polymorpha PMR1 gene affects cell viability and functioning of the secretory pathway. FEMS Yeast Res 7:1145–1152
Antebi A, Fink GR (1992) The yeast Ca(2+)-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell 3:633–654
Bates S, MacCallum DM, Bertram G, Munro CA, Hughes HB, Buurman ET, Brown AJ, Odds FC, Gow NA (2005) Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J Biol Chem 280:23408–23415
Cereghino JL, Cregg JM (2000) Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev 24:45–66
Chang SW, Lee GC, Shaw JF (2006) Codon optimization of Candida rugosa lip1 gene for improving expression in Pichia pastoris and biochemical characterization of the purified recombinant LIP1 lipase. J Agric Food Chem 54:815–822
Cortés JC, Katoh-Fukui R, Moto K, Ribas JC, Ishiguro J (2004) Schizosaccharomyces pombe Pmr1p is essential for cell wall integrity and is required for polarized cell growth and cytokinesis. Eukaryot Cell 3:1124–1135
Damasceno LM, Anderson KA, Ritter G, Cregg JM, Old LJ, Batt CA (2007) Cooverexpression of chaperones for enhanced secretion of a single-chain antibody fragment in Pichia pastoris. Appl Microbiol Biotechnol 74:381–389
Dürr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, Wolf DH, Rudolph HK (1998) The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell 9:1149–1162
Dux MP, Inan M (2006) Identification and characterization of calcium and manganese transporting ATPase (PMR1) gene of Pichia pastoris. Yeast 23:613–621
Gasser B, Maurer M, Rautio J, Sauer M, Bhattacharyya A, Saloheimo M, Penttilä M, Mattanovich D (2007) Monitoring of transcriptional regulation in Pichia pastoris under protein production conditions. BMC Genomics 8:179
Jahic M, Wallberg F, Bollok M, Garcia P, Enfors SO (2003) Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures. Microb Cell Fact 2:6
Ko JH, Hahm MS, Kang HA, Nam SW, Chung BH (2002) Secretory expression and purification of Aspergillus niger glucose oxidase in Saccharomyces cerevisiae mutant deficient in PMR1 gene. Protein Expr Purif 25:488–493
Lin H, Kim T, Xiong F, Yang X (2007) Enhancing the production of Fc fusion protein in fed-batch fermentation of Pichia pastoris by design of experiments. Biotechnol Prog 23:621–625
Melnick LM, Turner BG, Puma P, Price-Tillotson B, Salvato KA, Dumais DR, Moir DT, Broeze RJ, Avgerinos GC (1990) Characterization of a nonglycosylated single chain urinary plasminogen activator secreted from yeast. J Biol Chem 265:801–807
Murphy KP Jr, Gagne P, Pazmany C, Moody MD (1998) Expression of human interleukin-17 in Pichia pastoris: purification and characterization. Protein Expr Purif 12:208–214
Plantz BA, Sinha J, Villarete L, Nickerson KW, Schlegel VL (2006) Pichia pastoris fermentation optimization: energy state and testing a growth-associated model. Appl Microbiol Biotechnol 72:297–305
Schenk J, Balazs K, Jungo C, Urfer J, Wegmann C, Zocchi A, Marison IW, von Stockar U (2008) Influence of specific growth rate on specific productivity and glycosylation of a recombinant avidin produced by a Pichia pastoris Mut+ strain. Biotechnol Bioeng 99:368–377
Sinclair G, Choy FY (2002) Synonymous codon usage bias and the expression of human glucocerebrosidase in the methylotrophic yeast, Pichia pastoris. Protein Expr Purif 26:96–105
Smith RA, Duncan MJ, Moir DT (1985) Heterologous protein secretion from yeast. Science 229:1219–1224
Subramanian GM, Fiscella M, Lamousé-Smith A, Zeuzem S, McHutchison JG (2007) Albinterferon alpha-2b: a genetic fusion protein for the treatment of chronic hepatitis C. Nat Biotechnol 25:1411–1419
Surribas A, Stahn R, Montesinos JL, Enfors SO, Valero F, Jahic M (2007) Production of a Rhizopus oryzae lipase from Pichia pastoris using alternative operational strategies. J Biotechnol 130:291–299
Uccelletti D, Farina F, Mancini P, Palleschi C (2004) KlPMR1 inactivation and calcium addition enhance secretion of non-hyperglycosylated heterologous proteins in Kluyveromyces lactis. J Biotechnol 109:93–101
Wang J, Nguyen V, Glen J, Henderson B, Saul A, Miller LH (2005) Improved yield of recombinant merozoite Surface protein 3 (MSP3) from Pichia pastoris using chemically defined media. Biotechnol Bioeng 90:838–847
Werten MW, van den Bosch TJ, Wind RD, Mooibroek H, de Wolf FA (1999) High-yield secretion of recombinant gelatins by Pichia pastoris. Yeast 15:1087–1096
Woo JH, Liu YY, Mathias A, Stavrou S, Wang Z, Thompson J, Neville DM Jr (2002) Gene optimization is necessary to express a bivalent anti-human anti-T cell immunotoxin in Pichia pastoris. Protein Expr Purif 25:270–282
Woo JH, Liu YY, Stavrou S, Neville DM Jr (2004) Increasing secretion of a bivalent anti-T-cell immunotoxin by Pichia pastoris. Appl Environ Microbiol 70:3370–3376
Woo JH, Liu JS, Kang SH, Singh R, Park SK, Su Y, Ortiz J, Neville DM Jr, Willingham MC, Frankel AE (2008) GMP production and characterization of the bivalent anti-human T cell immunotoxin, A-dmDT390-bisFv(UCHT1) for phase I/II clinical trials. Protein Expr Purif 58:1–11
Xiao AF, Zhou XS, Zhou L, Zhang YX (2006) Detection of intracellular reactive oxygen species by flow cytometry in Pichia pastoris fermentation. Chinese J Biotechnol 22:273–277
Xue C, Zhao HL, Liu ZM (2005) A simple and practical densimetric scanning protocol with computer programs. Lett Biotechnol 16:284–286
Zhang W, Zhao HL, Xue C, Xiong XH, Yao XQ, Li XY, Chen HP, Liu ZM (2006) Enhanced secretion of heterologous proteins in Pichia pastoris following overexpression of Saccharomyces cerevisiae chaperone proteins. Biotechnol Prog 22:1090–1095
Zhang W, Inan M, Meagher MM (2007) Rational design and optimization of fed-batch and continuous fermentations. Methods Mol Biol 389:43–64
Zhao HL, Xue C, Wang Y, Li XY, Xiong XH, Yao XQ, Liu ZM (2007) Circumventing the heterogeneity and instability of human serum albumin-interferon-alpha2b fusion protein by altering its orientation. J Biotechnol 131:245–252
Zhao HL, Xue C, Wang Y, Duan QF, Xiong XH, Yao XQ, Liu ZM (2008) Disruption of Pichia pastoris PMR1 gene decreases its folding capacity on human serum albumin and interferon-alpha2b fusion protein. Yeast 25:279–286
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, H.L., Xue, C., Wang, Y. et al. Increasing the cell viability and heterologous protein expression of Pichia pastoris mutant deficient in PMR1 gene by culture condition optimization. Appl Microbiol Biotechnol 81, 235–241 (2008). https://doi.org/10.1007/s00253-008-1666-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1666-0