Abstract
N-carbamoyl-l-cysteine amidohydrolase (NCC amidohydrolase) was purified and characterized from the crude extract of Escherichia coli in which the gene for NCC amidohydrolase of Pseudomonas sp. strain ON-4a was expressed. The enzyme was purified 58-fold to homogeneity with a yield of 16.1% by three steps of column chromatography. The results of gel filtration on Sephacryl S-300 and SDS-polyacrylamide gel electrophoresis suggested that the enzyme was a tetramer protein of identical 45-kDa subunits. The optimum pH and temperature of the enzyme activity were pH 9.0 and 50°C, respectively. The enzyme required Mn2+ ion for activity expression and was inhibited by EDTA, Hg2+ and sulfhydryl reagents. The enzyme was strictly specific for the l-form of N-carbamoyl-amino acids as substrates and exhibited high activity in the hydrolysis of N-carbamoyl-l-cysteine as substrate. These results suggested that the NCC amidohydrolase is a novel l-carbamoylase, different from the known l-carbamoylases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
l-Cysteine is an important amino acid used as a food additive, as a component in nutritional infusions and cosmetics and as a chemical reagent in the food, cosmetics and pharmaceutical industries. l-Cysteine production has been carried out by extraction from hydrolysates of human hair (Hunt 1985). However, this method results in a low yield and has several problems, such as unpleasant odor during hydrolysis and a heavy cost for waste treatment. It was reported that some bacteria in the genus Pseudomonas, especially P. thiazolinophilum and P. desmolytica, could hydrolyze a chemically synthesized substrate, d,l-2-amino-Δ2-thiazoline-4-carboxylic acid (d,l-ATC) to produce l-cysteine (Sano et al. 1977, 1979; Sano and Mitsugi 1978). Thus, instead of the hydrolysis of hair, a microbial method from d,l-ATC to l-cysteine by Pseudomonas strains was developed (Sano et al. 1979; Ryu et al. 1997; Yamamoto et al. 2001). This method is favorable for l-cysteine production in industry, because d,l-ATC is easily synthesized chemically and the cost of the chemical synthesis of d,l-ATC is not high.
When we studied the bioconversion process of d,l-ATC to l-cysteine in Pseudomonas sp. ON-4a and other ATC-assimilating bacteria, l-cysteine was produced from d,l-ATC via N-carbamoyl-l-cysteine (l-NCC) formed as an intermediate (Tamura et al. 1998). This showed that only l-ATC in d,l-ATC is converted to l-cysteine by l-specific ATC hydrolase and NCC amidohydrolase in these bacterial strains. To investigate the molecular mechanism of the conversion process in strain ON-4a, we cloned and identified the genes for ATC hydrolase and NCC amidohydrolase involved in the process of d,l-ATC to l-cysteine (Ohmachi et al. 2002).
d,l-5′-Monosubstituted hydantoins are important starting materials for the preparation of amino acids by microbial conversion (hydantoinase method; Syldatk et al. 1992). The hydantoins are metabolized to amino acids by the following three successive reactions: (1) enzymatic (or chemical) racemization of the hydantoins, (2) hydrolysis of the hydantoins to N-carbamoyl amino acids and (3) hydrolysis of the N-carbamoyl amino acids to amino acids. Three enzymes, hydantoin racemase, hydantoinase and N-carbamoyl amino acid amidohydrolase, are involved in these three reactions, respectively. N-Carbamoyl amino acid amidohydrolase involved in the third reaction catalyzes the hydrolysis of N-carbamoyl amino acids to yield amino acids, ammonia and carbon dioxide according to the following equation:
In the conversion of d,l-5′-substituted hydantoins to the corresponding d-and/or l-amino acids, d-amino acids are produced from N-carbamoyl-d-amino acids by N-carbamoyl-d-amino acid amidohydrolase (d-N-carbamoylase; Ogawa et al. 1994; Ikenaka et al. 1998; Nanba et al. 1998); and l-amino acids are produced from N-carbamoyl-l-amino acids by N-carbamoyl-l-amino acid amidohydrolase (l-N-carbamoylase; Syldatk et al. 1992; Watanabe et al. 1992; Mukohara et al. 1993; Ishikawa et al. 1993, 1994; Batisse et al. 1997; Wilms et al. 1999). These d-N-carbamoylases and l-N-carbamoylases were purified and characterized from several microorganisms (Ogawa et al. 1993, 1994, 1995; Ogawa and Shimizu 1994; Ishikawa et al. 1996; Wilms et al. 1999).
Our previous studies (Tamura et al. 1998, Ohmachi et al. 2002) showed that the NCC amidohydrolase of Pseudomonas sp. ON-4a hydrolyzed only the l-form of d,l-NCC and that the amino acid sequence of NCC amidohydrolase had a significant similarity to those of the known l-N-carbamoylases; and we suggested that the enzyme is a member of the l-N-carbamoylase. In this paper, we present the purification and characterization of the NCC amidohydrolase expressed in the Escherichia coli transformant (pTrCM1) harboring the NCC amidohydrolase gene from Pseudomonas sp. ON-4a.
Materials and methods
Chemicals
N-Carbamoyl-d,l-aspartic acid, N-carbamoyl-d,l-serine, N-carbamoyl-l-histidine and N-carbamoylglycine were purchased from Sigma (St Louis, Mo., USA). Other N-carbamoyl derivatives were obtained from Nippon Rika Co. (Tokyo, Japan). Standard protein markers for gel filtration chromatography and for SDS-PAGE were obtained from Sigma and Pharmacia Biotech (UK), respectively. All other chemicals used in this work were commercially available.
Microorganisms and plasmid
Pseudomonas sp. strain ON-4a was grown at 30°C for 20 h in ATC medium, as described by Tamura et al. (1998). pTrCM1 is a derivative of pTrc99A (controlled by the trc promoter) containing the genes for the NCC amidohydrolase and ATC hydrolase of strain ON-4a (Ohmachi et al. 2002). E. coli DH5α harboring pTrCM1 was used for the purification of the NCC amidohydrolase.
Preparation of crude extract
E. coli harboring pTrCM1 was grown in 5 ml of Luria-Bertani (LB) medium containing 50 μg ml−1 ampicillin (Amp) at 37°C for 6 h with shaking. Cells collected by centrifugation (5,000 g, 5 min) were inoculated into 500 ml of LB medium (with 50 μg ml−1 Amp) containing 0.5 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) and cultivated at 37°C for 15 h with shaking. The cells were harvested by centrifugation and washed twice with 50 mM Tris-HCl (pH 8.0) and 1 mM EDTA. The cells were suspended in 20 mM Tris-HCl (pH 8.0) containing 0.2 mM phenylmethylsulfonyl fluoride, 1 mM MnSO4 and 10% glycerol and were disrupted by sonication (Ultrasonic disruptor UD-201; TOMY, Tokyo, Japan). The suspension was centrifuged at 10,000 g for 15 min to remove cell debris. The obtained supernatant (crude extract) was used for the purification of NCC amidohydrolase.
Assay of enzyme activity
The activity of NCC amidohydrolase was assayed by measurement of the l-cysteine formed from NCC as a substrate. The standard enzyme assay mixture contained 0.3 M Tris-HCl (pH 9.0), 5 mM NCC, 0.2 mM MnSO4 and an appropriate amount of enzyme in a total volume of 1.0 ml. After incubation at 50°C for 15 min, the reaction was stopped by the addition of 1.0 ml of 5% trichloroacetic acid. The l-cysteine formed was measured by the method of Gaitonde (1967), with a minor modification (States and Segal 1973). One unit of enzyme activity was defined as the amount of enzyme that produces 1 μmol min−1 of l-cysteine from NCC under the assay conditions.
Purification of NCC amidohydrolase
The crude extract of E. coli harboring pTrCM1 for purification was obtained from 500 ml of the culture broth, as described above. The NCC amidohydrolase was purified as described here. All procedures were done at 0–4°C.
Anion-exchange chromatography
The crude extract (540 mg protein) was dialyzed against buffer A (20 mM Tris-HCl, pH 8.0, 10% glycerol) overnight and applied to a DEAE-Cellulofine A-500 column (2.5×13.0 cm; Seikagaku Corp., Tokyo, Japan) equilibrated previously with the same buffer. After the column was washed with buffer A, the proteins retained were eluted with a linear gradient from 0 M to 0.3 M NaCl in buffer A at a flow rate of 0.5 ml min−1. NCC amidohydrolase activity was eluted from the column with 0.2–0.25 M NaCl. The fractions containing the enzyme activity were pooled.
Hydrophobic interaction chromatography
After the ammonium sulfate concentration of the pooled enzyme solution was adjusted to 0.8 M with solid ammonium sulfate, it was applied to a Butyl-Toyopearl 650M column (1.9×8.0 cm; Tosoh, Tokyo, Japan) equilibrated previously with buffer B (buffer A containing 0.8 M ammonium sulfate). The column was washed with buffer B and the bound proteins were eluted with buffer A containing ammonium sulfate on a linear gradient from 0.8 M to 0 M at a flow rate of 0.5 ml min−1. The enzyme fractions eluting from the column with 0.4 M to 0.2 M ammonium sulfate were pooled and combined. The fraction was then dialyzed against 10 mM sodium phosphate buffer (pH 7.5) and 10% glycerol overnight.
Hydroxyapatite chromatography
The dialyzed enzyme solution was applied to a Bio-Gel HTP column (1.6×12.0 cm; Bio-Rad, Calif., USA) equilibrated previously with 10 mM sodium phosphate buffer (pH 7.5) and 10% glycerol. After the column was washed with the same buffer, a linear gradient elution was carried out with 0.01 M to 0.2 M sodium phosphate buffer (pH 7.5). The enzyme detected in the flowthrough fraction was collected and concentrated to 1.0 ml by ammonium sulfate precipitation (80% saturation). The purified enzyme was characterized.
Analytical methods for NCC amidohydrolase
The molecular mass of the denatured enzyme was estimated by SDS-PAGE, using the method of Laemmli (1970). Standard proteins used for the estimation of the molecular mass were an electrophoresis calibration kit (Pharmacia Biotech). Proteins were visualized by Coomassie brilliant blue staining with Quick-CBB (Wako Pure Chemicals, Osaka, Japan). The molecular mass of the native enzyme was determined by gel filtration on a Sephacryl S-300 column (1.5×90.0 cm). The sample was applied to a column equilibrated previously with buffer A and eluted with the same buffer at a flow rate of 0.4 ml min−1. Protein standards used for molecular mass estimation were β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), bovine serum albumin (66 kDa) and carbonic anhydrase (29 kDa). The protein concentration was determined by the method of Lowry et al. (1951), using bovine serum albumin as a standard.
Substrate specificity
The relative activities of NCC amidohydrolase against several substrates (N-carbamoyl derivatives of amino acids) were determined by the standard enzyme assay. The concentration of amino acids liberated from N-carbamoyl derivatives of amino acids was measured by the ninhydrin method (Wilms et al. 1999) or by a model L8500 Amino acid analyzer (Hitachi, Tokyo, Japan). For the ninhydrin determination of amino acids, 100 μl of the reaction mixture (1 ml) were mixed with 100 μl of 2 M sodium acetate (pH 5.5) and heated at 60°C for 5 min. One hundred microliters of ninhydrin solution (consisting of 174 mg of ninhydrin and 174 mg hydrindantin·2H2O in 15 ml of ethylene glycol monomethyl ether) were added. After incubation at 60°C for 20 min, 1 ml of 50% isopropanol was added and the absorbance was measured at 570 nm. Standard curves were obtained using samples with defined amino acid concentrations.
Determination of kinetic parameters
Kinetic parameters of the purified enzyme were estimated for N-carbamoyl derivatives of l-cysteine, l-methionine, l-alanine and glycine, using concentration ranges from 0.1 mM to 10.0 mM. Activity was measured under standard enzyme assay conditions, as described above. Kinetic parameters were calculated from Lineweaver–Burk plots.
Overexpression of His-tagged NCC amidohydrolase in E. coli
The NCC amidohydrolase gene was amplified from plasmid pTrCM1 by PCR. For PCR amplification, the primers used were NdeNCCf [5′-GCGCCATATGAATGAGCGCTCACG-3′, containing the first 17 nucleotides (in italics) of the open reading frame of the NCC amidohydrolase gene from the initiation codon (Met)] and BamNCCr [5′-GCGGATCCAATTATCCGCCACCCATC-3′, containing the 16 nucleotides (in italics) complementary to the sequence upstream of the stop codon (TAA)]. These primers contain NdeI (CATATG) and BamHI (GGATCC) recognition sites, respectively, 5′ of the above-mentioned nucleotides. PCR amplification was performed in the presence of 5% dimethylsulfoxide with KOD Dash DNA polymerase (Toyobo, Japan) under the conditions described by the manufacturer. The amplified fragment was digested with NdeI and BamHI and then inserted between the NdeI and BamHI sites of the expression vector pET15b (Novagen) to create plasmid pETncc. pETncc was then used to transform E. coli BL21(DE3) to obtain the transformant BL21(DE3)pETncc. Cultivation of the transformant was carried out as described above, except for using 0.4 mM IPTG. NCC amidohydrolase was expressed in this transformant as an enzyme with a His-tag at the N-terminal end.
Results
Purification of NCC amidohydrolase
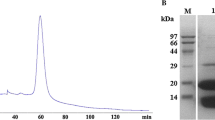
The NCC amidohydrolase expressed in E. coli harboring pTrCM1 was purified by three steps of column chromatography. The results of the purification of the enzyme are summarized in Table 1. The enzyme was purified 58-fold with a yield of 16.1% from the crude extract. The purified enzyme had a specific activity of 23.2 units mg −1 protein and showed a single band on a 12.5% SDS-PAGE gel (Fig. 1, lane 5), suggesting that the purified enzyme was homogeneous. The molecular mass of the enzyme protein was estimated to be approximately 45 kDa. This was in a good agreement with the calculated molecular mass (47,347 Da) based on the amino acid sequence deduced from the nucleotide sequence (Ohmachi et al. 2002). The apparent molecular mass of the native enzyme was estimated to be approximately 170 kDa by Sephacryl S-300 gel filtration (data not shown), indicating that the enzyme seems to be a tetramer consisting of identical subunits.
SDS-PAGE analysis of NCC amidohydrolase from E. coli harboring pTrCM1. SDS-PAGE was carried out with a 12.5% polyacrylamide gel. Lanes 1, 6 Standard proteins, lane 2 crude extract (20 μg protein), lane 3 DEAE-Cellulofine fraction (10 μg protein), lane 4 Butyl-Toyopearl fraction (5 μg protein), lane 5 Bio-Gel HTP fraction (2 μg protein). Standard proteins are phosphoryase b (94 kDa), bovine serum albumin (67 kDa), ovalbumin (43 kDa), carbonic anhydrolase (30 kDa), soybean trypsin inhibitor (20 kDa) and α-lactalbumin (14.4 kDa)
Effects of pH and temperature
The effects of pH and temperature on NCC amidohydrolase activity are shown in Fig. 2. The activity of the purified enzyme was highest at pH 9.0 (Fig. 2A). The enzyme activity decreased significantly under conditions below pH 7.0 and above pH 9.5. The optimum temperature for the enzyme activity was 50°C (Fig. 2B). Consequently, all standard assays were carried out at pH 9.0 and 50°C. As shown in Fig. 3A, the enzyme was stable at the narrow range pH 8.0–9.5. The enzyme was stable up to 50°C, but activity decreased to about 50% at 60°C and the enzyme was unstable at 60°C or higher (Fig. 3B).
Effects of A pH and B temperature on the activity of NCC amidohydrolase. A Enzyme activity was measured under standard enzyme assay conditions, except that the following buffers were used: 50 mM sodium citrate (pH 3.0–6.0, filled circles), 50 mM potassium phosphate (pH 6.0–8.0, filled squares), 50 mM Tris-HCl (pH 7.0–9.0, filled triangles) and 50 mM sodium carbonate (pH 9.0–11.0, open squares). B Assays were carried out at various temperatures under standard enzyme assay conditions. The relative activity is expressed as the percentage of the maximum activity attained under the conditions used
Effects of A pH and B temperature on the stability of NCC amidohydrolase. A The purified enzyme was incubated at 30°C for 30 min in the following buffers: 50 mM sodium citrate (pH 3.0–6.0, filled circles), 50 mM potassium phosphate (pH 6.0–8.0, filled squares), 50 mM Tris-HCl (pH 7.0–9.0, filled triangles) and 50 mM sodium carbonate (pH 9.0–11.0, open squares). The remaining activity was measured under standard enzyme assay conditions. B After the purified enzyme (8 milliunits) was incubated at the indicated temperatures for 30 min with 50 mM Tris-HCl (pH 8.0), the remaining activity was measured under standard enzyme assay conditions. The remaining activity is expressed as a percentage of the original enzyme activity
Effects of metal ions and inhibitors
The purified enzyme was treated with 10 mM EDTA at room temperature for 1 h and dialyzed against buffer A containing 1 mM EDTA at 4°C for 10 h to remove any contaminating metal ions. Then the enzyme was dialyzed again against the same buffer without EDTA at 4°C overnight. After the treatment, the activity decreased to below 10% of the initial enzyme activity. The enzyme was markedly activated about 8.9-fold and 9.9-fold by the addition of 0.1 mM and 1.0 mM Mn2+ ions, respectively (Table 2). Zn2+ and Mg2+ ions activated the enzyme slightly, while other divalent cations (Ni2+, Cu2+, Fe2+, Hg2+) at the low concentration (0.1 mM) were ineffective, although they caused an inhibitory effect at the high concentration. Especially, Hg2+ strongly inhibited the activity.
The effects of inhibitors on the activity of NCC amidohydrolase were investigated (Table 3). The metal chelating reagent, EDTA, inhibited the enzyme activity by over 90%. Sulfhydryl reagents (1.0 mM) [iodoacetic acid (IAA), N-ethyl-maleimide (NEM), p-chloromercuribenzoic acid (PCMB)] caused inhibition by 33.9, 1.7 and 12.6%, respectively; and these reagents almost completely inhibited the activity at the high concentration of 5 mM.
Substrate and stereospecificity
To investigate the substrate-specificity of the purified NCC amidohydrolase, enzyme activities toward N-carbamoyl amino acids were measured. As shown in Table 4, NCC amidohydrolase efficiently hydrolyzed l-NCC and d,l-NCC, but not d-NCC. Also, N-carbamoyl derivatives of l-methionine, l-alanine, d,l-serine and d,l-aspartic acid were hydrolyzed at a lower rate than l-NCC. Since no activity toward N-carbamoyl derivatives of d-amino acids tested was detected, it was shown that the NCC amidohydrolase seems to be strictly l-form stereospecific toward N-carbamoyl amino acids. Values for Km and Vmax were determined for l-NCC, N-carbamoyl-l-methionine, N-carbamoyl-l-alanine and N-carbamoyl-glycine, which were hydrolyzed in the initial studies. The Km value for NCC was the lowest (0.41 mM) among all substrates; and the Vmax value for NCC was the highest (20.8 μmol min−1 mg−1) among the substrates tested. The highest kcat and kcat/Km values were observed for l-NCC.
His-tagged NCC amidohydrolase.
When the transformant E. coli BL21 (DE3)pETncc was grown in the presence of IPTG as described above, NCC amidohydrolase was detected at the low activity level in the crude extract and the precipitate (inclusion bodies), which had activities of 0.65 units mg−1 and 0.11 units mg−1, respectively. These values were not as high as we expected. After solubilization and activation of the inclusion body fraction by the general method (Sambrook et al. 1989) using 8 M urea, about 65% of the protein from the inclusion bodies was solubilized and recovered; and the activity of NCC amidohydrolase increased to 15.9 units mg−1. The His-tagged NCC amidohydrolase from the activated inclusion body fraction was purified to a purity of higher than 90% by one step of metal affinity chromatography (data not shown).
Discussion
It has been shown that several bacterial genera, such as Alcaligenes, Arthrobacter, Bacillus, Flavobacterium and Pseudomonas, possess activity for l-amino acid production from the corresponding hydantoins and that hydantoinase and l-carbamoylase are involved in the process (Syldatk et al. 1992). In contrast, with regard to l-cysteine, several Pseudomonas strains having potent activities for the hydrolysis of d,l-ATC to l-cysteine have so far been isolated (Sano et al. 1977, 1979; Sano and Mitsugi 1978; Ryu and Shin 1991; Tamura et al. 1998). The production of l-cysteine from d,l-ATC by the microbial conversion method using some Pseudomonas strains has been developed and industrialized (Sano and Mitsugi 1978; Ryu et al.1997; Yamamoto et al. 2001). However, the mechanism of the conversion process was unclear. Recently, we identified NCC as an intermediate in the conversion process of d,l-ATC to l-cysteine in Pseudomonas strains (Tamura et al. 1998) and we isolated and identified the genes for ATC hydrolase and NCC amidohydrolase from strain ON-4a (Ohmachi et al. 2002). After our publication, Shiba et al. (2002) reported the isolation of the genes from Pseudomonas sp. SB corresponding to our genes.
In this paper, the purification and characterization of the NCC amidohydrolase of Pseudomonas sp. ON-4a expressed in E coli are described. The enzyme has been purified to homogeneity and characterized. As described above, NCC amidohydrolase has a homotetramer structure consisting of four identical subunits, each of which has a molecular mass of about 45 kDa. In contrast, all other l-carbamoylases purified and characterized so far have homodimers consisting of identical subunits of 44–45 kDa (Ogawa and Shimizu 1994; Ishikawa et al. 1996; Wilms et al. 1999), except for the 65-kDa subunits of the Alc. xylosoxidans enzyme (Ogawa et al. 1995).
The optimum temperature for NCC amidohydrolase was 50°C, as was that for l-carbamoylase from Art. aurescens DSM3747 (Wilms et al. 1999). The enzymes from Alc. xylosoxidans (Ogawa et al. 1995), Pseudomonas sp. NS671 (Ishikawa et al. 1996) and Fravonobacter sp. AJ-3912 (Yokozeki et al. 1987) had an optimum temperature of 35–40°C; and those from P. putida IFO12996 (Ogawa and Shimizu 1994) and B. kaustophilus CCRC11223 (Hu et al. 2003) had optima of 65°C and 70°C, respectively. The optimum pH of the NCC amidohydrolase was 9.0 (Fig. 2A). This is more to the alkaline side than those of l-carbamoylases from other bacterial strains, which range from pH 7.5 to pH 8.5. l-Carbamoylases from Alc. xylosoxidans (Ogawa et al. 1995), P. putida IFO12996 (Ogawa and Shimizu 1994), Pseudomonas sp. NS671 (Ishikawa et al. 1996) and B. kaustophillus (Hu et al. 2003) required divalent cations of Mn2+, Co2+ and Ni2+ for expression of their activity. NCC amidohydrolase required Mn2+ ions at a low concentration (0.1 mM) for its activity; and the other divalent cations tested did not activate or enhance the activity (Table 2). These results indicate that the properties of NCC amidohydrolase differ from those of all known l-carbamoylases from other bacterial strains.
With respect to substrate specificity, l-carbamoylases are classified into two groups. One group consists of the enzymes showing a broad substrate specificity toward l-N-carbamoyl derivatives of aliphatic and aromatic amino acids (Ishikawa et al. 1993, 1994; Ogawa and Shimizu 1995; Yamashiro et al. 1988); and the other group consists of those showing a narrow specificity toward l-N-carbamoyl derivatives of aromatic amino acids (Yokozeki et al. 1987; Wilms et al. 1999). So far, there is no report that these l-carbamoylases are able to hydrolyze l-NCC. However, as described in this paper, the NCC amidohydrolase from Pseudomonas sp. ON-4a hydrolyzed most efficiently l-NCC as a substrate; and N-carbamoyl derivatives of short-chain aliphatic (l-Ala) and hydrophilic (l-Ser, l-Asp) amino acids and Gly were hydrolyzed at a lower rate. But those of long-chain aliphatic (except for l-Met), aromatic (l-Phe) and basic (l-His) amino acids tested were not hydrolyzed. These results suggest that the NCC amidohydrolase from strain ON-4a is a novel l-carbamoylase acting on NCC with a narrow substrate specificity, which is different from the other l-carbamoylases characterized so far. When Km and Vmax were measured, the NCC amidohydrolase had values of kcat and kcat/Km for l-NCC higher than those for the other three substrates tested (Table 4). l-NCC proved to be the most favorable substrate for NCC amidohydrolase. However, this is not surprising, because it might be explained by the fact that the initial screening experiments were carried out using d,l-ATC as a substrate. NCC formed from d,l-ATC by ATC hydrolase is converted to l-cysteine by NCC amidohydrolase.
Although His-tagged NCC amidohydrolase overexpressed in the transformant E. coliBL21(DE3) was recovered in inclusion bodies as an insoluble form, the enzyme could be purified in an active form from the inclusion bodies by solubilization, activation and one-step chelate chromatography. This indicates that we would be able to obtain a large amount of purified active enzyme by a simple method and to use it for the preparation of l-cysteine from NCC. In l-cysteine production from d,l-ATC by the microbial conversion method, ATC hydrolase, like NCC amidohydrolase, is an important enzyme. Now, the purification and characterization of the ATC hydrolase from the transformant used in this study is in progress.
References
Batisse N, Weigel P, Lecocq M, Sakanyan V (1997) Two amino acid amidohydrolase genes encoding l-stereospecific carbamoylase and aminoacylase are organized in a common operon in Bacillus stearothermophilus. Appl Environ Microbiol 63:763–766
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Hu H-Y, Hsu W-H, Chien HR (2003) Characterization and phylogenetic analysis of a thermostable N-carbamoyl-l-amino acid amidohydrolase from Bacillus kaustophilus CCRC11223. Arch Microbiol 179:250–257
Hunt S (1985) Degradation of amino acids accompanying in vitro protein hydrolysis. In: Barrett GC (ed) Chemistry and biochemistry of the amino acids. Chapman & Hall, London, pp 376–398
Hunt S (1985) Degradation of amino acids accompanying in vitro protein hydrolysis. In: Barrett GC (ed) Chemistry and biochemistry of the amino acids. Chapman & Hall, London, pp 376–398
Ikenaka Y, Nanba H, Yamada Y, Yajima K, Takano M, Takahashi S (1998) Screening, characterization, and cloning of the gene for N-carbamyl-d-amino acid amidohydrolase from thermotolerant soil bacteria. Biosci Biotechnol Biochem 62:882–886
Ishikawa T, Watanabe K, Mukohara Y, Kobayashi S, Nakamura H (1993) Microbial conversion of dl-5-substituted hydantoins to the corresponding l-amino acids by Pseudomonas sp. strain NS671. Biosci Biotechnol Biochem 57:982–986
Ishikawa T, Mukohara Y, Watabe K, Kobayashi S, Nakamura H (1994) Microbial conversion of dl-5-substituted hydantoins to the corresponding l-amino acids by Bacillus stearothermophilus NS1122A. Biosci Biotechnol Biochem 58:265–270
Ishikawa T, Watanabe K, Mukohara Y, Nakamura H (1996) N-Carbmyl-l-amino acid amidohydrolase of Pseudomonas sp. strain NS671: purification and some properties of the enzyme expressed in Escherichia coli. Biosci Biotechnol Biochem 60:612–615
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mukohara Y, Ishikawa T, Watanabe K, Nakamura H (1993) Molecular cloning and sequencing of the thermostable N-carbamyl-l-amino acid amidohydrolase from Bacillus stearothermophilus strain NS 1122A. Biosci Biotechnol Biochem 57:1935–1937
Nanba H, Ikenaka Y, Ymada Y, Yajima K, Takano M, Takahashi S (1998) Isolation of Agrobacterium sp. strain KNK712 that produces N-carbamyl-d-amino acid amidohydrolase, cloning of the gene for this enzyme, and properties of the enzyme. Biosci Biotechnol Biochem 62:875–881
Ogawa J, Shimizu S (1994) β-Ureidopropionase with N-carbamoyl-α-l-amino acid amidohydrolase activity from an aerobic bacterium, Pseudomonas putida IFO 12996. Eur J Biochem 223:625–630
Ogawa J, Shimizu S, Yamada H (1993) N-Carbamoly-d-amino acid amidohydrolase from Comamonas sp. E222c. Purification and characterization. Eur J Biochem 212:685–691
Ogawa J, Chung MC-M, Hida S, Yamada H, Shimizu S (1994) Thermostable N-carbamoyl-d-amino acid amidohydrolase: screening, purification and characterization. J Biotechnol 38:11–19
Ogawa J, Miyake H, Shimizu S (1995) Purification and characterization of N-carbamoyl-l-amino acid amidohydrolase with broad substrate specificity from Alcaligenes xylosoxidans. Appl Microbiol Biotechnol 43:1039–1043
Ohmachi T, Nishino M, Kawata M, Edo M, Funaki H, Narita M, Mori K, Tamura Y, Asada Y (2002) Identification, cloning, and sequencing of the genes involved in the conversion of d,l-2-amino-Δ2-thiazoline-4-carboxylic acid to l-cysteine by Pseudomonas sp. strain ON-4a. Biosci Biotechnol Biochem 66:1097–1104
Ryu OH, Shin CS (1991) Analysis of the reaction steps in the bioconversion of d,l-ATC to l-cysteine. J Microbiol Biotechnol 1:50–53
Ryu OH, Yeong J, Shin CS (1997) Continous l-cysteine production using immobilized cell reactors and product extractors. Process Biochem 32:201–209
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, New York, Cold Spring Harbor, N.Y., pp 17.37–17.41
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y., pp 17.37–17.41
Sano K, Mitsugi K (1978) Enzymatic production of l-cysteine from dl-2-amino-Δ2-thiazoline-4-carboxylic acid by Pseudomonas thiazolinophilum: optimal conditions for the enzyme formation and enzymatic reaction. Agric Biol Chem 42:2315–2321
Sano K, Yokozeki Y, Tamura F, Yasuda N, Noda I, Mitsugi K (1977) Microbial conversion of d,l-2-amino-Δ2-thiazoline-4-carboxylic acid to l-cysteine and l-cystine: screening of microorganisms and identification of products. Appl Environ Microbiol 34:806–810
Sano K, Eguchi C, Yasuda N, Mitsugi K (1979) Metabolic pathway of l-cysteine formation from d,l-2-amino-Δ2-thiazoline-4-carboxylic acid by Pseudomonas. Agric Biol Chem 43:2373–2374
Shiba T, Takeda K, Yajima M, Tadano M (2002) Genes from Pseudomonas sp. strain BS involved in the conversion of l-2-amino-Δ2-thiazolin-4-carbonic acid to l-cysteine. Appl Environ Microbiol 68:2179–2187
States B, Segal S (1973) Quantitation of cyst(e)ine in human fibroblasts and separation of cysteinesulfinic acid, cysteic acid and taurine. Clin Chem Acta 43:49–53
Syldatk C, Muhler R, Pietzsch M, Wagner F (1992) Microbial and enzymatic production of l-amino acids from dl-5-monosubstituted hydantoins. In: Rozzell JD, Wagner F (eds) Biocatalytic production of amino acids and derivertives. Hanser, New York, pp 129–176
Syldatk C, Muhler R, Pietzsch M, Wagner F (1992) Microbial and enzymatic production of l-amino acids from dl-5-monosubstituted hydantoins. In: Rozzell JD, Wagner F (eds) Biocatalytic production of amino acids and derivertives. Hanser, New York, pp 129–176
Tamura Y, Nishino M, Ohmachi T, Asada Y (1998) N-Carbamoyl-l-cysteine as an intermediate in the bioconversion from d,l-2-amino-Δ2-thiazoline-4-carboxylic acid to l-cysteine by Pseudomonas sp. ON-4a. Biosci Biotechnol Biochem 62:2226–2229
Yamamoto Y, Fujita I, Horino I, Kouda T, Akashi K (2001) Enzymatic production of cystine in commercial plant (in Japanese). Nippon Nogeikagaku Kaishi 75:949–956
Yamashiro K, Kubota K, Yokozeki K (1988) Mechanism of stereospecific production of l-amino acids from the corresponding 5-monosubstituted hydantoins by Bacillus brevis. Agric Biol Chem 52:2857–2863
Yokozeki K, Hirose Y, Kubota K (1987) Mechanism of the asymmetric production of l-aromatic amino acids from the corresponding hydantoins by Flavobacterium spec. Agric Biol Chem 51:737–746
Watanabe K, Ishikawa T, Mukohara Y, Nakamura H (1992) Cloning and sequencing of the genes involved in the conversion of 5-substituted hydantoins to the corresponding l-amino acids from the native plasmid of Pseudomonassp. strain NS671. J Bacteriol 174:962–969
Wilms B, Wiese A, Syldatk C, Mattes R, Altenbuchner J, Pietzsch M (1999) Cloning, nucleotide sequence and expression of a new l-carbamoylase gene from Arthrobacter aurescens DSM3747 in E. coli. J Biotechnol 68:101–113
Acknowledgements
We are grateful to Drs. H. Nagayama and M. Muramatsu for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohmachi, T., Narita, M., Kawata, M. et al. A novel N-carbamoyl-l-amino acid amidohydrolase of Pseudomonas sp. strain ON-4a: purification and characterization of N-carbamoyl-l-cysteine amidohydrolase expressed in Escherichia coli. Appl Microbiol Biotechnol 65, 686–693 (2004). https://doi.org/10.1007/s00253-004-1687-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1687-2