Abstract
Ectoine is a compatible solutes that is diffusely dispersed in bacteria and archaea. It plays a significant role as protectant against various external pressures, such as high temperature, high osmolarity, dryness and radiation, in cells. Ectoine can be utilized in cosmetics due to its properties of moisturizing and antiultraviolet. It can also be used in the pharmaceutical industry for treating various diseases. Therefore, strong protection of ectoine creates a high commercial value. Its current market value is approximately US$1000 kg−1. However, traditional ectoine production in high-salinity media causes high costs of equipment loss and wastewater treatment. There is a growing attention to reduce the salinity of the fermentation broth without sacrificing the production of ectoine. Thus, heterologous production of ectoine in nonhalophilic microorganisms may represent the new generation of the industrial production of ectoine. In this review, we summarized and discussed the biological activities of ectoine on cell and human health protection and its heterologous production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most living cells, especially extremophiles, can adapt to a certain degree of fluctuations in environmental osmosis. Their ability to maintain internal osmotic pressure performs an important role throughout life. These microorganisms can manage the osmotic balance between inside and outside the cell by accumulating compatible solutes in the salt environment (Sajjad et al. 2018). The compatible solutes are intensely significant due to their ability to stabilize or disrupt the conformation of biomolecules without affecting the chemical structure (Oprzeska-Zingrebe et al. 2018). Thus, those microorganisms can live in harsh environments. The protective effect of the compatible soluble mass on cells can be illustrated through the “preferential exclusion model” (Hermann et al. 2020). This model indicates that the compatible solutes do not display direct interaction with macromolecules in cells. But the compatible solutes near macromolecules may be preferentially repelled to the bulk region, As a result, the situation highly increases the hydration of macromolecules and prevents macromolecules from denaturation (Yu et al. 2007). Generally, the compatible solutes are a group of low-weight molecules belong to different chemical families. This mainly includes polyols (glycerol, glucosyl-glycerol), sugars (sucrose, trehalose), amino acids and their descendants (acetyllysine, ectoine, hydroxyectoine) (Becker and Wittmann 2020; Zaccai et al. 2016).
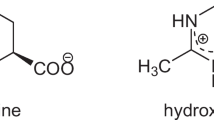
Ectoine (1,4,5,6-tetrahydro-2-methyl-4-pyrimidine carboxylic acid) is superior to other compatible solutes because of its excellent cell-protective properties (Fenizia et al. 2020). Ectoine is a physiologically inert compound that can stabilize the structure of large molecules, such as proteins and nucleic acids. It can maintain cell membranes and prevent cells from multiple environmental stimuli including salinity, refrigeration, desiccation and hyperthermia in vivo (Bownik and Stepniewska 2016; Hahn et al. 2017; Herzog et al. 2019; Ma et al. 2020; Pastor et al. 2010; Wittmar et al. 2020). In addition, recent studies have shown that ectoine can protect enzyme preparations and human tissues from allergens, ultraviolet light, dryness, and other effects in vitro. It can also apply in the pharmaceutical industry for the treatment of various diseases, such as Alzheimer’s, rhinoconjunctivitis symptom, lung inflammation, and respiratory illnesses (Bilstein et al. 2021; Buenger and Driller 2004; Kanapathipillai et al. 2005; Nayak et al. 2020; Salapatek et al. 2011; Sydlik et al. 2013). The recent studies have reviewed the biochemistry of ectoine/hydroxyectoine biosynthetic enzymes, and some of their available crystal structures were reviewed, the genetics of potential biosynthetic genes, and their transcriptional regulation were explored, and an extensive phylogenetic analysis of ectoine/hydroxyectoine biosynthetic genes was performed (Czech et al. 2018). Owing to the huge potential of applications of ectoine in biotechnology, cosmetics and medicine fields (Dao et al. 2019; Hseu et al. 2020; Tsai et al. 2020), it has opened up an enormous market with estimated sales values of approximately 1000 USD/kg (Strong et al. 2016). Therefore, people have attached more importance to the production of ectoine.
Ectoine was first identified by Galinski et al. in the halophilic Ectothiorhodospira halochloris (Galinski et al. 1985). Subsequently, its derivative hydroxyectoine was detected in Streptomyces parvulus in the next few years (Inbar and Lapidot 1988). Chemically, ectoine and hydroxyectoine are classified as amino acid derivatives. As the zwitterions, ectoine and hydroxyectoine can locally influence the hydrogen bonding (H-bond) network of water, which can bind to water through 7 strong H-bonds to generate a hydration shell in the water solution. The surrounding water can combine with COO− and NH+ groups of hydroxyectoine and ectoine, which illustrates the strong solubility and hydration properties of ectoine and hydroxyectoine (Hahn et al. 2016; Sahle et al. 2018).
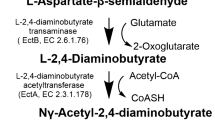
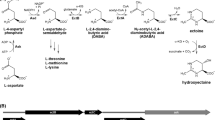
The biosynthesis way of ectoine begins with the L-aspartate-β-semialdehyde (ASA), which is accessed by the catalysis of an ATP-dependent L-aspartate kinase (Ask) and NADPH-dependent l-aspartate-β-semialdehyde dehydrogenase (Asd). The ASA is the metabolic center for multiple biosynthetic pathways (Czech et al. 2018; Lo et al. 2009). In the conversion process of the precursor ASA towards ectoine that is catalyzed by three enzymes: l-2,4-diaminobutyrate acetyltransferase (EctA), l-2,4-diaminobutyrate aminotransferase (EctB) and ectoine synthase (EctC) (Fig. 1) (Ono et al. 1999). EctB enzyme represents the initial and the rate-limiting enzyme in the biosynthesis route of ectoine (Hillier et al. 2020). EctB could reversibly transfer the amino group from the l-glutamate to the aldehyde group of the ASA (Czech et al. 2018; Richter et al. 2019). The catalysis of EctB can result in the formation of 2-oxoglutarate and l-2, 4-diaminobutyrate (DABA). EctA catalyzes the transfer of the acetyl-group from the co-substrate acetyl-coenzyme A to DABA (Ono et al. 1999), and the CoA and N-γ-acetyl-2, 4-diaminobutyrate (N-γ-ADABA) are acquired subsequently. The conversion of N-γ-ADABA to ectoine is the last step of the biosynthesis process, which is catalyzed by the EctC. The EctC enzyme catalyzes the cyclization of ectoine by removing a water molecule from the C=O-bond in N-γ-ADABA and generating an intramolecular imine bond (Czech et al. 2019a, b; Widderich et al. 2016a, b). Some natural ectoine producers contain the EctD enzyme, which is significant in the transformation of ectoine towards hydroxyectoine through a hydroxylation reaction (Bursy et al. 2007; Czech et al. 2016). The existence of oxygen is crucial for the catalysis of the EctD, which is stereoselectively and regionally selective (Hoppner et al. 2014; Reuter et al. 2010; Widderich et al. 2016a, b). In the recent years, it has been demonstrated that region- and stereoselective modification of homoectoine in recombinant E. coli strain by exploiting the substrate miscibility of ectoine hydroxylase to generate trans-5-hydroxyhomoectoine (Czech et al. 2019a, b). In addition, the genes encoding EctB, EctA and EctC stay together to form the ect gene cluster (ectBAC), sometimes accompanied by the EctD enzyme (Garcia-Estepa et al. 2006). The importance of ectA, ectB, and ectC genes for ectoine synthesis was revealed by cloning genes of the ectoine biosynthetic pathway from the Gram-positive moderate halophile Marinococcus halophilus expressed in E. coli. There are three enzymes involved in ectoine synthesis. ectB encodes l-2,4-diaminobutyric acid transaminase, the first enzyme in the synthetic ectoine pathway; ectA encodes l-2,4-diaminobutyric acid acetyltransferase, which is responsible for the acetylation step; and ectC encodes l-ectoine synthase, which converts Nγ-acetyldiaminobutyric acid into ectyrosine (Louis and Galinski 1997). Compared with ectoine, hydroxyectoine is more polar and has better solubility, which allows the macromolecular substances in the cell to maintain stability under the protection of hydroxyectoine. All ectoine/hydroxyectoine biosynthetic enzymes (EctABCD) have ben crystallized by now, either in their apo-forms or in complex with substrates or reaction products.
Routes for ectoine biosynthesis (Schwibbert et al. 2011). Ectoine is biosynthesized by three enzymes: l-2,4-diaminobutyrate acetyltransferase (EctA), l-2,4-diaminobutyrate aminotransferase (EctB) and ectoine synthase (EctC)
Furthermore, ectoine is not only used as a protectant against the stress in an environment of high salinity, but also can be degraded into carbon and nitrogen sources for cell growth when the high salt pressure is relieved (Hermann et al. 2020). The catabolism pathway of ectoine in Halomonas elongata DSM 2581T (H. elongate DSM 2581T) was first demonstrated by Schwibbert et al. (2011). The inverse conversion of ectoine to aspartate is catalyzed by the other four enzymes: ectoine hydrolase (DoeA), N-α-acetyl-l-2, 4-diaminobutyric acid deacetylase (DoeB), diaminobutyric acid transaminase (DoeD) and aspartate-semialdehyde dehydrogenase (DoeC). In the degradation pathway, ectoine is first hydrolyzed by DoeA, which forms the N-α-acetyl-l-2, 4-diaminobutyrate (N-α-ADABA) and N-γ-ADABA. The former can be deacetylated to generate diaminobutyric acid under the catalytic action of DoeB. However, the latter cannot be identified as the substrate for the DoeB, it can only be converted to ectoine by the catalysis of EctC in the ectoine biosynthetic pathway. DoeD further catalyzes the transition of diaminobutyric acid to ASA. Then, the aspartate is eventually synthesized under the oxidative dehydrogenation of DoeC with the substrate ASA (Fig. 1) (Reshetnikov et al. 2020). In addition to H. elongata, the catabolic pathway of ectoine has also been found in Ruegeria pomeroyi and Chromohalobacter salexigens (Correa and Jhonny 2013). In R. pomeroyi, the hydroxyectoine can be converted into ectoine by the catalysis of EutA, EutB, EutC and the ectoine is degraded to aspartate under the catalytic effect of DoeA, B, C, D in the ectoine catabolism pathway (Schulz et al. 2017). In addition to the Doe pathway, there is another ectoine/hydroxyectoine degradation route in R. pomeroyi. The ectoine/hydroxyectoine could be degraded into l-2, 4-diaminobutyrate (DABA)/hydroxy-l-2,4-diaminobutyrate (hydroxy-DABA) and acetate through the intermediate N-α-ADABA/hydroxy-N-α-acetyl-l-2,4-diaminobutyrate (hydroxy-N-α-ADABA) by the catalysis of EutD/EutE (Mais et al. 2020). In addition, the DABA can be converted into l-aspartate under the catalysis of the Atf aminotransferase and the Ssd dehydrogenase (Schwibbert et al. 2011).
Ectoine was successfully generated in the halophilic microorganism H. elongate through a bacterial milking process by Sauer et al. (Sauer and Galinski 1997). The bacterial milking process is a fermentation technique for the mass production of intracellular metabolites, which is wildly used in various bacteria, such as E. coli and Halorhodospira species. In the bacterial milking process, halophilic bacteria are first treated with the high-salinity media to accumulate the ectoine in cells. Subsequently, a low salinity medium is used to promote the release of intracellular ectoine by providing hypoosmotic stimulation. Bacteria will use mechanosensitive channels embedded in the cell membrane to avoid rupture under hypotonic conditions, and their transient opening allows rapid, nonspecific abandonment of low molecular weight solutes (ions and organic compounds). By relying on the opening and closing of mechanosensitive channels, cells can respond in a timely and graded manner to the severity of a suddenly imposed osmotic imbalance (Booth 2014; Cox et al. 2018; Pliotas and Naismith 2017; Rasmussen 2016). Then, the used cells are repeatedly cultivated in the high salinity environment and recycled to produce ectoine. The bacterial milking strategy is extensively employed for the industrial production of ectoine (Kunte et al. 2014).
Bioactivities of ectoine
Cell protection
Ectoine is known as a widespread compatible solute that prevents halophilic bacteria from high osmotic stress and protects the structure of protein and enzymes under abiotic stresses. Ectoine can be hydrated in a high concentration of salt solution due to its zwitterionic structure. It also can combine with the ions to form complex and shield coulomb interactions between ions in solution (Eiberweiser et al. 2015). The combination of hydration and ionic interaction is very significant in the protection against excessive ions and osmotic stress outside (Fedotova 2019).
The cell membrane provides a barrier to avoid the free entry of extracellular substances into the cell. This ensures the relative stability of cell circumstances and enables various biochemical reactions to proceed in an orderly manner. However, environmental factors, such as high temperature or poisonous substances may damage the cell membrane, resulting in destabilization. The cell membrane in water is stabilized by hydrophobic interactions of the apolar lipid tail and hydrophilic interactions of the polar lipid head groups. It was reported that ectoine can increase the fluidity of the membrane by enhancing these hydrophilic interactions. The high fluidity of the membrane can accelerate the repair mechanisms of the membrane and aid in the signaling process (Harishchandra et al. 2010). The previous studies have also shown that ectoine is excluded from the hydration layer on the membrane surface without affecting the molecular dynamics of the membrane. Ectoine can develop cell surface hydration by increasing the intermolecular spacing, which results in reducing the hydrophobicity of film and improving the liquidity of the lipid head groups in the cell membrane (Fig. 2) (Dwivedi et al. 2014; Zaccai et al. 2016). Consequently, ectoine can maintain cell stabilization and help cope with extreme conditions and different stressors by influencing the cell membrane (Herzog et al. 2019). Ectoine is the most effective solute to protect the zymogen from activation. ectoine reduced the formation of trypsin and chymotrypsin by 4% in the former and 23% in the latter compared to the control. Moreover, when studying the ability of solutes to maintain proteolytic activity during incubation, trypsin and chymotrypsin maintained approximately 50% residual activity in the presence of ectoine (Kolp et al. 2006).
Influence of water molecules alone (a) and aqueous solution of ectoine (b) on cell. Ectoine can increase the fluidity of membrane by enhancing the hydrophilic interactions; According to the preferential exclusion model, ectoine is expelled from the hydration shell of protein to promote the native conformation of protein; Ectoine could replace the water around DNA to reduce the generation of secondary particles and reduce the damage
According to the preferential exclusion model, ectoine is excluded from the hydration shell of protein, which facilitates the hydration and promotes the native conformation of the protein (Fig. 2) (Graf et al. 2008). The ectoine zwitterion molecule could combine with water molecules to form large and stable water–ectoine clusters. Thus, the clusters can protect protein stability and reduce the protein susceptibility to denaturation (Di Gioacchino et al. 2019; Goraj et al. 2019). The enthalpy–entropy compensation mechanisms assume the main responsibility for the effect of ectoine to stabilize the protein by the preferential exclusion. The formation of strong ectoine-water hydrogen bonds increases the ordering of water molecules, which induces a favorable enthalpy change. However, the weakening of water-water hydrogen bonds around ectoine leads to a favorable entropy change (Oprzeska-Zingrebe et al. 2018; Zaccai et al. 2016). In addition, ectoine was confirmed to strengthen the intramolecular interactions essential for the stability of the protein. Overall, the ectoine can protect the protein stability through directly and indirectly mechanisms, which may include exclusion from uncharged surfaces and modifications of the hydration shell (Hahn et al. 2015; Yu et al. 2007).
Ectoine is also an effective DNA protective substance against Ionizing and UV radiation. Marc Benjamin Hahn et al. demonstrated that ectoine could protect against DNA strand breaks caused by ionizing electron radiation (Schroter et al. 2017). Those secondary particles, such as secondary (kinetic) low energy electrons (LEE), OH-radicals and prehydrated electrons could cause the ionizing radiation-induced DNA injury. Those secondary particles could break the DNA strand via different damaging channels by reaching the sugar-phosphate backbone when they were generated within nanometer distances to DNA. Interestingly, this damage increases strongly with the imposition of the degree of DNA hydration. Ectoine can not only replace the water around DNA to reduce the damage, but also scavenge reactive oxygen species and decrease the energy of low-energy electrons (LEE) (Fig. 2). Therefore, ectoine is examined to be a highly effective DNA protective agent (Hahn et al. 2017).
Although ectoine was regarded as the stabilizer of DNA in the last decade, recent studies have indicated that ectoine could change the structure of DNA from a superhelical to an open-loop structure under certain conditions (Meyer et al. 2017). The DNA that was incubated in a nonionic aqueous solution with ectoine had more significant structural variations than DNA that was incubated in pure water without ectoine. This denature-like effect of ectoine attributes to the strong and nonspecific binding between ectoine and DNA, which is promoted by the electrostatic interactions associated with pronounced dispersion energies between the highly negative charge of the DNA and the zwitterionic (Oprzeska-Zingrebe et al. 2018). The preferential binding of ectoine to DNA forms a stable barrier around the DNA, which protect the nucleotides from irradiation damage.
Skin protection
The accumulated action of external factors, such as irradiation, wind, wetness, dryness and different temperatures results in accelerated skin aging (Rabe et al. 2006). Ectoine has been proved to provide an antiaging effect. For instance, the epidermal dendritic Langerhans cells are the crucial antigen-presenting cell population in the skin, which decreases its number in the aged skin (Beyer et al. 2000). Pretreatment with ectoine was confirmed to prevent a decrease of Langerhans cells caused by UV radiation. Ectoine was also confirmed to block the release of ceramides in the human keratinocytes under UV irradiation. This is because a high level of ceramides allows the activation of the intracellular signaling cascade and the consequent expression of the adhesion molecules (Grether-Beck et al. 2005). Furthermore, long-time exposure to sunlight can cause photoaged skin, which increases wrinkling, thickness and laxity. The release of the second messenger, activation of transcription factor AP-2, expression of the intercellular adhesion molecule 1, and mutation of mitochondrial DNA was induced by the UVA irradiation inherent in sunlight in skin cells. Buenger et al. indicated that ectoine can counteract this effect on skin age and provide valid protection against UVA radiation in vitro (Buenger and Driller 2004).
The stratum corneum on human skin acts as a barrier that can prevent moisture evaporation from the skin. Exposure to detergents containing surfactants, such as SDS, AES or extreme environments (dry air, low or high temperatures, air regulation) could break this barrier, leading to moisture loss. On account of the stabilization of intracellular biomacromolecules and protection of cell membrane (Harishchandra et al. 2011), ectoine can protect the skin barrier from being damaged with a reduction of surfactant-induced transepidermal water loss (TEWL) to 40% (Graf et al. 2008). In addition, when ectoine was added to the surfactant solution, it was confirmed to reduce the ability to dissolve the composition of the endocellular lipid components and solubilization of endocellular lipid components of surfactant (Bujak et al. 2020). The surfactant-induced removal of sebum on the skin layer and variation in the constitution of endocellular lipid resulted in skin irritation, eventually leading to the increase of TEWL (Mukherjee et al. 2010). Thus, ectoine could be a potential component in cleansing cosmetics for atopic skin. Moreover, ectoine can facilitate the sustentation of skin hydration with a long-duration effect. This allows ectoine to behave as a more valid moisturizer (Graf et al. 2008). The recent studies have shown that ectoine is effective in treating dermatitis (atopic dermatitis and retinoid dermatitis) caused by the impaired skin barrier. Moreover, the safety evaluation indicated that the cream containing 7% ectoine did not show a higher risk when compared with the control cream (Kauth and Trusova 2022).
It is a truism that the skin exposure to ultraviolet A (UV A)-irradiation leads to pigmentation. The UVA irradiation triggers the reactive-oxygen species (ROS) generation, which mediates the superabundant production of melanin in the skin cells. Ectoine and hydroxyectoine were considered to be safe and potential whitening agents as both ectoine and hydroxyectoine can suppress the expression of the melanogenesis-related gene (tyrosinase, TRP1, TRP2 and MITF) to inhibit the UV-induced melanogenesis in the B16F10 cells (Chung et al. 2019; Yao et al. 2013). In addition, ectoine can protect the skin from UV damage based on its antioxidant properties (Fig. 3). The HaCaT cells were pretreated with ectoine, and the expression of three antioxidant proteins (antioxidant heme oxygenase-1 (HO-1), NAD (P)H dehydrogenase [quinone 1] (NQO-1), and γ-glutamate-cysteine ligase catalytic subunit (γ-GCLC)) were upregulated via the nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) to inhibit the UV-induced ROS generation. The activation of Nrf2 was mediated by ectoine, which was accomplished through the p38, protein kinase B (PKB), protein kinase C (PKC), and casein kinase II protein kinase (CKII) pathways (Hseu et al. 2020). The Nrf2 pathway plays a significant role in ectoine-mediated antioxidant and antimelanogenic effects (Marrot et al. 2008). Ectoine pretreatment downregulated the expression of ROS-p53 mediated proopiomelanocortin (POMC) and its derived peptide α-melanocyte-stimulating hormone (α-MSH) in the UVA-irradiated HaCaT cells. POMC and α-MSH are important in the stimulation of the melanin synthesis and activation of the tyrosinase in melanin cells, respectively, which are the essential factors in the conversion of tyrosine into melanin (Rousseau et al. 2007). The previous study on the photoprotection assessment of ectoine, l-ergothioneine and mannitol also demonstrated that ectoine can reduce the genotoxicity on HaCaT cells caused by visible light/UVA (Botta et al. 2008). Ectoine is as effective as mannitol in scavenging the sodium salicylate test system because ectoine reacts with hydroxyl radicals to transform into two major products: N-acetimide aspartate and N-acetimide-β-alanine. In a previous study, a reaction mechanism was proposed in which the heterocycle of the compatible solute ectoine was cleaved and further oxidized at the C-terminus. The ectoine radical scavenging ability effectively explains the observed role of its acting as an anti-inflammatory compound in skin, lung, and intestinal diseases (Brands et al. 2019).
Application in medicine and pharmacy
Ectoine is also applied in medicine and pharmacy as it has the protective capability to biomacromolecule. For example, ectoine and hydroxyectoine were added to the organ storage solution to protect the liver during liver transplantation (Srinivasan et al. 2014). Ectoine was also used as the cryoprotectant for erythrocytes and umbilical cord blood cells due to its antifrozen capacity (Bissoyi and Pramanik 2013; El Assal et al. 2014). Indeed, ectoine provides more than protection. It was determined that ectoine could effectively relieve inflammation. In the cultured human bronchial cells and epithelial cells of rats, the nanoparticles are the main reason for the pro-inflammatory reactions. Ectoine could reduce the nanoparticles, restrain the nanoparticles-triggered cell signaling and limit the induced expression of IL-8, which plays a momentous role in the pathogenesis of tracheitis (Abdel-Aziz et al. 2013; Sydlik et al. 2009, 2013; Unfried et al. 2014). Furthermore, ectoine and hydroxyectoine were confirmed to be beneficial to the biophysical aspects of lung surfactants, which is a lipid-peptide monolayer functional for lowering the surface tension. Both ectoine and hydroxyectoine increase the efficiency and fluidity of the lung surfactants, promoting the continuous exchange of materials between the surfactant monolayer and the pulmonary hypophase. It suggests the feasible usage in inhalation therapy of ectoine (Harishchandra et al. 2011). Ectoine was also confirmed to be a promising antirelapse drug for IBD (intestinal epithelial barrier) due to its stabilization of the intestinal epithelial barrier (Bethlehem and Echten-Deckert 2021). In this regard, it seems that ectoine does not perform as well as its synthetic derivative homoectoine. Previous research indicated that both ectoine and homoectoine relieved the DSS (Dextran sulfate sodium)-induced intestinal epithelial barrier damage by decreasing the expression of proinflammatory cytokines, reducing the oxidative stress and maintaining the stability of junction architecture. Homoectoine comprehensively prevented the inflammatory claudin switch which was normally detected during colitis by improving the leak claudin-2 expression and reducing claudin-1 expression, while ectoine only decreased the expression of leak claudin-2 (Castro-Ochoa et al. 2019). Moreover, the recent studies displayed that ectoine could be added to the pharmaceutical preparation for allergic rhinitis, rhinoconjunctivitis, and dry eye syndrome. This illustrates that ectoine can become a reliable alternative to other drugs to treat the symptoms without side effects (Dwivedi et al. 2014; Eichel et al. 2014; Nosch et al. 2021; Sonnemann et al. 2014; Werkhauser et al. 2014). Ectoine could even inhibit the replication of HIV and prevent the formation of neurotoxic amyloid (Aβ42), which is the pathogenic protein of Alzheimer's disease (Kanapathipillai et al. 2005; Lapidot et al. 1995; Ryu et al. 2008).
Heterogeneous production of ectoine
Traditional ectoine biosynthesis is performed in high salinity substrate, which is corroded to traditional stainless-steel fermenters and connected facilities. It also increases the additional price of wastewater treatment (Lang et al. 2011; Zhao et al. 2019). In order to overcome the disadvantages attributed to high-salinity medium, ectoine production processes in low salinity medium have been developed. The ectoine biosynthetic genes were introduced into the low salinity host Corynebacterium glutamicum (C. glutamicum) and Escherichia coli (E. coli). The process has been made by increasing the ectoine production in a low-salinity medium (Fig. 4).
Corynebacterium glutamicum
In the last decades, C. glutamicum became the commercial host for the industrial production of amino acids and their derivatives, such as glutamate, lysine and homoserine due to its strong metabolic network and clear genetic background (Hirasawa and Shimizu 2016; Tsuge and Matsuzawa 2021). C. glutamicum is incapable of converting or degrading the ectoine as lacking the ectD and doeABCD gene (Kalinowski et al. 2003). In the previous lysine-producing strains, the feedback-resistant aspartokinase had been engineered to improve the supply of the precursor ASA, which is also the crucial precursor for the biosynthesis of ectoine (Kim et al. 2006). Consequently, the high-level lysine producer was considered the ideal initial strain for the heterologous ectoine production.
Becker et al. chose C. glutamicum LYS-1 as the initial strain for the heterologous ectoine production. This is because it has the mutated aspartate kinase (LysCT311I), which can release the feedback inhibition and produce extensive amino acid precursor ASA (Becker et al. 2011). The ectABCD gene cluster from Pseudomonas stutzeri was inserted into the origin of the ddh gene in C. glutamicum LYS-1 (Becker et al. 2013). The diaminopimelate dehydrogenase encoded by ddh was able to divert the carbon flux from the precursor ASA to the by-product lysine, so the carbon flux toward ectoine was increased. The strain that carried ectABCD under the control of the promoter Ptuf was named C. glutamicum ECT-1. The ECT-1 strain was able to produce ectoine but still secreted lysine as the main by-product. The lysE gene that encoded the exporter lysE was deleted, which can prevent the excretion of the by-product lysine. As a result, the lysE knocked out strain C. glutamicum ECT-2 blocked the excretion of lysine and increased the production of ectoine and intracellular accumulation of lysine. The final concentration of ectoine produced by ECT-2 in fed-batch fermentation was 4.5 g/L, with a productivity of 6.7 g/L/day (Table 1). Unexpectedly, the production of hydroxyectoine in C. glutamicum ECT-2 was much less as compared to that produced in P. stutzeri for an unknown reason (see Table 2).
In the previous study, the recombinant strain C. glutamicum DM1729 not only possessed the same feedback-resistant aspartate kinase LysCT311I, but also expressed mutational pyruvate carboxylase PycP458S as well as mutational homoserine dehydrogenase HomV59A (Georgi et al. 2005). The PycP458S exhibited an enhanced activity for providing more oxaloacetate precursor with the biosynthesis of l-lysine, while the mutational homoserine dehydrogenase HomV59A showed a decreased activity for the conversion of ASA towards methionine and threonine. The high lysine-producing strain C. glutamicum DM1729 could be a useful tool for the heterogeneous production of ectoine due to the common precursor ASA of the lysine and ectoine. Hence, the plasmid carries the ectABC gene cluster from C. salexigens DSM 3043 was expressed in DM1729. To make further improvement in the production of ectoine, the gene sugR and ldhA were knocked out, which encoded the transcription repressor and l-lactate dehydrogenase. As a result, the glycolytic gene was derepressed and the production of by-product l-lactate was decreased. Consequently, the gene-engineered strain named C. glutamicum Ecto5 exhibited increased glycolytic flux and the formation of precursor ASA. The ectoine production was 22 g/L in fed-batch fermentation with a productivity of 7.6 g/L/day (Perez-Garcia et al. 2017). However, the accumulation of the by-product l-lysine achieved 6.2 g/L, which was threefold lower than ectoine. Compared to C. glutamicum ECT-2, the deletion of lysE led to the substantial reduction of ectoine and impaired growth in C. glutamicum Ecto5. This phenomenon indicates that deletion of lysE in the strain C. glutamicum Ecto5 with high lysine flux and ASA supply might result in metabolic imbalance (Liu et al. 2021).
The best-engineered ectoine-produced strain C. glutamicum ectABCopt in decades started from another lysine producer C. glutamicum lysCfbr, which also expressed the feedback-resistant aspartate kinase. lysE gene was knocked out in the genome of C. glutamicum lysCfbr in order to reduce the secretion of the main by-product lysine. The codon-optimized ectABC gene cluster was subsequently introduced into C. glutamicum lysCfbr by ectABC library. In the created ectABC library (pEctABC-Lib), one of 19 different synthetic promoters and one of three bicistronic linkers were randomly linked to each code-optimized gene of the ectoine pathway from P. stutzeri to coordinate the transcription and co-expression. The C. glutamicum ectABCopt was then selected from 185,193 theoretical variants with a titer of 65 g/L and productivity of 1.16 g/L/h, which represents the highest level of ectoine production to date (Giesselmann et al. 2019). Meanwhile, the ectABC library revealed that the expression rate of ectA and ectB was crucial to the high ectoine production. The production increased with the rate between ectA and ectB until up to the highest production (65 g/L, ectA/ectB: 1/3.1). Then, the production decreased when the expression rate of ectA/ectB increased. This discovery signified that EctB was the rate-limiting enzyme through the ectoine pathway and promoted the development of further heterologous production of ectoine.
Escherichia coli
Escherichia coli is the most commonly used host in genetic engineering because of its clear genetic background, low growth cost, short generation time, mature technical operation methods, and good compatibility with heterologous proteins (Liu et al. 2021). The unnecessary ectoine consumption can be avoided as E. coli does not have the ectoine degradation pathway or hydroxyectoine biosynthetic pathway similar to C. glutamicum (Jebbar et al. 1992).
For the selection of a better ectoine producer, the endogenous ectABC gene cluster from H. elongata was introduced into the basic strain E. coli W3110, G1655 and K27. W3110 accumulated higher ectoine titles among them and exhibited the potential in ectoine production due to its strong ability to synthesize aspartate-derived products (Lee et al. 2007; Song et al. 2015). The code-optimized ectABC and strong promoter Ptrc were employed in the vector pTrc99a for the optimization of the heterologous expression of ectABC in E. coli W3110. The thrA gene encoding aspartokinasel/homoserine dehydrogenase was further knocked out to increase the carbon flux towards ectoine as threonine and ectoine shared the same precursor ASA. When the compensation for the damaged activity of aspartokinase is caused by the deletion of the thrA gene, the lysCG1A,C932T gene encodes the feedback-resistant aspartokinase from C. glutamicum was overproduced. For the further improvement of the precursor ASA supply, iclR encoding for aceBA transcriptional repressor was mutated to enhance the oxaloacetate production. In addition, the stronger promoter Ptrc was utilized to replace the promoter of the ppc gene, which coded the phosphoenolpyruvate carboxylase. The metabolic engineered E. coli W3110 strain named ECT05 turned out 25.1 g/L of ectoine with the productivity of 20.2 g/L/day in the fermenter, which employed glucose as a carbon source under low salt concentration (Ning et al. 2016). The high production of ECT05 indicated the importance of adequate substrate supply and large metabolic flux of product in the biosynthesis of ectoine.
As is reported that the EctB was the rate-limited enzyme in the ectoine synthetic pathway (Hillier et al. 2020). Thus, an advanced high-throughput screening method was exploited to screen the activity-enhanced EctB variants using the engineered regulatory protein AraC in E. coli. The mutated AraC could specifically recognize ectoine and activate the transcription of GFP (green fluorescent protein) in the presence of ectoine. The expression of downstream GFP was correlated with the production of ectoine. In this way, the EctB-mutated strain (D180V/F320Y/Q325R) with enhanced ectoine production was selected (Chen et al. 2015). The high-throughput screening method provided an efficient way for screening the ectoine hyper-producing strains and promoting the further directed evolution of the rate-limiting enzyme, optimizing the genetic network and metabolic pathway.
A new systematical fermentation regulation strategy was designed, which can promote the heterogeneous production of ectoine. The recombinant strain E. coli ET01 carried the ectABC operon from Halomonas venusta ZH and was used for producing ectoine in the fermentation. In the glucose feedback method, glucose was added according to the glucose consumption rate. However, excessively unused glucose could generate various organic acids including acetic acid, which inhibited the growth of recombinant strain ET01. A lower level of biomass was not conducive to the ectoine biosynthesis. Thus, another pH-feedback feeding way was applied to control the supplemented carbon source. In the pH-feedback, the biomass increased substantially but the ectoine production decreased because of lacking sufficient ammonia. Given that the biomass was accumulated in the early phase, while the ectoine was produced in the later phase. Those two strategies were combined. The pH-feedback feeding was employed to promote cell growth and the glucose feedback feeding method was used to stabilize the remaining glucose and provide sufficient nitrogen source in the form of ammonia. Further fermentation parameters under different dissolved oxygen (DO) levels illustrated that the ectoine production and biomass increased when the DO was kept at 20% and 40%. Thus, the optimized combined fermentation strategy was developed. During the first cell growth phase (0–24 h), the pH-feedback feeding way was used with 10 g/L initial glucose and 40% DO. In the later ectoine production phase (24–48 h), the glucose feedback feeding method was used with 20% DO. The engineered strain finally accumulated 47.8 g/L ectoine with the productivity of 1 g/L/h, which represented the highest title in E. coli to date (Dong et al. 2021).
The whole-cell biocatalysis represented the environmental and cost-effective method for large-scale industrial production of target products, which provided a selected way for further enhanced ectoine production. In the recombinant E. coli K-12 strain BW25113, ectoine gene cluster ectABC was likewise cloned from H. elongata under the control of the inducible promoter ara. Ectoine was accumulated up to 25.1 g/L with the productivity of 4048 mg/g DCW (dry cell weight) using whole-cell biocatalysis at the high cell density of 20 OD600nm. Aspartate and glycerol were used as substrates in the whole-cell biocatalysis. The former promoted the synthesis of precursor ASA, while the latter provided the acetyl group and energy for the biosynthesis of ectoine. The used bacterial cells could repeat the whole-cell biocatalysis for another two cycles with the ectoine titer of 21.1 g/L (second) and17.2 g/L (third) (He et al. 2015). In another whole-cell biocatalysis strategy, the ectABC gene was introduced into the engineered E. coli MG1655 strain the lysA gene was knocked out. The deletion of the lysA gene encoding the diaminopimelate decarboxylase could block the carbon flux from ASA towards the main by-product lysine. The lysA-deleted strain accumulated 12.7 g/L ectoine by whole-cell biocatalysis with a final yield of 1.27 g/g glycerol and sodium aspartate at the cell density of 15 OD600nm (Chen et al. 2020). It was worth mentioning that KCl was added in both whole-cell biocatalysis ways to improve the activity and stability of EctB.
Yeast
Yeast is a single-cell eukaryotic microorganism that contains mature genetic operation methods and is accessible to culture. In contrast to E. coli, yeast does not maintain endotoxin in the cell wall, which leads to adverse health outcomes (Shamsollahi et al. 2019). Therefore, yeast has been widely used in molecular modification.
Saccharomyces cerevisiae (S. cerevisiae) is an advantageous platform for the fermentation synthesis of ectoine. In the strain S. cerevisiae CEN.PK2‑1C, the codon-optimized ectABC gene cluster from H. elongata was introduced into the shuttle plasmid pEBS for expression (Yang et al. 2018). The double optimized strategy could enhance the expression of ectoine. The native ribosome-binding site (RBS) and promoter of the ectABC gene cluster were randomly replaced by one of three optimized RBS and one of three optimized promoters to obtain 45 optimized strains. The S. cerevisiae ‑AP1R3BC strain exhibited the highest ectoine production among them with an ectoine titer of 2.3 g/L in a shaking-flask fermentation.
In another methylotrophic yeast Hansenula polymorpha, the hydroxyectoine synthesis gene cluster (ectABCD) from H. elongata was inserted into the genome for hydroxylectoine production. The engineered H. polymorpha cells were cultivated with the supplemented methanol and sorbitol as a carbon source under the induction of methanol. Ultimately, hydroxyectoine accumulated up to 2.8 g/L, with the ectoine of 0.044 g/L as a by-product. The conversion efficiency of ectoine to hydroxyectoine was almost 100% (Eilert et al. 2013).
Conclusions
This review article demonstrated the crucial bioactivities of ectoine, which protects cells and human skin via stabilization of biomacromolecule. This generates a huge potential for application in the current medical industry. According to the development of ectoine in health protection, the worldwide requirement for ectoine has risen rapidly. Ectoine is mainly produced from the industrial high-salt fermentation broth of halophilic microorganisms, such as H. elongata. However, the high-salt medium is corrosive to the fermentation equipment and unfriendly to the environment. To solve the disadvantages in the fermentation of halophilic microorganisms, more and more strategies have been created in the recent decade. The engineered bacteria, such as E. coli and C. glutamicum, could accumulate ectoine with high productivity in the low-salty medium, which represents a new generation for the industrial production of ectoine. The techniques and genetically engineered strategies summarized in this review may also provide ideas for further investigation in ectoine yields.
References
Abdel-Aziz H, Wadie W, Abdallah DM, Lentzen G, Khayyal MT (2013) Novel effects of ectoine, a bacteria-derived natural tetrahydropyrimidine, in experimental colitis. Phytomedicine 20:585–591
Becker J, Wittmann C (2020) Microbial production of extremolytes—high-value active ingredients for nutrition, health care, and well-being. Cur Opin Biotechnol 65:118–128
Becker J, Zelder O, Hafner S, Schroder H, Wittmann C (2011) From zero to hero–design-based systems metabolic engineering of Corynebacterium glutamicum for L-lysine production. Metab Eng 13:159–168
Becker J, Schäfer R, Kohlstedt M, Harder BJ, Borchert NS, Stöveken N, Bremer E, Wittmann C (2013) Systems metabolic engineering of Corynebacterium glutamicum for production of the chemical chaperone ectoine. Microb Cell Fact 12:110
Bethlehem L, van Echten-Deckert G (2021) Ectoines as novel anti-inflammatory and tissue protective lead compounds with special focus on inflammatory bowel disease and lung inflammation. Pharmacol Res 164:105389
Beyer N, Driller H, Bünger J (2000) Ectoin—a innovative, multi-functional active substance for the cosmetic industry. Sofw Journal 126:27–29
Bilstein A, Heinrich A, Rybachuk A, Mosges R (2021) Ectoine in the treatment of irritations and inflammations of the eye surface. Biomed Res Int 2021:8885032
Bissoyi A, Pramanik K (2013) Effects of non-toxic cryoprotective agents on the viability of cord blood derived mncs. CryoLetters 34:453–465
Booth IR (2014) Bacterial mechanosensitive channels: progress towards an understanding of their roles in cell physiology. Curr Opin Microbiol 18:16–22
Botta C, Di Giorgio C, Sabatier AS, De Meo M (2008) Genotoxicity of visible light (400–800 nm) and photoprotection assessment of ectoin, l-ergothioneine and mannitol and four sunscreens. J Photochem Photobiol B 91:24–34
Bownik A, Stepniewska Z (2016) Ectoine as a promising protective agent in humans and animals. Arh Hig Rada Toksikol 67:260–265
Brands S, Schein P, Castro-Ochoa KF, Galinski EA (2019) Hydroxyl radical scavenging of the compatible solute ectoine generates two N-acetimides. Arch Biochem Biophys 674:108097
Buenger J, Driller H (2004) Ectoin: an effective natural substance to prevent UVA-induced premature photoaging. Skin Pharmacol Phys 17:232–237
Bujak T, Zagorska-Dziok M, Niziol-Lukaszewska Z (2020) Complexes of ectoine with the anionic surfactants as active ingredients of cleansing cosmetics with reduced irritating potential. Molecules 25:1433
Bursy J, Pierik AJ, Pica N, Bremer E (2007) Osmotically induced synthesis of the compatible solute hydroxyectoine is mediated by an evolutionarily conserved ectoine hydroxylase. J Biol Chem 282:31147–31155
Castro-Ochoa KF, Vargas-Robles H, Chanez-Paredes S, Felipe-Lopez A, Cabrera-Silva RI, Shibayama M, Betanzos A, Nava P, Galinski EA, Schnoor M (2019) Homoectoine protects against colitis by preventing a Claudin switch in epithelial tight junctions. Dig Dis Sci 64:409–420
Chen W, Zhang S, Jiang P-X, Yao J, He Y-Z, Chen L-C, Gui X-W, Dong Z-Y, Tang S-Y (2015) Design of an ectoine-responsive AraC mutant and its application in metabolic engineering of ectoine biosynthesis. Metab Eng 30:149–155
Chen J, Liu P-F, Chu X-H, Chen J-W, Zhang H-W, Rowley DC, Wang H (2020) Metabolic pathway construction and optimization of escherichia coli for high-level ectoine production. Curr Microbiol 77:1412–1418
Chung YC, Kim M-J, Kang EY, Kim YB, Kim BS, Park S-M, Hyun C-G (2019) Anti-melanogenic effects of hydroxyectoine via MITF inhibition by JNK, p38, and AKT pathways in B16F10 melanoma cells. Nat Prod Commun 14:1–6
Correa A, Jhonny E (2013) Hydroxyectoine metabolism in halomonas elongata. Universitäts-und Landesbibliothek Bonn 3190
Cox CD, Bavi N, Martinac B (2018) Bacterial Mechanosensors. Annu Rev Physiol 80:71–93
Czech L, Stoveken N, Bremer E (2016) EctD-mediated biotransformation of the chemical chaperone ectoine into hydroxyectoine and its mechanosensitive channel-independent excretion. Microb Cell Fact 15:126
Czech L, Hermann L, Stoveken N, Richter AA, Hoppner A, Smits SHJ, Heider J, Bremer E (2018) Role of the extremolytes ectoine and hydroxyectoine as stress protectants and nutrients: genetics, phylogenomics, biochemistry, and structural analysis. Genes (basel) 9:177
Czech L, Hoppner A, Kobus S, Seubert A, Riclea R, Dickschat JS, Heider J, Smits SHJ, Bremer E (2019a) Illuminating the catalytic core of ectoine synthase through structural and biochemical analysis. Sci Rep 9:364
Czech L, Wilcken S, Czech O, Linne U, Brauner J, Smits SHJ, Galinski EA, Bremer E (2019b) Exploiting substrate promiscuity of ectoine hydroxylase for regio- and stereoselective modification of homoectoine. Front Microbiol 10:2745
Dao VA, Overhagen S, Bilstein A, Kolot C, Sonnemann U, Mosges R (2019) Ectoine lozenges in the treatment of acute viral pharyngitis: a prospective, active-controlled clinical study. Eur Arch Otorhinolaryngol 276:775–783
Di Gioacchino M, Bruni F, Sodo A, Imberti S, Ricci MA (2019) Ectoine hydration, aggregation and influence on water structure. Mol Phys 117:3311–3319
Dong Y-S, Zhang H, Wang X-Y, Ma J-J, Lei P, Xu H, Li S (2021) Enhancing ectoine production by recombinant Escherichia coli through step-wise fermentation optimization strategy based on kinetic analysis. Bioproc Biosyst Eng 44:1557–1566
Dwivedi M, Brinkkotter M, Harishchandra RK, Galla HJ (2014) Biophysical investigations of the structure and function of the tear fluid lipid layers and the effect of ectoine. Part B: artificial lipid films. BBA-Biomembranes 1838:2716–2727
Eiberweiser A, Nazet A, Kruchinin SE, Fedotova MV, Buchner R (2015) Hydration and ion binding of the osmolyte ectoine. J Phys Chem B 119:15203–15211
Eichel A, Bilstein A, Werkhauser N, Mosges R (2014) Meta-analysis of the efficacy of ectoine nasal spray in patients with allergic rhinoconjunctivitis. J Allergy, p 292545
Eilert E, Kranz A, Hollenberg CP, Piontek M, Suckow M (2013) Synthesis and release of the bacterial compatible solute 5-hydroxyectoine in Hansenula polymorpha. J Biotechnol 167:85–93
El Assal R, Guven S, Gurkan UA, Gozen I, Shafiee H, Dalbeyler S, Abdalla N, Thomas G, Fuld W, Illigens BMW, Estanislau J, Khoory J, Kaufman R, Zylberberg C, Lindeman N, Wen Q, Ghiran I, Demirci U (2014) Bio-inspired cryo-ink preserves red blood cell phenotype and function during nanoliter vitrification. Adv Mater 26:5815–5822
Fedotova M (2019) Compatible osmolytes-bioprotectants: is there a common link between their hydration and their protective action under abiotic stresses? J Mol Liq 292:111339
Fenizia S, Thume K, Wirgenings M, Pohnert G (2020) Ectoine from bacterial and algal origin is a compatible solute in microalgae. Mar Drugs 18:42
Galinski EA, Pfeiffer HP, Troper HG (1985) 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur J Biochem 149:135–139
Garcia-Estepa R, Argandona M, Reina-Bueno M, Capote N, Iglesias-Guerra F, Nieto JJ, Vargas C (2006) The ectD gene, which is involved in the synthesis of the compatible solute hydroxyectoine, is essential for thermoprotection of the halophilic bacterium Chromohalobacter salexigens. J Bacteriol 188:3774–3784
Georgi T, Rittmann D, Wendisch VF (2005) Lysine and glutamate production by Corynebacterium glutamicum on glucose, fructose and sucrose: roles of malic enzyme and fructose-1,6-bisphosphatase. Metab Eng 7:291–301
Giesselmann G, Dietrich D, Jungmann L, Kohlstedt M, Jeon EJ, Yim SS, Sommer F, Zimmer D, Muhlhaus T, Schroda M, Jeong KJ, Becker J, Wittmann C (2019) Metabolic engineering of Corynebacterium glutamicum for high-level ectoine production: design, combinatorial assembly, and implementation of a transcriptionally balanced heterologous ectoine pathway. Biotechnol J 14:1800417
Goraj W, Stępniewska Z, Szafranek-Nakonieczna A (2019) Biosynthesis and the possibility of using ectoine and hydroxyectoine in health care. Adv Microbiol Postepy Mikrobiologii 58:339–349
Graf R, Anzali S, Buenger J, Pfluecker F, Driller H (2008) The multifunctional role of ectoine as a natural cell protectant. Clin Dermatol 26:326–333
Grether-Beck S, Timmer A, Felsner I, Brenden H, Brammertz D, Krutmann J (2005) Ultraviolet a-induced signaling involves a ceramide-mediated autocrine loop leading to ceramide de novo synthesis. J Invest Dermatol 125:545–553
Hahn MB, Solomun T, Wellhausen R, Hermann S, Seitz H, Meyer S, Kunte HJ, Zeman J, Uhlig F, Smiatek J, Sturm H (2015) Influence of the compatible solute ectoine on the local water structure: Implications for the binding of the protein G5P to DNA. J Phys Chem B 119:15212–15220
Hahn MB, Uhlig F, Solomun T, Smiatek J, Sturm H (2016) Combined influence of ectoine and salt: spectroscopic and numerical evidence for compensating effects on aqueous solutions. Phys Chem Chem Phys 18:28398–28402
Hahn MB, Meyer S, Schroter MA, Kunte HJ, Solomun T, Sturm H (2017) DNA protection by ectoine from ionizing radiation: molecular mechanisms. Phys Chem Chem Phys 19:25717–25722
Harishchandra RK, Wulff S, Lentzen G, Neuhaus T, Galla HJ (2010) The effect of compatible solute ectoines on the structural organization of lipid monolayer and bilayer membranes. Biophys Chem 150:37–46
Harishchandra RK, Sachan AK, Kerth A, Lentzen G, Neuhaus T, Galla HJ (2011) Compatible solutes: ectoine and hydroxyectoine improve functional nanostructures in artificial lung surfactants. BBA-Biomembranes 1808:2830–2840
He Y, Gong J, Yu H, Tao Y, Zhang S, Dong Z (2015) High production of ectoine from aspartate and glycerol by use of whole-cell biocatalysis in recombinant Escherichia coli. Microb Cell Fact 14:55
Hermann L, Mais CN, Czech L, Smits SHJ, Bange G, Bremer E (2020) The ups and downs of ectoine: structural enzymology of a major microbial stress protectant and versatile nutrient. Biol Chem 401:1443–1468
Herzog M, Dwivedi M, Harishchandra RK, Bilstein A, Galla HJ, Winter R (2019) Effect of ectoine, hydroxyectoine and beta-hydroxybutyrate on the temperature and pressure stability of phospholipid bilayer membranes of different complexity. Colloids Surf, B 178:404–411
Hillier HT, Altermark B, Leiros I (2020) The crystal structure of the tetrameric DABA-aminotransferase EctB, a rate-limiting enzyme in the ectoine biosynthesis pathway. FEBS J 287:4641–4658
Hirasawa T, Shimizu H (2016) Recent advances in amino acid production by microbial cells. Curr Opin Biotechnol 42:133–146
Hoppner A, Widderich N, Lenders M, Bremer E, Smits SH (2014) Crystal structure of the ectoine hydroxylase, a snapshot of the active site. J Biol Chem 289:29570–29583
Hseu Y-C, Chen X-Z, Vudhya Gowrisankar Y, Yen HR, Chuang J-Y, Yang H-L (2020) The skin-whitening effects of ectoine via the suppression of α-MSH-stimulated melanogenesis and the activation of antioxidant Nrf2 pathways in UVA-irradiated keratinocytes. Antioxidants (basel) 9:63
Inbar L, Lapidot A (1988) The structure and biosynthesis of new tetrahydropyrimidine derivatives in actinomycin D producer Streptomyces parvulus. Use of 13C- and 15N-labeled l-glutamate and 13C and 15N NMR spectroscopy. J Biol Chem 263:16014–16022
Jebbar M, Talibart R, Gloux K, Bernard T, Blanco C (1992) Osmoprotection of Escherichia coli by ectoine: uptake and accumulation characteristics. Journal of Bacteriololgy 174:5027–5035
Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L, Goesmann A, Hartmann M, Huthmacher K, Kramer R, Linke B, McHardy AC, Meyer F, Mockel B, Pfefferle W, Puhler A, Rey DA, Ruckert C, Rupp O, Sahm H, Wendisch VF, Wiegrabe I, Tauch A (2003) The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of l-aspartate-derived amino acids and vitamins. J Biotechnol 104:5–25
Kanapathipillai M, Lentzen G, Sierks M, Park CB (2005) Ectoine and hydroxyectoine inhibit aggregation and neurotoxicity of Alzheimer’s β-amyloid. FEBS Lett 579:4775–4780
Kauth M, Trusova OV (2022) Topical ectoine application in children and adults to treat inflammatory diseases associated with an impaired skin barrier: a systematic review. Dermatol Ther 12:295–313
Kim HM, Heinzle E, Wittmann C (2006) Deregulation of aspartokinase by single nucleotide exchange leads to global flux rearrangement in the central metabolism of Corynebacterium glutamicum. J Microbiol Biotechnol 16:1174–1179
Kolp S, Pietsch M, Galinski EA, Gutschow M (2006) Compatible solutes as protectants for zymogens against proteolysis. Biochim Biophys Acta 1764:1234–1242
Kunte HJ, Lentzen G, Galinski EA (2014) Industrial production of the cell protectant ectoine protection mechanisms, processes, and products. Curr Biotechnol 3:10–25
Lang Y, Bai L, Ren Y, Zhang L, Nagata S (2011) Production of ectoine through a combined process that uses both growing and resting cells of Halomonas salina DSM 5928T. Extremophiles 15:303–310
Lapidot A, Benasher E, Eisenstein M (1995) Tetrahydropyrimidine derivatives inhibit binding of a Tat-like, arginine-containing peptide, to HIV TAR RNA in vitro. FEBS Lett 367:33–38
Lee KH, Park JH, Kim TY, Kim HU, Lee SY (2007) Systems metabolic engineering of Escherichia coli for l-threonine production. Mol Syst Biol 3:149
Liu M-S, Liu H, Shi M, Jiang M-Y, Li L-L, Zheng Y-N (2021) Microbial production of ectoine and hydroxyectoine as high-value chemicals. Microb Cell Fact 20:76
Lo CC, Bonner CA, Xie G, D’Souza M, Jensen RA (2009) Cohesion group approach for evolutionary analysis of aspartokinase, an enzyme that feeds a branched network of many biochemical pathways. Microbiol Mol Biol Rev 73:594–651
Louis P, Galinski EA (1997) Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoreg u lated expression in Escherichia coli. Microbiology 143:1141–1149
Ma H, Zhao Y-Q, Huang W-Z, Zhang L-Z, Wu F-Q, Ye J-W, Chen G-Q (2020) Rational flux-tuning of Halomonas bluephagenesis for co-production of bioplastic PHB and ectoine. Nat Commun 11:3313
Mais CN, Hermann L, Altegoer F, Seubert A, Richter AA, Wernersbach I, Czech L, Bremer E, Bange G (2020) Degradation of the microbial stress protectants and chemical chaperones ectoine and hydroxyectoine by a bacterial hydrolase-deacetylase complex. J Biol Chem 295:9087–9104
Marrot L, Jones C, Perez P, Meunier JR (2008) The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma R 21:79–88
Meyer S, Schroter MA, Hahn MB, Solomun T, Sturm H, Kunte HJ (2017) Ectoine can enhance structural changes in DNA in vitro. Sci Rep 7:7170
Mukherjee S, Edmunds M, Lei X-G, Ottaviani MF, Ananthapadmanabhan KP, Turro NJ (2010) Stearic acid delivery to corneum from a mild and moisturizing cleanser. J Cosmet Dermatol 9:202–210
Nayak PK, Goode M, Chang DP, Rajagopal K (2020) Ectoine and hydroxyectoine stabilize antibodies in spray-dried formulations at elevated temperature and during a freeze/thaw process. Mol Pharm 17:3291–3297
Ning Y, Wu X, Zhang C, Xu Q, Chen N, Xie X (2016) Pathway construction and metabolic engineering for fermentative production of ectoine in Escherichia coli. Metab Eng 36:10–18
Nosch DS, Joos RE, Job M (2021) Prospective randomized study to evaluate the efficacy and tolerability of Ectoin® containing Eye Spray (EES09) and comparison to the liposomal Eye Spray Tears Again® (TA) in the treatment of dry eye disease. Cont Lens Anterior Eye 44:101318
Ono H, Sawada K, Khunajakr N, Tao T, Yamamoto M, Hiramoto M, Shinmyo A, Takano M, Murooka Y (1999) Characterization of biosynthetic enzymes for ectoine as a compatible solute in a moderately halophilic eubacterium, Halomonas elongata. Journal of Bacteriololgy 181:91–99
Oprzeska-Zingrebe EA, Meyer S, Roloff A, Kunte HJ, Smiatek J (2018) Influence of compatible solute ectoine on distinct DNA structures: thermodynamic insights into molecular binding mechanisms and destabilization effects. Phys Chem Chem Phys 20:25861–25874
Pastor JM, Salvador M, Argandona M, Bernal V, Reina-Bueno M, Csonka LN, Iborra JL, Vargas C, Nieto JJ, Canovas M (2010) Ectoines in cell stress protection: uses and biotechnological production. Biotechnol Adv 28:782–801
Perez-Garcia F, Ziert C, Risse JM, Wendisch VF (2017) Improved fermentative production of the compatible solute ectoine by Corynebacterium glutamicum from glucose and alternative carbon sources. J Biotechnol 258:59–68
Pliotas C, Naismith JH (2017) Spectator no more, the role of the membrane in regulating ion channel function. Curr Opin Struct Biol 45:59–66
Rabe JH, Mamelak AJ, Mcelgunn PJ, Morison WL, Sauder DN (2006) Photoaging: mechanisms and repair. J Am Acad Dermatol 55:1–19
Rasmussen T (2016) How do mechanosensitive channels sense membrane tension? Biochem Soc Trans 44:1019–1025
Reshetnikov AS, Rozova ON, Trotsenko YA, But SY, Khmelenina VN, Mustakhimov II (2020) Ectoine degradation pathway in halotolerant methylotrophs. PLoS ONE 15:0232244
Reuter K, Pittelkow M, Bursy J, Heine A, Craan T, Bremer E (2010) Synthesis of 5-hydroxyectoine from ectoine: crystal structure of the non-heme iron(II) and 2-oxoglutarate-dependent dioxygenase EctD. PLoS ONE 5:10647
Richter AA, Mais CN, Czech L, Geyer K, Hoeppner A, Smits SHJ, Erb TJ, Bange G, Bremer E (2019) Biosynthesis of the stress-protectant and chemical chaperon ectoine: biochemistry of the transaminase EctB. Front Microbiol 10:2811
Rousseau K, Kauser S, Pritchard LE, Warhurst A, Oliver RL, Slominski A, Wei ET, Thody AJ, Tobin DJ, White A (2007) Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J 21:1844–1856
Ryu J, Kanapathipillai M, Lentzen G, Park CB (2008) Inhibition of β-amyloid peptide aggregation and neurotoxicity by α-D-mannosylglycerate, a natural extremolyte. Peptides 29:578–584
Sahle CJ, Schroer MA, Jeffries CM, Niskanen J (2018) Hydration in aqueous solutions of ectoine and hydroxyectoine. Phys Chem Chem Phys 20:27917–27923
Sajjad W, Qadir S, Ahmad M, Rafiq M, Hasan F, Tehan R, McPhail KL, Shah AA (2018) Ectoine: a compatible solute in radio-halophilic Stenotrophomonas sp. WMA-LM19 strain to prevent ultraviolet-induced protein damage. J Appl Microbiol 125:457–467
Salapatek A, Bate M, Bilstein A, Patel D (2011) Ectoin®, a novel, non-drug, extremophile-based device, relieves allergic rhinoconjunctivitis symptoms in patients in an environmental exposure chamber model. J Allergy Clin Immun 127:AB202
Sauer T, Galinski EA (1997) Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol Bioeng 57:306–313
Schroter MA, Meyer S, Hahn MB, Solomun T, Sturm H, Kunte HJ (2017) Ectoine protects DNA from damage by ionizing radiation. Sci Rep 7:15272
Schulz A, Stoveken N, Binzen IM, Hoffmann T, Heider J, Bremer E (2017) Feeding on compatible solutes: a substrate-induced pathway for uptake and catabolism of ectoines and its genetic control by EnuR. Environ Microbiol 19:926–946
Schwibbert K, Marin-Sanguino A, Bagyan I, Heidrich G, Lentzen G, Seitz H, Rampp M, Schuster SC, Klenk HP, Pfeiffer F, Oesterhelt D, Kunte HJ (2011) A blueprint of ectoine metabolism from the genome of the industrial producer Halomonas elongata DSM 2581T. Environ Microbiol 13:1973–1994
Shamsollahi HR, Ghoochani M, Jaafari J, Moosavi A, Sillanpaa M, Alimohammadi M (2019) Environmental exposure to endotoxin and its health outcomes: a systematic review. Ecotox Environ Safe 174:236–244
Song CW, Lee J, Ko YS, Lee SY (2015) Metabolic engineering of Escherichia coli for the production of 3-aminopropionic acid. Metab Eng 30:121–129
Sonnemann U, Scherner O, Werkhauser N (2014) Treatment of rhinitis sicca anterior with ectoine containing nasal spray. J Allergy 2014:273219
Srinivasan PK, Fet N, Bleilevens C, Afify M, Doorschodt B, Yagi S, van Echten-Deckert G, Tolba RH (2014) Hydroxyectoine ameliorates preservation injury in deceased after cardiac death donors in experimental liver grafts. Ann Transplant 19:165–173
Strong PJ, Kalyuzhnaya M, Silverman J, Clarke WP (2016) A methanotroph-based biorefinery: potential scenarios for generating multiple products from a single fermentation. Bioresour Technol 215:314–323
Sydlik U, Gallitz I, Albrecht C, Abel J, Krutmann J, Unfried K (2009) The compatible solute ectoine protects against nanoparticle-induced neutrophilic lung inflammation. Am J Respir Crit Care Med 180:29–35
Sydlik U, Peuschel H, Paunel-Gorgulu A, Keymel S, Kramer U, Weissenberg A, Kroker M, Seghrouchni S, Heiss C, Windolf J, Bilstein A, Kelm M, Krutmann J, Unfried K (2013) Recovery of neutrophil apoptosis by ectoine: a new strategy against lung inflammation. Eur Respir J 41:433–442
Tsai T, Mueller-Buehl AM, Satgunarajah Y, Kuehn S, Dick HB, Joachim SC (2020) Protective effect of the extremolytes ectoine and hydroxyectoine in a porcine organ culture. Graefes Arch Clin Exp Ophthalmol 258:2185–2203
Tsuge Y, Matsuzawa H (2021) Recent progress in production of amino acid-derived chemicals using Corynebacterium glutamicum. World J Microb Biot 37:49
Unfried K, Kroker M, Autengruber A, Gotic M, Sydlik U (2014) The compatible solute ectoine reduces the exacerbating effect of environmental model particles on the immune response of the airways. J Allergy 2014:708458
Werkhauser N, Bilstein A, Sonnemann U (2014) Treatment of allergic rhinitis with ectoine containing nasal spray and eye drops in comparison with azelastine containing nasal spray and eye drops or with cromoglycic acid containing nasal spray. J Allergy 2014:176597
Widderich N, Czech L, Elling FJ, Konneke M, Stoveken N, Pittelkow M, Riclea R, Dickschat JS, Heider J, Bremer E (2016a) Strangers in the archaeal world: osmostress-responsive biosynthesis of ectoine and hydroxyectoine by the marine thaumarchaeon Nitrosopumilus maritimus. Environ Microbiol 18:1227–1248
Widderich N, Kobus S, Hoppner A, Riclea R, Seubert A, Dickschat JS, Heider J, Smits SH, Bremer E (2016b) Biochemistry and crystal structure of ectoine synthase: a metal-containing member of the cupin superfamily. PLoS ONE 11:0151285
Wittmar J, Meyer S, Sieling T, Kunte J, Smiatek J, Brand I (2020) What does ectoine do to DNA? A molecular-scale picture of compatible solute-biopolymer interactions. J Phys Chem B 124:7999–8011
Yang S, Liu Q-T, Zhang Y-F, Du G-C, Chen J, Kang Z (2018) Construction and characterization of broad-spectrum promoters for synthetic biology. ACS Synth Biol 7:287–291
Yao C-L, Lin Y-M, Mohamed MS, Chen J-H (2013) Inhibitory effect of ectoine on melanogenesis in B16–F0 and A2058 melanoma cell lines. Biochem Eng J 78:163–169
Yu I, Jindo Y, Nagaoka M (2007) Microscopic understanding of preferential exclusion of compatible solute ectoine. J Phys Chem B 111:10231–10238
Zaccai G, Bagyan I, Combet J, Cuello GJ, Deme B, Fichou Y, Gallat FX, Galvan Josa VM, von Gronau S, Haertlein M, Martel A, Moulin M, Neumann M, Weik M, Oesterhelt D (2016) Neutrons describe ectoine effects on water H-bonding and hydration around a soluble protein and a cell membrane. Sci Rep 6:31434
Zhao Q, Li S-N, Lv P-W, Sun S-M, Ma C-Q, Xu P, Su H-J, Yang C-Y (2019) High ectoine production by an engineered Halomonas hydrothermalis Y2 in a reduced salinity medium. Microb Cell Fact 18:184
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 32102099) and Natural Science Foundation of Zhejiang Province (Grant No. Q22C207891) and the China Postdoctoral Science Foundation (Grant No. 2021M702895). We highly appreciate Ziyi Cheng (University of Toronto) for the language polish and revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Z., Wu, C., Zhu, L. et al. Bioactivity profiling of the extremolyte ectoine as a promising protectant and its heterologous production. 3 Biotech 12, 331 (2022). https://doi.org/10.1007/s13205-022-03370-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03370-5