Abstract
One strain of Lactobacillus salivarius, two strains of Lactobacillus reuteri and Lactobacillus amylovorus, and two strains of Bifidobacterium thermacidophilum with antagonistic effect against Clostridium perfringens were isolated from porcine gastrointestinal tract. Isolates were assayed for their ability to survive in synthetic gastric juice at pH 2.5 and were examined for their ability to grow on agar plate containing porcine bile extract. There was a large variation in the survival of the isolates in gastric juice and growth in the medium containing 0.3% (w/v) bile. L. salivarius G11 and L. amylovorus S6 adhered to the HT-29 epithelial cell line. Cell-free supernatant of L. amylovorus S6 showed higher antagonistic activity as effective as the antibiotics such as neomycin, chlortetracycline, and oxytetracycline against bacterial pathogens including C. perfringens, Salmonella typhimurium, Staphylococcus aureus, Vibrio cholerae, Edwardsiella tarda, and Aeromonas salmonicida subsp. salmonicida.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clostridium perfringens is divided into five different types (types A–E) on the basis of production of lethal toxins. C. perfringens type A is consistently found in both the gastrointestinal tracts (GIT) of warm-blooded animals and the environment, while others (types B–E) are less common in the GIT of animals and can occasionally be found in places where disease caused by these pathogens is enzootic (Carter and Chengappa 1991; Niilo 1980; Songer 1996; Timoney et al. 1988). C. perfringens type A α-toxin (phospholipase C) is the cause of gas gangrene and necrotic enteritis in humans and animals. In necrotic enteritis, the lesions in the gut wall result in mortality of affected hosts after a clinical course of 6 to 12 h. Since described in 1961 (Parish 1961), this disease has been reported in most areas of world, where poultry are produced under intensive management conditions, and has been responsible for significant economic losses in poultry production (Van der Sluis 2000).
To control the incidence, antibiotic growth promoters such as penicillin G, tetracycline, bacitracin, and virginiamycin have been used in feed. However, the excessive use of antibiotics results in the development of antibiotic-resistant strains of human and animal pathogens (Emborg et al. 2004). Moreover, such antibiotics can be lethal to beneficial microorganisms in the GIT of human and animals, and they may also enter the food chain and accumulate in human body as undesirable chemical residues. Many European countries have banned the use of antibiotics including penicillin G, tetracycline, bacitracin, and virginiamycin because of the increasing public concern of the possible negative effect of antibiotics on the environment and human health. Therefore, there is a need for development of alternative control methods to the antibiotics used in feed to protect the animals from diseases such as NE. One of the alternative methods is biological control using antagonistic metabolites produced by microorganisms (probiotics) including lactic acid bacteria (Daeschel 1989; Jack et al. 1995; Jiraphocakul et al. 1990; Piard and Desmazeaud 1992).
Probiotics are defined as live microorganisms which contribute to the health and well-being of the hosts by maintaining or improving their intestinal microbial balance (Fuller 1989; Gatesoupe 1999). Lactobacillus spp., Bifidobacterium spp., and Bacillus subtilis can be used as probiotics that enhance and maintain beneficial bacteria in the GIT (Hoa et al. 2000; Saarela et al. 2000). Probiotic strains have been also reported that inhibit C. perfringens by production of antimicrobial agents (Barbosa et al. 2005; Kizerwetter-Swida and Binek 2005; La Ragione et al. 2004). To remain and exert probiotic potential within their host, two factors are usually considered. First, probiotic strains must possess the ability to overcome the extremely low pH of gastric juice and the detergent effect of bile salts and arrive at the site of action in a viable physiological state (Chou and Weimer 1999; Salminen et al. 1989, 1999). Second, they should be capable of adhering to the intestinal mucosa. The adherence to intestinal mucosa is indispensable for colonization of probiotics. Adhesion to and colonization of the mucosal surfaces have potential ability to prevent pathogens through competition for binding site and nutrients (Naidu et al. 1999; Westerdahl et al. 1991).
In an attempt to find probiotic strains that inhibit C. perfringens, we screened Lactobacillus and Bifidobacterium isolates from GIT of healthy pig. In this paper, we describe the processes of isolation and characterization of probiotics with anti-Clostridium effects. Probiotic strains that survived in the simulated GIT conditions and adhered to intestinal mucosa were further evaluated for their antagonistic effect against other human, animal, and fish pathogens.
Materials and methods
Sample collection
Fresh samples, large intestine, small intestine (proximal, middle, and distal), rectum, ileum, cecum, stomach, and feces, were collected immediately from healthy pig after slaughter at a commercial pig processing plant in Daejeon, Korea. Samples were placed separately in sterile disposable tubes (50 ml), kept on ice, and immediately transferred to an anaerobic chamber (Sheldon Manufacturing, Cornelius, OR, USA) under 85% N2, 10% H2, and 5% CO2, and the remaining samples were stored at −70 °C for further study.
Bacterial cultures and maintenance
Human and fish pathogens C.perfringens KCTC 3269, Salmonella typhimurium KCTC 1925, Staphylococcus aureus KCTC 1928, Vibrio cholerae KCTC 2715, Edwardsiella tarda KCTC 12267, and Aeromonas salmonicida subsp. salmonicida KCTC 12266 were kindly obtained from the Korean Collection for Type Cultures (KCTC, Daejeon, Korea). C. perfringens was incubated anaerobically in reinforced clostridial medium (RCM, Difco) at 37 °C. S. typhimurium, S. aureus, E. tarda, and A. salmonicida subsp. salmonicida were cultured in nutrient agar (Difco) medium. V. cholerae was grown in marine agar (Difco, USA) at 37 °C. The stock cultures of the isolates were stored in medium containing 20% glycerol at −70 °C.
Isolation of pig microflora
For isolation of bacteria from the pig intestinal compartments and feces, serial tenfold dilutions were made from each sample suspended in phosphate-buffered saline (PBS, pH 7.4). The samples were plated on the media including brain heart infusion (BHI), Man Rogosa Sharpe (MRS), glucose yeast extract peptone (GYP), Brucella blood agar (Brucella), and RCM. All plate media were prereduced overnight in the anaerobic chamber, and the aliquots (100 μl) were spread on the plates under anaerobic conditions and incubated anaerobically over 48 h at 37 °C. All isolated bacteria were stored at −70 °C in 20% glycerol stocks for further study.
Screening and identification of antagonists
Antagonistic effect of the isolates against C. perfringens was assayed by the following method. From the plates, over 400 isolates were picked and reinoculated onto an appropriate medium for growth. After incubation for 24 h, the colonies were overlaid with lawns of C. perfringens (ca. 1 × 107 CFU ml−1) and further incubated overnight at 37 °C. Among the isolates, seven strains (F1, L1, S6, M35, M40, G11, and G29) showing clear zones with a diameter of ≥5 mm were selected as the candidates. Three (S6, M35, and M40) and four strains (F1, L1, G11, and G29) were cultured in MRS and GYP broths, respectively, that showed optimal antagonistic effect under anaerobic condition at 37 °C for 48 h. To test antibacterial activity, their cell-free supernatants were collected by centrifugation at 1,000 × g for 15 min and by aseptic filtration (0.45 μm). Cells of C. perfringens were grown at 37 °C in RCM broth. The bacterial cells were seeded on 96-well microtiter plate (Greiner, Nurtingen, Germany) in RCM broth at a density of 1 × 105 cells (150 μl per well). Fifty microliters of the serially diluted supernatant was added to each well, and the cell suspension was incubated for 24 h at 37 °C. The turbidity of each well was measured at 550 nm using a microtitrator ELISA reader (Molecular Devices Emax, CA, USA). Also, antimicrobial spectrum was examined against S. typhimurium, S. aureus, V. cholerae, E. tarda, and A. salmonicida subsp. salmonicida by agar diffusion assay.
For the identification of 16S rRNA sequences, bacterial genomic DNA was isolated using the phenol extraction method (Sambrook et al. 1987). The 16S rRNA genes were amplified using polymerase chain reaction (PCR) with the universal primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGTYACCTTGTTGTTACGACTT-3′) (Lane 1991). The conditions of amplification were: 94 °C for 1 min followed by 30 cycles at 94 °C for 1 min, 50 °C for 1 min, and 72 °C for 1 min and 50 s, with a final 4-min extension at 72 °C. After amplification, the PCR products were purified (AccuPrep PCR purification kit, BIONEER, Daejeon, Korea) and sequenced with the ABI prism™ Bigdye™ Terminator Cycle sequencing ready reaction kit in an automated ABI 3730XL capillary DNA sequencer (Applied Biosystems, Foster City, CA, USA).
Survival in artificial gastric fluid
To examine survival rate of isolates under gastric conditions, bacterial cells grown overnight were washed with PBS (pH 7.4) and adjusted to about 108 to 109 CFU ml−1. One-hundred microliters of cells was transferred to 900 μl of synthetic gastric juice (consisting of 3.5 g d-glucose, 2.05 g NaCl, 0.6 g KH2PO4, 0.11 g CaCl2, 0.37 g KCl, 0.05 g porcine bile (Sigma, USA), 0.1 g lysozyme, and 13.3 g pepsin/l). The pH was adjusted to pH 2.5 using HCl and incubated anaerobically for 0 min, 30 min, and 2 h at 37 °C (Casey et al. 2004). After incubation, viable bacterial cells were counted by plating serial dilutions of the culture in PBS (pH 7.4) on MRS and GYP agar.
Resistance of isolates to porcine bile
MRS and GYP plates containing 0.3, 1.0, and 5.0% (w/v) porcine bile were prepared. These plates were prereduced overnight in the anaerobic chamber before use. Bacterial cells grown overnight were streaked with an inoculating loop onto the surface of individual plates and incubated anaerobically for 48 h at 37 °C. Bile tolerance was e stimated by comparing bacterial growth onto individual agar plates (Casey et al. 2004).
Cell adherence assay
The HT-29 cell line was obtained from the Korea Cell Line Bank (KCLB, Seoul, South Korea). The cells were routinely cultured in RPMI 1640 medium (Gibco BRL, USA) supplemented with 10% heat-inactivated fetal bovine serum. Before the attachment assay, the confluence HT-29 monolayers on 12-well plate (BD Science, USA) were washed three times in prewarmed PBS (25 °C) to remove any culture medium. Overnight bacterial cultures were washed twice with PBS and resuspended in RPMI 1640 with no serum. Samples of prepared bacterial strains (ca. 109 CFU ml−1) were added to the cells and incubated at various times at 37 °C in a 5% CO2 atmosphere. After 2 h attachment, the monolayers were washed six times with PBS to remove nonattached bacteria. The cells were lysed with 0.1% Triton X-100 in PBS. Serial dilutions of the mixtures were then plated onto MRS agar (Difco, USA) and incubated for 72 h at 37 °C. The attachment ability was determined by counting of CFU per milliliter.
Statistical analysis
All experiments were replicated duplicates. Statistical significance was assessed by ANOVA followed by Duncan’s test in SAS software package (version 9.1). The level of significance was defined at p < 0.01.
Comparison of antagonistic effect with antibiotics
To compare antimicrobial activity of cell-free supernatant with several antibiotics, a strain S6 showing the strongest activity was incubated in MRS broth at 37 °C for 48 h. Cell-free supernatant was collected by centrifugation at 1,000 × g for 15 min and filter-sterilized using 0.45 μm filter. Neomycin, chlortetracycline, and oxytetracycline were used as comparative chemical antibiotics. Each antibiotic was dissolved in distilled water and adjusted to a final concentration of 100 ppm. C. perfringens KCTC 3269 was used as a target pathogen and was added to the well of a flat-bottomed, 96-well microtiter plate (Greiner, Nurtingen, Germany) containing Muller-Hinton (Difco) broth. Cell-free supernatants and antibiotics diluted serially were added to each well, and the cell suspension was incubated at 37 °C for 24 h. The turbidity of each well was measured by absorbance at 550 nm using a microtitrator ELISA reader (Molecular Devices Emax, CA, USA). Positive control was normal cells incubated at 37 °C for 24 h, and Muller-Hinton broth without cell suspension was used as negative control. All assays were performed in duplicates.
Results
Screening and identification of antagonists
For the selection of antagonists having potent ability to suppress C. perfringens KCTC 3269, over 400 bacteria were isolated from feces, large intestine, small intestine, cecum, ileum, and rectum of the pig. Among these isolates, seven strains that inhibited C. perfringens growth and produced clear zones in Clostridium lawns with a diameter of ≥5 mm were selected as probiotic candidates. After broth cultivation for 48 h, antagonistic effects were examined with cell-free supernatants by serial dilution in 96-well microplates. All tested probiotic supernatants were found to significantly inhibit the growth of C. perfringens. In addition, 32-fold diluted sample of a strain S6 showed strong antagonistic effect against these bacterium (Fig. 1). Using PCR amplification with universal bacterial 16S rDNA primers (27F and 1492R), PCR products of approximately 1,400 bp were obtained. The sequences of 16S rDNA from probiotic strains were aligned using nucleotide–nucleotide BLAST (blastn) program in the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). According to 16S rDNA sequences and phylogenetic analysis, S6 and M35 had 100% homology with L. amylovorus DSM 20531, G11 had 100% homology with L. salivarius RA2115, and F1 and L1 had 99% homology with Lactobacillus reuteri LU3. M40 and G29 also had 97% homology with Bifidobacterium thermacidophilum B12 (data not shown).
Survival in artificial gastric juice
To examine their ability to survive in gastric juice (pH 2.5), the probiotic isolates were incubated for 0 min, 30 min, and 2 h at 37 °C and assayed by viable cell counting. Survival rates of the probiotic isolates varied during incubation (Table 1). L. amylovorus S6 showed higher survival rate than any other isolates and followed by L. reuteri F1 and L1. These three isolates survived in acidic condition (pH 2.5) for 2 h with cell numbers of 5.0 × 105, 3.7 × 105, and 2.1 × 106 CFU ml−1, although survival rates significantly declined from 0.01 to 0.2%. On the other hand, L. amylovorus M35 and L. salivarius G11 exhibited low viable cell numbers (≤2.0 × 102 CFU ml−1) and survival rates (<0.001%), and none of the two strains of B. thermacidophilum could survive in synthetic gastric juice after incubation for 30 min.
Bile tolerance
Probiotic isolates were also examined for their ability to grow plate containing porcine bile. All isolates grew at concentrations of at least 0.3% (w/v) bile extract, and the maximum value of bile resistance varied from 0.3 to 5.0% (w/v, Table 2). L. reuteri L1 and L. amylovorus S6 showed the maximum resistance at 5.0% concentrations and formed cloudy zones of precipitate in the growth media by producing bile-salt hydrolase (BSH) (Dashkevicz and Feighner 1989).
Adherence assay
Of the seven probiotic strains tested, L. salivarius G11 and L. amylovorus S6 exhibited significant adhesion to the HT-29 cell line compared with the Lactobacillusrhamnosus GG used as positive control (Kontula et al. 1999; Lehto and Salminen 1997). However, three strains, L. amylovorus M35, L. reuteri F1, and L. reuteri L1, adhered to the epithelial cell line in little numbers (Fig. 2). In addition, adhesion assay to the HT-29 cell line of the two Bifidobacterium strains was not determined because they could not grow under aerobic culture condition (data not shown).
Comparison of inhibitory activities of bacterial supernatants with chemical antibiotics
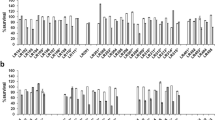
The results of comparison of antagonistic effect of cell-free supernatant of L. amylovorus S6 and chemical antibiotics are shown in Fig. 3. Cell-free supernatant diluted up to 16-fold inhibited growth of C. perfringens as effectively as several antimicrobial agents tested and 32-fold diluted supernatant also suppressed proliferation of these bacterium and retarded the growth by 50%. Furthermore, crude extract of L. amylovorus S6 exhibited inhibitory activities against various pathogens such as S. typhimurium, S. aureus, V. cholerae, E. tarda, and A. salmonicida subsp. salmonicida (data not shown).
Comparison of antagonistic effect of cell-free supernatant of L. amylovorus S6 and chemical antibiotics against Clostridium perfringens. Positive control (filled diamond), negative control (filled square), neomycin (asterisk), chlortetracycline (multiplication symbol), oxytetracycline (filled circle), cell-free supernatant of L. amylovorus S6 (filled triangle)
Antimicrobial spectrum
As shown in Table 3, the antimicrobial spectra of the probiotic isolates were examined against S. typhimurium, S. aureus, V. cholerae, E. tarda, and A. salmonicida subsp. salmonicida. Among the isolates, L. amylovorus S6 had the most inhibitory activity and produced the largest inhibition zones ranging from 6.0 to 14.6 mm and was followed by B. thermacidophilum G29 and L. reuteri F1. However, the supernatant of some isolates, L. reuteri L1, L. salivarius G11, and B. thermacidophilum M40, displayed a relatively little activity, and L. amylovorus M35 did not inhibit growth of all fish pathogens.
Discussion
The objective of our research was the isolation of beneficial microbes inhabiting various compartments of the pig GIT, which can be used as potential probiotics against C. perfringens. As with other investigators, we screened the bacteria and they were identified as L. reuteri (Chang et al. 2001; Rodriguez et al. 2003; Roos and Jonsson 2002), L. amylovorus (Konstantinov et al. 2004), L. salivarius (Nemcova et al. 1997; Robredo and Torres 2000) and B. thermacidophilum (Dong et al. 2000).
We also examined the survival rates of the probiotic strains in artificial gastric juice (pH 2.5) by incubating for 0 min, 30 min, and 2 h and found that the probiotic strains could survive the gastric acidity which enables them to reach the action site of the intestine (Table 1). Bile-salt resistance is the next major challenge for the microorganisms which are expected to survive in the GIT. Gilliland et al. (1984) reported that intestinal bile acid concentration in human GIT is 0.3% (w/v), but there is no information about the concentration of bile acid in animal intestine. Therefore, in present study, the tolerance of the isolates was tested in three different concentrations of 0.3, 1.0, and 5.0% (w/v) of bile salts. L. reuteri L1 and L. amylovorus S6 demonstrated superior tolerance to the other isolates (Table 2). These strains formed cloudy zones by producing BSH which is consistent with a report of strong BSH activity from porcine intestinal bacteria (Corzo and Gilliland 1999). Also, all probiotic strains were able to grow under conditions of at least 0.3% (w/v) bile salt for 48 h (Table 2). The next essential factor for probiotic preparation is the ability to adhere to the intestinal epithelia cell line. Adhesion of the probiotic strains to the intestinal mucus is considered a prerequisite for successful colonization and is important for antagonistic activity against enteropathogens (Ouwehand 1998; Ouwehand et al. 1999). Upon entering the gastrointestine of the host, the probiotic strains have to attach to the brush border of microvilli or adhere to the mucus layer to prevent sweep from the colon by peristalsis.
In the agreement with previous report (Jacobsen et al. 1999), some variations of adhesion ability were observed among Lactobacillus strains. Compared with L. rhamnosus GG, L. salivarius G11 and L. amylovorus S6 significantly possessed high ability to adhere to the HT-29 epithelial cell, independent of species (Fig. 2; p < 0.01). In addition, the adhesion ability of L. amylovorus also varied between strains. The adhesion ability of S6 strain was higher than M35 in the present experiments. Many scientists reported that multiple components such as proteins, carbohydrates (possibly glycoproteins), and divalent cations act on adhesion mechanism between bacteria and epithelial cells (Bernet et al. 1993; Chauviére et al. 1992; Coconnier et al. 1992; Greene and Kleanhammer 1994). S-layer proteins form crystalline layer around the bacterial cells, and it has been proposed that these proteins function as cell protection, adhesion, and surface recognition (Sleytr and Messner 1983). Some of Lactobacillus strains showed bacterial adhesion to epithelial cell lines by producing the S-layer proteins (Frece et al. 2005; Hynonen et al. 2002). Polysaccharides located on bacterial cell surface mediate adherence by interacting between the bacteria and the extracellular adhesion-promoting proteinaceous factors. The proteinaceous factors were secreted into the culture medium, and they play an important role in adherence by forming divalent bride between the bacteria and the eukaryotic cell receptors (Hood and Zottola 1989). Many scientists reported about this mechanism of adhesion to intestinal mucus by polysaccharides produced from probiotics (Conway and Kjelleberg 1989; Ruas-Madiedo et al. 2006a,b). Divalent cations affecting the adherence of probiotics to intestinal epithelial cells were investigated by several scientists (Craven and Williams 1998; Zarate et al. 2002). Attachment of the probiotics to intestinal epithelial cells was commonly enhanced by calcium and was dependent on other divalent cations such as barium and manganese. The nature of adhesion determinants and some factors (divalent cations and chelating agents) affecting the adherence of probiotics used in present study will be further studied.
Antimicrobial spectra of the seven probiotics used in this study were also tested against S. typhimurium, S. aureus, V. cholerae, E. tarda, and A. salmonicida subsp. salmonicida (Table 3). Three strains, L. amylovorus S6, B. thermacidophilum G29, and L. reuteri F1, had antimicrobial activity against all pathogens, with strain L. amylovorus S6 showing the strongest activity. Also, we compared the antagonistic effects between cell-free supernatant of L. amylovorus S6 and chemical antibiotics. Cell-free supernatants diluted up to 16-fold showed powerful activity equally as effective as chemical antimicrobial agents against C. perfringens (Fig. 3) and other pathogens (data not shown). From these results, we suggest that the probiotic strains showing a broad spectrum can be used as biological agents to substitute chemical antibiotics in animal and fish-feed industry.
On the basis of previous reports, the potential probiotic strains provide many beneficial effects in animal feeds (Gusils et al. 1999). These beneficial effects include: (1) competitive inhibition of pathogenic bacteria such as Escherichiacoli (Watkins et al. 1982), Campylobacter jejuni (Morishita et al. 1997), Salmonella enterica serovar Enteritidis (Pascual et al. 1999), and C.perfringens (Teo and Tan 2005), (2) growth promotion and maintenance of beneficial intestinal microbes (Hosoi et al. 2000), and (3) enzymatic digestion and absorption of nutritive elements (Thomke and Elwinger 1998).
Considering the probiotic properties of isolates used in this study, a strain L. amylovorus S6 satisfied all the requirements as a useful probiotic candidate. This strain displayed the best anti-Clostridium activity and was as effective as various antimicrobial agents. In addition, it survived in gastric juice, tolerated bile salt, and adhered to the mucus. We will progress future works such as: (1) test of the in vivo survival of some of the isolates when administered to pig and fish as feed additive, and (2) antagonistic effect of the isolates against various pathogens under these circumstances.
References
Barbosa TM, Serra CR, La Ragione RM, Woodward MJ, Henriques AO (2005) Screening for Bacillus isolates in the broiler gastrointestinal tract. Appl Environ Microbiol 71:968–978
Bernet MF, Brassart D, Neeser JR, Servin AL (1993) Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Appl Environ Microbiol 59:4121–4128
Carter GR, Chengappa MM (1991) Essentials of veterinary bacteriology and mycology. Lea and Febiger, Philadelphia, PA
Casey PG, Casey GD, Gardiner GE, Tangney M, Stanton C, Ross RP, Hill C, Fitzgerald GF (2004) Isolation and characterization of anti-Salmonella lactic acid bacteria from the porcine gastrointestinal tract. Lett Appl Microbiol 39:431–438
Chang YH, Kim JK, Kim HJ, Kim WY, Kim YB, Park YH (2001) Selection of a potential probiotic Lactobacillus strain and subsequent in vivo studies. Antonie Van Leeuwenhoek 80:193–199
Chauviére G, Coconnier MH, Kerneis S, Fourniat S, Servin AL (1992) Adhesion of human Lactobacillus acidophilus strain LB to human enterocyte-like Caco-2 cells. J Gen Microbiol 138:1689–1696
Chou LS, Weimer B (1999) Isolation and characterization of acid-and bile-tolerant isolates from strains of Lactobacillus acidophilus. J Dairy Sci 82:23–31
Coconnier MH, Klaenhammer TR, Kernéis S, Bernet MF, Servin AL (1992) Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Appl Environ Microbiol 58:2034–2039
Conway PL, Kjelleberg (1989) Protein-mediated adhesion of Lactobacillus fermentum strain 737 to mouse stomach squamous epithelium. J Gen Microbiol 135:1175–1186
Corzo G, Gilliland SE (1999) Bile salt hydrolase activity of three strains of Lactobacillus acidophilus. J Dairy Sci 82:472–480
Craven SE, Williams DD (1998) In vitro attachment of Salmonella typhimurium to chicken cecal mucus: effect of cations and pretreatment with Lactobacillus spp. isolated from the intestinal tracts of chickens. J Food Prot 61:265–271
Daeschel MA (1989) Antimicrobial factors from lactic acid bacteria for use as food preservatives. Food Technol 43:484–490
Dashkevicz MP, Feighner SD (1989) Development of a differential medium for bile salt hydrolase-active Lactobacillus spp. Appl Environ Microbiol 55:11–16
Dong X, Xin Y, Jian W, Liu X, Ling D (2000) Bifidobacterium thermacidophilum sp. nov., isolated from an anaerobic digester. Int J Syst Evol Microbiol 50:119–125
Emborg HD, Andersen JS, Seyfarth AM, Wegener HC (2004) Relations between the consumption of antimicrobial growth promoters and the occurrence of resistance among Enterococcus faecium isolated from broilers. Epidemiol Infect 132:95–105
Frece J, Kos B, Svetec IK, Zgaga Z, Mrša V, Šuškovi J (2005) Importance of S-layer proteins in probiotic activity of Lactobacillus acidophilus M92. J Appl Microbiol 98:285–292
Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66:365–378
Gatesoupe FJ (1999) The use of probiotics in aquaculture. Aquaculture 180:147–165
Gilliland SE, Staley TE, Bush LJ (1984) Importance of bile tolerance of Lb. acidophilus used as a dietary adjunct. J Dairy Sci 67:3045–3051
Greene JD, Kleanhammer TR (1994) Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl Environ Microbiol 50:4487–4494
Gusils C, Gonzalez SN, Oliver G (1999) Some probiotic properties of chicken lactobacilli. Can J Microbiol 45:981–987
Hoa NT, Baccigalupi L, Huxham A, Smertenko A, Van PH, Ammendola S, Ricca E, Cutting SM (2000) Characterization of Bacillus species used for oral bacteriotherapy and bacterio-prophylaxis of gastrointestinal disorders. Appl Environ Microbiol 66:5241–5247
Hood SK, Zottola EA (1989) An electron microscopic study of the adherence of Lactobacillus acidophilus to human intestinal cells in vitro. Food Microstruct 8:91–97
Hosoi T, Ametani A, Kiuchi K, Kaminogawa S (2000) Improved growth and viability of lactobacilli in the presence of Bacillus subtilis (natto) or subtilin. Can J Microbiol 46:892–897
Hynonen U, Westerlund-Wikstrom B, Palva A, Korhonen TK (2002) Fibronectin-binding function in the SlpA surface protein of Lactobacillus brevis. J Bacteriol 184:3360–3367
Jack RW, Tagg JR, Ray B (1995) Bacteriocins of gram-positive bacteria. Microbiol Rev 59:171–200
Jacobsen CN, Rosenfeldt NV, Hayford AE, Moller PL, Michaelsen KE, Paerregaard A, Sandstrom B, Tvede M, Jakobsen M (1999) Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol 65:4949–4956
Jiraphocakul S, Sullivan TW, Shahani KM (1990) Influence of a dried Bacillus subtilis culture and antibiotics on performance and intestinal microflora in turkeys. Poult Sci 69:1966–1973
Kizerwetter-Swida M, Binek M (2005) Selection of potentially probiotic Lactobacillus strains towards their inhibitory activity against poultry enteropathogenic bacteria. Pol J Microbiol 54:287–294
Konstantinov SR, Awati A, Smidt H, Williams BA, Akkermans AD, de Vos WM (2004) Specific response of a novel and abundant Lactobacillus amylovorus-like phylotype to dietary prebiotics in the guts of weaning piglets. Appl Environ Microbiol 70:3821–3830
Kontula P, Suihko ML, Von Wright A, Mattila-Sandholm T (1999) The effect of lactose derivatives on intestinal lactic acid bacteria. J Dairy Sci 82:249–256
La Ragione RM, Narbad A, Gasson MJ, Woodward MJ (2004) In vivo characterization of Lactobacillus johnsonii FI9785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry. Lett Appl Microbiol 38:197–205
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Academic, Chichester, UK, pp 115–175
Lehto E, Salminen S (1997) Adhesion of two Lactobacillus strains, one Lactococcus and one Propionibacterium strain to cultured human intestinal Caco-2 cell line. Biosci Microflora 16:13–17
Morishita TY, Aye PP, Harr BS, Cobb CW, Clifford JR (1997) Evaluation of an avian-specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers. Avian Dis 41:850–855
Naidu AS, Bidlack WR, Clemens RA (1999) Probiotic spectra of lactic acid bacteria (LAB). Crit Rev Food Sci Nutr 38:13–26
Nemcova R, Laukova A, Gancarcikova S, Kastel R (1997) In vitro studies of porcine lactobacilli for possible probiotic use. Berl Munch Tierarztl Wochenschr 110:413–417
Niilo L (1980) Clostridium perfringens in animal disease: a review of current knowledge. Can Vet J 21:141–148
Ouwehand AC (1998) Antimicrobial components from lactic acid bacteria. In: Salminen S, von Wright A (eds) Lactic acid bacteria: microbiology and functional aspects. Marcel Dekker, New York, pp 139–160
Ouwehand AC, Kirjavainen PV, Gronlund MM, Isolauri E, Salminen SJ (1999) Adhesion of probiotic micro-organisms to intestinal mucus. Int J Food Microbiol 64:119–126
Parish WE (1961) Necrotic enteritis in the fowl (Gallus gallus domesticus). I. Histopathology of the disease and isolation of a strain of Clostridium welchii. J Comp Pathol 71:377–393
Pascual M, Hugas M, Badiola JI, Monfort JM, Garriga M (1999) Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens. Appl Environ Microbiol 65:4981–4986
Piard JC, Desmazeaud M (1992) Inhibiting factors produced by lactic acid bacteria. 2. Bacteriocins and other antimicrobial factors. Lait 72:113–142
Robredo B, Torres C (2000) Bacteriocin production by Lactobacillus salivarius of animal origin. J Clin Microbiol 38:3908–3909
Rodriguez E, Arques JL, Rodriguez R, Nunez M, Medina M (2003) Reuterin production by lactobacilli isolated from pig faeces and evaluation of probiotic traits. Lett Appl Microbiol 37:259–263
Roos S, Jonsson H (2002) A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148:433–442
Ruas-Madiedo P, Gueimonde M, de los Reyes-Gavilan CG, Salminen S (2006a) Short communication: effect of exopolysaccharide isolated from “villi” on the adhesion of probiotics and pathogens to intestinal mucus. J Dairy Sci 89:2355–2358
Ruas-Madiedo P, Gueimonde M, Margolles A, de los Reyes-Gavilan CG, Salminen S (2006b) Exopolysaccharides produced by probiotic strains modify the adhesion of probiotics and enteropathogens to human intestinal mucus. J Food Prot 69:2011–2015
Saarela M, Mogensen G, Fonden R, Matto J, Mattila-Sandholm T (2000) Probiotic bacteria: safety, functional and technological properties. J Biotechnol 84:197–215
Salminen S, Bouley C, Boutron-Ruault M-C, Cummings JH, Frank A, Gibson GR, Isolauri E, Moreau M-C, Roberfroid M, Rowland I (1989) Functional food science and gastrointestinal physiology and function. Br J Nutr 80:147–171
Salminen S, Ouwehand AC, Benno Y, Lee YK (1999) Probiotics: how should they be defined? Trends Food Sci Technol 10:107–110
Sambrook J, Fritsch EF, Maniatis T (1987) Molecular cloning—a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sleytr U, Messner P (1983) Crystalline surface layers on bacteria. Annu Rev Microbiol 37:311–339
Songer JG (1996) Clostridial enteric diseases of domestic animals. Clin Microbiol Rev 9:216–234
Teo AY, Tan HM (2005) Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens. Appl Environ Microbiol 71:4185–4190
Thomke A, Elwinger K (1998) Growth promotants in feeding pigs and poultry. Ø. Alternatives to antibiotic growth promotants. Ann Zootech (Paris) 47:245–271
Timoney JF, Gillespie JH, Scott FW, Barlough JE (1988) Hagan and Bruner’s microbiology and infectious diseases of domestic animals. Comstock, Ithaca, NY
Van der Sluis W (2000) Necrotic enteritis (3): clostridial enteritis is an often underestimated problem. World Poult 16:42–43
Watkins BA, Miller BF, Neil DH (1982) In vivo effects of Lactobacillus acidophilus against pathogenic Escherichia coli in gnotobiotic chicks. Poult Sci 61:1298–1308
Westerdahl A, Olsson JC, Kjelleberg S, Conway PL (1991) Isolation and characterization of turbot (Scophthalmus maximus)-associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol 57:2223–2228
Zarate G, Morata De Ambrosini V, Perez Chaia A, Gonzalez S (2002) Some factors affecting the adherence of probiotic Propionibacterium acidipropionici CRL 1198 to intestinal epithelial cells. Can J Microbiol 48:449–457
Acknowledgment
This study was supported by the Technology Development Program for Agriculture and Forestry, Ministry of Agriculture and Forestry, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, P.I., Jung, M.Y., Chang, YH. et al. Probiotic properties of Lactobacillus and Bifidobacterium strains isolated from porcine gastrointestinal tract. Appl Microbiol Biotechnol 74, 1103–1111 (2007). https://doi.org/10.1007/s00253-006-0741-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0741-7





 )
)
