Abstract
Two sets of Saccharomyces cerevisiae strains were compared for their physiological responses to different stress conditions. One group is composed of three strains adapted to controlled laboratory conditions (CEN.PK, LR88 and RS58), whereas the other consisted of five industrial strains (IND1101, SuperStart, LO24, LO41 and Azteca). Most industrial strains showed higher tolerance to heat shock and to an oxidative environment than laboratory strains. Excluding CEN.PK, a similar behavior was observed regarding ethanol production in high sugar concentrations (180 g/l glucose). Addition of acetate (10 g/l) or furfural (2 g/l), in concentrations similar to those found in sugar cane bagasse hydrolysates, decreased cell mass formation and growth rate in almost all strains. CEN.PK and SuperStart showed the highest sensitivity when grown in furfural-containing medium. Acetic acid treatment severely affected cell mass formation and reduced growth rate in all strains; CEN.PK and LO24 were the most resistant. The specific ethanol production rate was not affected by furfural addition. However, specific ethanol production rates decreased in response to acetic acid in four industrial strains, and increased in all laboratory strains and in LO24. No significant correlation was found between the stress tolerance of the strains tested and the transcript accumulation of genes selected by their involvement in the response to each of the stressful environments applied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The yeast Saccharomyces cerevisiae is preferred for industrial ethanol production, because it is an efficient fermentation microorganism. This fact has allowed the selection of industrial strains with acquired traits that make them superior ethanol producers, more tolerant to fermentation by-products and with greater process hardiness, as compared to S. cerevisiae strains adapted to controlled laboratory conditions. The constant environmental changes to which industrial yeast strains are exposed nowadays include the wide variety of feedstocks, which leads to different media composition used in ethanol production processes. Additional environmental changes include temperature, ethanol accumulated along the process, solute concentration, medium ionic strength, and reactive oxygen species (Kensall and Lyons 1999). Furthermore, toxins in culture media (for instance furans—furfural and hydroxymethylfurfural, phenolics and acetic acid—that arise from hemicellulose hydrolysis from agricultural residues can also limit fermentation (Taherzadeh et al. 1997a; Martinez et al. 2000).
In this work we compared the abilities of different S. cerevisiae strains adapted to industrial processes or to a controlled laboratory environment to tolerate various stress conditions. The stress conditions tested included high reactive oxygen species levels, heat shock, hyperosmosis, freezing, and high ionic strength, using a glucose rich-medium. Because of the wide interest in fuel ethanol production from lignocellulose hydrolysates, we also tested the capacity of these strains to convert glucose to ethanol in the presence of toxins present in diluted acid hemicellulose hydrolysates.
Materials and methods
Strains used and inocula development
The S. cerevisiae strains used in this work were classified in two groups, those obtained from an industrial source and those routinely used in scientific research laboratories. The so-called industrial strains were: Azteca, used in the bakery industry (isolated from compressed baker yeast, Levadura Azteca, Mexico D.F.); IND1101, used in local sugar-ethanol factories in Mexico (Azucarera Independencia, Veracruz, Mexico); LO24 and LO41, used in Tequila production processes (CIATEJ, Jalisco, Mexico; Pinal et al. 1997) and SuperStart (SS; Alltech, Santa Ma. La Rivera, Mexico D.F.) widely used in ethanol production (Kensall and Lyons 1999; Thomas et al. 2002). The laboratory strains group included RS58 (Garay-Arroyo and Covarrubias 1999), LR88 (Petitjean et al. 1990) and CEN.PK-113-7D (CEN.PK; van Dijken et al. 2000).
All industrial strains used in this work were obtained directly from the industries that use them. These yeast strains were not selected through designed improvement programs, rather they were selected over time from a complex inocula. Strains were maintained on solid YPD 2%, per liter: 10 g Difco yeast extract, 20 g Difco bacto-peptone, 20 g dextrose (Baker, Phillipsburg, N.J.) and 15 g agar. Glucose was autoclaved separately. Inocula were incubated for 24 h at 30°C and 250 rpm (C24C incubator-shaker, New Brunswick Scientific, New Brunswick, N.J.), in 500-ml conical flasks containing 200 ml medium (YPD 2%). Enough inoculum was spun down to obtain an initial OD660nm of 3.55 [roughly equivalent to 1 g dry cell weight (DCW)/l) in all cultures.
Culture conditions and media composition
Furfural and acetic acid were used at concentrations of 2 g/l and 10 g/l, respectively, according to the average concentration values obtained from these hydrolysates (Taherzadeh et al. 1997a, 2000; Larsson et al. 1999; Martinez et al. 2000). These inhibitory compounds were not added for the corresponding batch control experiments. To study the effects of acetic acid or furfural on growth and ethanol production by yeast cultures, we used a complex medium (YPD) containing glucose as carbon source. Cultures were carried out in Fleakers mini-fermentors (Beall et al. 1991) containing 200 ml YPD 2%, without aeration, at 30°C and 100 rpm. Before inoculation, the pH was adjusted to 5 with diluted HCl or NaOH, and was kept constant at pH 5 during cultivation by automatic addition of 2 N KOH. When ethanol production tests were performed at high glucose concentration, 250-ml conical flasks containing 100 ml YPD plus 18% dextrose at pH 5 were used, incubated at 30°C and 120 rpm. All experiments were carried out in duplicate.
Growth conditions for stress studies
Cells were grown as batch cultures in YPD 2%, containing 30 µg/ml adenine, to an OD660 of 0.5(±0.1). At this point, different stress conditions were applied. Oxidative stress was imposed by adding H2O2 to a final concentration of 10 mM. Heat shock was applied by incubating cultures at 50°C. Freezing shock treatments were imposed by freezing yeast cells at −20°C for 2 days and thawing at room temperature. Osmotic and ionic stress were measured by plating appropriate dilutions of cells on YPD plates containing 1.5 M sorbitol or 1.0 M NaCl, respectively. Samples were taken at different times to determine viability and to obtain total RNA. In all cases, viability was measured by plating the appropriate dilution of cells on YPD plates and expressed as a percentage of the initial colony forming units (cfu) measured before application of stress treatment. The number of cfu was determined using COVASIAM (Corkidi et al. 1998). All experiments were performed in duplicate and reproduced at least three times.
Isolation of genes, RNA purification and RNA blot hybridization analysis
Full-length gene sequences were obtained by PCR amplification using specific primers designed according to the known sequences. Primers used were (5′-TTG TGG CCG CAG CCT CCG GC-3′) and (5′-CCC ACC CCA GCA CGC CGG GG-3′) for SOD1, (5′-CCA CAC CTG GGT AGT CTG GC-3′) and (5′-GCT GGG TGA AGG CGG CTT AAG-3′) for HSP26, (5′-GAT AGC AGT ATC ACA CGC CC-3′) and (5′-CAG CTT TTC TTG TGA TGT GTT GC-3′) for GRE2, (5′-GGA TCC CTC GCT TTG GTA CC-3′) and (5′-CGC TTC GAA TGG ATC GCG C-3′) for ENA1, and (5′-TCA ATT GGA GCA TCT GCC TTT-3′) and (5′-AGA CAA ATT GGT GCC AAG AAC A-3´) for IPP1. PCR reactions using genomic DNA as template were as follows: 35 cycles of 30 s at 94°C, 30 s at 50–55°C and 45 s at 72°C. PCR products were used as probes for RNA blot hybridizations. GPD1, HSP104 and CTT1 probes were obtained from genomic clones kindly donated by R. Gaxiola, J. Nieto (both of Instituto de Biotecnología, Universidad Nacional Autónoma de México), and A. Gonzalez (Instituto de Fisiología Celular, Universidad Nacional Autónoma de México), respectively.
Total RNA was extracted as indicated by Collart and Oliviero (1995). RNA concentration was determined spectrophotometrically. For RNA blot hybridization, 10 µg total RNA was separated by electrophoresis in formaldehyde 1.2% (w/v) agarose gel and blotted to a Hybond N+ membrane (Amersham, Piscataway, N.J.) following standard protocols. Probes were labeled with a commercial random kit (Dupont, Boston, Mass.) using [α32P] (3,000 Ci/mmol; Amersham). High stringency conditions were used for all hybridizations and washes. The IPP (inorganic pyrophosphatase) gene was used as a loading control because its transcript does not fluctuate under different stress conditions (Rep et al. 2001). Transcript levels were quantified using NIH image 1.62 software (http://rsb.info.nih.gov/).
Analytical methods
Growth was determined spectrophotometrically as optical density (OD) at 660 nm (Lambda 11, Perkin Elmer, Bodenseewerk, Germany) and converted to DCW per liter using a calibration curve (1 OD =0.282 g DCW/l). Samples were centrifuged and cell-free culture broth was frozen until analysis. Acetic acid, furfural, and glucose concentrations were determined by HPLC analysis. The separation system consisted of an Aminex HPX-87H ion exclusion column (300×7.8 mm; Bio-Rad, Hercules, Calif.), using 5.0 mM H2SO4 solution as mobile phase (0.5 ml/min) at 60°C. Glucose was detected with a 2410 differential refractive index detector; acetic acid and furfural with a 996 photodiode array detector at 210 nm (Waters, Millipore, Milford, Mass.). Ethanol was analyzed by gas chromatograph using n-butanol as internal standard (6850 Series GC System, Agilent, Wilmington, Del.).
Results
We compared the growth and ethanol production performance of laboratory and industrial S. cerevisiae strains in the presence of furfural (2 g/l) and acetic acid (10 g/l) in rich medium with glucose (20 g/l). At different elapsed culture times, the yield of glucose conversion to ethanol was above 80% of the theoretical value for the eight strains used under all conditions tested, i.e., with or without inhibitors (data not shown). However, cell mass formation, growth rate and ethanol production rates were severely affected (Fig. 1). The use of complex media in this kind of experiment allows us to discriminate the effects of acetic acid and furfural on cell mass formation, growth rate and ethanol production. This strategy avoids the problem of changes in physiological properties caused by genetic differences between strains that carry auxotrophic markers, which require the addition of amino acids, purine or pyrimidine bases to the medium.
Effect of furfural and acetic acid on kinetic parameters
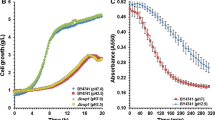
In the absence of inhibitors, maximum cell mass accumulation levels were different between the strains tested (Fig. 1a). CEN.PK showed the highest DCW (5.2 g/l) whereas the lowest levels of cell mass accumulation were obtained for strains RS58 and IND1101 (2.8±0.1 g DCW/l). The remaining strains, LR88, Azteca, LO24, SS and LO41, reached a DCW average of 4.2 g/l. Furfural addition led to different levels of cell mass formation in the strains tested when compared to growth conditions in the absence of this compound. Whereas for RS58 and LO41, cell mass formation was not affected, LR88, IND1101, Azteca and LO24 showed a 15–20% decrease; a larger reduction in cell mass accumulation was obtained for CEN.PK (30%) and SS (60%). In the case of acetic acid addition, cell mass formation decreased in all strains, ranging from 25 to 50%, CEN.PK and IND1101 being the less affected strains (Fig. 1a). In absolute values, the highest biomass accumulation levels in the presence of inhibitors were obtained for LO41 when furfural was added to the growth medium, and for CEN.PK grown in medium containing acetic acid.
In the absence of furfural or acetic acid the highest specific growth rate obtained was for SS (0.34 h−1); LR88, CEN.PK, LO41, IND1101, Azteca and LO24 showed intermediate values (0.25±0.03 h−1), whereas RS58 exhibited the lowest specific growth rate (0.16±0.02 h−1) (Fig. 1b). Growth rate was not affected by furfural addition in RS58 and LO41; however, for LR88, IND1101, Azteca and LO24 a decrease of 15–25% was observed. CEN.PK and SS strains showed a more dramatic reduction in growth rate: 45 and 76%, respectively. Acetic acid also led to a large decrease in this growth parameter: for LR88, RS58, Azteca, LO41, IND1101 and SS, growth rate was reduced in a range from 42 up to 88%, while for CEN.PK and LO24 strains decrements of 11 and 25% were obtained, respectively. Overall, in absolute values, in the absence of inhibitors the highest specific growth rate was obtained for SS. When furfural was added to the medium, LR88, Azteca, LO24 and LO41 showed the highest growth rates, whereas in the presence of acetic acid, CEN.PK and LO24 had the best performance.
Under control conditions, without inhibitors, most strains (LR88, RS58, CEN.PK, Azteca, LO24 and LO41) exhibited similar specific ethanol production rates, in the range 0.45–0.6 g ethanol g DCW−1 h−1 (Fig. 1c). IND1101 and SS showed the highest ethanol production rate under these conditions, at 0.83 and 0.85 g ethanol g DCW−1 h−1, respectively. For all strains tested, furfural addition reduced this rate (up to 20%). In contrast, the effect of acetic acid addition on ethanol production rate varied in the different strains. A significant increase was detected in RS58, LR88, CEN.PK, IND1101 and LO24 (15–45%), while a large decrease (>55%) in this parameter was observed for Azteca and LO41 cultures. In the case of strain SS, acetic acid addition did not lead to significant changes in specific ethanol production rate. In both growth conditions, the highest ethanol production rate (above 0.8 g ethanol g DCW−1 h−1) was obtained by SS and IND1101 strains without inhibitors, IND110 in the presence of furfural, and LR88, SS and LO24 in the presence of acetic acid. Figure 1d shows the linear relationship between the specific rates of ethanol production and glucose consumption for all conditions tested.
Neither ethanol nor acetic acid consumption was detected under the conditions and times assessed (data not shown). Furthermore, HPLC data shows that acetic acid was not produced by any of the yeast strains evaluated. Furfural was metabolized by all strains during the exponential growth phase, and remained constant for 3 days in control medium (without inoculum), incubated under the same experimental conditions as those used for yeast evaluation (data not shown). Hence, the observed differences in yeast behavior can be ascribed to acetate tolerance and, for furfural, to the combined effect of tolerance and metabolism.
Growth and ethanol production in high sugar concentrations
Excluding CEN.PK, results in medium with high sugar concentrations showed that laboratory strains tested are not efficient ethanol producers (Fig. 2a). This is because although cell mass formation is similar for industrial and laboratory strains (at 12 h of culture elapsed time; Fig. 2b), volumetric productivity is higher for those strains commonly used in ethanol-production-related processes (Fig. 2a). A comparison of specific ethanol production rates showed that RS58, SS and IND1101 performance is the most affected when cultures are grown in high sugar concentration medium (see Fig. 2c).
Stress tolerance of different yeast strains
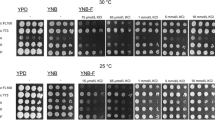
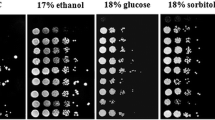
As shown in Fig. 3, the major differences between strains were detected following oxidative- and heat-shocks, where it was evident that industrial strains (LO24, Azteca, IND1101, LO41, and SS) showed a higher tolerance to these stress treatments than those adapted to controlled laboratory environments (CEN.PK, LR88 and RS58). Contrasting differences were also detected in the tolerance level following ionic stress, where most industrial strains tested (LO24, Azteca and LO41) showed a higher survival under this stress condition than laboratory strains, except for RS58, a laboratory strain that showed the highest tolerance. Additional experiments indicated that this strain is particularly tolerant to ionic stress, compared to any other wild type strain, since it is able to tolerate up to 700 mM LiCl (data not shown). IND1101 and SS presented survival levels similar to those detected for CEN.PK and LR88 (Fig. 4a). Freeze-thaw and osmotic treatments did not reveal any particular difference between these two groups of strains. A high tolerance to hyperosmosis of two of the industrial strains (IND1101 and SS) was noted.
Stress tolerance and transcript accumulation of eight yeast strains in response to H2O2, high temperature and freezing treatments. a Stress tolerance expressed as percentage of survival upon adverse treatments as indicated. b Northern blot experiments showing transcript accumulation levels of different genes in response to stress treatments. c Quantification of the accumulated transcript levels. Transcript levels were normalized relative to the intensity of inorganic pyrophosphatase (IPP) and control condition transcripts. The highest relative mRNA level of any transcript in a given condition was set to 100
Stress tolerance and transcript accumulation of eight yeast strains in response to osmotic and ionic treatments. a Stress tolerance. b Northern blot experiments. c Quantification of the accumulated transcript levels. See Fig. 3 for details
RNA accumulation patterns of different stress genes
To find out whether there is any correlation between RNA accumulation patterns of stress genes and tolerance levels of the different strains examined, we carried out Northern blot experiments using as probes DNA fragments from genes whose response to different stress conditions have been well characterized. The genes used were ENA1 (proton pump ATPase), a gene responsive to ionic stress (Serrano 1996); GPD1 and GRE2, which are typically induced by osmotic stress (Hohmann 2002); CTT1 and SOD1, which respond to oxidative stress (Lee et al. 1999); and HSP26 and HSP104, whose transcripts accumulate in response to heat shock (Amoros and Estruch 2001). Since oxidative stress seems to be common to different stress conditions, CTT1 and SOD1 RNA accumulation patterns were obtained not only from H2O2 treatments but also from some other treatments such as hostile hyperosmotic and ionic conditions. Results in Figs. 3 and 4 show that all transcripts selected are responsive to the stress treatments applied in the different strains tested. In particular, upon oxidative shock, two of the industrial strains, LO41 and SS, accumulated the highest SOD1 transcript levels. RS58, one of the laboratory strains that showed the lowest survival under this stress condition, accumulated high SOD1 and CTT1 transcript levels. A similar phenomenon occurs in the case of LR88, which is unable to survive oxidant treatment. Data in Fig. 3 showed that even though industrial strains are more thermotolerant than laboratory strains, there is no correlation between this characteristic and high HSP104 transcript levels as a consequence of heat shock. The highest levels were detected in LR88, LO41 and IND1101 (Fig. 3b, c). Upon hyperosmosis, three of the industrial strains (LO24, Azteca and IND1101) showed higher transcript levels for the stress genes analyzed when compared to those accumulated in laboratory strains (Fig. 4b, c). Even though ENA1 is known to play a key role in the adaptive response under high ionic strength, NaCl treatment did not led to higher ENA1 transcript accumulation in RS58—the strain that showed the highest percentage of cell survival—when compared to strains with a lower survival index. In contrast, with the exception of LR88, those strains that did not survive this stress condition (IND1101, SS and CEN.PK) showed the highest ENA1 transcript accumulation. IND1101 also exhibited the highest accumulation of CTT1 and GRE2 transcripts (Fig. 4).
Discussion
Furfural and acetic acid reduce biomass yield and growth rate in yeast strains, but specific ethanol production rate is not always boosted in the presence of acetic acid
Acetic acid (10 g/l) severely reduced cell mass formation and specific growth rates in the eight strains tested, with the exception of CEN.PK and LO24. Several studies have demonstrated that volumetric and specific ethanol production rates are boosted in the presence of acetic acid, at a pH that allows growth (Taherzadeh et al. 1997b; Palmqvist et al. 1999), but others have shown the opposite effect (Narendranath et al. 2001). However, for the strains tested in this work both behaviors were observed. All laboratory and LO24 strains showed an increase in specific ethanol production rate, whereas no significant change was obtained for the Alltech strain (SS) and a decrease was detected for three of the industrial strains analyzed (Azteca, LO41 and IND1101). The increase in specific ethanol production rate in the presence of acetic acid for some strains can be explained by the rise in ATP production needed to keep the intracellular pH constant. The requirement for higher ATP levels will increase glycolytic flux, thereby enhancing the rate of ethanol formation (Taherzadeh et al. 1997b; Palmqvist et al. 1999). Therefore, it is likely that the ATPase activity differs considerably in the strains tested, which is in agreement with the physiological variability detected among them.
Furfural caused a small negative effect on cell mass formation and growth rate, with the exception of the two reference strains (CEN.PK and SS). Nonetheless, in all strains, furfural had only a small effect on the specific rates of glucose consumption and ethanol production. These data suggest that furfural addition does not affect glycolysis performance. Overall, the analysis of the physiological data in this work strongly indicates that, for all conditions tested (control, furfural and acetic acid), ethanol production rate is controlled by the rate of glucose consumption.
Interestingly, the analysis of ethanol production when yeast cells were cultivated with high glucose concentration (18%) allowed us to distinguish laboratory strains from industrial strains. Higher ethanol volumetric production rates were obtained with the industrial strains, in accordance with the selective pressure to which they have been exposed during process selection. However, when specific ethanol production rate was analyzed with respect to glucose concentration (2% and 18%), it is evident that some strains (RS58, IND1101 and SS) that are highly efficient when they are grown in 2% glucose, do not show the same specific ethanol production rate in high glucose (18%). In the case of IND1101 and SS (two industrial strains), this negative effect on ethanol production by high sugar concentrations seems to be compensated by their growth performance under these conditions, which justifies their use in some ethanol production factories. With the exception of RS58, the other two laboratory strains (CEN.PK and LR88) showed acceptable specific ethanol production rates at both glucose concentrations tested.
Industrial yeast strains show higher tolerance to oxidative and heat stress conditions
Given the changing environment to which industrial strains are exposed, it may be hypothesized that they can tolerate different stress conditions. Results in this work indicate that the group of industrial strains showed the highest survival rates following oxidative- and heat-shock. This was not so evident when hyperosmosis was imposed. Under the latter condition, two of the industrial strains (IND1101 and SS) showed the best performance whereas the remaining strains presented only 30–35% survival. Ionic stress treatment severely affected strain survival with the exception of RS58, which, in contrast to the other laboratory strains, was highly tolerant to this adverse condition. Chilling stress led to a low survival in most strains (35%) with SS and RS58 the most affected.
Northern analyses showed that transcripts of all selected stress genes accumulate in response to adverse situations, although the accumulation levels are not the same in every case, thus indicating that strains used have the capacity to detect and respond to the applied stimulus. Even though some of the genes analyzed have been showed to be relevant for stress adaptation, we were unable to find a correlation between transcript accumulation levels and stress tolerance. In some cases, such as the CTT1 and ENA1, transcript analysis in response to oxidative and ionic stress, respectively, an inverse correlation was detected. Some of the strains that showed the lowest cell survival present the highest mRNA accumulation levels, which suggests that the stress levels imposed in this study could be more severe than those commonly encountered by these microorganisms. Therefore, even if the synthesis of the protective proteins encoded by these genes is induced (as shown in the RNA blot experiments), their level might not be sufficient to offer protection against the stress. Alternatively, these strains might lack additional adaptive mechanisms needed for survival under these conditions. In contrast, some other strains did not show this gene induction response, even though they were able to resist the adverse environment imposed, which supports the multifactorial nature of the adaptive response to stressful milieu.
The wide variability of responses to the different environmental stress conditions tested shows that no general rules can be assumed for different S. cerevisiae strains, and that these responses are highly dependent on their genetic and environmental background. In a recent work, using a cocktail of different lignocellulose derived inhibitors, it was also concluded that tolerance of S. cerevisiae to specific inhibitors is strain-dependent (Martín and Jönsson 2003). The results of our work confirm the versatility of CEN.PK as a research platform for growth and physiological studies in ethanol production (van Dijken et al. 2000; Martín and Jönsson 2003).
References
Amoros M, Estruch F (2001) Hsf1p and Msn2/4p cooperate in the expression of Saccharomyces cerevisiae HSP26 and HSP104 in a gene and stress type-dependent manner. Mol Microbiol 39:1523–1532
Beall DS, Ohta K, Ingram LO (1991) Parametric studies of ethanol production from xylose and other sugars by recombinant Escherichia coli. Biotechnol Bioeng 38:296–303
Collart M, Oliviero S (1995) In: Ausubel FM et al (eds) Current protocols in molecular biology. Wiley, New York, pp 13.12.1–13.12.4
Corkidi G, Diaz-Uribe R, Folch-Mallol JL, Nieto-Sotelo J (1998) COVASIM: an image analysis method that allows detection of confluent sizes for automated counting. Appl Environ Microbiol 64:1400–1404
Dijken JP van et al (2000) An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb Technol 26:706–714
Garay-Arroyo A, Covarrubias AA (1999) Three genes whose expression is induced by stress in Saccharomyces cerevisiae. Yeast 15:879–892
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeast. Microbiol Mol Biol Rev 66:300–372
Kensall DR, Lyons TP (1999) Management of fermentations in the production of alcohol: moving toward 23% ethanol. In: Jacques KA, Lyons TP, Kelsall DR (eds) The alcohol textbook: a reference for the beverage, fuel and industrial alcohol industries, 3rd edn. Nottingham University Press, Nottingham, UK, pp 26–38
Larsson S, Palmqvist E, Hahn-Hägerdal B, Tengborg Ch, Stenberg K, Zacchi G, Nilvebrant N-O (1999) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Technol 24:151–159
Lee J, Godon C, Lagniel G, Spector D, Garin J, Labarre J, Toledano MB (1999) Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J Biol Chem 274:16040–16046
Martín C, Jönsson LJ (2003) Comparison of the resistance of industrial and laboratory strains of Saccharomyces and Zygosaccharomyces to lignocellulose-derived fermentation inhibitors. Enzyme Microb Technol 32:386–395
Martinez A, Rodríguez ME, York SW, Preston JF, Ingram LO (2000) Effects of Ca(OH)2 treatments ("overliming") on the composition and toxicity of bagasse hemicellulose hydrolysate. Biotechnol Bioeng 69:526–536
Narendranath NV, Thomas KC, Ingledew WM (2001) Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J Ind Microbiol 26:171–177
Palmqvist E, Grade H, Meinander NQ, Hahn-Hägerdal B (1999) Main and interaction effects of acetic acid, furfural, and p-hydroxybenzoic acid on growth and ethanol productivity of yeast. Biotechnol Bioeng 63:46–55
Petitjean A, Hilger F, Tatchell K (1990) Comparison of thermosensitive alleles of the CDC25 gene involved in the cAMP metabolism of Saccharomyces cerevisiae. Genetics 124:797–806
Pinal L, Cedeño M, Gutiérrez H, Alvarez-Jacobs J (1997) Fermentation parameters influencing higher alcohol production in the Tequila process. Biotechnol Lett 19:45–47
Rep M, Proft M, Remize F, Tamás M, Serrano R, Thevelein J M, Hohmann S (2001) The Saccharomyces cerevisiae Sko1p transcription factor mediates HOG pathway-dependent osmotic regulation of a set of genes encoding enzymes implicated in protection from oxidative damage. Mol Microbiol 40:1067–1083
Serrano R (1996) Salt tolerance in plants and microorganisms: toxicity, targets and defense responses Int Rev Cytol 165:1–52
Taherzadeh MJ, Eklund R, Gustafsson L, Niklasson C, Lidén G (1997a) Characterization and fermentation of dilute-acid hydrolyzates from wood. Ind Eng Chem Res 36:4659–4665
Taherzadeh MJ, Niklasson C, Lidén G (1997b) Acetic acid—friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae? Chem Eng Sci 52:2653–2659
Taherzadeh MJ, Niklasson C, Lidén G (2000) On-line control of fed-batch fermentation of dilute-acid hydrolyzates. Biotechnol Bioeng 69:330–338
Thomas KC, Hynes SH, Ingledew WM (2002) Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic and lactic acids. Appl Environ Microbiol 68:1616–1623
Acknowledgements
Strains were provided by Dr. Peter Köter (CEN.PK), Dr. José A. Peña (Azteca), Ignacio Lazcano (IND1101), Maria de la Luz Nuñez (LO24 and LO41), and Dra. Gladys Hoyos from Alltech Mexico (SuperStart). Technical support for HPLC analysis from G. Hernández is gratefully acknowledged. We also thank E. López and P. Gaytan for oligonucleotide synthesis. This work was supported by grant Z-003 from the Consejo Nacional de Ciencia y Tecnología-México (CONACyT). I. Niño held a scholarship from CONACyT-México.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garay-Arroyo, A., Covarrubias, A.A., Clark, I. et al. Response to different environmental stress conditions of industrial and laboratory Saccharomyces cerevisiae strains. Appl Microbiol Biotechnol 63, 734–741 (2004). https://doi.org/10.1007/s00253-003-1414-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1414-4