Abstract
A central composite experimental design leading to a set of 16 experiments with different combinations of pH and temperature was performed to attain the optimal activities of xylose reductase (XR) and xylitol dehydrogenase (XDH) enzymes from Candida mogii cell extract. Under optimized conditions (pH 6.5 and 38°C), the XR and XDH activities were found to be 0.48 U/ml and 0.22 U/ml, respectively, resulting in an XR to XDH ratio of 2.2. Stability, cofactor specificity and kinetic parameters of the enzyme XR were also evaluated. XR activity remained stable for 3 h under 4 and 38°C and for 4 months of storage at −18°C. Studies on cofactor specificity showed that only NADPH-dependent XR was obtained under the cultivation conditions employed. The XR present in C. mogii extracts showed a superior K m value for xylose when compared with other yeast strains. Besides, this parameter was not modified after enzyme extraction by aqueous two-phase system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Xylose reductase (XR) enzyme is responsible for the first step in the xylose metabolism of yeasts (Chiang and Knight 1960). In a reaction catalyzed by this enzyme, xylose is reduced to xylitol that can be oxidized into xylulose by the xylitol dehydrogenase (XDH) enzyme or released to the environment, depending on the culture conditions and on the microorganism utilized (Kern et al. 1997; Ho et al. 1990; Bolen et al. 1986). Xylitol is a sugar–alcohol of economic interest due to its sweetening power and its dietetic and anticariogenic properties (Hyvönen and Koivistoinen 1982). Studies on extraction and purification of XR from yeast cells are being conducted aimed at either characterizing the enzyme to improve the fermentative process or obtaining a purified XR solution for direct use in enzymatic conversion of xylose into xylitol (Mayerhoff et al. 2001; Cortez et al. 2001).
Enzymes are sensitive molecules unable to resist a variety of stresses when released from their natural protective environment. Once the tissue is disrupted, intracellular enzymes are released from their safe surroundings into a hostile medium in which proteolytic enzymes are also released, and so they are exposed to threat, which may lead to loss of activity (Scopes 1994). One of the problems of enzyme purification is establishing a reliable and reproducible assay to relate the amounts of enzyme in different fractions during the process.
Candida mogii has been selected among 31 yeast species as a good xylitol producer (Mayerhoff et al. 1997). XR purification using this microorganism has been investigated in our laboratories (Mayerhoff et al. 2004). Although the enzymes XR and XDH are well characterized for various species of yeasts, little information for either enzyme specifically related to C. mogii is available. In the present work, different values of pH and temperature were tested to determine the optimal XR and XDH activity conditions as well as optimal XR to XDH ratio. Experimental design using response surface methodology (RSM) was used in this stage. The XR stability was also investigated for temperatures of storage and handling. Kinetic parameters were determined for the XR present both in C. mogii extract without purification and in extract purified by an aqueous two-phase system (ATPS) composed of polyethylene glycol and phosphate salts.
Materials and methods
Chemicals
d-Xylose, β-mercaptoethanol and the cofactors NADPH, NADH and NAD+ were purchased from Sigma, St. Louis, MO, USA. The other reagents were of analytical grade.
Microorganism cultivation
The yeast C. mogii NRRL Y-17032 obtained from the Northern Regional Research Laboratory (Peoria, IL) was kept at 4°C on malt-extract agar slants. Cells were cultivated in medium prepared from rice straw hemicellulosic hydrolysate diluted with distilled water to an initial xylose concentration of 50 g/l (Mayerhoff et al. 2001). Cultivation was carried out in Erlenmeyer flasks, and the ratio of flask volume to medium volume was 5:1. The flasks were incubated in a rotary shaker at 200 rpm and 30°C for 48 h with an initial cell concentration of 1 g of cell dried weight per litre. At the end of fermentation, the cells were harvested by centrifugation at 1,100×g, washed with 0.1 M potassium phosphate buffer, centrifuged and resuspended with the same buffer. The final suspension (about 15 g dry weight cell per litre) was stored at −18°C.
Obtainment of cell-free extracts
The cell suspension was thawed and disrupted by sonication in 1-s pulses for a period of 35 min with 1-s intervals using a disrupter (VC-100; Sonics and Materials, Newton, CT) at a frequency of 60 Hz. During the disruption, the cell suspension was kept cooled at 4°C to prevent overheating. Samples were centrifuged for 10 min under 6,000×g in a Jouan MR1812 centrifuge. Cell-free extracts were collected and analysed under given conditions.
XR extraction
The enzyme was extracted in an ATPS composed of 19.3% (w/w) PEG 1000 and 12.4% (w/w) sodium/potassium phosphate (Mayerhoff et al. 2004). A 10-g system was prepared by adding 2 ml of cell extract, and the final mass was made up with deionized water. The systems were agitated in magnetic stirrer, and after thorough homogenization, transferred to graduate centrifuged tubes. Separation was promoted by centrifugation at 1,600×g for 5 min. The top phase was collected for the determination of XR kinetic parameters.
Determination of optimal enzymatic activities and kinetic parameters
Optimal enzymatic activities were investigated by running enzymatic analyses according to an experimental design with three different temperatures (20, 40 and 60°C) and four pH values (5.0, 6.0, 7.0 and 8.0). For each temperature, reagents were maintained for 5 min in a water bath. The pH values were achieved using appropriate phosphate buffer solutions. In the XR analyses, NADH and NADPH were used separately as cofactors, so that their specificity could be determined. To evaluate the XR stability, the optimum pH value was used and extract samples were kept at −18, 4 and 38°C. The samples at 4 and 38°C were analysed at 30- or 60-min intervals for 5 h. The frozen samples were analysed after 60 and 120 days. Kinetic parameters were studied by varying the substrate and the cofactor concentrations in the reaction mixtures of the analyses.
XR activities were determined by the change of absorbance at 340 nm due to the oxidation of the coenzyme (NADPH or NADH). Assays were run in a 1-ml cuvette containing 50 μl 1 M phosphate buffer, 100 μl 0.1 M mercaptoethanol, 100 μl 1.2 mM NADPH, 100 μl 0.5 M d-xylose and 650 μl cell-free extract. The reaction was started up by the addition of d-xylose. The activity of XDH was determined in a similar manner using 0.5 M xylitol and 1.2 mM NAD+ as the substrate and the cofactor, respectively. Total protein concentration was determined according to Bradford’s method using bovine serum albumin as the standard. One unit of XR activity (U) was defined as the amount of enzyme catalyzing the oxidation of 1 μmol of NADPH per minute.
Statistical analysis
The results of experimental design were analysed using the software Design-Expert 5 from Stat-Ease Corporation, USA. The coefficients were generated by regression analysis. The fit of the models was evaluated by the determination coefficients (R 2) and the F test (ANOVA).
Results
Optimal activity
A central composite experimental design leading to a set of 16 experiments with different combinations of pH and temperature was performed to attain the optimal conditions for XR and XDH activities (Table 1). Data for NADH-dependent XR are not shown since no activity could be detected using this cofactor.
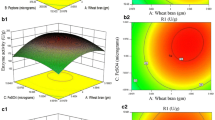
XR and XDH activities varied markedly with the conditions evaluated, ranging from 0.0 to 0.52 U/ml and from 0.01 to 0.32 U/ml, respectively. The highest values of XR and XDH activity were attained when intermediary levels of pH and temperature were used (assays 12 to 16). Multiple regression analysis of the experimental data was performed to fit the response function to a second-order polynomial model. Two models expressed by Eqs. 1 and 2 were generated, representing the XR and XDH activities as a function of the pH and temperature. The statistical significance of the second-order model was evaluated by the F test analysis of variance (Table 2). A detailed presentation of the optimum values predicted from the models using the isoresponse contour is given in Figs. 1 and 2.
and
in which XR and XDH are the enzyme activities (units per millilitre) and X 1 and X 2 are coded values for pH and temperature, respectively.
The maximum XR and XDH activity values predicted by the models were 0.48 and 0.34 U/ml, respectively, corresponding to the point defined by pH 6.5 at 38°C for XR and pH 8.0 at 36°C for XDH.
Based on the models obtained, a new working region (pH 6.5 at 38°C) was selected in which it is possible to reach a higher XR activity (0.48 U/ml) and lower XDH activity (0.22 U/ml), resulting in an XR to XDH ratio of 2.2. These predicted values were confirmed experimentally, having achieved levels of 0.52 U/ml for XR and 0.24 U/ml for XDH.
XR stability at storage and handling temperatures
Figure 3 shows the results of the XR stability study. For temperatures of 4 and 38°C, the behaviour of the enzyme activity was the same, remaining stable for 180 min. A slight variation within the analytical error (6.6%) can be observed. The relative XR activity dropped to about 80% after 240 min and 50% after 300 min. XR activity remained stable for 4 months of storage at −18°C, and after thawing, it decreased about 10% after 180 min at 4°C (data not shown).
Kinetic parameters
The effects of xylose and NADPH concentrations on the activity of XR enzyme present in C. mogii cell extract are shown in Figs. 4a and 5a. As can be seen, the NADP formation rate increases with the increase in the concentrations of both substrates up to a certain point, after which it tends to be constant. In fact, the rate of catalysis by enzymes varies with the substrate concentration so that a maximum value (V max) is reached. Linear methods are used for determining the V max and the Michaelis constant (K m) in enzymatic catalysis. The Lineweaver–Burk plot was used to determine XR kinetic constants using xylose and NADPH as substrate (Figs. 4a and 5a). The V max and K m values found for XR of C. mogii cell extract were 0.485 μmol NADP/min and 63 mM and 0.390 μmol NADP/min and 0.032 mM for xylose and NADPH, respectively.
The effect of the initial substrate concentration on the activity of the XR present in the top phase of cell extract purified in an ATPS was also investigated (Fig. 6a). Table 3 shows XR activity and total protein content from C. mogii extracted by partitioning in an ATPS composed of polyethyleneglycol (PEG) and phosphate salts. Under this condition, the purification factor (PF) in the top phase was about 1.90. The values of K m and V max for xylose calculated using the Lineweaver–Burk plot (Fig. 6b) were 65 mM and 0.247 μmol NADP/min, respectively. The dilution of the enzyme in the ATPS could explain the reduction in V max since this parameter is dependent on the enzyme concentration.
Discussion
In this study, response surface methodology (RSM) was used as an approach for the maximization of the xylose reductase (XR) and xylitol dehydrogenase (XDH) activities as a function of pH and temperature. The objective of this work was also to establish operational conditions that allow a desirable working region where a high XR to XDH ratio can be attained. The high XR to XDH ratio is important for the biotechnological process of xylitol production because the XDH catalyzes the oxidation of xylitol to xylulose, leading to the decrease in the overall xylitol yield.
Recently, RSM has been used to determine the kinetic constants of enzymatic reactions as well as for the optimization of reactions. Boyaci (2005) used RSM and the conventional method to determine the kinetic constants of glucose oxidase as a function of reaction temperature and pH. The accuracy of the RSM for determining K m and V max was tested by comparing the results of these two methods. According to the author, reasonable results in the range of tested parameters were reached. A quite good correlation between the kinetic constants of horse liver alcohol dehydrogenase obtained from conventional methods and those obtained from RSM was also reported by Andersson and Adlercreutz (1999).
In the present work, mathematical models to represent XR and XDH optimal activities as a function of pH and temperature were obtained. The analysis of variance (Table 2) shows that the models adjusted for the responses of XR and XDH activity are adequate since a good determination coefficient (R 2) of 0.86 and 0.71 for XR and XDH, respectively, was achieved. This indicates that 86 and 71% of the variability in the response could be explained by the model obtained. In addition, the model did not show lack of fit, and the F test demonstrates high statistical significance (p<0.01) at 99% confidence level. The maximum XR activity value predicted by the model corresponded to the point defined by pH 6.5 at 38°C, whereas for XDH, the maximum value was attained at pH 8.0 and 36°C. Similar optimal pH for both XR and XDH activities from various yeasts has been related in the literature (Woodyer et al. 2005; Gírio et al. 1996). On the other hand, the optimum temperature for these enzymes of C. mogii was shown to be lower than those reported for other XRs and XDHs. Takamizawa et al. (2000) characterized the XDH from Candida tropicalis IFO 0618 and showed that the optimal pH and temperature values for this enzyme were pH 8.0 and 50°C, respectively. According to Neuhauser et al. 1997, the optimum pH and temperature of aldose (xylose) reductase from the yeast Candida tenuis were found to be 6.0 and 50°C, respectively.
As can be seen in Fig. 1, the maximum predicted value for the XR activity (0.48 U/ml) was attained at pH 6.5 and 38°C. Under this condition, the predicted value for XDH activity is 0.22 U/ml (Fig. 2), resulting in an XR to XDH ratio of 2.2. This result was experimentally confirmed, having achieved an XR to XDH ratio of 2.16. Similar values have been found for xylitol-producing yeasts such as Candida boidinii (Vandeska et al. 1995) and Debaryomyces hansenii (Gírio et al. 1994). For these microorganisms, XR to XDH ratio varied from 1.10 to 2.10 and from 1.14 to 2.26, respectively.
The kinetic parameters determined in the present work were compared with results found in the literature for different yeast strains (Table 4). As can be seen, C. mogii had a higher specificity for xylose when compared to other yeasts since its K m values were the lowest (63 and 65 mM). The effect of the initial substrate concentration on the XR purified in an ATPS composed of polyethylene glycol and phosphate salts was also determined. According to our results, XR enzyme extracted in ATPS presented a similar specificity for xylose, suggesting that the pre-purification of the enzyme did not modify this parameter.
This study demonstrated that response surface analysis is a methodology quite adequate for the maximization of XR and XDH activities as a function of pH and temperature. This methodology also makes it possible to determine a desirable working region where a high XR to XDH ratio can be attained. XR activity was shown to be exclusively NADPH-dependent, and the period of time in which XR can be used or stored without activity loss was also determined in this work. These data are very useful for the XR purification experiments. The XR present in C. mogii extracts showed a superior K m value for xylose when compared to other yeast strains. Besides, this parameter was not modified after enzyme extraction by ATPS.
References
Andersson M, Adlercreutz P (1999) Evaluation of simple enzyme kinetics by response surface modeling. Biotechnol Tech 13:903–907
Chiang C, Knight SG (1960) Metabolism of d-xylose by moulds. Nature 188:79–81
Bolen PL, Roth KA, Freer SN (1986) Affinity purifications of aldose reductase and xylitol dehydrogenase from the xylose-fermenting yeast Pachysolen tannophilus. Appl Environ Microbiol 52:660–664
Boyaci IH (2005) A new approach for determination of enzyme kinetic constants using response surface methodology. Biochem Eng J 25:55–62
Cortez EV, Felipe MGA, Roberto IC, Pessoa Jr A, Vitolo M (2001) Extraction by reversed micelles of the intracellular enzyme xylose reductase. Appl Biochem Biotechnol 91/93:753–759
Ditzelmüller G, Kubicek CP, Wöhrer W, Röhr M (1984) Xylose metabolism in Pachysolen tannophilus: purification and properties of xylose reductase. Can J Microbiol 30:1330–1336
Gírio FM, Roseiro JC, Sá-Machado P, Duarte-Reis AR, Amaral-Collaço MT (1994) Effect of oxygen transfer rate on levels of key enzymes of xylose metabolism in Debaryomyces hansenii. Enzyme Microb Technol 16:1074–1078
Gírio FM, Pelica F, Amaral-Collaço MT (1996) Characterization of xylitol dehydrogenase from Debaryomyces hansenii. Appl Biochem Biotechnol 56:79–87
Granström T, Leisola M (2002) Controlled transient changes reveal differences in metabolite production in two Candida yeasts. Appl Microbiol Biotechnol 58:511–517
Ho NWY, Lin FP, Huang S, Andrews PC, Tsao GT (1990) Purification, characterization, and amino terminal sequence of xylose reductase from Candida shehatae. Enzyme Microb Technol 12:33–39
Hyvönen L, Koivistoinen P (1982) Food technological evaluation of xylitol. Adv Food Res 28:373–403
Kern M, Haltrich D, Nidetzky B, Kulbe DK (1997) Induction of aldose reductase and xylitol dehydrogenase activities in Candida tenuis CBS 4435. FEMS Microbiol Lett 149:31–37
Mayerhoff ZDVL, Roberto IC, Silva SS (1997) Xylitol production from rice straw hemicellulose hydrolysate using different yeast strains. Biotechnol Lett 19:407–409
Mayerhoff ZDVL, Roberto IC, Franco TT (2001) Activity of xylose reductase from Candida mogii grown in media containing different concentrations of rice straw hydrolysate. Appl Biochem Biotechnol 91–93:729–736
Mayerhoff ZDVL, Roberto IC, Franco TT (2004) Purification of xylose reductase from Candida mogii in aqueous two-phase systems. Biochem Eng J 18:217–223
Neuhauser W, Haltrich D, Kulbe KD, Nidetzky B (1997) NAD(P)H-dependent aldose reductase from the xylose-assimilating yeast Candida tenuis. Isolation, characterization and biochemical properties of the enzyme. Biochem J 326:683–692
Nidetzky B, Klimacek M, Mayr P (2001) Transient-state and steady-state kinetic studies of the mechanism of NADH-dependent aldehyde reduction catalyzed by xylose reductase from the yeast Candida tenuis. Biochemistry 40:10371–10381
Scopes RK (1994) Protein purification: principles and practice. Springer, Berlin Heidelberg New York
Takamizawa K, Uchida S, Hatsu M, Suzuki T, Kawai K (2000) Development of a xylitol biosensor composed of xylitol dehydrogenase and diaphorase. Can J Microbiol 46:350–357
Vandeska E, Kuzmanova S, Jeffries TW (1995) Xylitol formation and key enzyme activities in Candida boidinii under different oxygen transfer rates. J Ferment Bioeng 80:513–516
Woodyer R, Simurdiak M, van der Donk WA, Zhao H (2005) Heterologous expression, purification, and characterization of a highly active xylose reductase from Neurospora crassa. Appl Environ Microbiol 71:1642–1647
Acknowledgements
The authors gratefully acknowledge financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mayerhoff, Z.D.V.L., Roberto, I.C. & Franco, T.T. Response surface methodology as an approach to determine the optimal activities of xylose reductase and xylitol dehydrogenase enzymes from Candida Mogii . Appl Microbiol Biotechnol 70, 761–767 (2006). https://doi.org/10.1007/s00253-005-0304-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0304-3