Abstract
Erysipelothrix rhusiopathiae, the causative agent of swine erysipelas, was cultivated in a 5-L stirred and aerated bioreactor under different dissolved oxygen tensions (0%, 5%, and 30% of saturation) for evaluation of the influence of oxygen on cell growth as well as on the production of the main antigenic component of the vaccine against erysipelas, a 64–69 kDa protein (SpaA). The microorganism presented different growth profiles for different aeration conditions. However, at the end of the batch cultivations, similar cell concentrations were obtained under the studied conditions. In order to maximize biomass titers and antigen production, the microorganism was cultivated in fed-batch operation mode under aerobic conditions. Under this condition, there was a fivefold increase in biomass production in comparison to the results attained in batch cultivations. To follow up antigen expression, samples collected during batch cultivations were concentrated and treated with choline for antigen extraction. Antigen expression was then assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and by murine immunization tests. It was observed a direct influence of oxygen availability upon antigen expression, which is favored in the presence of oxygen. Analysis of the samples collected throughout the fed-batch process also revealed that antigen production is growth associated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Erysipelothrix rhusiopathiae is a Gram-positive and facultative anaerobic rod that causes erysipelas besides many other illnesses, such as sheep polyarthritis and human erysipeloid (Wood 1992).
Swine erysipelas is among the diseases that cause great worldwide losses in swine culture (Makino et al. 1998), and it has been combated by the use of vaccines containing attenuated live microorganisms (Japan) or inactivated microorganisms (other countries). Despite the lack of a detailed comprehension concerning the interaction between microorganism and host (Shimoji 2000), these vaccines have satisfactorily combated the disease in its acute and subacute forms; however, they are incapable of preventing the chronic form (Wood 1992).
So far, most of the studies regarding this microorganism have focused almost exclusively on the elucidation of its antigens, with the purpose of developing an antigenic subunit vaccine against this pathogen.
The main antigenic agent is a protein of 64–69 kDa (designated SpaA) present in the culture medium supernatant (Lachmann and Deicher 1986) as well as adhered to the cell surface through interactions with choline residues of the teichoic and lipoteichoic acids present in the cell wall (Makino et al. 2000). Doses of 500 and 100 μg of this purified antigen provided a degree of protection equivalent to vaccines with the attenuated microorganism in immunization tests performed in swine (Yamazaki et al. 1999). A study using 15 strains of E. rhusiopathiae demonstrated that antigen production is strain-dependent and immunization test results in mice indicated a direct correlation between the concentration of this antigen and the level of protection offered by different prepared bacterins (vaccines with the inactivated microorganism, Kitajima et al. 2000).
However, antigen production is not only associated with the strain but also with the cultivation conditions used for its production. In spite of that, no studies regarding optimization of E. rhusiopathiae cultivation in bioreactors are found in the literature, not even concerning the influence of the oxygenation levels upon the growth of the microorganism and antigen production.
In fact, all results previously reported in the literature were obtained in batch experiments, which were carried out in incubators with the use of flasks, agitated or not, and, therefore, with no adequate control of variables important for cell growth as well as product formation, such as pH and dissolved oxygen concentration. Nevertheless, it is necessary for the definition of optimum cultivation conditions the use of bioreactors equipped with accessories for measurement and control of temperature, pH, dissolved oxygen supply, and agitation speed. Besides, the industrial production of the vaccine is carried out in bioreactors, but there is not any information available about the best conditions to operate them in order to maximize the production.
Aiming to contribute to the elucidation of these important questions, the present work reports the study of the growth of this pathogen as well as the influence of oxygen supply upon antigen expression using aerated-stirred tank-type bioreactors in experiments conducted under different dissolved oxygen concentrations.
Materials and methods
Microorganism and culture media
Strains NCTC 11004 and 11002 of E. rhusiopathiae were used in this research. The latter strain, more virulent, was used as the contamination agent in the immunological tests of bacterins in mice.
Different culture media used in the experiments were based on the modified Feist medium (Groschup and Timoney 1990) with changes in the concentration of a few nutrients. A culture medium containing 9.0 g.L−1 glucose, 7.5 g.L−1 proteose peptone n°2 (Difco, France), 7.5 g.L−1 yeast extract (Acumedia, Michigan), 0.75 g.L−1 L-arginine, and 0.75 mL.L−1 Tween 80 dissolved in 20 mM Na2HPO4.12H2O/KH2PO4 (95/5 v/v) buffer, pH 8.0 (reagents with analytical degree of purity) was used for the simple batch cultivations. For the fed-batch experiment, the feed stream was prepared in the same buffer and consisted of 150 g.L−1 of glucose, with all other nutrients in proportionally increased concentrations.
Cultivations in bioreactor
Simple batch
Cultivations were performed for three levels of oxygen availability: (a) aerobiosis (dissolved oxygen concentration at 30% of its saturation limit), (b) limited oxygenation (dissolved oxygen at 5% of its saturation limit), and (c) anaerobiosis. The gas supplied to the bioreactor was air in cultivations (a) and (b) and nitrogen in (c). In all experiments, the pressure in the bioreactor was maintained at 770 mm Hg and the temperature of the gas inflow was approximately 25°C.
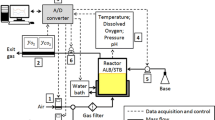
The experiments were performed in a 5.0-L bioreactor model Bioflo II (New Brunswick Sci. Co. Inc., USA) equipped with a carbon dioxide analyzer for the outlet gas (Rosemount model 880), a mass flow meter (Cole Parmer model EW33116-10) and a dissolved oxygen meter (Metler Toledo model InPro 6050). All accessories were connected to a data control and acquisition system (PLC GE-Fanuc). Air or nitrogen flow rates were kept at 1.0 L.min−1 (NCPT) for experiments (a) and (c), while for experiment (b), air flow rate was reduced to 0.5 L.min−1. The stirrer speed was varied between 100 and 400 rpm in order to maintain the dissolved oxygen concentration at the desired levels. All experiments were performed at 37°C and pH 8.0 by the addition of a 4.0 M NaOH solution.
The experiments started with the activation of a cryotube containing 3.0 mL of cell suspension, which was transferred to 27 mL of fresh medium (0.2 M Na3PO4.12H2O buffer, pH 8.0) and incubated at 37°C for 17 to 20 h, at rest. After the broth reached an optical density (OD) of 1.5 (at λ = 420 nm) 30 mL of activated broth were used for the preparation of 300 mL of inoculum. After reaching an OD of 1.5, the inoculum was then transferred to the bioreactor containing 2.7 L of medium.
Fed-batch operation
With the objective of estimating the feed flow rate to be implemented in the fed-batch and also the required glucose concentration in the feed for supporting cell growth, many profiles of cell concentration (CX), substrate concentration (CS), and acid lactic concentration (CP) were sequentially simulated with the software AnaBio 1.0 (Silva et al. 2003). A simple unstructured mathematical model was set up, consisting of the mass balance Eqs. 1–3 and the Andrews-Levenspiel expression (Shuler and Kargi 2002), with mixed inhibition by substrate and product, for description of the growth kinetics (Eq. 4).
Parameters and other symbols used in Eqs. 1 to 4 are defined as follows:
V (L) = bioreactor working volume; F (L.h−1) = flow rate of nutrient feed; C SF = glucose concentration in the nutrient feed; μ = specific growth rate (h−1); μ max (h−1) = maximum specific growth rate; K S (g.L−1) = saturation constant; K IS (g.L−1) = substrate inhibition constant; Cp* (g.L−1) = critic product concentration for product inhibition; n = parameter from the Levenspiel model with product inhibition; α \(\left( {g_{lactate} \cdot g_{DCW}^{ - 1} } \right)\) = pseudo-stoichiometric coefficient for lactate formation associated to growth; \(Y_{{X \mathord{\left/ {\vphantom {X S}} \right. \kern-\nulldelimiterspace} S}} {\text{ }}\left( {g_{DCW} .{\text{ }}g_{glucose}^{ - 1} } \right)\) = cell mass yield coefficient; \(Y_{{P \mathord{\left/ {\vphantom {P S}} \right. \kern-\nulldelimiterspace} S}} {\text{ }}\left( {g_{lactate} .{\text{ }}g_{glucose}^{ - 1} } \right)\) = product yield coefficient; k D (h−1) = cell death coefficient (h−1); C S 1 = substrate concentration from which cell death starts. All concentrations are given in gram per liter.
The mean values of the experimental data from the batch aerobic cultivations carried out in triplicate were used for parameter estimation. Only the data corresponding to the exponential growth phase (first 7.5 h of cultivation) were used for parameter estimation once the simulations were employed to choose feed profiles that extended the exponential growth during fed-batch operation. The values for the yield coefficients and maximum specific growth rates were determined directly from experimental data. The remaining parameters were estimated by setting F to zero and visually fitting the model to the experimental data referred previously. The model was then used for simulating fed-batch operations at different values of F and C SF in order to identify the feeding condition that supported intense growth while maintaining predicted glucose concentration between 4.0 and 8.0 g.L−1 during the entire fed-batch operation. Based on the simulation results, a feeding strategy in two stages was adopted: the addition of supplementary medium began after 7.5 h of simple batch run, with a flow rate of 0.05 L.h−1 and lasted 4 h (stage 1); the flow rate was then raised to 0.12 L.h−1 and kept at this value for 8.5 h (stage 2). The concentration of glucose in the nutrient medium was chosen as 150 g/L.
Analytical methods
Determination of cell concentration
Cell concentration was followed through the measurement of the absorbance of the culture broth at 420 nm (optical density) and by the dry cell weight method. Cell viability was evaluated by the determination of colony forming units (CFU).mL−1: culture samples of 0.1 mL were sequentially diluted in a sterilized solution of NaCl 0.9% and spread on the surface of tryptose phosphate agar plates. The colony counting was performed in triplicate for dilutions of 105 to 107 after an incubation period of 48 h.
Determination of glucose and metabolite concentrations
These analyses were performed by HPLC using an Aminex HPX-87H column (Bio-Rad) and a 5 mM sulfuric acid solution at a flow rate of 0.6 mL.min−1 as mobile phase. The temperature was 50°C. Organic acids were detected at 210 nm (Waters 486 UV-detector), while ethanol and glucose were measured with a refraction index detector (Waters 410).
Antigen extraction and SDS-PAGE analysis
Samples collected during the fed-batch run and in all batch cultures, whenever the broth OD reached 3.0 were inactivated with the addition of 0.3% formalin (v/v; Zarkasie et al. 1996). Cells and supernatant were separated by centrifugation at 10,000×g, 4°C for 20 min. Following that, the cells were resuspended in a 0.9% NaCl and 2% choline chlorine solution (w/v) in a volume ten times smaller than the original sample volume, and left overnight under mild agitation at 4°C. Subsequently, the solutions were filtered in a 0.22 μm membrane and concentrated tenfold by ultrafiltration (Amicon Ultra system–Millipore, 30 kDa NMWL; Makino et al. 2000, modified). The samples were then analyzed by 15% polyacrylamide gel electrophoresis under denaturing conditions (Laemmli 1970).
Immunological procedures and analyses
Bacterin preparation
The cultures were interrupted with the addition of 0.3% formalin (v/v; Zarkazie et al. 1996) and the bacterin formulation was made with the addition of aluminum hydroxide gel as described by Eamens et al. (2006). Comparison tests between the bacterins prepared under different oxygenation conditions (always in duplicate) used formulations with 2.0 × 108 CFU.mL−1. For the immunization tests with the aerobic bacterin compared to commercial vaccines, 2.0 × 109 CFU.mL−1 were employed.
Vaccination and challenge
Female mice of the Swiss lineage with 17 to 20 g were used. The animals were divided into groups (vaccine tests and negative control) with ten mice each. Vaccination was performed subcutaneously in a single dose of 1/50 the dose for swine, and 21 days after the vaccination the challenge was performed with a 0.3-mL intraperitoneal injection of virulent E. rhusiopathiae culture (challenge strain), corresponding to 1,000 DL50. The animals were observed for 8 days and the number of deaths was registered for each group.
Results
Influence of the dissolved oxygen concentration on growth and metabolite formation in E. rhusiopathiae cultures
The experimental results obtained in the simple batch cultures under controlled conditions of pH and dissolved oxygen concentrations are shown in Fig. 1.
Similar cell concentrations were obtained for the three studied oxygenation conditions as shown in Fig. 1. No significant lag phase was observed for any of the experiments. However, the condition DO-5% of saturation displayed a somehow slower growth rate.
After the exponential phase, the differences between the studied conditions became more evident: a long stationary phase appeared in the absence of oxygen and growth persisted in the aerated conditions, however less intensely, until the cellular death phase started.
The maximum cell concentration achieved in anaerobiosis was the lowest among the three studied conditions. Such a fact may be explained by the inhibition effect caused by the accumulation of lactic acid, which would be more toxic in the absence of oxygen. Indeed the highest maximum cell concentration was reached in the DO-5% culture, despite the fact that lactate concentrations reached similar values in the anaerobic and DO-5% experiments (Table 1).
Besides lactic acid, the microorganism also produced small quantities of acetic acid, formic acid, and traces of ethanol from glucose in the three investigated conditions. The carbon balance for each condition was performed using the results from the metabolite analyses of all experiments (Table 2).
A small deficit of 5.2% was obtained in the aerobic condition and a difference of 1.2% in the anaerobic cultivation, indicating that the main catabolites were measured. In the 5% dissolved O2 saturation culture, apparently, there was a greater consumption of other nutrients of the medium as carbon sources. In this case, because the energy produced by the deficient respiration was not sufficient to maintain growth, other carbon sources started to be consumed, while different fermentation pathways were activated.
Despite the fact that microbial growth was slightly favored in the conditions with low or no oxygen, the aerobic condition was studied in more detail and was chosen as the condition to be adopted for fed-batch process. This choice was based on the observation that any strategy to maximize biomass production in the cultivation of this microorganism must overcome the growth-inhibition problem caused by the accumulation of lactic acid. This effect is markedly reduced in aerobiosis. Thus, the study of this condition was carried out in triplicate, through three independent experiments performed at identical conditions.
A high reproducibility was observed in this study (Fig. 2). Furthermore, a good agreement between the measures using optical density and the gravimetric method was observed for following microbial growth. Cell concentrations in the order of 1.8 gDCW.L−1 were reached, and, at the maximum cell growth point, an average of 3.6 × 109 CFU.mL−1 was obtained.
Evaluation of the immunization level of the vaccine prepared from the aerobic cultivation
For evaluation of the protection level of the bacterins prepared under the conditions here established, a vaccine was formulated using the culture broth from one of the aerobic cultivations. This formulation was compared with three commercially available vaccines in immunization tests using mice, and the results are shown in Fig. 3.
The administered doses contained 2.0 × 109 CFU.mL−1 and offered an excellent level of protection against the 1,000 LD50 challenges of a virulent E. rhusiopathiae strain. The results showed that the prepared formulation presented a very good immunization capacity against swine erysipelas, which was similar to the protection provided by the vaccines currently available in the market.
Fed-batch culture
Fed-batch operation is a well-known cultivation strategy to cope with inhibition by high glucose concentrations and to reach high biomass concentration through the periodic addition of the depleting nutrients. The experimental results of E. rhusiopathiae fed-batch cultivation are displayed in Fig. 4. As expected, this operation mode favored larger biomass formation, and the glucose concentration remained within adequate limits so that inhibition effects or intense cell death did not take place during the experiment. It is important to notice that the fed-batch experiment was carried out according to the feeding strategy suggested by the simulation results, as described “Fed-batch operation.” The predicted values for biomass, lactic acid, and glucose concentrations obtained through the model simulations for fed-batch operation were also included at Fig. 4. The values of the parameters used in these simulations are listed in Table 3 for each of the two predicted feeding stages. Despite the simple approach used for modeling and parameter estimation, an excellent fit of the simulated results to the experimental data, especially for cell growth and lactic acid formation, can be observed at Fig. 4.
Furthermore, the feed profile recommended by the simulations performed quite well on keeping the glucose concentration at the desired range. Thus, as predicted, after 7.5 h of cultivation, the concentration of this substrate fell below 5.0 g.L−1, and the fed-batch operation started. The flow rate of concentrated culture medium used in this first stage was enough to sustain the same pattern of cell growth of the simple batch during 4 h, when glucose concentration once again fell below 5.0 g.L−1. At this moment, the feeding inflow was raised, initiating the second stage of the fed-batch that allowed the continuity of the biomass growth, up to the point where lactic acid inhibition became important. Glucose accumulation was observed in this stage of the experiment, however, never above 12.0 g.L−1. Besides lactic acid, small concentrations of acetic and formic acids, up to 2.2 and 0.5 g.L−1, respectively, accumulated in the medium at the end of fed-batch run.
In Fig. 4, it is observed that the employed conditions enabled a constant and intense cell growth up to nearly 9.0 gDCW.L−1 of cells, where lactate inhibition began when its concentration approached 10 g.L−1. From this point on, a stationary phase was reached where the consumed glucose was used for maintenance of the cell population tolerant to the current lactate concentration. The nutrient feed supply lasted for 20h. But, the residual glucose present in the medium continued to be consumed, being mainly converted to lactic acid and other metabolites until the experiment was interrupted after 21.5 h of cultivation. At the end, the cell concentration was 9.9 gDCW.L−1, corresponding to an optical density reading of 18.0 and to a counting of 1.7 × 1010 CFU.mL−1.
Figure 5 shows the increment of biomass reached with the fed-batch strategy compared to the simple batch cultures, along with the lactic acid production during the cultivations.
The electrophoresis analyses of the samples collected during the fed-batch process revealed that antigen production is associated to cell growth. The concentration of the SpaA antigen raised significantly with time, following the increment in cell concentration throughout the cultivation (Fig. 6).
Electrophoresis of the samples collected throughout the fed-batch run. Lanes 1 and 6, Molecular mass standards (BenchMark Protein Ladder–Invitrogen); 2 to 5, choline-extracted surface proteins of samples at 6.5, 12.5, 17.5, and 20 h, respectively. Samples collected at 6.5 and 12.5 h revealed with silver nitrate; Samples collected at 17.5 and 20 h revealed with Coomassie Brilliant Blue
Influence of dissolved oxygen concentration in antigen expression
Antigen expression, under the tested aeration conditions, was assessed through the comparison of the protection levels offered by the bacterins prepared from the culture broths obtained from these experiments. For bacterin formulation, interruption of cell growth occurred when the optical density of the broth was approximately 1.5 for all experiments, corresponding to a cell concentration of about 2.0 × 108 CFU.mL−1. As shown in Fig. 7, the vaccines prepared in the anaerobic condition presented the worst performance in the immunization tests.
Besides the immunization tests, the influence of dissolved oxygen concentration upon antigen expression was further investigated through electrophoresis. Culture samples were collected between the intermediary and the final phase of growth, when the optical density was about 3.0 and processed as described in the antigen extraction and SDS-PAGE analysis section. The comparison of the SpaA protein band observed in the electrophoresis gels confirmed the results obtained in the immunization tests. Although the bacteria grew well either in the absence or in the presence of oxygen, antigen production was smaller in the former case, and it was not possible to observe this band in the concentrated sample from the anaerobic cultivation, as shown in Fig. 8.
Electrophoresis of concentrated extracts containing the antigen in choline solution, in the three studied bioreactor aeration conditions. Lane 1, molecular mass standards (Benchmark Protein Ladder–Invitrogen); Lane 2, 30% of oxygen saturation; Lane 3, 5% of saturation; Lane 4, anaerobic, 0% of saturation
Discussion
Different aspects of E. rhusiopathiae cultivation were evaluated here for the first time, leading to a better knowledge about the growth and antigen expression of this pathogen. These findings are very important for the establishment of an optimized culture condition of this microorganism for the preparation of an effective vaccine to combat swine erysipelas.
The different aeration conditions studied during the cultivation of the bacterium showed to have an important influence on the growth pattern and especially on antigen expression by the microorganism.
In respect to the growth kinetics, among the three different aeration conditions studied, in general, the condition of 5% dissolved O2 tension presented an intermediate behavior between the aerobic and anaerobic conditions. In this condition, smaller rates of carbonic dioxide production and higher rates of lactic acid and other metabolites production were observed, bringing the results of this cultivation closer to those obtained in anaerobiosis. However, the DO-5% maximum specific growth rate was closer to the aerobic condition one, while its maximum OD was the highest (Table 1).
Contradicting the expected behavior, the maximum specific growth rate in the absence of oxygen was as higher as in aerobiosis. This observation indicates possible deficiencies in the oxidative phosphorylation or in the electron transport system of E. rhusiopathiae because this microorganism is unable to efficiently use oxygen as energy source, although it is capable of consuming it.
Concerning the limiting growth conditions, low glucose concentrations (approximately 2.0 g.L−1 or less) are associated with a sudden reduction of cell viability in the end of the cultivations (Table 1). This effect was taken as responsible for the interruption of cell growth in the aerobic batch cultures. Similarly, the low glucose concentration at the end of cultivation was also identified as the factor limiting microbial growth in the condition of DO-5%. On the other hand, the lactic acid inhibition was responsible for the deceleration of the growth rate after approximately 6 h of cultivation in the anaerobic condition (Fig. 1).
In the fed-batch culture, as observed in the previous aerobic experiments, fermentative pathways of the microorganism were activated even in aerobic conditions. A great accumulation of lactic acid was observed, and the concentration limit of this metabolite that causes effective cell-growth inhibition was reached. After reaching 10 g.L−1 of lactate, as already said, an interruption in the raise of biomass production occurred, and a phase where cell concentration remained practically constant was reached, despite the continuous increment in the quantity of metabolites present in the medium, as shown in Fig. 5a.
The comparison of the results obtained in all cultivations (Fig. 5b) indicates that the fed-batch presented an almost fivefold raise in the maximum OD in a period of time only two times larger than the simple batch experiments. Production of lactic acid followed the same profile of the aerobic cultures during the simple batch stage and subsequently accompanied the growth of the microorganism. In the simple batch fermentations with little or no oxygen, the production of this organic acid occurred more intensely, indicating that fed-batch, in these conditions, would not be an efficient strategy for obtaining high cell densities.
About the influence of oxygen in antigen expression, the highest levels of protection in the in vivo tests were observed with bacterins prepared from the cultivations performed in the presence of oxygen, as well as higher concentrations of antigen were observed in the samples of this experiment by the sodium dodecyl sulfate polyacrylamide gel electrophoresis analyses. This observation is in agreement with the fact that environmental changes, such as oxygen supply, induce pathogenic microorganisms to adapt, sometimes leading to phenotypic (Weiser et al. 1994) and metabolic alterations.
In the case of SpaA, the influence of the different oxygen availability conditions are observed in the expression of this surface protein, which possibly acts on cell adhesion mechanisms and has an important role in the infection processes caused by E. rhusiopathiae (Makino et al. 2000). A similar role is played by proteins RspA and RspB, also identified as antigens (Shimoji 2003).
The achieved results highlight the importance of adequate control of culture conditions for the production of more immunogenic bacterins from E. rhusiopathiae. The observation that antigen production is directly associated to cell growth justifies the use of the fed-batch operation mode for obtaining larger biomass and, consequently, larger antigen concentrations per batch. Furthermore, the adequate choice of the oxygenation level also proved to be crucial. The entirely aerobic condition (at 30% of O2 saturation, with air inflow) is indicated for vaccine production because it allows cultivation of the microorganism in fed-batch with minimum accumulation of lactic acid (an inhibitor product). In addition, aerobic condition favors antigen expression, as observed in immunization tests using bacterins prepared in different aerating conditions. These results were crosschecked through the electrophoresis of antigen-rich extracts from these cultivations.
References
Eamens GJ, Chin JC, Turner B, Barchia I (2006) Evaluation of Erysipelothrix rhusiopathiae vaccines in pigs by intradermal challenge and immune responses. Vet Microbiol 116:138–148
Groschup MH, Timoney JF (1990) Modified Feist broth as a serum-free alternative for enhanced production of protective antigen of Erysipelothrix rhusiopathiae. J Clin Microbiol 28:2573–2575
Kitajima T, Oishi E, Amimoto K, Ui S, Nakamura H, Oda K, Katayama S, Izumida A, Shimizu Y (2000) Quantitative diversity of 67 kDa protective antigen among serovar two strains of Erysipelothrix rhusiopathiae and its implication in protective immune response. J Vet Med Sci 62:1073–1077
Lachmann PG, Deicher H (1986) Solubilization and characterization of surface antigenic components of Erysipelothrix rhusiopathiae T28. Infect Immun 52:818–822
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Makino S, Yamamoto K, Murakami S, Shirahata T, Uemera K, Sawada T, Wakamoto H, Morita H (1998) Properties of repeat domain found in a novel protective antigen, SpaA, of Erysipelothrix rhusiopathiae. Microb Pathog 25:101–109
Makino S-I, Yamamoto K, Asakura H, Shirahata T (2000) Surface antigen, SpaA, of Erysipelothrix rhusiopathiae binds to Gram-positive bacterial cell surfaces. FEMS Microbiol Lett 186:313–317
Shimoji Y (2000) Review: Pathogenicity of Erysipelothrix rhusiopathiae: virulence factors and protective immunity. Microb Infect 2:965–972
Shimoji Y, Ogawa Y, Osaki M, Kabeya H, Maruyama S, Mikami T, Sekizaki T (2003) Adhesive surface proteins of Erysipelothrix rhusiopathiae bind to polystyrene, fibronectin, and type I and IV collagen. J Bacteriol 185:2739–2748
Shuler ML, Kargi F (2002) Bioprocess engineering: basic concepts. Prentice Hall, Englewood Cliffs
Silva FH, Moura LF, Badino Jr AC (2003) AnaBio 1.0: Um Programa para Análise de Biorreatores, XIV Simpósio Nacional de Fermentação, anais. Florianópolis, Santa Catarina
Weiser JN, Austrian R, Screenivasan PK, Masure HR (1994) Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun 62:2582–2589
Wood RL (1992) Erysipelas. In: Leman AD et al (ed) Disease of swine. Iowa State University Press, Iowa, pp 475–486
Yamazaki Y, Sato H, Sakakura H, Shigeto K, Nakano K, Saito H, Maehara N (1999) Protective activity of the purified protein antigen of Erysipelothrix rhusiopathiae in pigs. J Vet Med B 46:47–55
Zarkasie K, Sawada T, Yoshida T, Takahashi I, Takahashi T (1996) Growth ability and immunological properties of Erysipelothrix rhusiopathiae. J Vet Med Sci 58:87–90
Acknowledgements
The authors thank Vallée S.A. for funding this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, A.J., de Baptista-Neto, Á., do Carmo Cilento, M. et al. Bioreactor aeration conditions modulate growth and antigen expression during Erysipelothrix rhusiopathiae cultivation. Appl Microbiol Biotechnol 79, 23–31 (2008). https://doi.org/10.1007/s00253-008-1399-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1399-0