Abstract
Sphingomonas paucimobilis SYK-6, which can degrade various low molecular weight compounds derived from plant polyphenols such as lignin, lignan, and tannin, metabolizes these substances via 2-pyrone-4,6-dicarboxylic acid (PDC). We focused on this metabolic intermediate as a potential raw material for novel, bio-based polymers. We cloned the ligAB and ligC genes of SYK-6, which respectively encode protocatechuate 4,5-dioxygenase and 4-carboxy-2-hydroxymuconate-6-semialdehyde dehydrogenase, into a broad host range plasmid vector, pKT230MC. The resulting plasmid, pDVABC, was introduced into the PpY1100 strain of Pseudomonas putida, and we found that PDC could be stably produced from protocatechuate and accumulated. In addition, we examined the efficiency of production of PDC from protocatechuate on a 5-L scale in a Luria–Bertani medium containing 100 mM glucose and determined that PDC was stably produced from protocatechuate to yield 10 g/L or more.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignin is the most abundant natural aromatic biomass. In trees, high levels of lignin are synthesized in wood and account for 15–36% of the dry weight of wood. However, because lignin forms a highly complicated three-dimensional network in which the monolignols synthesized in plant cells are randomly polymerized, an advanced system for the utilization of lignin as biomass has not been established. In addition, the chemical treatment system of lignin, such as aqueous alkaline oxidation, can decompose high molecular lignin to low molecular compounds, and the various low molecular compounds were produced. However, only few parts of these compounds are utilized for aroma chemicals, etc., and almost these compounds are burned or discarded (Parke et al. 2000). On the other hand, various soil microorganisms in nature degrade these various aromatic substances via complicated metabolic pathways and utilize it as an energy source. Sphingomonas paucimobilis SYK-6 that was isolated from pulping waste liquor is able to grow on various dimeric lignin compounds, including β-aryl ether, biphenyl, and diarylpropane, as sole carbon and energy sources (Masai et al. 1999), and we have characterized the enzymes and genes involved in β-aryl ether cleavage (Masai et al. 1991; 1993) and biphenyl degradation (Peng et al. 1998; 1999) by this bacterium. The unique, specific degradation enzymes for various lignin derivatives in SYK-6 would be suitable tools for the conversion of lignin to useful intermediate metabolites.
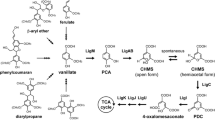
The metabolic pathways of degradation of low molecular lignin compounds by SYK-6, which have been identified in previous studies, are shown in Fig. 1. By these metabolic pathways, various low molecular weight lignin compounds are all converted within SYK-6 cells into such compounds as vanillin, vanillate, and syringate. After conversion into the metabolic intermediate 2-pyrone-4,6-dicarboxylic acid (PDC) via the protocatechuate 4,5-cleavage pathway, they are then completely degraded (Nishikawa et al. 1998; Noda et al. 1990; Hara et al. 2000; Sonoki et al. 2000). Therefore, in SYK-6 cells, various low molecular weight lignin compounds converge for transformation into PDC and are subsequently degraded. We focused here on the study of PDC, a substance with a unique structure containing a pyran ring with two carboxylic acid groups. Artificial organic synthesis of this structure for mass production is desirable but has not yet been achieved. Also, because PDC has two carboxylic acid groups, the conversion of these groups into derivatives would make it a potential raw material for various novel, bio-based polymers (Fig. 2). In fact, Shigehara et al. succeeded in producing PDC polyamide as a novel polymer chemicals via the 2-pyrone-4,6-dicarbonyl dichloride derivative (PDC dichloride; Shigehara et al. 1998; Okamura et al. 1999; Kawai et al. 2001; Sato et al. 2003). Therefore, if we could establish a method for the production of PDC using metabolic engineering technology, we could make a significant contribution to the establishment of an advanced technique for utilizing aromatic biomasses such as lignin—a task that has so far proven difficult—and to the production of a raw material for novel polymers by combination with chemical reaction as pretreatment of high molecular lignin decomposition. To establish a system for the production of PDC by means of the metabolic pathways in SYK-6, it was essential to introduce the metabolic functions of SYK-6, which is very slow growing and hard to manipulate, into a fast-growing and easily manipulated host strain and to achieve a high expression level within this strain. In the present study, to see whether PDC could be stably produced by means of metabolic engineering, we started by introducing the genes ligA, ligB (Noda et al. 1990), and ligC (Masai et al. 2000) into the mutant strain Pseudomonas putida PpY1100 (Katayama et al. 1987). These three genes respectively encode protocatechuate 4,5-dioxygenase and 4-carboxy-2-hydroxymuconate-6-semialdehyde (CHMS), which convert protocatechuate into PDC (Noda et al. 1990; Masai et al. 2000). P. putida PpY1100 is able to take up protocatechuate but is incapable of metabolizing it (Katayama et al. 1987); hence, we investigated whether the resulting transformant strain could accumulate PDC and therefore be used to enhance PDC production.
Metabolic pathway of lignin-related compounds by S. paucimolbilis SYK-6. By these metabolic pathways, various low molecular weight lignin compounds are all converted within SYK-6 cells into vanillin, vanillate (VA), and syringate (SA). After conversion into the metabolic intermediate PDC via the protocatechuate 4,5-cleavage pathway, they are then completely degraded. ligA, ligB, and ligC, which are used in this study, are shown inside the box
Materials and methods
Strains and media
E.coli DH5α was used as the cloning strain. P. putida PpY1100 was used as the cloning and ligABC expression strain. E. coli and P. putida strains were routinely grown in Luria–Bertani (LB) medium (Bacto–Tryptone, 10 g/L; yeast extract, 5 g/L; NaCl, 5 g/L) at 37 and 28°C, respectively. When succinic acid and other compounds were used as carbon sources, each was added to W medium (Peng et al. 1998) at a final concentration of 0.2% (w/v). Kanamycin and nalidixic acid were added to the selective medium at final concentrations of 25 mg/L for P. putida strains. For E. coli, kanamycin and ampicillin were added to the selective medium at final concentrations of 50 mg/L.
Substrates, enzymes, and reagents
Protocatechuate and succinate were purchased from Sigma Co. (Tokyo, Japan). All restriction enzymes, T4 DNA ligase, T4 DNA polymerase, and E. coli Klenow fragment were obtained from Takara Shuzo Co. (Kyoto, Japan). All antibiotics were purchased from Wako Pure Chemical Industries (Saitama, Japan).
Plasmid constructs
Plasmids pUC119 (Takara Shuzo Co.) and pKT230MC (Sonoki et al. 2000) as well as vectors were used for the preparation of constructs to the ligA, ligB, and ligC genes in E. coli and P. putida strains. A genomic region containing the ligA, ligB, ligC, ligI, and other ORFs relating protocatechuate metabolic pathway was cloned to pVA01 from the genomic library of S. paucimobilis SYK-6 in a previous study (Katayama et al. 1987). To obtain only the ligA, ligB, and ligC region from pVA01, pVA01 was digested by XbaI and then partially digested by EcoRI. The resulting fragment was treated by the Klenow fragment (Takara Shuzo Co.) and ligated. The resulting plasmid was named pVABC, containing the genomic region of ligA, ligB, and ligC. pVABC was partially digested by EcoRI. The resulting fragment containing ligA, ligB, and ligC was subcloned at the EcoRI site of pUC119, which was named pHN200. pHN200 was digested by XbaI, and the resulting fragment containing the ligA, ligB, and ligC genes was cloned at the XbaI site of pKT230MC. This plasmid was named pDVABC.
Assay of protocatechuate 4,5-dioxygenase (ligA and ligB) and CHMS hydrolase (ligC) activity
The activity of protocatechuate 4,5-dioxygenase and CHMS hydrolase was determined by the detection of the product of 2-pyran-4,6-dicarboxylic acid (PDC) from protocatechuate. Reaction mixtures were acidified to pH 2 by the addition of 12 N HCl and extracted three times with 300 μL of ethyl acetate. Then, PDC was detected by thin-layer chromatography on silica gel 60 plates with a fluorescent indicator, using the solvent system chloroform/ethylacetate/formic acid (10:8:1, v/v/v). In addition, the extract was dried on a rotary evaporator (REN-1, Iwaki Glass Co. Ltd., Iwaki, Japan) with a vacuum controller (FTP-10; Asahi Techno Glass, Japan). The residue was dissolved in 20 μg of pyridine and treated with bistrimethylsilyl-trifluoroacetamide (BSTFA; Tokyo Kasei Co., Tokyo, Japan) to prepare trimethylsilyl derivatives. Then, 1 μg of the solution of these derivatives was subjected to gas chromatography (GC; model 390; GL Science, Tokyo, Japan) and GC–mass spectrometry (MS; Auto Mass System II; JEOL, Tokyo, Japan). A fused silica capillary column (CP-Sil 5CB; 25 m×0.32 mm i.d.; 0.25 μm; Chrompack, The Netherlands) was used as the stationary phase. The temperature of the eluent was increased at 5°C/min from 100 to 300°C. On further analysis, the extract was dissolved in dimethyl sulfoxide (DMSO)–d 6 and then analyzed by the 1H and 13C nuclear magnetic resonance spectrum system (Lambda 400; JEOL).
Efficient production system of PDC
P. putida PpY1100/pDVABC was cultured in W medium containing 0.2% glucose as a carbon source or PDC production media containing 10 g of Bacto–Tryptone, 5 g of yeast extract, 5 g of NaCl, and 18 g of glucose per liter via Jar farmentor system (Mini Jar Farmentor TSC-A10L; Takasugi Seisakusyo Co. Ltd., Tokyo, Japan). A 10% (w/v) protocatechuate solution was then added at each point in Fig. 7 to the medium via a peristaltic pump over 6 h to give a total protocatechuate amount of 50 g, and pH was adjusted at 6.5 with the addition of 5 N NaOH. The monitoring of the conversion from protocatechuate into PDC was measured at an absorbance of 310 nm by absorption spectrometer (U2000; Hitachi Co. Ltd., Tokyo, Japan) and thin-layer chromatography. The resulting culture solution was centrifuged at 4,000×g at 15 min and removed the bacterial cells. The supernatant was acidified by the addition of 12 N HCl and extracted three times with the same volume of ethylacetate. The extract was then dried on a rotary evaporator (REN-1, Iwaki Glass Co. Ltd.). The residue was dissolved in DMSO–d 6 and analyzed by 1H and 13C nuclear magnetic resonance spectrum (Lambda 400; JEOL).
Results and discussion
Construction of high expression level vector involved in ligA, ligB, and ligC
The fragment containing the ligA, ligB, and ligC genes was prepared from pVA01, which was obtained in our previous study (Katayama et al. 1987), and was subcloned into pUC119 (see “Materials and methods”). The resulting plasmid was designated pHN200. The fragment containing the ligA, ligB, and ligC genes in pHN200 was then cloned into a broad host range plasmid vector, pKT230MC, and thus the plasmid pDVABC was obtained (Fig. 3). In this plasmid, the ligA, ligB, and ligC genes were transcribed as operons under the β-lactamase promoter and were noninducibly expressed at high levels.
Construction of high expression vector containing ligA, ligB, and ligC. The fragment containing the ligA, ligB, and ligC genes was cloned at the XbaI site of a broad host range plasmid vector, pKT230MC. This plasmid was named pDVABC. In pDVABC, the ligA, ligB and ligC genes were transcribed as operons under the β-lactamase promoter and were noninducibly expressed at high levels
Production of PDC from protocatechuate by transformed bacterium
We adopted P. putida PpY1100 as a host strain to express protocatechuate 4,5-dioxygenase and CHMS dehydrogenase. P. putida PpY1100 is a mutant strain whose cells are able to take up protocatechuate but are incapable of metabolizing it. The transformant strain obtained by introducing pDVABC into P. putida PpY1100 was designated P. putida PpY1100/pDVABC. P. putida PpY1100/pDVABC was able to grow with succinate as the sole carbon source. Cells of this transformant strain were precultured in W medium containing 0.2% succinate and then further cultured with the addition of 15 mM protocatechuate. We found that a substance with maximum absorption at a wavelength of 310 nm was accumulated. The accumulated substance was purified and analyzed by 1H NMR and thus confirmed to be PDC (Fig. 4). We concluded that the protocatechuate 4,5-dioxygenase and CHMS dehydrogenase encoded on pDVABC were expressed in the active form in the P. putida PpY1100/pDVABC cells, and that they could produce and accumulate PDC from protocatechuate. Not all of the protocatechuate added to the medium was, however, converted into PDC, and conversion stopped within 24 h (Fig. 5).
The conversion of protocatechuate to PDC by the time course of P.putida PpY1100/pDVABC culture. P.putida PpY1100/pDVABC were precultured in W medium containing 0.2% carbon source (solid line, succinate; dotted line, glucose) and then further cultured with the addition of 15 mM protocatechuate. Diamonds and triangles show the amount of protocatechuate (mM); squares and crosses show the amount of PDC (mM)
We considered this failure to achieve complete conversion of protocatechuate into PDC in the medium with succinate as the sole carbon source to be caused by the poor activity of the enzymes. Maintenance of enzymatic activity requires cofactors as well as stable expression of proteins. We previously determined that protocatechuate 4,5-dioxygenase (LigAB) can function with oxygen alone. In contrast, we also determined that CHMS dehydrogenase (LigC) uses NADP as a cofactor (Noda et al. 1990).
We considered the enhancement of turnover of NADP in P. putida PpY1100/pDVABC cells to be achievable by the activation of the glycolytic pathway. P. putida PpY1100/pDVABC cells were therefore precultured in medium with glucose as the sole carbon source and then further cultured with the addition of 15 mM protocatechuate. Despite not observing the change at the growth rate, whether in succinic acid or glucose culture (data not shown), we found in the glucose culture that all of the added protocatechuate was completely metabolized and converted into PDC within 24 h (Fig. 5). This result shows that the enhancement of turnover of NADP in the glycolytic pathway activated within the cells using glucose as the carbon source caused complete conversion of protocatechuate into PDC.
Efficient production system of PDC
We then investigated whether it was possible to mass produce PDC at least 10 g/L on a larger scale. Precultured P. putida PpY1100/pDVABC cells were cultured in 5 L of W medium containing glucose as the carbon source until its OD 660 nm reached 1.0 ABS. A 10% (w/v) protocatechuate solution was then added all at once to the medium to give a total protocatechuate amount of 50 g. We monitored the conversion from protocatechuate into PDC with an absorption spectrometer and found that most of the added protocatechuate was seldom converted into PDC even if 24 h passed after the start of the addition of protocatechuate (Fig. 6, diamond). In this result, we considered that the abundantly added protocatechuate gave the fatal effect for P. putida PpY1100/pDVABC cells. Parke et al (2000) described that protocatechuate was somewhat toxic for microorganisms. To clear this problem, we added the protocatechuate solution to the medium via a peristaltic pump over 6 h to give a total protocatechuate amount of 50 g, and we found the accumulated PDC from protocatechuate in the media. However, only 10% of protocatechuate was converted to PDC, and the reactions were stopped within 12 h (Fig. 6, triangle). Additionally, we investigated the most optimal substrate addition time. Figure 7a shows the growth rate of P. putida PpY1100/pDVABC cells in 5 L of W medium containing glucose as the carbon source. So far, the substrate was being added in the initial logarithmic growth phase of the microorganism (Fig. 7a, point A). However, there was the drastic improvement at conversion efficiency, when protocatechuate was added before the resting stage of P. putida PpY1100/pDVABC cells (Fig. 7a, point B), and it succeeded in converting into the PDC about 50% of adding protocatechuate (Fig. 6 triangle). In addition, to raise the growth efficiency of P. putida PpY1100/pDVABC cells, we cultured in a nutrient-rich medium—LB medium containing 100 mM glucose. As shown in Fig. 7b, the growth of P. putida PpY1100/pDVABC cells drastically increased to 13.0 ABS, and the added protocatechuate was perfectly converted into the PDC within 36 h after the start of the addition of protocatechuate (Figs. 7b, point C and 8). In these results, it becomes clear that the following facts are important for the mass conversion from protocatechuate to PDC by P. putida PpY1100/pDVABC cells: (1) addition of the glucose to the medium to enhance NADP turnover; (2) gradually dropping so that it may not excessively accumulate in the culture because protocatechuate is toxic; (3) dense cultivation of P. putida PpY1100/pDVABC cells by the nutrient-rich medium to efficiently convert from protocatechuate to PDC. By solving these problems, it succeeded in constructing the system that converts protocatechuate to PDC to yield 10 g/L or more within 36 h using the transformed bacterium P. putida PpY1100/pDVABC (Fig. 8).
The conversion of protocatechuate to PDC by P. putida PpY1100/pDVABC in various conditions. P. putida PpY1100/pDVABC were cultured in 5 L of W medium containing glucose as the carbon source. A 10% (w/v) protocatechuate solution was added all at once to the medium to give a total protocatechuate amount of 50 g in Fig. 7a, point A (diamond). A 10% (w/v) protocatechuate solution was added to the medium via a peristaltic pump to give a total protocatechuate amount of 50 g in Fig. 7a, point A (square) or point B (triangle)
Growth curve of P. putida PpY1100/pDVABC. aP. putida PpY1100/pDVABC cultured in 5 L of W medium containing glucose as the carbon source. bP. putida PpY1100/pDVABC cultured in 5 L of LB medium containing 100 mM of glucose (nutrient-rich medium). Points A, B, and C show the addition point of protocatechuate (Figs. 6 and 8)
High-level production of PDC from protocatechuate by jar farmentor system. P. putida PpY1100/pDVABC were cultured in 5 L of LB medium containing 100 mM glucose until its OD 660 nm reached 13.0 ABS (Fig 7b, point C). Then, a 10% (w/v) protocatechuate solution was added to the medium via a peristaltic pump to give a total protocatechuate amount of 50 g. Squares show PDC amount, and diamonds show protocatechuate amount
We achieved enhanced production of PDC from protocatechuate with a yield of 10 g/L or more via biotechnological methods using the metabolic function of S. paucimobilis SYK-6. PDC has a unique structure whose method of synthesis had not been established by artificial chemical reactions. Now that we have mass-produced PDC for the first time, various polymer studies and applied research can now proceed. Using the PDC as raw material, it is expected to contribute in the development of novel PDC-based plastics, fibers, etc., such as petrochemicals. In addition, PDC is one of the major intermediates of aromatic compound metabolic pathway in various soil bacteria. Therefore, it is considered that the PDC-based materials are biodegradable materials. Now that we have established a system of producing PDC from protocatechuate, it will be possible to organize systems of PDC production from various other compounds found in biomass through further application of the metabolic pathways of SYK-6. We expect that, in the future, we will be able not only to produce PDC from polyphenols such as lignin, lignan, and tannin that are abundantly present in plant biomass but to produce bio-based polymers in further studies as well.
References
Hara H, Masai E, Katayama Y, Fukuda M (2000) The 4-oxalomesaconate hydratase gene, involved in the protocatechuate 4,5-cleavage pathway, is essential to vanillate and syringate degradation in Sphingomonas paucimobilis SYK-6. J Bacteriol 182:6950–6957
Katayama Y, Nishikawa S, Nakamura M, Yano K, Yamasaki M, Morohoshi N, Haraguchi T (1987) Cloning and expression of Pseudomonas paucimobilis SYK-6 genes involved in the degradation of vanillate and protocatechuate in P. putida. Mokuzai Gakkaishi 33:77–79
Kawai H, Matsumoto M, Shigehara K, Katayama Y, Nishikawa S (2001) Synthesis of aromatic polyamides with pyranone unit. Polymer Preprints, Japan (English edn) 50:356
Masai E, Katayama Y, Kawai S, Nishikawa, S, Yamasaki M, Morohoshi N (1991) Cloning and sequencing of the gene for Pseudomonas paucimobilis enzyme that cleaves β-aryl ether. J Bacteriol 173:7950–7955
Masai E, Katayama Y, Kubota S, Kawai S, Yamasaki M, Morohoshi N (1993) A bacterial enzyme degrading the model lignin compound β-etherase is a member of the glutathione-S-transferase superfamily. FEBS Lett 323:135–140
Masai E, Katayama Y, Nishikawa S, Fukuda M (1999) Characterization of Sphingomonas paucimobilis SYK-6 genes involved in degradation of lignin-related compounds. J Ind Microbiol Biotech 23:364–373
Masai E, Momose K, Hara H, Nishikawa S, Katayama Y, Fukuda M (2000) Genetic and biochemical characterization of 4-carboxy-2-hydroxymuconate-6-semialdehyde dehydrogenase and its role in the protocatachuate 4,5-cleavage pathway in Sphingomonas paucimobilis SYK-6. J Bacteriol 182:6651–6658
Nishikawa S, Sonoki T, Kasahara T, Obi T, Kubota S, Kawai S, Morohoshi N, Katayama Y (1998) Cloning and sequencing of Sphingomonas (Pseudomonas) paucimobilis gene essential for the O demethylation of vanillate and syringate. Appl Environ Microbiol 64:836–842
Noda Y, Nishikawa S, Shiozuka K, Kadokura H, Nakajima H, Yoda K, Katayama Y, Morohoshi N, Haraguchi T, Yamasaki M (1990) Molecular cloning of the protocatechuate 4,5-dioxygenase gene of Pseudomonas paucimobilis. J Bacteriol 172:2704–2709
Okamura N, Matsumoto M, Shigehara K, Seki C, Nishikawa S (1999) Synthesis of polymer with 2H-pyran-2-one-4,6-dicarboxylic acid (PDC) nuclei. Polymer Preprints, Japan (English edn) 48:502
Parke D, D'argenio DA, Ornston LN (2000) Bacteria are not what they eat: that is why they are so diverse. J Bacteriol 182:257–263
Peng X, Egashira T, Hanashiro K, Masai E, Nishikawa S, Katayama Y, Kimura K, Fukuda M (1998) Cloning of a Sphingomonas paucimobilis SYK-6 gene encoding a novel oxygenase that cleaves lignin-related biphenyl and characterization of the enzyme. Appl Environ Microbiol 64:2520–2527
Peng X, Masai E, Katayama Y, Fukuda M (1999) Characterization of the meta-cleavage compound hydrolase gene involved in degradation of lignin-related biphenyl structure by Sphingomonas paucimobilis SYK-6. Appl Environ Microbiol 65:2789–2793
Sato M, Inoue H, Matsumoto M, Shigehara K, Katayama Y, Nishikawa S, Kimura T (2003) Mechanical properties and degradability of polyesters carrying PDC nuclei. Polymer Preprints, Japan (English edn) 52:359
Shigehara K, Okamura N, Matsumoto M, Seki C, Katayama Y, Nishikawa S (1998) Synthesis of polyamide with 2H-pyran-2-one-4,6-dicarboxylic acid (PDC) nuclei. Polymer Preprints, Japan (English edn) 47:415
Sonoki T, Obi T, Kubota S, Higashi M, Masai E, Katayama Y (2000) Coexistence of two different O demethylation systems in lignin metabolism by Sphingomonas paucimobilis SYK-6: cloning and sequencing of the lignin biphenyl-specific O-demethylase (ligX) gene. Appl Environ Microbiol 66:2125–2132
Xiang Q, Lee YY (2001) Production of oxychemicals from precipitated hardwood lignin. Appl Biochem Biotechnol 91–93:71–80
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Otsuka, Y., Nakamura, M., Shigehara, K. et al. Efficient production of 2-pyrone 4,6-dicarboxylic acid as a novel polymer-based material from protocatechuate by microbial function. Appl Microbiol Biotechnol 71, 608–614 (2006). https://doi.org/10.1007/s00253-005-0203-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0203-7